Abstract
Breathing high concentrations of oxygen (hyperoxia) causes lung injury and is associated with lung diseases such as bronchopulmonary dysplasia (BPD), respiratory distress syndrome (RDS) and persistent pulmonary hypertension of the newborns (PPHN). Hyperoxia (95–100%O2) causes DNA damage and growth arrest of lung cells and consequently cells die by apoptosis or necrosis. Although supplemental oxygen therapy is clinically important, the level and duration of hyperoxic exposure that would allow lung cells to reenter the cell cycle remains unclear. We hypothesized that cells exposed lower concentrations of hyperoxia will retain the capacity to enter cell cycle when recovered in room air. We employed varying concentrations of oxygen (21–95%) to determine the response of lung cells to hyperoxia. Our results indicate that cells were growth arrested and failed to reenter the cell cycle when exposed to greater than 60% oxygen. Cell cycle checkpoint proteins were increased in a biphasic manner, increasing until 70% oxygen, but declined in greater than 90% oxygen. Microarray analysis shows that there is significant decrease in the abundance of Cdks 6–8 and retinoblastoma protein (Rb), p107 and p130 in exposure to 90% oxygen for 48 hours. We further tested the effect of clinically relevant as needed oxygen [(pro-re-nada (prn)] in premature infant (125d and 140d) baboon model of bronchopulmonary dysplasia (BPD). The microarray results show that 6 or 14d PRN oxygen exposed animals had induced expression of chromosomal maintenance genes (MCMs), genes related to anti-inflammation, proliferation and differentiation.
Introduction
Although supplemental oxygen is clearly beneficial in clinical situations, prolonged breathing of high concentrations of oxygen induces lung injury in human and animal models. Hyperoxia induced lung damage is of great clinical interest due to the use of oxygen therapy in the care and management of infants and adults with respiratory failure. Additionally, hyperoxia (30–100%) is frequently used in combination with volatile anesthetics such as sevoflurane for several hours in surgical procedures [1]. Animal studies have described the chronic and acute effects of elevated oxygen tension on the pulmonary alveolus [2–8]. Cell culture models using 95% oxygen as hyperoxia are being widely used to study various aspects of cell cycle regulation. However, exposure of cultured cells to 95% oxygen results in growth arrest of cells and cells die predominantly via necrosis [9]. Although a large amount of data has been generated using 95% oxygen as hyperoxia, the effect of lesser concentrations of oxygen on cell cycle regulatory proteins, cell proliferation and cell death has not been clearly elucidated. It is critically important to determine the threshold of hyperoxic exposure that would allow cells to re-enter the cell cycle following withdrawal of hyperoxia. The re-entry of cells to the cell cycle allows cell growth that is vital for repair of the respiratory epithelium damaged due to high oxygen concentration. Further, the degree of hyperoxia and the duration of exposure that would allow cells to recover; and conversely, the level and duration that would inhibit recovery of cells has not been clearly established.
Progression of the cell cycle requires sequential activation of cyclins and cdks that control the cell cycle transition through G1/S and G2/M phase boundaries [4]. The activation of Rb and its family members such as p107 and p130 are required for G1/S phase transition [4]. These proteins are also required for embryonic development [10]. Further, Rb and p130 are maintained in high levels in the adult lung [10]. Rb, p130 and p107 are also required for Clara and ciliated cell differentiation in mice [10]. The central and rate-limiting function in the transition from G2 into M phase is performed by cyclin B1 and cdk1 complex. The expression and activities of these proteins in hyperoxia affects entry of cells to G2 phase of cell cycle and interferes with G2/M transition. Cell cycle checkpoints, such as checkpoint kinase −1 and 2 (Chk1 & Chk2) are activated in response to DNA damaging agents including hyperoxia [11, 12]. Increased expression of transcription factor p53 and its downstream target protein p21 results in arrest of cell cycle, and increased p53 invokes a DNA repair pathway [12]. The progression of cell cycle is stopped to repair the damaged genetic material when these checkpoint proteins are expressed. In the event of extensive irreparable DNA damage, the cells are allowed to undergo apoptosis. However, contradictory data are presented in the literature regarding necrotic or apoptotic cell death in hyperoxia [9, 13, 14].
Bronchopulmonary dysplasia is a disease of prematurity due to exposure of pre-term infants to varying oxygen tension. In contrast to lower animals such as rat or mice, primates such as baboons can be supported with varying concentration of oxygen with extreme prematurity at birth, but they do develop significant lung injury [6]. Oxygen insult during lung development in premature infants poses complications during therapy and causes significant morbidity following oxygen exposure. Because high concentrations of oxygen inhibits cell cycle progression which in turn affects lung development, it is important to determine the effect of varying concentrations of oxygen on cell cycle regulatory genes in a disease model such as BPD.
Our study is designed to test the hypothesis that exposure of cells to lower concentrations of oxygen would allow cells to reenter the cell cycle for subsequent growth in room air following withdrawal of hyperoxia. Additionally, we hypothesize that pre-term baboons exposed to low levels of oxygen (as needed, prn) would survive due to induced expression of genome maintenance genes. Our study demonstrates that there is a marked difference in the response of cell cycle regulatory proteins to 60 or 75% oxygen compared to 95% oxygen in cultured lung cells. The expression of Rb, p107 and p130 is severely decreased in hyperoxia. Further, the levels of Cdk6, 7 and 8 are also significantly downregulated in hyperoxia. Whereas less than 75% oxygen exposure retains the ability of the cells to reenter the cell cycle, the exposure of cells to 95% oxygen inhibits reentry of cells to growth phase and is associated with significant cell death by necrosis. Additionally, using microarray analysis this study indicates that premature baboons (125d and 140d) exposed to varying concentrations of oxygen induce chromosome maintenance, anti-inflammatory and proliferation inducing genes.
Materials and Methods
Reagents and Antibodies
All chemicals were purchased from Sigma Chemical Co (St. Louis, MO). The antibodies were obtained from the following vendors: Trx, p21, and Chk1 antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Chk2 antibody was obtained from Millipore (Billerica, MA); poly-ADP ribose polymerase (PARP), p53, phospho-p53 (ser15), and pChk1 antibodies were obtained from Cell Signaling Technologies (Beverly, MA). Secondary HRP-conjugated antibodies for various IgGs were obtained from Santa Cruz Biotechnology or Cell Signaling Technologies.
