Abstract
Estrogen is a steroid hormone that has been implicated in a variety of cellular and physiological processes and in the development of diseases such as cancer. Here we show a remarkable widespread microRNA (miRNA) downregulation in the zebrafish (Danio rerio) liver following 17β-estradiol (E2) treatment. This unique miRNA expression signature in the fish liver was further supported by a combination of computational predictions with gene expression microarray data, showing a significant bias toward upregulation of miRNA target genes after E2 treatment. Using pathway analysis of target genes, their involvement in the processes of cell cycle, DNA replication, and proteasome was observed, suggesting that miRNAs are incorporated into robust regulatory networks controlled by estrogen. In oviparous vertebrates, including fish, the formation of yolky eggs during a process known as vitellogenesis is regulated by estrogen. Microarrays were used to compare miRNA expression profiles between the livers of vitellogenic and nonvitellogenic zebrafish females. Among the upregulated miRNAs in vitellogenic females, were five members of the miR-17-92, a polycistronic miRNA cluster with a role in cell proliferation and cancer. Furthermore, a number of miRNA target genes related to fish vitellogenesis were revealed, including vtg3, a putative target of miR-122; the most abundant miRNA in the liver. Moreover, several of the differentially expressed miRNAs were only conserved in oviparous animals, which suggest an additional novel level of regulation during vitellogenesis by miRNAs and consequently, improves our knowledge of the process of oocyte growth in egg-laying animals.
Introduction
In vertebrates, 17β-estradiol (E2) regulates a large array of physiological processes such as growth, differentiation, functioning of the reproductive, skeletal, cardiovascular, and central nervous systems and is also involved in a variety of diseases such as cancer.1–3 These functions are executed through estrogen receptors (ERα and ERβ), which are ligand-activated transcription factors that share common structure domains and regulate genes in steroid-responsive tissues.4 In the classical mechanism of estrogen action, E2 diffuses into the cell and binds to ERs, which are mostly located in the nucleus of target cells.5 After ligand binding, ERs form dimmers that bind to inverted palindromic estrogen response element sequences in the promoter region of estrogen-responsive genes.6 However, other mechanisms also exist, such as interactions of ERs with other transcription factors and also nongenomic estrogenic pathways mediated by cell membrane receptors, which are relatively rapid and do not depend on RNA and protein synthesis.7,8
In oviparous animals, including teleost fish, E2 is a key hormone in the regulation of the vitellogenesis process within the liver.9 Vitellogenesis is a process that results in the formation of yolky eggs from oocytes. This occurs before the spawning season of fish and consists of highly regulated pathways that involve the brain, liver, and ovary. During vitellogenesis, synthesis of the egg yolk protein precursor vitellogenins (Vtgs) occur in the liver, and after their secretion and transport in the plasma, they are incorporated by developing oocytes via specific Vtg receptors in the ovary.10,11 The high levels of plasma Vtg, in conjunction with the rapid rates of Vtg sequestration, allow the considerable oocyte growth during vitellogenesis.12 Vtg molecules are large and complex calcium-binding phospho-lipo-glicoproteins. They are sequestered by receptor-mediated endocytosis into the developing oocytes, where they are cleaved by cathepsin D to form the yolk proteins (lipovitellin and phosvitin), which accumulate and enable the proper development of the embryo.13 Seven different vtg genes have been characterized so far in the zebrafish.14
microRNAs (also termed miRNAs) are endogenous noncoding segments of RNA, 18–25 nucleotides (nt) in length, that negatively regulate gene expression at the post-transcriptional level and fine-tune gene functions.15
According to computational miRNA target prediction programs, each miRNA can potentially regulate the expression of hundreds of different genes and it is becoming increasingly apparent that miRNAs are involved in almost every cellular process investigated so far.16
Compelling evidence continues to accumulate that miRNAs are involved in estrogen signaling and metabolism and there are several examples of estrogen regulation of miRNAs and several more examples of estrogen-related processes that are regulated by miRNAs.17,18 We previously showed the involvement of E2 in the regulation of miRNA expression in whole body and tissue-specific expression profiles of the zebrafish.19 Later on, several studies have shown global miRNA regulation by E2 in human MCF-7 breast cancer cells, using the microarray approach.20–24
Here we investigated, using miRNA and gene expression data, the possible involvement of miRNAs in estrogen regulation and during the vitellogenesis process within the zebrafish liver and identified their potential target genes and related pathways.
Materials and Methods
Animals
Adult zebrafish (3 months old) were purchased from a local zebrafish supplier (A & H holdings, Israel Ltd.) and raised under standard conditions of 25°C with a light/dark cycle of 14/10 h. All fish were anesthetized with Tricaine (Sigma) before experimental procedures,25 adhering to institutional ethics regulations.
E2 treatment and sample collection
Male zebrafish were exposed to 17β-estradiol (E2) (Sigma) by immersion.26 The concentration used was 5 μg/L (18 nM), because it was the most affective concentration in previous studies and was also determined to be the E2 natural concentration in the plasma of adult vitellogenic female zebrafish.19,27 The reason for using zebrafish males in E2 treatment experiments is because levels of E2 are higher in the plasma of vitellogenic females, therefore, the E2 impact may be more pronounced in males and its effect on gene expression can be achieved by using the physiological level of the hormone. Ten fish were kept in each aquarium (5 L in volume) under static conditions. E2 (dissolved in 50% ethanol) was added and tissue samples were collected at each time point (4, 12, 24, and 48 h) after treatment. The concentration of ethanol was 5 μL per liter (0.0005%). Water was changed and E2 was refreshed once a day during the incubation period. Only ethanol (25 μL in total) was added to the control (0 h) fish aquarium. Plasma samples were collected using heparinized capillary tubes. The concentration of 17β-estradiol in the plasma was determined by the enzyme-linked immunosorbent assay (ELISA, Estradiol EIA kit; Cayman Chemicals), according to the manufacturer's instructions. The sex of each sampled fish was confirmed by dissection. Samples were frozen instantly in liquid nitrogen and stored at −80°C.
