Abstract
Dendritic cells (DCs) are professional antigen-presenting cells specifically targeted during Plasmodium infection. Upon infection, DCs show impaired antigen presentation and T-cell activation abilities. In this study, we aimed to evaluate whether cellular extracts obtained from Plasmodium berghei-infected erythrocytes (PbX) modulate DCs phenotypically and functionally and the potential therapeutic usage of PbX-modulated DCs in the control of experimental autoimmune encephalomyelitis (EAE, the mouse model for human multiple sclerosis). We found that PbX-treated DCs have impaired maturation and stimulated the generation of regulatory T cells when cultured with naive T lymphocytes in vitro. When adoptively transferred to C57BL/6 mice the EAE severity was reduced. Disease amelioration correlated with a diminished infiltration of cytokine-producing T cells in the central nervous system as well as the suppression of encephalitogenic T cells. Our study shows that extracts obtained from P. berghei-infected erythrocytes modulate DCs towards an immunosuppressive phenotype. In addition, the adoptive transfer of PbX-modulated DCs was able to ameliorate EAE development through the suppression of specific cellular immune responses towards neuro-antigens. To our knowledge, this is the first study to present evidence that DCs treated with P. berghei extracts are able to control autoimmune neuroinflammation.
Keywords: dendritic cells, experimental autoimmune encephalomyelitis, neuroinflammation, Plasmodium extracts
Introduction
Dendritic cells (DCs) are professional antigen-presenting cells that activate naive T cells and help to dictate the course of the adaptive immune response through the stimulation of T helper type 1, type 2, type 17 cells or regulatory T (Treg) cells.1–4 Because of this potential, DCs are frequent targets during infection. For instance, it was shown that DCs harbour bacteria and parasites in Salmonella and Plasmodium infections, respectively.5–8 The impairment of DC function also dampens the immune system and slows down or even abrogates the elimination of the pathogen. This can be observed in DCs from malaria patients, which are modulated towards an anti-inflammatory phenotype: lower expression of co-stimulatory molecules, secretion of interleukin-10 (IL-10) and stimulation of Treg cells.9,10 The mechanisms by which some strains of Plasmodium suppress DCs are not yet fully understood. However, it was previously demonstrated that hemozoin, the Plasmodium pigment, is able to arrest human and mouse DC maturation.10–12
The modulation of DCs has proven to be a promising field for the treatment of inflammatory diseases. For instance, it was previously demonstrated that DCs treated with synthetic drugs or pathogen-derived extracts acquire immature phenotype and upon adoptive transfer suppress the severity of collagen-induced arthritis, mouse kidney grafts and experimental autoimmune encephalomyelitis (EAE).13–19–22 The mechanisms of DC-induced suppression are mostly dependent on the generation of Treg cells in vivo.17 EAE is a T-cell-dependent neuroinflammation that, in many aspects, mimics human multiple sclerosis. CD4+ T cells play a pivotal role in disease development with the stimulation of accessory cells and production of inflammatory mediators, which leads to inflammation and neuronal damage.23,24 Hence, suppression of the cellular response is desirable for the control of EAE.
The interplay between DCs and Plasmodium parasites must be better characterized and, when possible, directed towards new approaches in inflammatory conditions. In this study, we aimed to evaluate whether extracts from Plasmodium berghei, the causative agent of malaria, modulate DCs towards a regulatory phenotype, aimed at its use for the control of inflammatory conditions. We found that DCs treated with P. berghei extracts stimulated the generation of Treg cells in vitro. Upon adoptive transfer, modulated DCs reduced the severity of EAE through the reduction of the inflammation in the central nervous system (CNS) and suppressed the proliferation of autoreactive T lymphocytes.
Materials and methods
Mice
Female C57BL/6 mice, 6–8 weeks old, from the Multidisciplinary Centre for Biological Research, University of Campinas, were used in this study. Mice were kept in specific-pathogen-free conditions, in an environement with controlled temperature and photoperiod, with free access to autoclaved food and water throughout the experiment. All protocols involving laboratory animals were approved and performed in accordance with the guidelines of the State University of Campinas′ Committee on the Use and Care of Animals (Comissão de Ética no Uso de Animais – CEUA, # 2687-1).
Plasmodium berghei NK65 infection, enrichment of infected erythrocytes and P. berghei extract preparation
Malaria-infected red blood cells (iRBCs; 1 × 106) were obtained from a source mouse and injected intraperitoneally into naive C57BL/6 mice. On the 14th day of infection, mice were killed and blood samples were collected in heparinized tubes. Mice with parasitaemia > 30% were used for the enrichment of iRBCs, according to a previously described method.25 Briefly, blood samples were centrifuged over a Percol 65% gradient (300 g, 20 min, 4° with no brakes) and the cells from the interface were collected and washed with PBS 0·02 m, pH 7·2. This suspension is mainly iRBCs (95%) as confirmed by Giemsa staining in blood smears. For the preparation of P. berghei extracts (PbX), the iRBC-enriched suspension was submitted to 20 cycles of freeze–thawing in liquid nitrogen and a warm bath (37°), as previously described.26 As controls, normal RBC underwent the same freeze–thaw process. The protein concentration was determined using the Bradford Protein Assay following the manufacturer′s instructions (Sigma-Aldrich, St Louis, MO).