Animal experimental procedure
All animal experiments were performed according to the Guide for the Care and Use of Laboratory Animals as previously described [4–7, 15]. The protocols for animal husbandry and surgery were approved by the Institutional Animal Care and Use Committee (IACUC) of the Southwest Foundation for Biomedical Research, San Antonio, Texas. Fetal baboons of 125d and 140d gestational ages (±2 days) were delivered by hysterectomy. Gestational ages were determined by timed mating as previously described (8). Control gestational fetal baboons were immediately killed and peripheral lung was immersed in Waymouth’s medium and shipped to our laboratory by overnight mail. The 125d 14d prn and 140d 6d prn treatment groups were delivered at 125d±2d and 140±2 d of gestation and immediately placed on positive pressure ventilation with an inspired oxygen tension as needed (PRN, pro re nata a) to maintain paO2 at 60–70 torr. The FIO2 ranged from 0.21 to 0.8 necessary to maintain PaO2 of 60–70 mm Hg. Following treatment pentobarbital was used to kill the animals. The lungs were perfused with saline and immersed in Waymouth’s media and shipped to our laboratory (University of Arkansas for Medical Sciences, Little Rock, author’s previous institution)
Cell Culture and Exposure to Hyperoxia
A lung alveolar type II cell line (A549) was obtained from ATCC (Manassas, VA) and grown in F-12K media supplemented with 10% FBS and 100 units each of penicillin and streptomycin. Cells in tissue culture dishes containing 10–12 ml of media were exposed to hyperoxia (95% oxygen + 5% CO2) at a flow rate of 10 L/min for 10 min in humidified modular exposure chambers (Billups-Rothenburg, CA) following which the chamber was sealed and incubated in a 37°C incubator. Control cells were maintained in room air containing 5% CO2 (normoxia, 21% oxygen) in a CO2 incubator (Stericult, Forma Scientific) for 24, 48 or 72 hrs. At the end of the incubation, cells were either processed for total cell lysates preparation for western analysis or for flow cytometry. Exposure of cells to hyperoxia was performed in the log phase of cell growth, and cells were seeded at low density to prevent any contact inhibition at the end of the exposure period. Cells were counted using cell counter (ViCell, Beckman Coulter)
Flow Cytometry
A549 cells were seeded in 60 mm2 tissue culture dishes and after 24 h; cells were cultured in conditions of normoxia (21% O2 and 5% CO2) or hyperoxia (30–90% O2 + 5% CO2+ balance N2) for 24 h in a Hereas tissue culture incubator with variable setting for oxygen concentrations. Cells were trypsinized and were washed twice in phosphate-buffered saline and fixed in cold 70% ethanol until staining and analysis. For the DNA content analysis, cells were suspended in freshly prepared propidium iodide (PI) staining solution (0.1% BSA containing phosphate-buffered saline, 0.1% RNase A, and 50 µg/ml propidium iodide) for 30 min in the dark. Cell cycle analysis was carried out with the help of flow cytometry core facility (University of Arkansas for Medical Sciences; the author’s previous institution). Data analysis was performed with ModFit LT (Verity Software House Inc., Topsham, ME).
Microarray Analysis of cell cycle regulatory genes
A549 cells were exposed to normoxia or hyperoxia for 24 h and RNA was isolated using an RNeasy Mini Kit (Qiagen, Valencia, CA). RNA from baboon BPD lung was also isolated using RNeasy Mini Kit. We used a GEArray human cell cycle checkpoint assay kit (Super Array Inc., Bethesda, MD) for analysis of cell cycle checkpoint genes. Briefly, 10 µg of total RNA was reverse transcribed with GEAprimer mix using 5 µl (10 mCi/ml) [α-32P] dCTP and 2 µl (50U/µl) MMLV RT (Promega, WI) at 42°C for 20 min. The reaction was stopped, and the probe was denatured at 68°C with the appropriate buffers. The labeled cDNA probe was then added to the GEArray hybridization solution and incubated for 16–18 h at 68°C with continuous agitation. The washed, wet membrane was sealed in a hybridization bag and exposed to x-ray film at −70°C until sufficient exposure was achieved. The developed spots were identified with the grid card provided in the kit.
Western Analysis
Total cellular lysate was prepared in lysis buffer (50 mM Tris-HCl at pH 7.5, 5 mM EDTA, 150 mM NaCl, 1% Triton X-100, 50 mM NaF, 10 mM sodium pyrophosphate, 25 mM β-glycerophosphate, 1 mM PMSF, 30 µL/mL aprotinin [Sigma Chemical Company, St. Louis, MO], and 1 mM Na3VO4). For Chk1 or phospho-Chk1 Western blotting, 30 µg of protein was resolved by 10% SDS-PAGE and electroblotted onto a nitrocellulose membrane. The membrane was blocked in 5% nonfat dry milk in Tris-buffered saline (TBS) containing 0.1% Tween 20 (Bio-Rad, Hercules, CA). After washes, the membrane was incubated with gentle shaking overnight with primary antibody or phosphospecific antibodies (Cell Signaling Technologies, Beverly, MA) in TBS containing 0.1% Tween 20 and 5% BSA. Other proteins including β-actin (used as a loading and transfer control) were detected using 10 to 20µg total lysate.
Statistical Analysis
The statistical evaluations were performed with analysis of variance (ANOVA) in Graph Pad Prism software. ANOVA was used for analysis of more than 3 means. Where necessary post-test was performed with Tukey’s test following ANOVA. The minimum number of n=3 was employed in all experiments performed with cell culture experiments including flow cytometry. Two independent lung samples for each 125d, 125d 14d PRN samples and similar 140d samples were analyzed for the microarray analysis. A representative microarray blot is provided with tetra spots. The density of all four spots was pulled for preparation of graphs normalized to GAPDH or b-actin. The results were considered significant at p<0.05.