Other samples used in the current study were those from vitellogenic (group Vit 2) and nonvitellogenic (group NV) females of Experiment 2, described in detail in Levi et al.27. In brief, juvenile zebrafish were maintained under the regular photoperiod cycle of 14 h light/10 h dark (Vit 2). Nonvitellogenic females (NV) were obtained by exposing the fish to photoperiod conditions of 6 h light/18 h dark. The two groups were kept at a temperature of 25°C until they reached the age of 2 months. Finally, liver RNA samples of vitellogenic and nonvitellogenic females were used to compare gene expression profiles using microarray experiments.27
RNA extraction, polyadenylation, and reverse transcription
RNA extraction was carried out using the TRI reagent (Sigma). One milliliter of the reagent was added to liver tissues and the samples were homogenized. Polyadenylation and reverse transcription of RNA were carried out as described before by Shi and Chiang.28 Total RNA (2 μg) was treated with RQ1 DNase I (Promega) and the treated RNA was polyadenylated by poly(A) polymerase at 37°C for 1 h, using the A-plus Poly(A) polymerase Tailing Kit (Epicentre). The RNA was reverse transcribed using 200 U of SuperScript II Reverse Transcriptase (Invitrogen) and 1 μg of a poly(T) adapter: GCGAGCACAGAATTAATACGACTCACTATAGGTTTTTTTTTTTTVN at 42°C for 1 h.
Semiquantitative reverse transcriptase-polymerase chain reaction
Total RNA was reverse transcribed using oligo(dT) primer (dT 23 VN) and Bio-RT Reverse Transcriptase (Bio-Lab Ltd.). Amplification of cDNAs was carried out with specific primer sets (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/zeb), using Ready Mix (Bio-Lab Ltd.), for 35 cycles with a final annealing temperature of 60°C. Polymerase chain reaction (PCR) products were analyzed on an ethidium bromide-stained 1.5% agarose gel.
Primer design and real-time reverse transcriptase-polymerase chain reaction
For miRNA analysis, total RNA was polyadenylated and reverse transcribed, while for mRNA analysis, total RNA was reverse transcribed as described above. In both cases, the PCR mixture consisted of 1 μL of the cDNA sample, 70 nM of each primer, and 12.5 μL of SYBR Green mix (ABgene), in a final volume of 25 μL. Amplification was carried out in a StepOnePlus Real-Time PCR System (Applied Biosystems), under the following conditions: initial denaturation at 95°C for 15 min, followed by 40 cycles of denaturation at 95°C for 15 s, and annealing extension at 60°C for 1 min. Amplification of cDNAs was performed in triplicates and the standard deviation of mean values was lower than 0.16 in all determinations. The relative gene expression was normalized to the amount of the elongation factor 1α29 (ef1α) using the formula 2−ΔCt with ΔCt=(Ct mRNA−Ct ef1α). The relative miRNA expression was calculated using ΔCt=(Ct miRNA−Ct reference RNA), while in addition to ef1α, small nuclear RNAs U11 and 7SK also served as reference genes, as more than one reference gene has the benefit of more accurate calculated normalization than a single gene.30 ΔCt corresponds to the difference between the cycle threshold (Ct) measured for the target gene and the Ct measured for the reference gene. Analysis of real-time reverse transcriptase-polymerase chain reaction (RT-PCR) data was performed using the REST-384 beta version 2 software.31 For the detection of mature miRNAs, the forward-specific primer sequence was the same as the mature miRNA sequence.28 Sequences were derived from the Sanger Institute miRBase database and from Kloosterman et al.32 The reverse primer (GCGAGCACAGAATTAATACGAC) in the amplification was fixed in all the reactions.28 The list of primers is shown in Supplementary Table S1. Primers (purchased from Sigma) were designed by Primer3 program (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi). They were validated for amplification efficiency and resulted in a single band by gel electrophoresis. PCR products and 10 bp OrangeRuler marker (Fermentas) were analyzed on the ethidium bromide-stained 12% polyacrylamide gel.
Gene expression microarrays of male zebrafish livers after E2 treatment
Total RNA was purified by YM-30 Microcon filters (Millipore) and checked for RNA integrity by 2100 Bioanalyzer (Agilent Technologies). RNA from untreated and E2-treated zebrafish was hybridized to dual-sample oligo microarray chips containing 43,603 probes on the array (V2, 4x44K; Agilent Technologies) and was analyzed using the Feature Extraction software at the Department of Biological Services, Weizmann Institute of Science. Microarray data were deposited in the Gene Expression Omnibus database (accession number GSE45562). For assay experimental design, RNA from one of the three different E2-treated fish (representing three biological replicates) of each time point was hybridized with RNA that was pooled from liver samples of 15 untreated fish (control pool) on one chip. Another chip hybridized two control pools together and served as a 0 h chip (Fig. 1B). Dye swap was used to eliminate labeling-related biases.
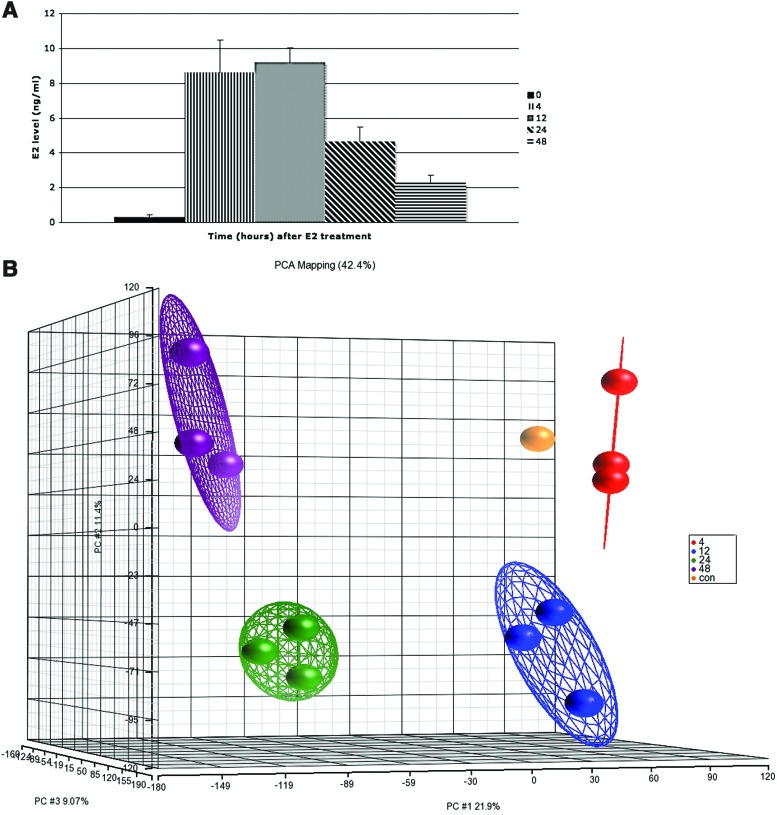
FIG. 1.