Generation of DCs, in vitro modulation and adoptive transfer
Bone-marrow-derived precursors were used in the generation of DCs, according to a previous report.27 Briefly, femurs were collected and the bone marrow cells were flushed out with RPMI-1640 medium supplemented with 2-mercaptoethanol (2 mm), fetal bovine serum (10% volume/volume) and gentamycin (50 μg/ml) – referred to as complete medium. Cells (5 × 106) were seeded in 24-well culture plates containing complete medium supplemented with granulocyte–macrophage colony-stimulating factor (10 µg/ml). Fresh medium was added at days 3 and 6 of culture. This culture method results in DC generation of 85–95% purity, assessed by flow cytometry. The DCs were used in transfer or co-culture experiments. For DC modulation, the cells were treated with PbX (100 μg/ml) for 18 hr in the presence of an activating factor – lipopolysaccharide from Escherichia coli O111:B4 (1 µg/ml; Sigma-Aldrich) and pulsed overnight with 10 μg/ml of myelin oligodendrocyte glycoprotein peptide (MOG35–55; Genemed Synthesis Inc., San Antonio, TX) or ovalbumin (50 μg/ml, OVA; Sigma-Aldrich). The RBC extracts were used as a control reagent; 1·5 × 106 cells were adoptively transferred intravenously by the retro-orbital route 3 days before (prophylactic) and 14 days after (therapeutic approach) EAE induction.
Antigen presentation assays and cytokine dosages
The following assay was performed as previously described.28 Briefly, 5 × 105/well DCs treated as above were seeded in U-bottom 96-well plates. Splenocytes were enriched in lymphocytes by centrifugation in Percoll gradient, following a previously published protocol.29 Total T lymphocytes were isolated using Dynabeads following manufacturer′s instructions (Mouse Pan T-cell isolation kit; Life Technologies, Austin, TX). Responder T cells from EAE-inflicted mice were stained with carboxyfluorescein succinimidyl ester (CFSE, 2·5 μm; Sigma-Aldrich) following the manufacturer′s instructions. T cells were seeded together with DCs at a ratio of 1 : 1 (DC : T) in complete RPMI-1640 medium containing MOG35–55 (10 μg/ml; Genemed Syn). As controls, T cells were grown in the absence of DCs. The plates were incubated at 37° for 96 hr. The proliferation in these sets of experiments was measured by the decay of the dye in a flow cytometer. Culture supernatants were collected and assayed for cytokines [IL-10, IL-17, interferon (IFN-γ) and tumour necrosis factor-α] secretion using the Cytometric Bead Array (CBA; BD Biosciences, Franklin Lakes, NJ) according to the manufacturer′s instructions.
EAE induction and evaluation
EAE was induced and evaluated in mice according to a previous published method.30 Briefly, each mouse was injected with 200 μg MOG35–55 (Genemed Syn) emulsified with complete Freund′s adjuvant (Sigma-Aldrich). Pertussis toxin (300 µg; Sigma-Aldrich) was administered intraperitoneally at 0 and 48 hr after antigen challenge. Clinical signs were followed and graded daily according to the following score method: 0, no sign; 1, flaccid tail; 2, hind limbs weakness; 3, hind limbs paralysis; 4, hind paralysis and fore-limb weakness; 5, full paralysis/dead. At the indicated time-points after antigen challenge mice were killed and their spinal cords were removed, dehydrated in serial alcohol and xylene solutions and paraffin embedded; 10-μm thin slices were made and stained with haematoxylin & eosin.
Lymphoproliferative response and cytokine dosage
Splenic cells from EAE mice were aseptically collected after 10 days of antigen administration. Cells (5 × 105/well) were diluted in RPMI-1640 medium supplemented with fetal calf serum (10% volume/volume), guaramicine (50 μg/ml) and 2-mercaptoethanol (2 mm). Media were supplemented with MOG35–55 (10 μg/ml), myelin basic protein (50 μg/ml) or OVA (50 μg/ml). The cells were plated in flat-bottom plates and incubated at 37° in 5% CO2 for 96 hr. As controls, cells were grown in the absence of antigen. After the incubation period, the cells were treated with 10 μl of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, 5 mg/ml; Sigma Aldrich) for 4 hr and the formazan crystals were dissolved with 100 μl isopropanol. Absorbances were read at 540 nm by spectrophotometer (VersaMax; Molecular Devices, Sunnyvale, CA).
Analysis of cellular infiltration in the central nervous system
Fourteen days after EAE induction, mice were anaesthetized, perfused with ice-cold PBS and their CNS tissue was prepared for the enrichment of infiltrating leucocytes according to a previously described methodology and analysed by flow cytometry.31
Flow cytometry
Fluorochrome-conjugated monoclonal antibodies were used to stain leucocytes. Cells were surface stained with anti-CD4/phycoerythrin (PE)-Cy5 (clone GK1.5), anti-CD8/allophycocyanin (APC) (clone 53-6.7), anti-CD3/APC-Cy7 (clone 145-2C11), CD11c/APC (clone N418), MHC-II/PE-Cy7 (clone M5/114.15.2), CD80/FITC (clone 16-10A1) and CD86/PE (clone GL-1). For intracellular staining, cells were cultured for 4 hr in the presence of PMA (50 µg/ml; Sigma) and ionomycin (100 µg/ml). Brefeldin A (10 μg/ml) was added as well. Later, the cells were fixed/permeabilized (fixation/permeabilization buffers) according to the manufacturer′s recommendations, and monoclonal anti-Foxp3/APC (clone FJK-16s), IL-10/PE (clone JES5-16E3), IFN-γ/PE (clone XMG1.2) and IL-17/APC (clone eBIO17B7) were added to cells. Isotype controls were also used. All antibodies were purchased from BD Biosciences. Preparations (50 000 events/sample, unless otherwise stated) were acquired with a Gallios flow cytometer (Beckman Coulter, Brea, CA) and data were analysed using flowjo 7.6 (Tree Star Inc., Ashland, OR). The analysis of cytokine-producing cells was performed using the Fluorescence Minus One methodology to help to identify gating boundaries, in which cells are stained with all surface antibodies but those for intracellular staining.32
Statistical analysis
Clinical score comparisons between control and experimental groups were performed by two-way analysis of variance (anova) and post-tested with Bonferroni. Other analyses among two and three (or more) groups were carried out with Student′s t-test and one-way anova, respectively. The statistical analyses were performed in graphpad prism software (v.5; GraphPad Software, Inc., La Jolla, CA). Results are expressed as mean ± standard error mean (SEM) and P < 0·05 value were defined as significant.