Results
Effect of oxygen concentrations and duration on lung cell growth
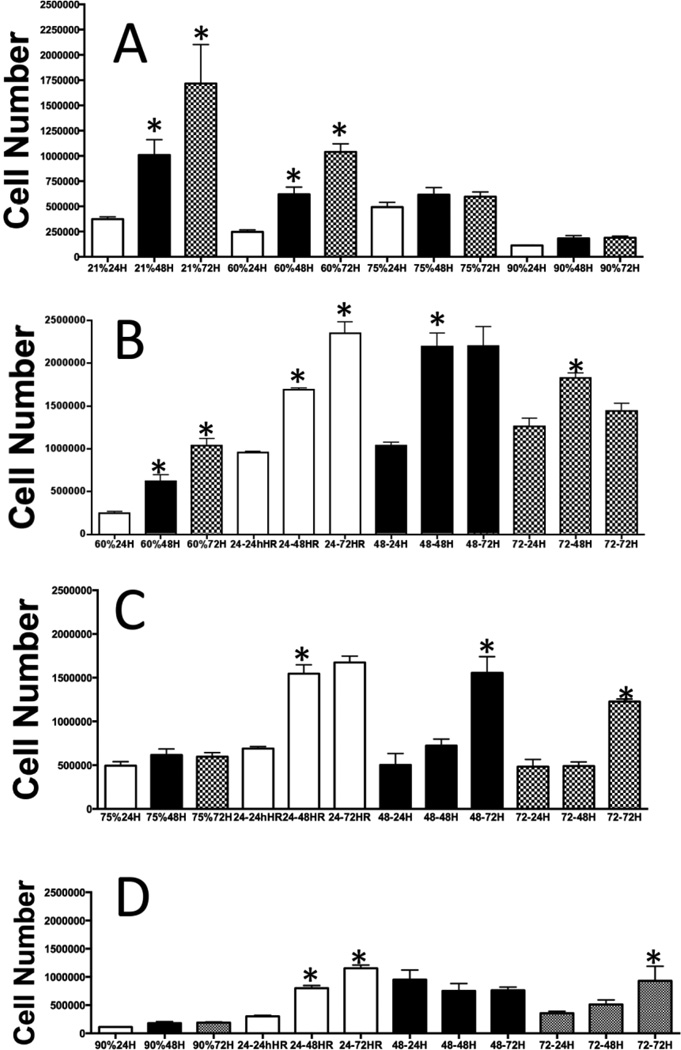
A vast majority of cell culture studies use 95% oxygen to determine the effect of hyperoxia on cell growth and other metabolic parameters, and compare these data to normoxia (21% oxygen). We determined the effect of 21, 60, 75 or 90% oxygen exposure for 24, 48 or 72 hours on cell growth to understand the threshold of oxygen level for normal cellular metabolism. We used A549 cells in our studies because it provides correlation and understanding of hyperoxia-mediated growth response in these cells in the context of extensive data already published using this cell line [11–14, 16–21]. As shown in Fig 1A, cell growth was linearly increased over time in room air (21% oxygen) as expected. Exposure of cells to 60% oxygen for 24, 48 or 72 hours significantly decreased the cell growth at all time points. However, there was a significant increase in cell number between 24 and 48 hours. In a similar manner cell growth form 48 hours to 72 hours was significant, demonstrating that cells retain the capacity to grow, albeit with reduced propensity. When the cells were exposed to 75% oxygen there was no significant growth of cells from 24 to 72 hours exposure, demonstrating that the ability to undergo cell division was arrested in 75% oxygen exposure. Finally, we exposed cells to 90% oxygen for 24, 48 or 72 hours. As shown in Fig 1A, the cell number was significantly decreased compared to 75% oxygen exposed cells, and there was no significant difference in cell numbers in 24, 48 or 72 hours, demonstrating severe growth inhibition and cell death in 90% oxygen exposure at all time points.
Figure 1.
(A) Effect of duration and levels of hyperoxia on cell growth: A549 cells were grown in tissue culture plates and exposed to 60, 75 or 90% oxygen in modular chambers as described in the experimental procedure. After exposure to 24, 48 or 72 hours cell numbers were counted and presented as a bar graph. (B) Cells were exposed to 60% oxygen for 24, 48 or 72 hours. After exposure to these time-points cells were allowed to grow in room air for 24, 48 or 72 hours with regular media and supplements. (C) Cells were exposed to 75% oxygen for 24, 48 or 72 hours. After exposure to these time-points cells were allowed to grow in room air for 24, 48 or 72 hours with regular media and supplements. (D) Cells were exposed to 90% oxygen for 24, 48 or 72 hours. After exposure to these time-points cells were allowed to grow in room air for 24, 48 or 72 hours with regular media and supplements. All experiments were performed in triplicate.
Next, we determined the potential of cells to recover in room air after exposure to a specific level and duration of oxygen. As demonstrated in Fig 1B, cells exposed to 60% oxygen for 24 or 48 hours were able to recover in room air with significant increase in cell number. However, cells exposed to 72 hours of 60% oxygen showed decreased proliferation in room air with no significant increase of cells in 72 hours compared to 24 hours, demonstrating that prolonged duration of 60% oxygen exposure affects the proliferation potential of cells. As shown in Fig 1C, recovery of cells in ambient air previously exposed to 75% oxygen for 24, 48 or 72 hours showed significant decrease in cell growth. Cells exposed to 24 hours in 75% oxygen were significantly increased when recovered for 48 hours in room air. In contrast, there was no difference in cell numbers between 48 or 72 hours exposure in room air, suggesting that the growth potential of 75% (24h) oxygen exposed cells decreased over time. Further, as shown in Fig 1D, the recovery of cell growth was drastically reduced in cells exposed to 90% oxygen for 24, 48 or 72 hours. We next determined whether oxygen levels lower than 60% modulate cell cycle progression.