Alterations in gene expression profiles in the zebrafish liver after E2 treatment. (A) E2 concentrations in the plasma of control and E2-treated fish (mean±standard error of the mean [SEM], n=3). (B) Principal component analysis (PCA) plot of gene expression microarray results after E2 treatment. Each dot represents a chip of the different time points (4, 12, 24, 48 hrs). Another chip hybridized two control pools together and served as a control chip in the PCA plot. Performed using Partek-Genomics-Suite software. (C) Heatmap representation of regulated genes at different time points after E2 treatment. The range of expression values is from −3-fold to +3-fold. The rectangles on the left represent chips. Microarray results were hierarchically clustered using Partek-Genomics-Suite software. (D) Venn diagram of differentially expressed genes of gene expression microarrays after E2 treatment: 12 h (N), 24 h (O), 48 h (P). (E) Enriched gene ontology terms in gene expression microarray results after E2 treatment, as were identified using the DAVID functional annotation tool. Shown are significant-log2 Benjamini p-values of biological process terms (level 4) of differentially expressed genes at 24 and 48 h. Color images available online at www.liebertpub.com/zeb
miRNA microarray of male zebrafish livers after E2 treatment
miRNA microarray analysis was performed by Exiqon. The quality of the total RNA was verified by an Agilent 2100 Bioanalyzer (Agilent Technologies). One hundred fify nanogram total RNA from samples and references was labelled with the Hy3™ and Hy5™ fluorescent label, respectively, using the miRCURY™ LNA Array power labelling kit (Exiqon) following the procedure described by the manufacturer. The Hy3-labeled samples and a Hy5-labeled reference RNA sample were mixed pairwise and hybridized to the miRCURY LNA Other Species array version 11.0 (Exiqon), which contains capture probes targeting all miRNAs, but human, mouse, or rat registered in the miRBase database version 13.0 at the Sanger Institute. Sequences and chip content were submitted to ArrayExpress EMBL-EBI (accession number E-MEXP-3866). The hybridization was performed according to the miRCURY LNA array manual using a Tecan HS4800 hybridization station (Tecan). Hybridizations were performed using a common reference design. The common reference was a pool of RNA composed of 10 samples: four control samples, three samples of 24 h after E2 treatment, and three samples of 48 h after E2 treatment. The same samples were also used for the hybridizations of gene expression microarrays, as described above, to receive microarray expression data of miRNAs and mRNAs from the same biological samples. Each individual sample was then hybridized separately with the common reference. After hybridization, the microarray slides were scanned and stored in an ozone-free environment to prevent potential bleaching of the fluorescent dyes. The miRCURY LNA array microarray slides were scanned using the Agilent G2565BA Microarray Scanner System (Agilent Technologies) and the image analysis was carried out using the ImaGene 8.0 software (BioDiscovery).
miRNA microarray of vitellogenic and nonvitellogenic female zebrafish livers
miRNA microarray analysis was performed by LC Sciences. The quality of the total RNA was verified by an Agilent 2100 Bioanalyzer (Agilent Technologies). Hybridization experiments were performed with total RNA that was extracted from the livers of vitellogenic and nonvitellogenic females (group Vit 2 and group NV in Levi et al.27). Five micrograms of total RNA from untreated and E2-treated zebrafish were size fractionated (<300 nt) by YM-100 Microcon centrifugal filter (Millipore), and poly(A) tails were added to the RNA sequences at the 3′ ends using a poly(A) polymerase, and nucleotide tags were then ligated to the poly(A) tails for fluorescent dye staining later. Dual-sample array assays were used, in which, two sets of RNA sequences were added with tags of two different sequences, and then combined together. The RNA sequences were hybridized overnight in a μParaflo microfluidic miRNA microarray chip using a microcirculation pump. Tag-specific Cy3 and Cy5 staining dyes were then circulated through the microfluidic chip to complete labeling. The hybridization melting temperatures were balanced by chemical modifications of the detection probes.
In this study, each miRNA microarray chip contained 319 probes for all known zebrafish mature miRNAs. Array sequence contents were derived from the Sanger Institute miRBase database and from Kloosterman et al.32 Each miRNA probe had 10 repeats. Sequences and chip content were submitted to ArrayExpress EMBL-EBI (accession number E-MEXP-1572). Differentially color-labeled RNA from each of the four different pools (each consisting of eight livers) of vitellogenic females was hybridized with RNA from one of the four pools (consisting of eight livers) of nonvitellogenic females, to one miRNA chip. After hybridizations, the images were collected using a laser scanner (GenePix 4000B; Molecular Device) and digitized using Array-Pro image analysis software (Media Cybernetics). The dye swap design approach was used to eliminate labeling-related biases between samples.
Statistical data analysis
Gene expression microarray data (Agilent Technologies) were analyzed using Partek-Genomics-Suite (PGS) v6.6 software.
Normalization of microarray data was performed by LOWESS33 (LOcally WEighted Scatterplot Smoothing), followed by one-way analysis of variance (ANOVA) with the false discovery rate (fdr) <0.05.
miRNA microarray analysis (LC Sciences) included local background subtraction and LOWESS normalization of log2-transformed intensities. A miRNA to be listed as detectable must meet at least two conditions: signal intensity higher than 3×(background standard deviation) and spot coefficient of variation <0.5, where coefficient of variation is calculated as (standard deviation/signal intensity). In addition, where signals were detected for <3 of the repeats, they were considered unreliable and excluded from sets of detected miRNAs. p-Values of the t-test were calculated. Differentially detected signals were those with p<0.01 (for log2-ratio of Cy5/Cy3 signals).
miRNA microarray data (Exiqon) were analyzed using PGS v6.6 software. After reducing the background median values from signal median values, log2(Hy3/Hy5) ratios were used to reveal the variation across sample groups versus the control. One-way ANOVA was performed with the fdr <0.05.
To reveal miRNAs that have more E2 upregulated or downregulated putative targets than are expected by chance, a Z-test was performed on results obtained by PGS v6.6 software (enrichedAssociations outputs presented in Table 2) and p-values were calculated.
Table 2.