Results
Dendritic cells acquire an immature phenotype after contact with P. berghei extracts, stimulating the generation of regulatory T cells and changing the profile of T-cell-derived cytokines in vitro.
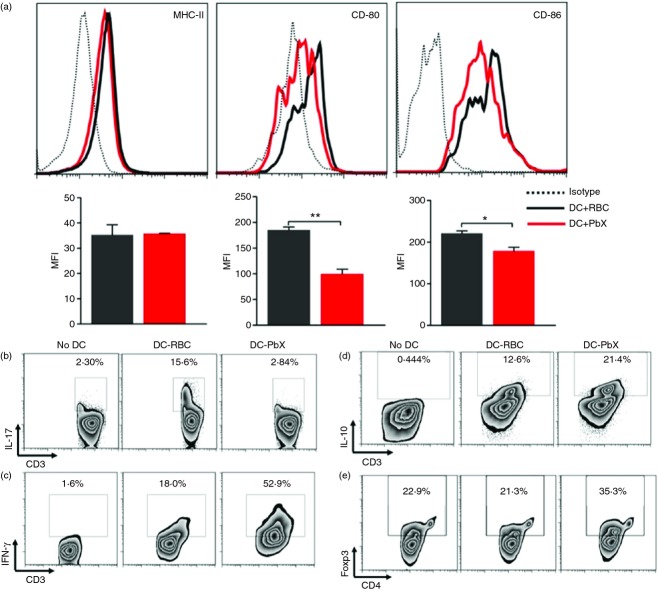
It has been previously shown that splenic DCs from Plasmodium-infected patients and mice present impaired antigen presentation.9,26 In addition, treatment of DCs with Plasmodium falciparum extracts drives DCs towards a permissive state.9 In this context, we aimed to evaluate whether the contact of DCs with crude extracts from P. berghei-infected erythrocytes (PbX) would promote changes in the maturation phenotype of DCs. Our results showed that after stimulation with lipopolysaccharide, DCs treated with PbX presented a significant reduction in the expression of CD86 and CD80, but not in MHC-II, when compared with cells treated with non-infected RBC extracts (Fig. 1a).
Figure 1.
Dendritic cells (DCs) treated with Plasmodium berghei extracts (PbX) acquire an immature phenotype and stimulate the generation of regulatory T cells. Bone marrow-derived DCs were stimulated with lipopolysaccharide (LPS; 1 µg/ml) for 18 hr in the presence of PbX (100 μg/ml). As control, cells were stimulated in the presence of non-infected red blood cell extracts (RBC, 100 μg/ml). (a) The cells were collected and stained with fluorochrome-conjugated antibodies for MHC-II, CD80 and CD86. The mean fluorescence intensity (MFI) was calculated from 500 000 acquired events. Dendritic cells were treated with PbX (100 μg/ml) or non-infected RBC extracts (100 μg/ml), stimulated with LPS (1 µg/ml) and pulsed with myelin oligodendrocyte glycoprotein peptide (MOG35–55) peptide (10 μg/ml) for 18 hr. Then cells were seeded in 96-well U-bottom plates (5 × 105 cells/well). T lymphocytes were isolated from spleens of naive mice using Dynabeads and seeded with DCs (1 DC : 1 T). The cells were allowed to grow for 96 hr in the presence or absence of MOG peptide (10 μg/ml). As controls, T cells were cultivated without DCs. At the end of the culture period, the supernatant was collected and the cells were treated with PMA and Ionomycin together with Brefeldin-A for 4 hr. (b–d) Intracellular interleukin-17 (IL-17), interferon-γ (IFN-γ) and IL-10 in CD3+ cells was determined, respectively. (e) The generation of Foxp3+ regulatory T cells was also determined. Data from three separate experiments with similar results. *P < 0·05 and **P < 0·01.
Our next goal was to investigate whether the PbX-treated DCs (DC-PbX) were modulated towards a suppressive phenotype. For that purpose, T cells were isolated from naive mice and co-cultured with MOG-pulsed DC-PbX or MOG-pulsed non-infected RBC-treated DCs (DC-RBC) as control. The data obtained show that DC-RBC, but not DC-PbX, stimulated the generation of IL-17-producing T cells (Fig. 1b). Also, DC-RBC were able to induce the production of IFN-γ in naive T cells, but not as efficiently as DC-PbX (Fig. 2c). DC-PbX were able to stimulate T cells to produce IL-10 (Fig. 2d). Interestingly, further analysis showed that DC-PbX induced IFN-γ/IL-10 double-producing T cells (data not shown). As the differentiation of naive T cells into regulatory T cells is a desirable ability in the immunosuppressive phenotype of DCs, we then investigated whether DC-PbX were able to induce Treg cells. Co-cultures conducted in the presence of DC-PbX had higher frequencies of Foxp3+ Treg cells than cultures conducted in the presence of DC-RBC (Fig. 2e).
Figure 2.