Effect of levels and duration of hyperoxia on cell cycle progression
To understand the effect of clinically relevant concentrations of oxygen on progression of cell cycle, we exposed cells to 21, 30, 45, 60, 75 or 90% oxygen and determined the number of cells in G1, S or G2. As shown in Fig 2A, there was no change in the number of cells in G1 from 21 to 45% oxygen exposure. However, the G1 population of cells was decreased in a consistent fashion from 45 to 90% oxygen exposure, with significantly fewer number of cells in G1 in 90% oxygen. Further, analysis of S-phase cell population show that cells exposed to 60 or 90% oxygen accumulate in S-phase demonstrating a S-phase arrest of cell cycle in these concentration ranges (Fig 2B). In addition, the number of cells was significantly increased in 90% oxygen in G2, demonstrating a G2 cell cycle arrest in cells exposed to 90% oxygen (Fig 2C). Analysis of Sub-G0 phase (indicator of apoptosis) show that cells exposed to 30 or 45% oxygen underwent significant apoptosis compared to 60 to 90% oxygen exposure (Fig 2D). These data show that cells might be dying by necrosis in the high concentration of oxygen, but the apoptotic mode of cell death occurs at lower concentrations of oxygen. Exposure of cells from 21 to 45% oxygen did not cause significant change in the growth or cell cycle progression of A459 cells in culture. Since exposure of cells to 45% oxygen and above resulted in perturbations in cell cycle progression we next determined the response of critical cell cycle transition and checkpoint proteins in various levels of hyperoxia.
Figure 2. Effect of various concentrations of oxygen on cell cycle progression.
A549 cells were exposed to 30, 45, 60, 75 or 90% oxygen for 24 hours followed by processing of cells for flow cytometric analysis as described in the experimental procedure: (A) Percentage of cells in G1; (B) Percentage of cells in S; (C) Percentage of cells in G2; (D) Percentage of cells undergoing apoptosis as measured by Sub-G0 phase. All experiments were performed in triplicate
Effect of levels and duration of hyperoxia on Cell cycle and checkpoint proteins
We determined the effect of various levels of oxygen on the critical cell cycle regulatory proteins and checkpoint proteins expression to understand the mechanisms of hyperoxia-mediated growth arrest of cells. As demonstrated in Fig 3 (A–B), the expression of pChk1 and pChk2 were increased in hyperoxia indicating the activation of checkpoint proteins. Additionally, the expression of p53 was also increased with increasing concentrations of oxygen Fig 3 (E–F). Further, the expression of proteins that control the transition of cells from G2 to M phase such as p34cdc2 and Cdc25C was increased in hyperoxia (Fig 3 G–J). However, the expression of Cyclin B1 was decreased.
Figure 3. Effect of various concentrations of oxygen on cell cycle checkpoint and cell cycle transitions proteins.
A549 cells were exposed to 21, 30, 45, 60, 75 or 90% oxygen for 24 hours as described in the experimental procedure. Cell lysate was prepared and western analysis of cell cycle checkpoint and transition proteins: (A) pChk2, (B) Chk2, (C) pChk1, (D) Chk1, (E) p53Ser15, (F) p53, (G) Cdc25C, (H) Cyclin B1, (I) p-p34cdc2, (J) p34cdc2, (K) p21 and (L) β-actin was performed as described in the experimental procedure. The densitometry was performed on proteins in Fig 3A–L, and data normalized to β-actin. The relative density of each protein was plotted against oxygen concentrations. (M) pChk2/Chk2, (N) pChk1/Chk1, (O) pp53(Ser15)/p53, (P) p53 and p-p53(Ser15), (Q) p21/ β-actin, (R) Cdc25C/β-actin, (S) p34/β-actin, (T) p-p34cdc2/p34cdc2, (U) Cyclin B1.
On close examination, when we plotted the relative densities of proteins, we found that pChk2 or pChk1 was increased until about 50–60% oxygen exposure, but sharply declined at 90% oxygen exposure (Fig 3M–N). In contrast, the expression phospho-p53 was continuously increased in 30 to 80% oxygen (Fig 3O). This increase was maintained at similar level as 90% exposure (Fig 3P). The expression of p53 and the p-p53 (Ser15) increased until 50% oxygen exposure, and after that it maintained at same level as 90% oxygen exposure (Fig 3P). The expression of p21 that is a p53-dependent protein increased until 60% exposure, but declined at 90% exposure (Fig 3Q). These data show that the severity of hyperoxia determines whether the cells would undergo apoptosis or induce DNA repair pathway. For example, 90% oxygen exposure could cause irreparable DNA damage requiring cells to undergo apoptosis or necrosis rather than a halt in cell cycle for repair of genetic material. Further, the proteins of G2 checkpoint control such as p34cdc2 and Cdc25C were increased until 50 to 70% oxygen exposure, but are sharply declined in cells exposed to 90% oxygen, suggesting that G2 control is lost in 90% hyperoxia (Fig 3R–S). However, the sharp decline in cyclin B1 expression occurred in cells exposed to as low as 30% oxygen, and this decline continued to 90% exposed cells (Fig 3U). Because threshold levels of cyclin B1 is necessary for the formation of maturation promoting factor (MPF) [22], our data show that G2 transition is affected in as low as 30% hyperoxia, and cells would accumulate in G2 phase as shown in our flow cytometric analysis (Fig 2). Next, we determined the response of cell cycle regulatory genes in response to most commonly used oxygen concentration of 95% using microarray analysis.
Microarray analysis of cell cycle regulatory gene in hyperoxia
We analyzed the expression of cell cycle regulatory genes using a 112-gene cell cycle microarray (Super Array Inc.,) after exposure of cells to 95% oxygen for 25 hours (Fig 4A–B). We found that the genes related to checkpoint control, apoptosis or proliferations are differentially regulated at the level of 95% oxygen. This study demonstrates that hyperoxia decreases the expression of Rb, p107 and p130 proteins (Fig 4C), suggesting that lung epithelial cell differentiation could be compromised in high oxygen concentration, which could impair the recovery of lung cells following hyperoxia [10]. Additionally, the expression of cyclin B1 was decreased and the expression of p21, bax and Gadd45 were increased in hyperoxia (Fig 4D). As shown in Fig 4E, our results show that the expression of cdk6, cdk7 and cdk8 were significantly decreased in cells exposed to hyperoxia. Because p21 activation inhibits cell cycle progression at G1/S boundary, we next determined whether recovery of cells in hyperoxia dependent on modulation of p21 expression.
Figure 4. Microarray analysis of 112 cell cycle genes in hyperoxia.
(A) Autoradiogram showing response of cell cycle regulatory genes in normoxia and hyperoxia. We used a GEArray human cell cycle checkpoint assay kit (SuperArray Inc, Bethesda, MD 20827) for analysis of cell cycle checkpoint genes. The hybridization and detection were performed as described in the materials and methods section.(A) Cells exposed to 95% oxygen for 48 hours; (B) cells in room air for 48 hours; (C) relative density of Rb, p107 and p130 proteins; (D) relative abundance of Cdk6, cdk7 and cdk8; (E) relative abundance of cyclin B1, p21, Gadd45 and Bax.