Most Over-Represented miRNA Target Sets After E2 Treatment Partek-Genomics-Suite Software Output Results Describing the 20 Most Over-Represented miRNA Target Sets at 24 and 48 h After E2 Treatment
| microRNA | Enrichment p-value | Number of significant gene argets | Number of upregulated significant targets | Number of downregulated significant targets |
|---|---|---|---|---|
| 24 h | ||||
| 184 | 1.26e-12 | 74 | 55 | 19 |
| 146a | 2.35e-12 | 81 | 51 | 30 |
| 430c | 2.47e-12 | 72 | 47 | 25 |
| 460-3p | 8.31e-12 | 80 | 61 | 19 |
| 489 | 5.32e-11 | 72 | 53 | 19 |
| 124 | 1.04e-10 | 78 | 46 | 32 |
| 1 | 4.26e-10 | 67 | 44 | 23 |
| 16a | 1.60e-09 | 66 | 44 | 22 |
| 181b | 2.24e-09 | 72 | 47 | 25 |
| 462 | 6.67e-09 | 56 | 31 | 25 |
| 451 | 1.37e-08 | 64 | 40 | 24 |
| 732 | 2.08e-08 | 63 | 40 | 23 |
| 130c | 4.09e-08 | 69 | 40 | 29 |
| 142b-5p | 4.34e-08 | 58 | 37 | 21 |
| 216b | 6.85e-08 | 73 | 50 | 23 |
| 194b | 8.29e-08 | 64 | 37 | 27 |
| 101a | 9.58e-08 | 70 | 42 | 28 |
| 17a | 9.73e-08 | 68 | 51 | 17 |
| 199 | 1.14e-07 | 63 | 39 | 24 |
| 7b | 1.22e-07 | 61 | 37 | 24 |
| 48 h | ||||
| 184 | 9.82e-19 | 112 | 77 | 35 |
| 457b | 9.29e-17 | 99 | 58 | 41 |
| 16b | 2.58e-16 | 104 | 69 | 35 |
| 146a | 2.86e-14 | 113 | 66 | 47 |
| 1 | 6.29e-13 | 97 | 61 | 36 |
| 133c | 7.88e-13 | 105 | 64 | 41 |
| 462 | 1.34e-12 | 84 | 49 | 35 |
| 460-3p | 1.41e-12 | 109 | 71 | 38 |
| 124 | 1.78e-11 | 107 | 58 | 49 |
| 155 | 2.13e-11 | 94 | 58 | 36 |
| 152 | 3.91e-11 | 101 | 57 | 44 |
| 430c | 4.07e-11 | 92 | 55 | 37 |
| 499 | 1.40e-10 | 89 | 51 | 38 |
| 181a | 1.46e-10 | 98 | 66 | 32 |
| 206 | 2.33e-10 | 87 | 54 | 33 |
| 723 | 3.32e-10 | 95 | 64 | 31 |
| let-7d | 3.45e-10 | 105 | 53 | 52 |
| 725 | 3.74e-10 | 95 | 52 | 43 |
| 148 | 4.10e-10 | 97 | 52 | 45 |
| 200a | 7.50e-10 | 111 | 71 | 40 |
Enrichment p-value represents the Fisher's exact test p-value.
Bioinformatics data analysis
All miRNA sequences were obtained from the Sanger Institute miRBase database34 (http://microrna.sanger.ac.uk/sequences/). The MicroCosm Targets v5 program (http://ebi.ac.uk/enright-srv/microcosm/htdocs/targets/v5/) was used to reveal putative target genes of miRNAs. Gene ontology (GO) terms were obtained from the Zebrafish Information Network database35 (http://zfin.org/). A DAVID v6 functional annotation tool36 (http://david.abcc.ncifcrf.gov/) was used to identify enriched GO terms. Zebrafish 3′ untranslated region (3′UTR) sequences were obtained from the UCSC genome browser37 (http://genome.ucsc.edu/). Gene expression analysis of microarray data was performed using PGS v6.6 software. Pathway analysis was performed using Advanced Pathway Painter v2.29. ClustalW program was used for multiple sequence alignments of zebrafish sequences38 (http://ebi.ac.uk/Tools/msa/clustalw2/). The miRanda software v1 was used for finding miRNA target sites in the 3′UTR sequences.39
Results
E2-responsive genes in the zebrafish liver are affected in a temporal manner as revealed by transcriptome profiling
A time-course experiment was conducted exposing adult zebrafish males (n=3) to E2 for 0, 4, 12, 24, and 48 h and microarrays (Agilent Technologies zebrafish chips) were performed to reveal changes in the gene expression level in the liver. The E2 level in the plasma was measured by ELISA to confirm the efficacy of the treatment. Mean values for E2 in the plasma of fish ranged from 0.3 ng/mL (controls) to 9.1 ng/mL, in treated fish (Fig. 1A). Principal component analysis (PCA) shows the distinct clustering of the microarray data according to the time point of the experiment (Fig. 1B). To evaluate the kinetic of gene expression in the zebrafish liver, hierarchical clustering was performed, revealing two phases of the E2 effect during early (4–12 h) and late (24–48 h) time points (Fig. 1C). Statistical analysis (one-way ANOVA, p<0.05, fold