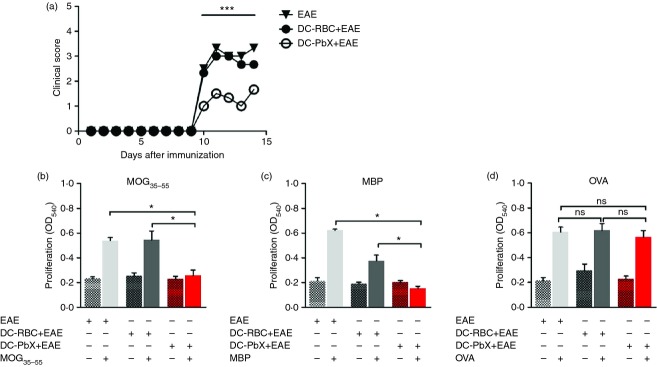
Reduced experimental autoimmune encephalomyelitis (EAE) severity in mice that received Plasmodium berghei extract (PbX) -modulated dendritic cells (DCs) correlates with decreased cellular response towards neuro-antigens. DCs were stimulated with lipopolysaccharide (LPS; 1 µg/ml) then treated with PbX (100 μg/ml) and pulsed with myelin oligodendrocyte glycoprotein peptide (MOG35–55) peptide (10 μg/ml) for 18 hr and adoptively transferred (1·5 × 106 cells/mouse) into C57BL/6 mice (n = 6/group) before the induction of EAE. (a) The clinical score of DC-transferred and untreated EAE-effected mice was monitored daily; ***P < 0·001. The spleens of EAE and DC-transferred EAE [both DC-red blood cells (DC-RBC and DC-PbX] mice were collected and total leucocytes (5 × 105/well) were cultured in the presence of MOG35–55 peptide (b), Myelin basic protein (c) and ovalbumin (d) (10, 50 and 50 μg/ml, respectively) for 96 hr. The proliferation was analysed using the MTT method; *P < 0·05. Representative data of three independent experiments.
Transfer of DCs treated with P. berghei extracts (DC-PbX) reduces EAE through the suppression of antigen-specific cellular responses
To evaluate whether DCs treated with P. berghei extracts show a therapeutic potential, we transferred DC-PbX to naive C57BL/6 mice 3 days before the immunization with MOG35–55 peptide as a prophylactic approach. As shown in Fig. 2, mice receiving DC-RBC (controls) showed an aggravated disease course whereas the transfer of DC-PbX significantly reduced the clinical signs of EAE (Fig. 2a). It is well known that the MOG-induced EAE is dependent on the cellular immune response towards the CNS tissue.33 In this context, we compared the reactivity of splenic leucocytes from EAE-inflicted mice towards MOG35–55 peptide and whole myelin basic protein with the reactivity of splenic leucocytes from mice adoptively transferred with DC-PbX or DC-RBC. Our data showed that splenocytes from EAE mice that received DC-PbX proliferated significantly less than cells derived from DC-RBC or untreated EAE-affected mice when cultured in the presence of neuro-antigens (Fig. 2b,c). Interestingly, splenocytes from MOG35–55-immunized mice cultivated in the presence of OVA proliferated at the same level as the cells cultivated in the presence of MOG35–55 and myelin basic protein. This indicates that some antigen presentation occurs in vitro due to the presence of antigen-presenting cells in the culture, but it also shows that the cellular immune response towards an unrelated antigen was unaffected (Fig. 2d).
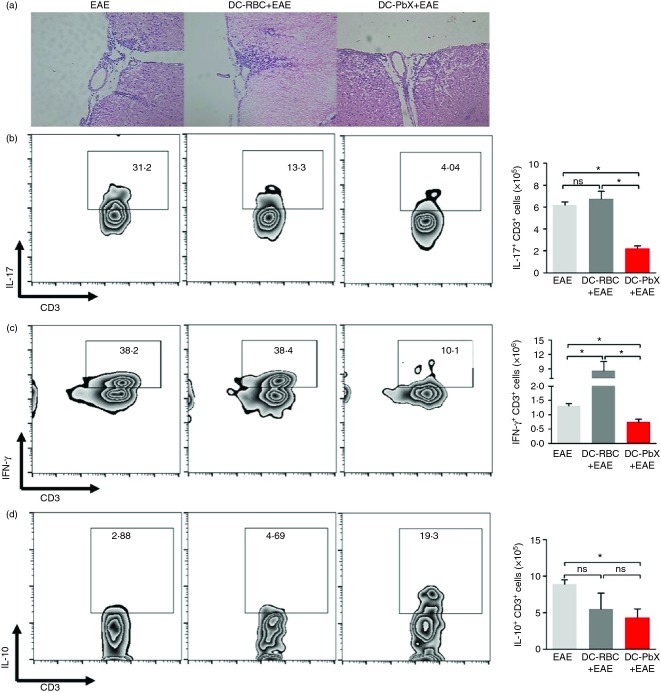
Next, we investigated whether the infiltration of inflammatory cells in the CNS was changed. Our data showed that DC-RBC recipient and untreated EAE-effected mice presented high infiltration of leucocytes, whereas the transfer of DC-PbX reduced the cellular entry to the CNS (Fig. 3a). In addition, the profile of the invading T cells was altered. We observed that both the frequencies and the absolute numbers of IL-17-producing and IFN-γ-producing T cells were reduced in mice that received DC-PbX compared with DC-RBC and the untreated EAE group (Fig. 3b,c, respectively). The frequency of IL-10-producing T cells was significantly augmented in the DC-PbX-transferred group compared with the DC-RBC and un-transferred EAE-effected mice; however, because of the lower infiltration of T cells in the CNS from DC-RbX-transferred mice (Fig. 3a), the absolute numbers of infiltrating IL-10-producing cells was significant reduced in comparison to the EAE group (Fig. 3d).
Figure 3.
Changes of infiltrating cells in the central nervous system (CNS) from dendritic cell (DC) -transferred mice. DCs were stimulated with lipopolysaccharide (LPS; 1 µg/ml) then treated with Plasmodium berghei extracts (PbX; 100 μg/ml) and pulsed with myelin oligodendrocyte glycoprotein peptide (MOG35–55) peptide (10 μg/ml) for 18 hr and adoptively transferred (1·5 × 106 cells/mouse) into C57BL/6 mice (n = 6/group) before the induction of experimental autoimmune encephalomyelitis (EAE). (a) At day 14 after immunization, the CNS tissue of EAE-effected mice was removed and processed for histopathology analysis by haematoxylin & eosin staining. Magnification: 200×. In addition, the CNS tissue of EAE mice (both DC-transferred and untreated) was removed at day 14 post-immunization and the infiltrating leucocytes were enriched through gradient centrifugation using Percol reagent. Cells were stimulated with PMA + ionomycin in the presence of Brefeldin-A for 4 hr before staining with fluorochrome-conjugated antibodies. The frequencies of T cells producing interleukin-17 (IL-17) (b), interferon-γ (IFN-γ) (c) and IL-10 (d) were evaluated by flow cytometry. Data from three independent experiments. *P < 0·05.