The expression of p21 does not decrease in cells recovered following 90% oxygen exposure, but decreases in cells exposed to 75% oxygen
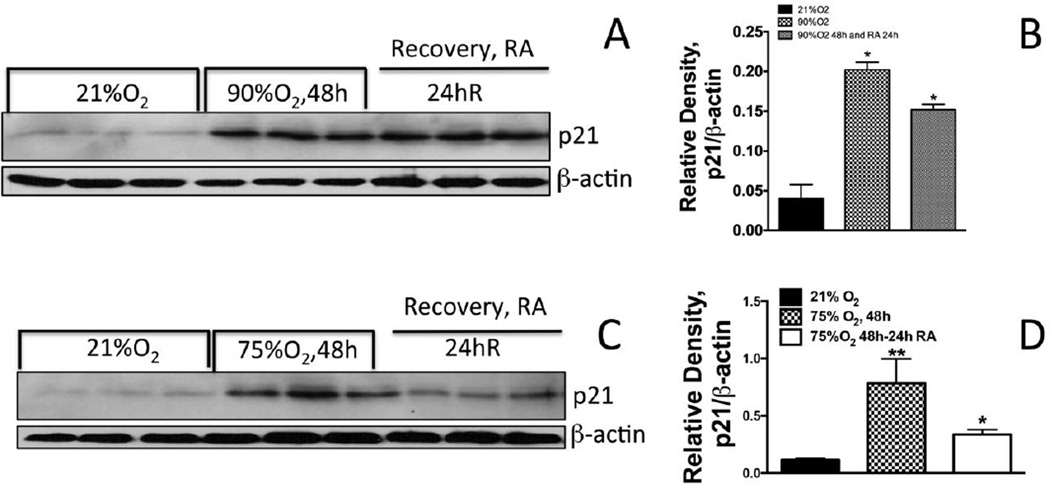
Our cell cycle data (Fig 1D) shows that cells exposed to 90% oxygen followed by recovery in the room air do not show any increase in cell number. However, cells exposed to 75% oxygen followed by recovery in room air result in increased cell numbers. We reasoned that p21 expression could be a limiting factor in the recovery of cells due to either 90 or 75% oxygen exposure. As demonstrated in Fig 5A&B the expression of p21 remains elevated in cells exposed to 90% oxygen for 48 hours followed by recovery in room air for 24-hours. The expression of p21 was increased in exposure of cells to 48 hours in 75% oxygen, however, the expression of p21 declined when these cells were recovered in room air for 24 hours (Fig 5C & D). These data show that the threshold of oxygen concentration for re-entry to cell cycle should be 75% or lower for A549 cells. In contrast, 90% oxygen exposure does not allow recovery of cells in room air due to increased expression of p21 during room air recovery.
Figure 5. Effect of oxygen exposure and recovery on cyclin-dependent kinase inhibitor p21 expression.
A549 cells were exposed to 75% or 90% oxygen followed by recovery for 24, 48 or 72 hours in room air (RA). P21 expression was analyzed in the cell lysates. (B) Densitometry or p21/β-actin ratio. ** Significantly higher compared to 21% oxygen exposed cells; * Significantly lower that 75% oxygen exposed cells for 48 hours.
The expression of p34cdc2 decreased in cells exposed to hyperoxia, however, the level was restored in recovery in room air
Since G2 transition is dependent on threshold of cyclin B1 and Cdk1 (p34), we determined whether recovery in room air would depend on the levels of cyclin B1-Cdk1. Cyclin B1 expression was decreased in a dose-dependent manner beginning with 30% oxygen exposure, and expression is completely lost in 90% oxygen. Further, the phosphorylation of p34cdc2 was maximal in 75% oxygen (Fig 4G–I), but the level was sharply declined in 90% oxygen. As demonstrated in Fig 6, exposure of A549 cells to hyperoxia down regulated the p34 protein expression. However, when cells were returned to normoxia for 48 hours, p34 protein levels began to recover suggesting that the decrease in p34 protein in hyperoxia is a reversible process (Fig 6A & B). These results suggest that although hyperoxia profoundly affects MPF expression, cells recovered in room air could progress to M phase, but the expression of cyclin B1 could be a limiting factor.
Figure 6. Effect of oxygen exposure and recovery on p34cdc2 expression.
A549 cells were exposed to 90% oxygen for 48 hours followed by 24 hours of recovery. The expression of p34cdc2 was determined by western analysis: (A) Western analysis of p34cdc2 (B) Ratio of densities of p34cdc2 and β-actin.
Microarray analysis of cell cycle regulatory genes in baboon BPD
Although extremely premature primates could be managed with prolonged supplemental oxygen inspiration they invariably develop severe lung injury and subsequent morbidity. We previously reported that p53, p21 and cyclins or cdks are upregulated in baboon model of BPD in response to “prn” oxygen[4, 5]. Based on our findings with lower levels of oxygen exposure, we hypothesized that these premature primates would survive due to increase response to genome maintenance genes as that would prevent DNA damage-related apoptosis of lung cells. The lung cells could be growth arrested during the exposure, but would enter the cell cycle after withdrawal of hyperoixa that would allow them to continue lung development. Therefore, we used two gestational ages, 125d and 140d baboons (baboon term delivery 185±2d) either exposed to normoxia or 6–14 days of as needed oxygen (varies from 30 to 80%) to determine relative response of lung to cell cycle-related genes. As shown in Fig 7 A, B and E the most pronounced expression of MCM6, p16, p19A and UBC genes occurs in 125d 14d PRN oxygen exposed animals compared to GC animals. Additionally, as shown in Fig 7 C–D and F, the expression of MCM4, MCM6, p16, UCB, TIMP3, Cyclin D and E2F4 was increased in 140d 6d animals compared to 140d GC animals. We did not observe any increase in other cell cycle regulatory proteins as shown in A549 cells exposed to 95% oxygen for 48 hours (Fig 4). These data show a significant difference in the response of lungs to lower concentrations of oxygen when compared with cell culture studies that employ high concentration of oxygen.