change <−1.7 or >1.7) revealed 360 differentially expressed genes that were changed at 12 h, 2540 at 24 h, and 3905 at 48 h. Overall, results revealed 186 genes that were upregulated and 174 genes that were downregulated 12 h after E2 treatment, 1382 genes were upregulated and 1158 genes were downregulated at 24 h, and 2149 genes were upregulated and 1756 genes were downregulated during 48 h after E2 treatment. Microarray results were confirmed by evaluating the expression of mRNAs using real-time PCR (Pearson correlation of 85%). The overlap between differentially expressed transcripts at different time points (12, 24, 48 h) of gene expression microarray results after E2 treatment was compared using a Venn diagram (Fig. 1D). GO enrichment analysis was created by the DAVID program to reveal enriched biological processes in lists of differentially expressed genes of 24 and 48 h after E2 treatment (Fig. 1E). Among them are dozens of genes related to the processes of ribosome biogenesis and translation that were upregulated during the different time points (Supplementary Table S2). Microarray results identified a large number of upregulated genes coding for ribosomal proteins (RPs), genes involved in rRNA and tRNA transcription, modification, and processing; components of RNA polymerase I (polr1a, Polr1c) and RNA polymerase III (polr3b, polr3d), genes coding for subunits of RNA processing complexes such as RNase P/RNase MRP (rpp21, pop5), the exosome (exosc9, exosc10), and the processome (utp13, utp20). In addition, included are genes involved in modifications of rRNA and tRNA precursors, such as methyltransferases and pseudouridine synthases (trdmt1, trm5, pus1). Other upregulated genes related to ribosome biogenesis are the DEAD-box family of RNA helicases and genes important for nuclear export and transport of the ribosome subunits (tsr1, ltv1). Some of the genes mentioned above were already induced during the early time points (12 h) and remained upregulated during late time points (24–48 h), while the induction observed in RP genes and genes involved in translation such as initiation and elongation factors and tRNA synthetases was mainly during later time points (24–48 h) after E2 treatment. Several of the genes that were upregulated after E2 treatment in the liver of male zebrafish were also induced in livers of vitellogenic female zebrafish (Supplementary Table S2), indicating a possible role for these genes in the reproduction process of zebrafish.27
E2 treatment leads to comprehensive miRNA repression in the zebrafish liver
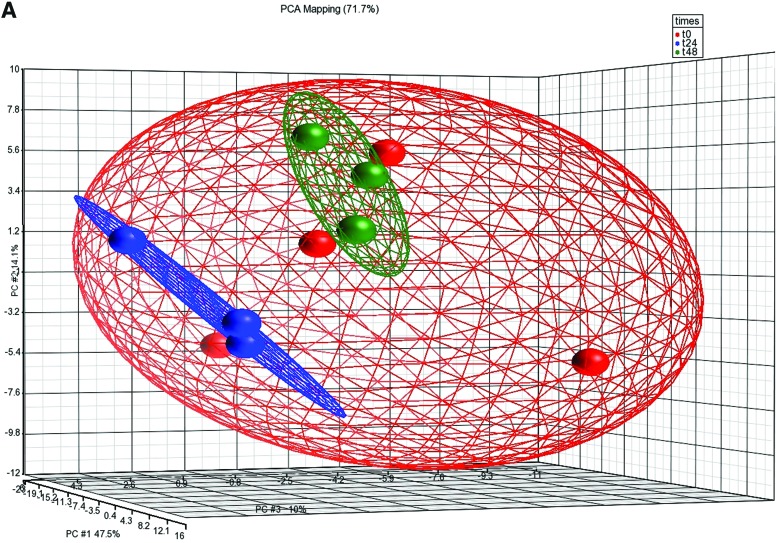
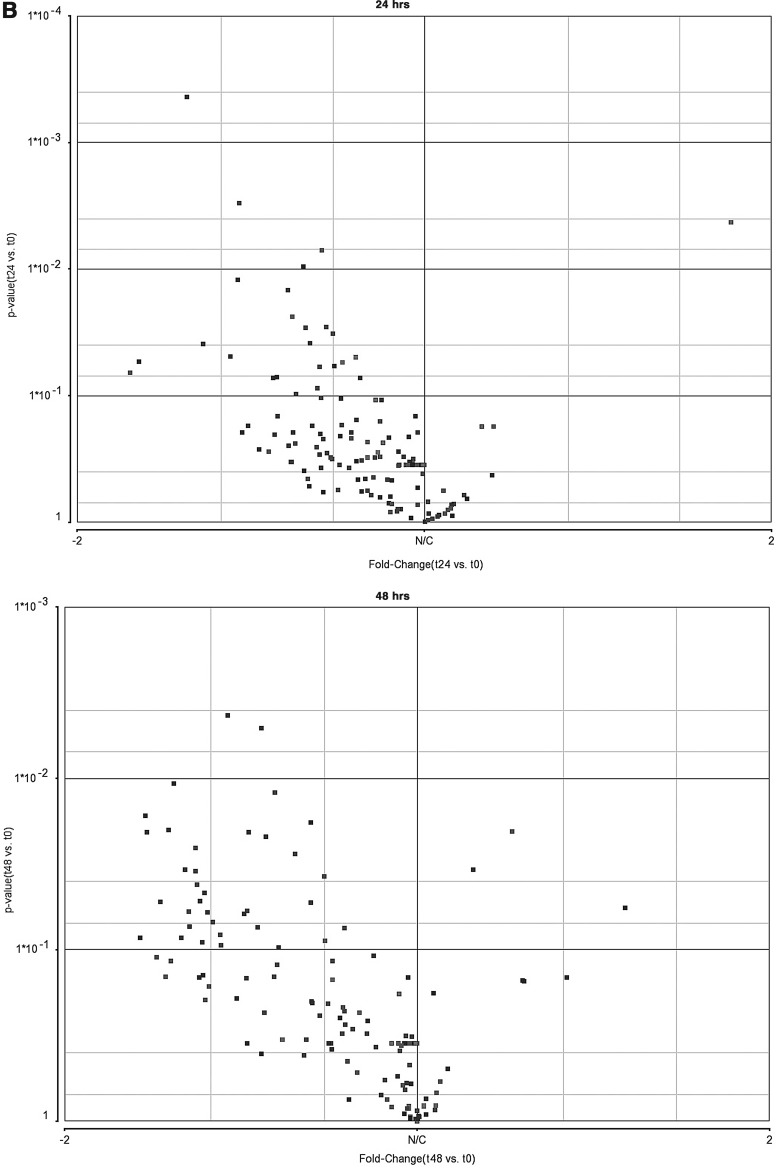
As mentioned above, the liver is the main tissue that responds to E2 and synthesizes Vtgs. miRNA expression patterns were previously revealed in different zebrafish tissues after E2 treatment and most interestingly, the expression level of most miRNAs in the liver was reduced after E2 treatment.19 To reveal whether downregulation of miRNA expression in the liver after exposure to E2 is a more general phenomenon, using the same total RNA that was also used for gene expression microarrays, RNA samples of 0, 24, and 48 h after E2 treatment were used for miRNA microarrays (Exiqon). The expression was validated by real-time PCR (Pearson correlation of 89%). PCA was performed on miRNA expression data showing the distinguished distribution of samples from different groups (Fig. 2A). Volcano plots of miRNA microarray indicate a considerable bias toward downregulation of miRNAs at 24 and 48 h after E2 treatment (Fig. 2B). Similarly, the list of differentially expressed miRNAs (one-way ANOVA, p<0.05, fold change <−1.4 or >1.4) show that the vast majority of miRNAs are downregulated: five out of six miRNAs at 24 h and all 10 miRNAs at 48 h after E2 treatment (Table 1).
FIG. 2.