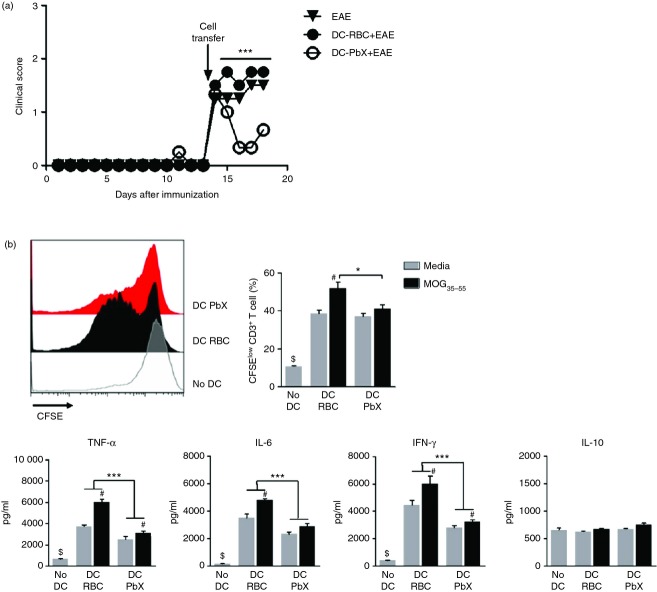
Dendritic cells treated with extracts from P. berghei suppress ongoing EAE and encephalitogenic T cells
In this study, we show that the adoptive transfer of DC-PbX reduced the clinical course of EAE. Our data demonstrated, in Figs 2 and 3, that the cellular immune response towards neuro-antigens as well as the frequency of inflammatory T cells in the CNS was reduced in EAE-inflicted mice that received DC-PbX (DC-PbX+EAE) in comparison with EAE-inflicted mice that received DC-RBC (DC-RBC+EAE). However, in this set of experiments, the DCs encountered naive T cells by the time of adoptive transfer, because cells were transferred before EAE induction. To investigate whether DC-PbX would suppress the clinical course of ongoing EAE, we adoptively transferred these cells at disease onset. Results showed that the transfer of DC-PbX significantly reduced the clinical course of EAE (Fig. 4a). In addition, the suppressive effect of DC-PbX over encephalitogenic T cells was evaluated. For that purpose, we co-cultured DC-PbX with splenic T cells from MOG35–55-immunized mice. Our results showed that the proliferation of MOG-specific T cells was significantly suppressed in the presence of DC-PbX, but not when cells were co-cultured with DC-RBC (Fig. 4b). Besides, in the culture supernatants, levels of TNF-α, IL-6 and IFN-γ were reduced in DC-PbX co-cultures in comparison with the cultures conducted with DC-RBC. There were no statistical differences in IL-10 levels between the groups (Fig. 4b).
Figure 4.
Dendritic cells (DCs) treated with Plasmodium berghei extracts suppress ongoing experimental autoimmune encephalomyelitis (EAE) and encephalitogenic T cells. DCs were stimulated with lipopolysaccharide (LPS; 1 µg/ml) then treated with Plasmodium berghei extracts (PbX; 100 μg/ml) and pulsed with myelin oligodendrocyte glycoprotein peptide (MOG35–55 peptide; 10 μg/ml) for 18 hr and adoptively transferred (1·5 × 106 cells/mouse) into C57BL/6 mice (n = 6/group) at the onset of EAE (day 14 after immunization). (a) The clinical score of DC-transferred and untreated EAE mice was monitored daily; ***P < 0·001. DCs were modulated with PbX (100 μg/ml) stimulated with LPS (1 µg/ml) and pulsed with MOG peptide (10 μg/ml) for 18 hr. Splenic T cells from MOG35–55-immunized mice were isolated at the 10th day after immunization, CFSE-stained and co-cultured with DC-PbX (1 : 1, DC : T) in the presence or absence of MOG35–55 (10 μg/ml) for 96 hr. As controls, cells were grown with DC-RBC or alone. (b) The proliferation of T cells was measured by the decay of the dye at the end of the culture period by flow cytometry. Cytokine levels in culture supernatants were determined by Cytometric Bead Assay. ***P < 0·001; *P < 0·05 (in comparison to the DC-RBC co-culture); #P < 0·05 (in comparison to co-cultures without antigen in the same DC regimen); $P < 0·05 (in comparison to all DC co-cultures). Representative data from two independent experiments with similar results.
Discussion
It has been demonstrated that DCs in malaria patients and mice are immature and non-functional.10–12,34 Although in malaria infection the paralysed DC is a problem, in autoimmune inflammation this is a desired phenomenon. In this study, we show, for the first time to our knowledge, that DCs modulated by extracts of P. berghei-infected erythrocytes protect mice from the development of EAE.