Figure 7. Microarray analysis of 112 cell cycle regulatory genes in 125d GC, 125d 14d PRN, 140d GC and 140d 6d PRN BPD model.
Premature infant baboons were delivered and exposed to PRN oxygen (pro-re nata) for 6 or 14 days as described in our previous publication [2, 3]. RNA was isolated and microarray analysis was performed as described in the experimental procedure. (A) Autoradiogram of tetra spot microarray (SuperArray Biosciences) of 125d GC and (B) 125d 14d PRN baboon; (C) autoradiograph of 140d GC and (D) 140d 6d PRN baboons. The average tetra spot density was normalized to GAPDH density, and the graphs were plotted (E) 125d GC & 125d 14d PRN; (F) 140d GC & 140d 6d PRN baboons. The experiment was performed on two independent microarrays, and the result repoted using two tetra-spots of microarray.
Discussion
Our study demonstrates that cells exposed to greater than 60% oxygen are growth inhibited and fail to reenter the cell cycle. In contrast, cells exposed to less than 60% oxygen are capable of reentering the cell cycle and continue to grow in ambient air. Baboons of different gestational ages with varying concentrations of oxygen induce chromosome maintenance, proliferation and anti-inflammatory genes that enable them to survive the oxygen insult.
We show that cells undergo division and grow in 60% oxygen for 24, 48 or 72 hours, but with decreased efficiency. After exposure to 60% oxygen these cells reenter the cell cycle when maintained in room air. However, the growth of cells exposed to 75% oxygen is significantly retarded, and these cells grow very slowly in room air after exposure to 75% oxygen. Cells exposed to 90% oxygen are severely growth inhibited and fail to recover. We show that progression of the cell cycle continues following 30 or 45% oxygen exposure for 24 hours. Conversely, cells were arrested in S and G2 phases when exposed to 60 to 90% oxygen. The expression of cell cycle and checkpoint regulatory proteins pChk1, pChk2, pCdc25C, and p34cdc2 were increased in 30 to 75% oxygen exposure, but declined in 90% oxygen. Further, the expression of p53 increased consistently until 60% oxygen, after which it remained elevated at a level similar to 90% oxygen. Using a microarray analysis of cell cycle regulatory genes we demonstrate for the first time that the expression of cdk5, cdk6 and cdk7 are severely decreased in 90% oxygen in addition to Rb, p103 and p107. Further, we show for the first time that in severely premature infant baboons exposed to 6d prn oxygen showed increased expression of MCM4, UBC, E2F and p16 expression. The premature 140d baboons exposed to prn oxygen for 6-days increased expression of MCM4, MCM6, p16, UCB, TIMP3, Cyclin D3 and E2F4.
Rb and its associated proteins, p107 and p130, form complexes in quiescent cells with the E2F family of transcription factors [23]. Phosphorylation of Rb and p130 releases E2F proteins, which then act as transcription factors for G1/S phase genes [23]. The amount of Rb and its associated proteins p107 and p130, as well as the extent of phosphorylation, is crucial for progression of cells toward the G1/S phase transition. The fact that hyperoxia decreases the expression of these proteins suggests that protein levels may be a limiting factor for cell cycle progression. Additionally, Rb is an essential regulator of lung epithelial development [10] that underscores the importance of decreased Rb levels in epithelial repair following hyperoxia. In support of this idea, we found that premature infant baboons in respiratory distress have decreased levels of Rb [24]. Additionally, Rb family proteins are also required for the differentiation of lung epithelial cells such as clara and ciliated cells [10]. Loss of Rb in hyperoxia in premature infants could compromise cellular differentiation impairing lung function during recovery and beyond. Previous reports have noted decreased expression of cyclin B1 protein in cells in hyperoxic environments [21, 25]. Our microarray analysis shows that hyperoxia decreases the mRNA expression of cyclin B1. Because threshold levels of cyclinB1 is required for G2/M phase transition of the cell cycle, the loss of cyclin B1 in hyperoxia could be a limiting factor in hyperoxia-mediated growth arrest of cells. Cdks 6–8 activates various cyclins by binding to them once the threshold level of cyclin synthesis is achieved [23]. A decrease in either cdks or cyclins would adversely affects the kinase function of cyclin-cdk complex, which in turn, would retard cell cycle progression. Our microarray data show for the first time that Cdk6, cdk7 and cdk8 are decreased in hyperoxia possibly contributing a major mechanism of cell growth arrest in hyperoxia.
We employed cell lines to test the hypothesis that lower concentrations of oxygen exposure would permit cells to re-enter the cell cycle. As an extension of this hypothesis applied to a disease state, we hypothesized that pre-term baboon lungs exposed to low oxygen concentrations would induce genome maintenance genes likewise permitting re-entry into the cell cycle. Although the majority of cell culture experiments employ 95% oxygen exposure to study hyperoxia-mediated growth arrest, it is unknown how lower concentrations of oxygen affect cell growth and cell cycle transitions. Mostly, humans are exposed to lower concentrations of supplemental oxygen for treatment of adult respiratory distress syndrome (ARDS), bronchopulmonary dysplasia (BPD) or other obstructive or restrictive lung diseases. Moreover, countless patients worldwide undergoing general anesthesia are exposed to brief periods of 100% oxygen administration prior to anesthetic induction and during anesthetic emergence. Likewise, varying oxygen concentrations are employed perioperatively to maintain hemoglobin oxygen saturation and enhance tissue oxygen delivery. In previous studies using a baboon BPD model we have shown that cell cycle checkpoint proteins, cyclins and cdks are modulated in PRN oxygen exposures ranging from 30% to 70% [4]. In the present study, we found that at lower levels of oxygen exposure the cell cycle checkpoint proteins such as Chk1 or Chk2 are increased (up to 60% oxygen exposure), after which the level of these proteins sharply declines to a minimal level found at 90% oxygen concentration. These data are consistent with our cell cycle data demonstrating that cell cycle arrest occurs following 45% oxygen exposure, and that cells continue to be growth arrested in 90% oxygen. Further, exposure of cells to 90% oxygen decreased the cell number to 50% of the cell number of cells in 21% oxygen reflecting significant cell death in 90% oxygen. Moreover, there was very little apoptosis seen in cells exposed to 90% oxygen demonstrating that necrosis is likely the major mode of cells death following 90% hyperoxia exposure. These data confirms the study of Horowitz et al. [13, 14]. However, the apoptotic mode of cell death was increased in cells exposed to 30 or 45% oxygen.