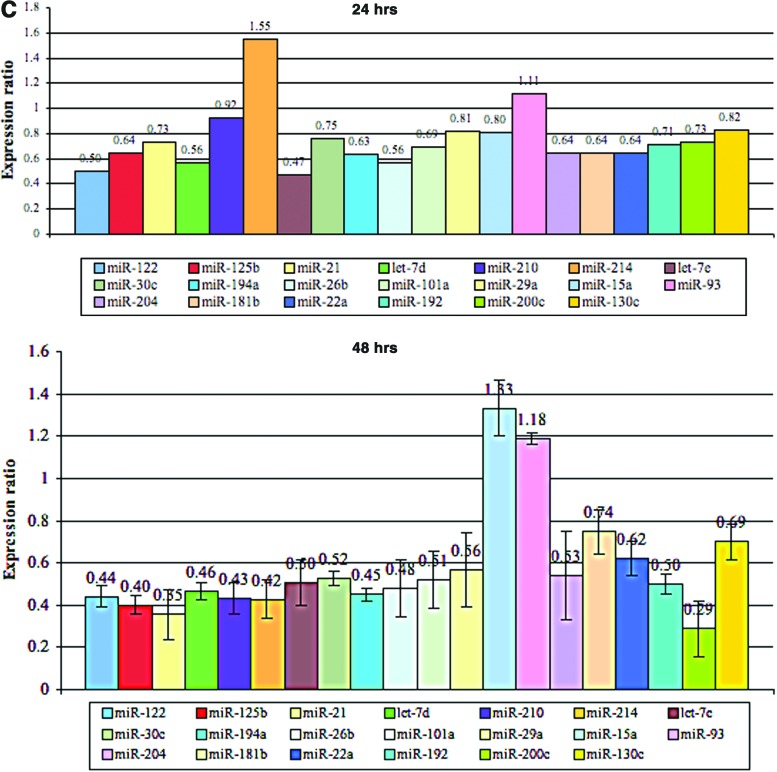
Alterations in microRNA (miRNA) expression profiles in the zebrafish liver after E2 treatment. (A) PCA plot of miRNA microarray results after E2 treatment: each dot represents a chip of the different time points (0, 24, 48 hrs). Performed using Partek-Genomics-Suite software. (B) Volcano plots of miRNA microarray results after E2 treatment at 24 and 48 h. Performed using Partek-Genomics-Suite software. (C) Relative real-time reverse transcriptase-polymerase chain reaction (RT-PCR) results of 20 miRNAs in the male zebrafish liver after E2 treatment at 24 h (mean, n=2) and 48 h (mean±SEM, n=3). Color images available online at www.liebertpub.com/zeb
Table 1.
microRNA Microarray Results After E2 Treatment
| microRNA | p-Value | Fold change |
|---|---|---|
| 24 h | ||
| miR-107 | 0.0004 | −1.60 |
| miR-338 | 0.003 | −1.44 |
| miR-733 | 0.004 | 1.84 |
| miR-126* | 0.01 | −1.45 |
| let-7d | 0.03 | −1.55 |
| miR-126 | 0.04 | −1.47 |
| 48 h | ||
| miR-125a | 0.004 | −1.45 |
| miR-145 | 0.01 | −1.61 |
| miR-143 | 0.01 | −1.70 |
| miR-126 | 0.01 | −1.63 |
| miR-125b | 0.02 | −1.70 |
| miR-199 | 0.02 | −1.54 |
| let-7d | 0.03 | −1.57 |
| miR-26b | 0.03 | −1.54 |
| miR-26a | 0.04 | −1.54 |
| miR-101b | 0.04 | −1.51 |
microRNA microarray (Exiqon) results at 24 and 48 h after E2 treatment in male zebrafish livers.
Lists of differentially expressed miRNAs (one-way analysis of variance, p<0.05, fold change <1.4 or >1.4) were achieved using Partek-Genomics-Suite software.
Evaluation of relative expression levels of 20 miRNAs, which was examined by real-time PCR, shows a decrease in the expression level of most miRNAs that were tested (except for miR-93 and miR-214) 24 h after E2 treatment, relative to control (Fig. 2C). Intriguingly, the mean expression level of most of these miRNAs was further decreased 48 h after E2 treatment (exceptions are miR-93 and miR-15a) (Fig. 2C).
A significant bias toward upregulation of miRNA putative target genes after E2 treatment in the zebrafish liver
Numerous miRNA-mRNA targeting associations were reported after estrogen exposure of breast cancer cells, by integration of gene expression profiling with miRNA target predictions.40 PGS software provides a method, based on Creighton et al.40 bioinformatics tools, for analyzing and integrating microarray gene expression data and a list of genes retrieved from a specified database, in the case of this study, the MicroCosm Targets database, to identify miRNAs that putatively regulate these genes in a statistically significant manner. Using the option, “Finding over-represented miRNA target sets from gene expression data” in the PGS software, a Fisher's exact test p-value was calculated for enrichment of a set of targets for a particular miRNA within the differentially expressed genes of each time point after E2 treatment. Numerous miRNAs that were over-represented (Fisher's exact test, p<0.05) were found in the differentially expressed gene sets and the 20 most enriched miRNA targeting associations are listed for 24 and 48 h after E2 treatment (Table 2). From these results, the numbers of significantly upregulated and downregulated genes, targeted by a particular miRNA, were used to reveal miRNAs with significantly more upregulated or downregulated putative targets after E2 treatment. Two statistical tests were performed for each time point, using the ratios between upregulated and downregulated genes in the differentially expressed gene lists, to reveal miRNAs that have a significantly (Z-test, p<0.05) higher percentage of upregulated putative targets or downregulated putative targets than are expected by chance. The results show a bias toward miRNAs that have more upregulated putative targets at 12 h after E2 treatment: 34 miRNAs show a significantly higher percentage of upregulated putative targets versus 4 miRNAs that show a significantly higher percentage of downregulated putative targets (Supplementary Table S3). Similarly, a clear bias was also observed for the late time points: 89 versus 0, at 24 h, and 53 versus 0, at 48 h (Supplementary Table S3).