During the Plasmodium infection, the parasite develops tropism towards DCs, which phagocytose the infected RBCs.35,36 Inside DCs, the parasite produces hemozoin, a product of haemoglobin degradation, ultimately leading to impaired DC maturation.12 In vitro hemozoin-treated DCs acquire immunosuppressive abilities such as reduced expression of co-stimulatory molecules CD80 and CD86, secretion of IL-10 and induction of Treg cells.10,34 Semi-mature DCs are so called because they express low-to-intermediate levels of co-stimulatory molecules and have the ability to induce the differentiation of Treg cells when co-cultured with naive T cells (reviewed in refs 37,38). Our data showed that extracts from P. berghei-infected erythrocytes (PbX) changed the maturation status of DCs, with lower expression of antigen-presenting molecules, which may characterize these cells as semi-mature DCs. Indeed, we observed that these modulated DCs also stimulated the production of anti-inflammatory cytokines by T cells and were able to induce the conversion of naive T cells into Treg cells. The DCs treated with PbX stimulated the production of IFN-γ by naive T cells, suggesting that the induced anti-inflammatory profile of DC-PbX is dependent on the stimulation of an IFN-γ-biased milieu. A similar mechanism of DC modulation can be observed with Schistosoma japonicum-derived Sj16 protein, which induces IL-10- and IFN-γ-producing Treg cells.39 Also, it was recently shown that P. falciparum schizont extracts also modulate human DCs, that acquire the ability to induce Treg cells from naive autologous T cells.25 On the other hand, it was demonstrated that extracts of Plasmodium yoelii-infected erythrocytes induced DC maturation and inflammatory activation.40 These data, in conjunction with our results, indicate that diverse Plasmodium species are able to promote distinct effects on DCs.
Here we have demonstrated that transfer of DC-PbX into EAE-effected mice reduced the clinical signs of the disease. The peripheral immune response towards neuro-antigens was also suppressed while splenic T cells were able to proliferate against an unrelated antigen, OVA. This finding suggests that the transfer of modulated DCs not only reduced the neuroinflammation but also stimulated the generation of an antigen-specific immunosuppression.
Moreover, when DCs treated with PbX were co-cultured with previously activated encephalitogenic T cells the specific proliferation was reduced as well as the supernatant′s inflammatory cytokine levels. These observations might correlate with the amelioration of the disease. Although the precise mechanism for this observation is unknown, an increasing amount of evidence suggests that Plasmodium-derived molecules modulate the immune system. It was previously demonstrated that P. falciparum soluble extracts potentiate the induction and function of human regulatory T cells through a transforming growth factor-β-dependent mechanism.41 In Plasmodium chabaudi infection of mice, there is an increase in the frequency of peripheral Treg cells, which modifies the course of EAE.42
Interestingly, DCs modulated with PbX presented different results when cultured with naive and encephalitogenic T cells regarding the secretion of IFN-γ. It is uncertain how these IFN-γ/IL-10-double-producing T cells, generated from naive cells, suppress the establishment of EAE (in the prophylactic approach), although the IFN-γ/IL-10 double-producing Treg cells have been previously reported when helminth-derived products were used to modulate T-cell activation.39 These observations suggest that PbX-modulated DCs possess a dual role: (i) to stimulate the differentiation of Treg cells from naive T cells and (ii) to suppress the proliferation of inflammatory T cells.
Modulation of DCs with pathogen-derived molecules is notorious. For instance, it was previously demonstrated that eggs from Schistosoma mansoni arrests DC maturation towards an immature phenotype.43,44 In addition, soluble egg antigens from S. mansoni is enriched in omega-1, which modulates DCs and induces the expression of Foxp3 in T cells in vitro.45,46 Although the restrained immune response in malaria and schistosomiasis is a problem, the impairment in DC function may become useful for the control of chronic inflammatory conditions. Likewise, DCs exposed to soluble egg antigens and omega-1 acquire modulatory properties and induces Treg cells in CD4+ T cells from non-obese diabetic (NOD) mice, reducing the incidence of spontaneous diabetes.47 Other groups have also reported that the administration of helminth products, such as those from Trichuris suis, reduced the clinical manifestation of EAE, probably through the modulation of antigen-presenting cells.48
As DCs dictate the course of the immune response, it is reasonable to assume that they are targets during infections, which change the maturation/activation profile of these cells towards an immunosuppressive phenotype. Taken together the results presented herein show that DCs modulated with P. berghei-derived extracts are suppressive and control EAE. Further elucidations are necessary to define the mechanisms that control the modulation of DCs.
Acknowledgments
This work was supported by the Sao Paulo Funding Agency (FAPESP, grant #2011/17965-3). RT, LKI, RdG and TAC received a FAPESP scholarship (grants: #2011/13191-3, #2012/08303-0, #2013/01401-9 and #2012/22131-7, respectively). ITF received a CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) scholarship. MACH, FTMC and LV are fellows of the National Council of Technological and Scientific Development (CNPq). We are in debted to Marcos César Meneghetti for his help with animal care.
Authors' contributions
RT, FTMC and LV conceived and designed the experiments. RT, LKI, TAdC, RDG, ITF, CR and SCPL performed the experiments. RT, MACH, FTMC and LV analysed the data. LV, FTMC and MACH contributed reagents/materials/analysis tools and RT and LV wrote the paper.
Disclosures
The authors declare no competing interest.