Previous studies have shown that in baboon BPD (125d and 140d) the expression of p21 and p53 protein increases in 6d prn exposed animals [5]. In our microarray analysis we observed that MCM6, UBC, p19A and p16 expression are increased in 125d 14d prn model, whereas the expression of MCM4, MCM6 TIMP3, UBC, p16 and E2F4 is increased in 140d 6d prn exposure. E2F family of transcription factors are involved in gene regulation of proliferative genes. Studies have shown that this factor is required for normal development of airway epithelium in mice [26]. MCM4 and MCM6 essentially function as DNA helicase to unwind the DNA duplex at the replication forks [27]. Therefore, MCM proteins are important for chromosomal integrity. Our data show that in more premature BPD (125d) MCM6 is increased whereas 140d animals both MCM4 and 6 are increased. These data suggest that more premature animals have decreased potential to increase these important chromosome maintenance proteins. This could contribute to the loss of genetic integrity in hyperoxia. Our data demonstrate that hyperoxia induces the E2F4 gene in the baboon lung in 140d prn oxygen, but not in 125d prn oxygen, suggesting that more premature baboons fail to respond to increase E2F4 in hyperoxia. This could result in decreased airway ciliated cells [26]. UBC is required for fetal development as UBC knockout mice die in utero [28]. The role of UBC in lung development remains unknown. Our data show that UBC is strongly induced in the 140d prn animals compared to 125d 14d prn animals, demonstrating that oxygen positively regulates this protein. Severely premature baboons could be more sensitive to prn oxygen due to lack of adequate UBC. The expression of p19A, also known as S-phase kinase 1A, is increased in 125d 14d prn group, which could degrade G1 cyclins and cdks [29].The expression of TIMP3 is increased in 140d 6d prn baboons compared to GCs. However, there was no change in the expression of TIMP3 in 125d 14d prn animals compared to GCs. Studies have shown that the level of TIMP3 does not change in bleomycininduced acute lung injury [30]. TIMP3 is expressed in the lung and is stored in the lung matrix [31]. Upregulation of TIMP3 is associated with decreased lung inflammation in addition to decreasing MMPs [30]. Our data suggest that 140d prn exposed baboons had higher levels of TIMP3 that would decrease lung inflammation in these animals compared to 125d animals in 6d prn. Taken together, our microarray data reveals that severely premature baboons (125d) respond differently to 14d prn oxygen compared to 140d animals in 6d prn.
Acknowledgement
Research reported in this publication was supported by National Heart, Lung And Blood Institute of the National Institutes of Health under Award Number R01HL 071558, HL1R01HL107885 and HL1R01HL109397. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health. We acknowledge the superb and dedicated assistance of numerous physician, nurses, technicians, and other personnel at the Broncho-pulmonary Dysplasia (BPD) Resources Center (San Antonio, TX). Excellent technical assistance of Harish Muniyappa and Ravi Dashnamoorthy is acknowledged.
Abbreviations
- MCM4
Minichromosome maintenance deficient 4
- MCM6
Minichromosome maintenance deficient 6
- TIMP3
Tissue inhibitor of metalloproteinase 3
- E2F4
E2F Transcription factor 4
- UBC
Ubiquitin C
- Cyd3
Cyclin D3
- P19A
S-phase kinase 1A
- P16
Cyclin dependent kinase inhibitor 2A, p16INK4
Literature Cited
- 1.Schober P, Schwarte LA. From system to organ to cell: oxygenation and perfusion measurement in anesthesia and critical care. J Clin Monit Comput. 2012;26:255–265. doi: 10.1007/s10877-012-9350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Das KC, Guo XL, White CW. Induction of thioredoxin and thioredoxin reductase gene expression in lungs of newborn primates by oxygen. Am J Physiol. 1999;276:L530–L539. doi: 10.1152/ajplung.1999.276.3.L530. [DOI] [PubMed] [Google Scholar]
- 3.Das KC, Pahl PM, Guo XL, White CW. Induction of peroxiredoxin gene expression by oxygen in lungs of newborn primates. Am J Respir Cell Mol Biol. 2001;25:226–232. doi: 10.1165/ajrcmb.25.2.4314. [DOI] [PubMed] [Google Scholar]
- 4.Das KC, Ravi D. Altered expression of cyclins and cdks in premature infant baboon model of bronchopulmonary dysplasia. Antioxid Redox Signal. 2004;6:117–127. doi: 10.1089/152308604771978426. [DOI] [PubMed] [Google Scholar]
- 5.Das KC, Ravi D, Holland W. Increased apoptosis and expression of p21 and p53 in premature infant baboon model of bronchopulmonary dysplasia. Antioxid Redox Signal. 2004;6:109–116. doi: 10.1089/152308604771978417. [DOI] [PubMed] [Google Scholar]
- 6.Coalson JJ, Kuehl TJ, Escobedo MB, Hilliard JL, Smith F, Meredith K, Null DM, Jr, Walsh W, Johnson D, Robotham JL. A baboon model of bronchopulmonary dysplasia. II. Pathologic features. Exp Mol Pathol. 1982;37:335–350. doi: 10.1016/0014-4800(82)90046-6. [DOI] [PubMed] [Google Scholar]
- 7.Coalson JJ, Winter VT, Gerstmann DR, Idell S, King RJ, Delemos RA. Pathophysiologic, morphometric, and biochemical studies of the premature baboon with bronchopulmonary dysplasia. Am Rev Respir Dis. 1992;145:872–881. doi: 10.1164/ajrccm/145.4_Pt_1.872. [DOI] [PubMed] [Google Scholar]
- 8.Freeman BA, Crapo JD. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J Biol Chem. 1981;256:10986–10992. [PubMed] [Google Scholar]
- 9.Horowitz S. Pathways to cell death in hyperoxia. Chest. 1999;116:64S–67S. doi: 10.1378/chest.116.suppl_1.64s. [DOI] [PubMed] [Google Scholar]