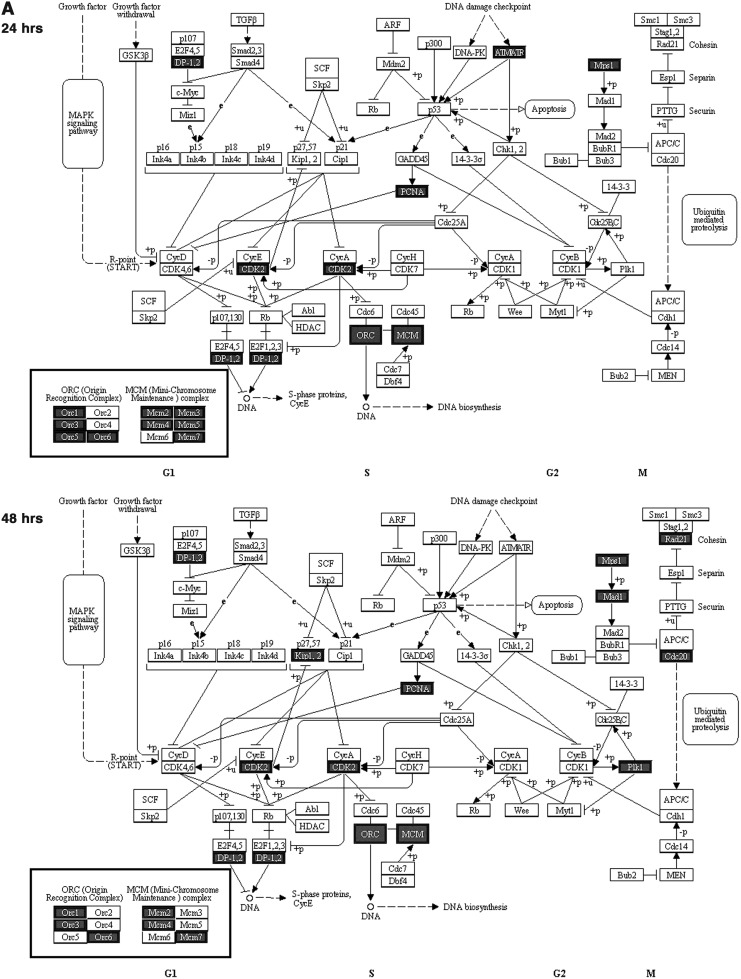
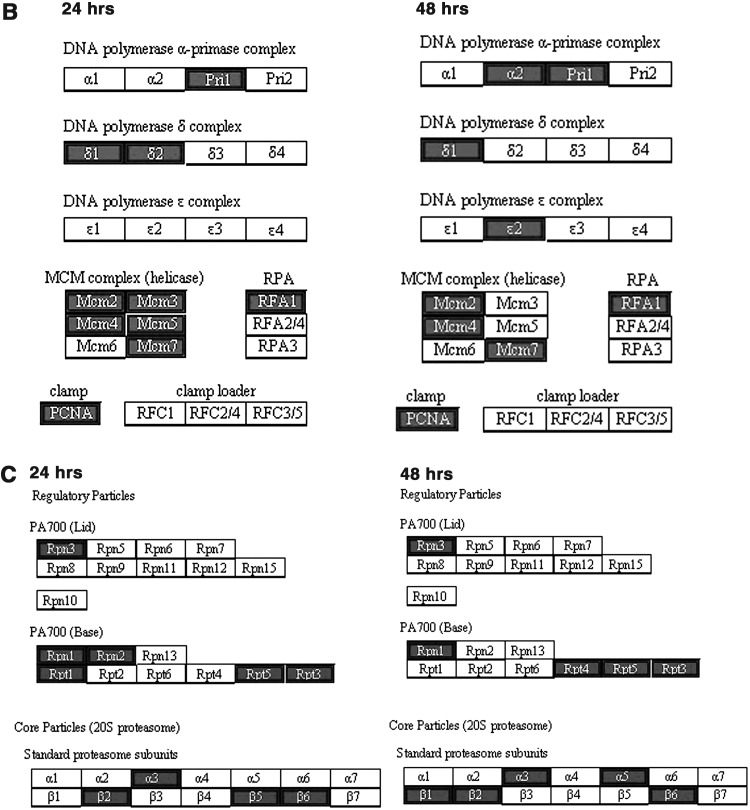
To reveal which processes the miRNAs and their putative targets are involved, the Advanced Pathway Painter program was used to find enriched pathways, based on the humam Kyoto encyclopedia of genes and genomes pathway database. Putative target sets of the 20 most over-represented miRNAs with most significant enrichment p-values at 24 and 48 h after E2 treatment (Table 2) were loaded into the program. The results reveal three enriched pathways: cell cycle, DNA replication, and proteasome (Fig. 3).
FIG. 3.
Enriched pathways of the most over-represented miRNA targets after E2 treatment. (A) Cell cycle. (B) DNA replication. (C) Proteasome. Differentially expressed upregulated genes are marked in dark color. Performed by the Advanced Pathway Painter program using the 20 most over-represented miRNAs target sets at 24 and 48 h after E2 treatment (Table 2).
Hepatic miRNA expression profiles of vitellogenic compared to nonvitellogenic female zebrafish
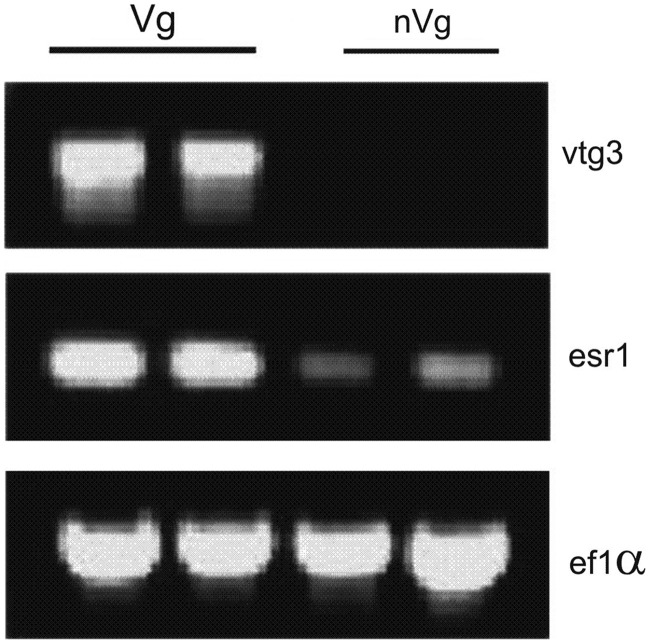
Liver samples of vitellogenic females show a prominent expression of esr1 and vtg3 by RT-PCR, in contrast to nonvitellogenic females (Fig. 4). To identify miRNAs expressed during vitellogenesis, hepatic miRNA expression profiles of vitellogenic females were compared with those of nonvitellogenic females, using miRNA microarrays (LC Sciences). Among the 44 differentially expressed miRNAs (t-test, p<0.01), were five members of the miR-17-92 cluster: miR-17a, miR-19a, miR-19b, miR-20a, and miR-92a and all of them were highly expressed in vitellogenic females (Table 3[A]). miRNAs showing lower expression levels in vitellogenic females include members of the miR-200 superfamily: miR-141, miR-200a, miR-200b, miR-200c, miR-429, and the liver-specific miR-122 (Table 3[B]). Among the differentially expressed miRNAs are also additional members of the miRNA families: miR-23a/b, miR-26a/b, miR-27a/b/c, miR-146a/b, and miR-148/152. Microarray results were verified by real-time RT-PCR (Pearson correlation of 87%). Among the differentially expressed miRNAs are several miRNAs specific to oviparous vertebrates: ZF_nl_249 (Danio rerio), miR-462 (D. rerio, Oryzias latipes), miR-738 (D. rerio, Cyprinus carpio), ZF_nl_302 (D. rerio, Fugu rubripes, Tetradon nigroviridis), miR-456 (D. rerio, Petromyzon marinus, Taeniopygia guttata, Anolis carolinensis, Gallus galus), miR-457a (D. rerio, C. carpio, A. carolinensis), and miR-458 (D. rerio, F. rubripes, T. nigroviridis, O. latipes, T. guttata, A. carolinensis, G. galus, Ornithorynchus anatinus).