References
- 1.Baxter AG, Jordan MA. Plasticity is the differentiated state of CD4 T cells. Cell Mol Immunol. 2013;10:375–8. doi: 10.1038/cmi.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leung S, Liu X, Fang L, Chen X, Guo T, Zhang J. The cytokine milieu in the interplay of pathogenic Th1/Th17 cells and regulatory T cells in autoimmune disease. Cell Mol Immunol. 2010;7:182–9. doi: 10.1038/cmi.2010.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 5.van der Velden AW, Velasquez M, Starnbach MN. Salmonella rapidly kill dendritic cells via a caspase-1-dependent mechanism. J Immunol. 2003;171:6742–9. doi: 10.4049/jimmunol.171.12.6742. [DOI] [PubMed] [Google Scholar]
- 6.Bueno SM, Gonzalez PA, Carreno LJ, et al. The capacity of Salmonella to survive inside dendritic cells and prevent antigen presentation to T cells is host specific. Immunology. 2008;124:522–33. doi: 10.1111/j.1365-2567.2008.02805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niedergang F, Sirard JC, Blanc CT, Kraehenbuhl JP. Entry and survival of Salmonella typhimurium in dendritic cells and presentation of recombinant antigens do not require macrophage-specific virulence factors. Proc Natl Acad Sci USA. 2000;97:14650–5. doi: 10.1073/pnas.97.26.14650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wykes MN, Kay JG, Manderson A, et al. Rodent blood-stage Plasmodium survive in dendritic cells that infect naive mice. Proc Natl Acad Sci USA. 2011;108:11205–10. doi: 10.1073/pnas.1108579108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urban BC, Ferguson DJ, Pain A, et al. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature. 1999;400:73–7. doi: 10.1038/21900. [DOI] [PubMed] [Google Scholar]
- 10.Giusti P, Urban B, Frascaroli G, et al. Plasmodium falciparum-infected erythrocytes and β-hematin induce partial maturation of human dendritic cells and increase their migratory ability in response to lymphoid chemokines. Infect Immun. 2011;79:2727–63. doi: 10.1128/IAI.00649-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Millington OR, Di Lorenzo C, Phillips RS, Garside P, Brewer JM. Suppression of adaptive immunity to heterologous antigens during Plasmodium infection through hemozoin-induced failure of dendritic cell function. J Biol. 2006;5:5. doi: 10.1186/jbiol34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urban BC, Todryk S. Malaria pigment paralyzes dendritic cells. J Biol. 2006;5:4. doi: 10.1186/jbiol37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ko HJ, Cho ML, Lee SY, et al. CTLA4-Ig modifies dendritic cells from mice with collagen-induced arthritis to increase the CD4+CD25+Foxp3+ regulatory T cell population. J Autoimmun. 2010;34:111–20. doi: 10.1016/j.jaut.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Sofronic-Milosavljevic L, Radovic I, Ilic N, Majstorovic I, Cvetkovic J, Gruden-Movsesijan A. Application of dendritic cells stimulated with Trichinella spiralis excretory-secretory antigens alleviates experimental autoimmune encephalomyelitis. Med Microbiol Immunol. 2013;202:239–49. doi: 10.1007/s00430-012-0286-6. [DOI] [PubMed] [Google Scholar]
- 15.Tarbell KV, Yamazaki S, Olson K, Toy P, Steinman RM. CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J Exp Med. 2004;199:1467–77. doi: 10.1084/jem.20040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terness P, Oelert T, Ehser S, et al. Mitomycin C-treated dendritic cells inactivate autoreactive T cells: toward the development of a tolerogenic vaccine in autoimmune diseases. Proc Natl Acad Sci USA. 2008;105:18442–7. doi: 10.1073/pnas.0807185105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Unger WW, Laban S, Kleijwegt FS, van der Slik AR, Roep BO. Induction of Treg by monocyte-derived DC modulated by vitamin D3 or dexamethasone: differential role for PD-L1. Eur J Immunol. 2009;39:3147–59. doi: 10.1002/eji.200839103. [DOI] [PubMed] [Google Scholar]
- 18.van Halteren AG, Tysma OM, van Etten E, Mathieu C, Roep BO. 1α,25-dihydroxyvitamin D3 or analogue treated dendritic cells modulate human autoreactive T cells via the selective induction of apoptosis. J Autoimmun. 2004;23:233–9. doi: 10.1016/j.jaut.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Emmer PM, van der Vlag J, Adema GJ, Hilbrands LB. Dendritic cells activated by lipopolysaccharide after dexamethasone treatment induce donor-specific allograft hyporesponsiveness. Transplantation. 2006;81:1451–9. doi: 10.1097/01.tp.0000208801.51222.bd. [DOI] [PubMed] [Google Scholar]
- 20.Moser M, De Smedt T, Sornasse T, et al. Glucocorticoids down-regulate dendritic cell function in vitro and in vivo. Eur J Immunol. 1995;25:2818–24. doi: 10.1002/eji.1830251016. [DOI] [PubMed] [Google Scholar]
- 21.Rea D, van Kooten C, van Meijgaarden KE, Ottenhoff TH, Melief CJ, Offringa R. Glucocorticoids transform CD40-triggering of dendritic cells into an alternative activation pathway resulting in antigen-presenting cells that secrete IL-10. Blood. 2000;95:3162–7. [PubMed] [Google Scholar]
- 22.Roelen DL, Schuurhuis DH, van den Boogaardt DE, et al. Prolongation of skin graft survival by modulation of the alloimmune response with alternatively activated dendritic cells. Transplantation. 2003;76:1608–15. doi: 10.1097/01.TP.0000086340.30817.BA. [DOI] [PubMed] [Google Scholar]