- 10.Wikenheiser-Brokamp KA. Rb family proteins differentially regulate distinct cell lineages during epithelial development. Development. 2004;131:4299–4310. doi: 10.1242/dev.01232. [DOI] [PubMed] [Google Scholar]
- 11.Das KC, Dashnamoorthy R. Hyperoxia activates the ATR-Chk1 pathway and phosphorylates p53 at multiple sites. Am J Physiol Lung Cell Mol Physiol. 2004;286:L87–L97. doi: 10.1152/ajplung.00203.2002. [DOI] [PubMed] [Google Scholar]
- 12.Kulkarni A, Das KC. Differential roles of ATR and ATM in p53, Chk1, and histone H2AX phosphorylation in response to hyperoxia: ATR-dependent ATM activation. Am J Physiol Lung Cell Mol Physiol. 2008;294:L998–L1006. doi: 10.1152/ajplung.00004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kazzaz JA, Horowitz S, Li Y, Mantell LL. Hyperoxia in cell culture. A non-apoptotic programmed cell death. Ann N Y Acad Sci. 1999;887:164–170. doi: 10.1111/j.1749-6632.1999.tb07930.x. [DOI] [PubMed] [Google Scholar]
- 14.Kazzaz JA, Xu J, Palaia TA, Mantell L, Fein AM, Horowitz S. Cellular oxygen toxicity. Oxidant injury without apoptosis. J Biol Chem. 1996;271:15182–15186. doi: 10.1074/jbc.271.25.15182. [DOI] [PubMed] [Google Scholar]
- 15.Das KC, Guo XL, White CW. Hyperoxia induces thioredoxin and thioredoxin reductase gene expression in lungs of premature baboons with respiratory distress and bronchopulmonary dysplasia. Chest. 1999;116:101S. [PubMed] [Google Scholar]
- 16.Ahmad S, White CW, Chang LY, Schneider BK and Allen CB. Glutamine protects mitochondrial structure and function in oxygen toxicity. Am J Physiol Lung Cell Mol Physiol. 2001;280:L779–L791. doi: 10.1152/ajplung.2001.280.4.L779. [DOI] [PubMed] [Google Scholar]
- 17.Allen CB, White CW. Glucose modulates cell death due to normobaric hyperoxia by maintaining cellular ATP. Am J Physiol. 1998;274:L159–L164. doi: 10.1152/ajplung.1998.274.1.L159. [DOI] [PubMed] [Google Scholar]
- 18.Budinger GR, Tso M, McClintock DS, Dean DA, Sznajder JI, Chandel NS. Hyperoxia-induced apoptosis does not require mitochondrial reactive oxygen species and is regulated by Bcl-2 proteins. J Biol Chem. 2002;277:15654–15660. doi: 10.1074/jbc.M109317200. doi:10.1074/jbc.M109317200 M109317200 [pii] [DOI] [PubMed] [Google Scholar]
- 19.Das KC. Hyperoxia Decreases Glycolytic Capacity, Glycolytic Reserve and Oxidative Phosphorylation in MLE-12 Cells and Inhibits Complex I and II Function, but Not Complex IV in Isolated Mouse Lung Mitochondria. PLoS One. 2013;8:e73358. doi: 10.1371/journal.pone.0073358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gardner PR, Nguyen DD and White CW. Aconitase is a sensitive and critical target of oxygen poisoning in cultured mammalian cells and in rat lungs. Proc Natl Acad Sci U S A. 1994;91:12248–12252. doi: 10.1073/pnas.91.25.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGrath-Morrow SA and Stahl J. Growth arrest in A549 cells during hyperoxic stress is associated with decreased cyclin B1 and increased p21(Waf1/Cip1/Sdi1) levels. Biochim Biophys Acta. 2001;1538:90–97. doi: 10.1016/s0167-4889(00)00142-7. [DOI] [PubMed] [Google Scholar]
- 22.Innocente SA, Abrahamson JL, Cogswell JP and Lee JM. p53 regulates a G2 checkpoint through cyclin B1. Proc Natl Acad Sci U S A. 1999;96:2147–2152. doi: 10.1073/pnas.96.5.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morgan DO. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- 24.Chehab NH, Malikzay A, Stavridi ES, Halazonetis TD. Phosphorylation of Ser-20 mediates stabilization of human p53 in response to DNA damage. Proc Natl Acad Sci U S A. 1999;96:13777–13782. doi: 10.1073/pnas.96.24.13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Das KC, White CW. Detection of thioredoxin in human serum and biological samples using a sensitive sandwich ELISA with digoxigenin-labeled antibody. J Immunol Methods. 1998;211:9–20. doi: 10.1016/s0022-1759(97)00163-4. [DOI] [PubMed] [Google Scholar]
- 26.Danielian PS, Bender Kim CF, Caron AM, Vasile E, Bronson RT and Lees JA. E2f4 is required for normal development of the airway epithelium. Dev Biol. 2007;305:564–576. doi: 10.1016/j.ydbio.2007.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bagley BN, Keane TM, Maklakova VI, Marshall JG, Lester RA, Cancel MM, Paulsen AR, Bendzick LE, Been RA, Kogan SC, Cormier RT, Kendziorski C, Adams DJ, Collier LS. A dominantly acting murine allele of Mcm4 causes chromosomal abnormalities and promotes tumorigenesis. PLoS Genet. 2012;8:e1003034. doi: 10.1371/journal.pgen.1003034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryu KY, Maehr R, Gilchrist CA, Long MA, Bouley DM, Mueller B, Ploegh HL, Kopito RR. The mouse polyubiquitin gene UbC is essential for fetal liver development, cell-cycle progression and stress tolerance. Embo J. 2007;26:2693–2706. doi: 10.1038/sj.emboj.7601722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vodermaier HC. APC/C and SCF: controlling each other and the cell cycle. Curr Biol. 2004;14:R787–R796. doi: 10.1016/j.cub.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 30.Gill SE, Huizar I, Bench EM, Sussman SW, Wang Y, Khokha R, Parks WC. Tissue inhibitor of metalloproteinases 3 regulates resolution of inflammation following acute lung injury. Am J Pathol. 2010;176:64–73. doi: 10.2353/ajpath.2010.090158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gill SE, Parks WC. Metalloproteinases and their inhibitors: regulators of wound healing. Int J Biochem Cell Biol. 2008;40:1334–1347. doi: 10.1016/j.biocel.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]