- 23.Chu CQ, Wittmer S, Dalton DK. Failure to suppress the expansion of the activated CD4 T cell population in interferon γ-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J Exp Med. 2000;192:123–8. doi: 10.1084/jem.192.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Domingues HS, Mues M, Lassmann H, Wekerle H, Krishnamoorthy G. Functional and pathogenic differences of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. PLoS ONE. 2010;5:e15531. doi: 10.1371/journal.pone.0015531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clemente AM, Fadigati G, Caporale R, et al. Modulation of the immune and inflammatory responses by Plasmodium falciparum schizont extracts: role of myeloid dendritic cells in effector and regulatory functions of CD4+ lymphocytes. Infect Immun. 2013;81:1842–51. doi: 10.1128/IAI.01226-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finney OC, Lawrence E, Gray AP, Njie M, Riley EM, Walther M. Freeze-thaw lysates of Plasmodium falciparum-infected red blood cells induce differentiation of functionally competent regulatory T cells from memory T cells. Eur J Immunol. 2012;42:1767–77. doi: 10.1002/eji.201142164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lutz MB, Kukutsch N, Ogilvie AL, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 28.Thome R, Issayama LK, Digangi R, et al. Dendritic cells treated with chloroquine modulate experimental autoimmune encephalomyelitis. Immunol Cell Biol. 2014;92:124–32. doi: 10.1038/icb.2013.73. [DOI] [PubMed] [Google Scholar]
- 29.Thome R, Fernandes LG, Mineiro MF, Simioni PU, Joazeiro PP, Tamashiro WM. Oral tolerance and OVA-induced tolerogenic dendritic cells reduce the severity of collagen/ovalbumin-induced arthritis in mice. Cell Immunol. 2012;280:113–23. doi: 10.1016/j.cellimm.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 30.Thome R, Moraes AS, Bombeiro AL, et al. Chloroquine treatment enhances regulatory T cells and reduces the severity of experimental autoimmune encephalomyelitis. PLoS ONE. 2013;8:e65913. doi: 10.1371/journal.pone.0065913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peron JP, Yang K, Chen ML, et al. Oral tolerance reduces Th17 cells as well as the overall inflammation in the central nervous system of EAE mice. J Neuroimmunol. 2010;227:10–7. doi: 10.1016/j.jneuroim.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Herzenberg LA, Tung J, Moore WA, Parks DR. Interpreting flow cytometry data: a guide for the perplexed. Nat Immunol. 2006;7:681–5. doi: 10.1038/ni0706-681. [DOI] [PubMed] [Google Scholar]
- 33.Dittel BN, Visintin I, Merchant RM, Janeway CA., Jr Presentation of the self antigen myelin basic protein by dendritic cells leads to experimental autoimmune encephalomyelitis. J Immunol. 1999;163:32–9. [PubMed] [Google Scholar]
- 34.Skorokhod OA, Alessio M, Mordmuller B, Arese P, Schwarzer E. Hemozoin (malarial pigment) inhibits differentiation and maturation of human monocyte-derived dendritic cells: a peroxisome proliferator-activated receptor-γ-mediated effect. J Immunol. 2004;173:4066–74. doi: 10.4049/jimmunol.173.6.4066. [DOI] [PubMed] [Google Scholar]
- 35.Cockburn IA, Tse SW, Radtke AJ, et al. Dendritic cells and hepatocytes use distinct pathways to process protective antigen from plasmodium in vivo. PLoS Pathog. 2011;7:e1001318. doi: 10.1371/journal.ppat.1001318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mauduit M, See P, Peng K, Rénia L, Ginhoux F. Dendritic cells and the malaria pre-erythrocytic stage. Immunol Res. 2012;53:115–26. doi: 10.1007/s12026-012-8269-7. [DOI] [PubMed] [Google Scholar]
- 37.Lutz MB. Therapeutic potential of semi-mature dendritic cells for tolerance induction. Front Immunol. 2012;3:123. doi: 10.3389/fimmu.2012.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–9. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 39.Sun XJ, Li R, Sun X, et al. Unique roles of Schistosoma japonicum protein Sj16 to induce IFN-gamma and IL-10 producing CD4+CD25+ regulatory T cells in vitro and in vivo. Parasite Immunol. 2012;34:430–9. doi: 10.1111/j.1365-3024.2012.01377.x. [DOI] [PubMed] [Google Scholar]
- 40.Bettiol E, Carapau D, Galan-Rodriguez C, Ocana-Morgner C, Rodriguez A. Dual effect of Plasmodium-infected erythrocytes on dendritic cell maturation. Malar J. 2010;9:64. doi: 10.1186/1475-2875-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clemente A, Caporale R, Sannella AR, et al. Plasmodium falciparum soluble extracts potentiate the suppressive function of polyclonal T regulatory cells through activation of TGFβ-mediated signals. Cell Microbiol. 2011;13:1328–38. doi: 10.1111/j.1462-5822.2011.01622.x. [DOI] [PubMed] [Google Scholar]
- 42.Farias AS, Talaisys RL, Blanco YC, et al. Regulatory T cell induction during Plasmodium chabaudi infection modifies the clinical course of experimental autoimmune encephalomyelitis. PLoS ONE. 2011;6:e17849. doi: 10.1371/journal.pone.0017849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Osada Y, Shimizu S, Kumagai T, Yamada S, Kanazawa T. Schistosoma mansoni infection reduces severity of collagen-induced arthritis via down-regulation of pro-inflammatory mediators. Int J Parasitol. 2009;39:457–64. doi: 10.1016/j.ijpara.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 44.Everts B, Adegnika AA, Kruize YC, Smits HH, Kremsner PG, Yazdanbakhsh M. Functional impairment of human myeloid dendritic cells during Schistosoma haematobium infection. PLoS Negl Trop Dis. 2010;4:e667. doi: 10.1371/journal.pntd.0000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steinfelder S, Andersen JF, Cannons JL, et al. The major component in schistosome eggs responsible for conditioning dendritic cells for Th2 polarization is a T2 ribonuclease (omega-1) J Exp Med. 2009;206:1681–90. doi: 10.1084/jem.20082462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Everts B, Perona-Wright G, Smits HH, et al. Omega-1, a glycoprotein secreted by Schistosoma mansoni eggs, drives Th2 responses. J Exp Med. 2009;206:1673–80. doi: 10.1084/jem.20082460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zaccone P, Burton OT, Gibbs SE, et al. The S. mansoni glycoprotein omega-1 induces Foxp3 expression in NOD mouse CD4+ T cells. Eur J Immunol. 2011;41:2709–18. doi: 10.1002/eji.201141429. [DOI] [PubMed] [Google Scholar]
- 48.Kuijk LM, Klaver EJ, Kooij G, et al. Soluble helminth products suppress clinical signs in murine experimental autoimmune encephalomyelitis and differentially modulate human dendritic cell activation. Mol Immunol. 2012;51:210–8. doi: 10.1016/j.molimm.2012.03.020. [DOI] [PubMed] [Google Scholar]