FIG. 4.
Relative mRNA levels in livers of vitellogenic (Vg) and nonvitellogenic (nVg) female zebrafish. Results shown are of semiquantitative RT-PCR reactions, using primer sets for esr1, vtg3, and ef1α. Each lane contains pooled samples of eight fish. PCR products were analyzed on the ethidium bromide-stained 1.5% agarose gel.
Table 3.
microRNA Microarray Results of Vitellogenic Versus Nonvitellogenic Females
| miRNA | Vg/nVg (fold) |
|---|---|
| (A) Upregulated miRNAs | |
| miR-738 | 5.75 |
| miR-458 | 3.89 |
| miR-19a | 3.83 |
| miR-19b | 3.3 |
| ZF_n1_249 | 3.27 |
| miR-148 | 3.26 |
| miR-19d | 3.22 |
| miR-20a | 2.04 |
| miR-457a | 2.01 |
| miR-92b | 1.9 |
| miR-93 | 1.75 |
| miR-17a | 1.74 |
| miR-456 | 1.74 |
| miR-19c | 1.74 |
| miR-92a | 1.71 |
| miR-152 | 1.69 |
| miR-20b | 1.65 |
| miR-30e | 1.55 |
| miR-16b | 1.42 |
| (B) Downregulated miRNAs | |
| miR-146a | 0.32 |
| miR-200c | 0.34 |
| miR-200b | 0.36 |
| miR-462 | 0.43 |
| miR-141 | 0.45 |
| miR-221 | 0.45 |
| ZF_n1_302 | 0.46 |
| miR-200a | 0.47 |
| miR-146b | 0.48 |
| miR-126 | 0.48 |
| miR-27c | 0.48 |
| miR-24 | 0.49 |
| miR-107 | 0.51 |
| miR-27b | 0.51 |
| miR-23a | 0.53 |
| miR-214 | 0.53 |
| miR-23b | 0.54 |
| miR-21 | 0.55 |
| miR-429 | 0.57 |
| miR-150 | 0.58 |
| miR-145 | 0.59 |
| miR-27a | 0.61 |
| miR-26b | 0.67 |
| miR-26a | 0.74 |
| miR-122 | 0.84 |
Microarray analysis (LC Sciences) results showing differentially expressed miRNAs (t-test, p<0.01) in livers of vitellogenic (Vg) compared to nonvitellogenic (nVg) female zebrafish (pooled samples of 32 fish).
Shown in bold are members of the miR-17-92 cluster (upregulated) and miR-200 superfamily (downregulated).
Putative miRNA target genes with a potential role in vitellogenesis
miRNA microarrays were performed from the same total RNA of vitellogenic and nonvitellogenic female zebrafish, which was previously used for gene expression microarray experiments described in detail in Levi et al.27 Using bioinformatics data retrieved from the MicroCosm Targets database, the expression of predicted target genes was integrated with the expression profiles of their corresponding miRNAs. Integration of the results revealed 55 anticorrelated putative target genes for their corresponding 10 miRNAs (Supplementary Table S4).
Among the biological processes of the potential target genes were ribosome biogenesis and translation: rpl8 (ribosomal protein L8), rps10 (ribosomal protein S10), rrbp1 (ribosome binding protein 1), tsr1 (20s rRNA accumulation), ddx51 (DEAD box polypeptide 51), rrp1 (ribosomal RNA processing 1), and rpp30 (ribonuclease P/MRP 30 kDa subunit) (Supplementary Table S4).
Other interesting putative target genes are related to steroidogenesis: hsd3b7 (hydroxy-delta-5-steroid dehydrogenase, 3 beta- and steroid delta-isomerase 7), hsd11b3 [hydroxysteroid (11-beta) dehydrogenase 3], and lipid metabolism: apoeb (apolipoprotein Eb precursor), agpat4 (1-acylglycerol-3-phosphate O-acyltransferase 4), and degs2 (degenerative spermatocyte homolog 2, lipid desaturase).
During the evaluation of vitellogenin genes as predicted target genes for miRNAs, vtg3 was identified as a putative target gene for miR-122 and miR-107 (Table 4). Moreover, two different putative target sites for miR-122, separated by 44 nt, were detected in the 3′UTR sequence of vtg3 (Table 5). These target sites were not found in other zebrafish vtg gene sequences, highlighting the distinctively unique sequence of vtg3 and its potential function as a miR-122 target gene. Intriguingly, another pair of miR-122 target sites was also identified in the vtg3 coding sequence (Supplementary Fig. S1). These target sites, separated by only 8 nt, contain a full miR-122 recognition motif (CACTCC seed match), which were not found in other zebrafish vtg gene sequences.
Table 4.
Putative vtg miRNA Target Genes
| Putative target gene | ENSEMBL_ID | miRNAs |
|---|---|---|
| Vtg1 | ENSDART00000105263 | 33, 367, let-7, 183, 15a* |
| vtg 2 | ENSDART00000061165 | 214, 459* |
| vtg3 | ENSDART00000103418/ENSDART00000103413 | 1, 19, 223, 206, 122, 133/103, 107, 456, 1, 100, 489, 99 |
| vtg4 | ENSDART00000105237 | 34, 138, 15, 460-5p |
| vtg5 | ENSDART00000105247 | 98, let-7, 19* |
| vtg6 | ENSDART00000105228 | 23, 727*, 202* |
| vtg7 | ENSDART00000105208 | 499, 107, 182, 126* |
A list of zebrafish vtg genes (vtg1–vtg7) that were predicted as miRNA targets (derived from MicroCosm Targets). Shown in bold are anticorrelated miRNAs that were differentially expressed in livers of vitellogenic compared to nonvitellogenic female zebrafish (Table 3).
Table 5.
Putative vtg miRNA Target Genes of Differentially Expressed miRNAs
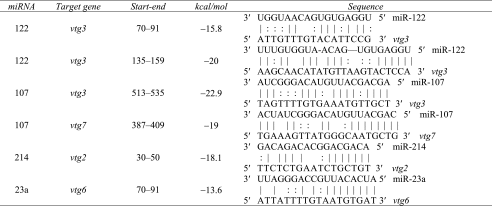 |
A list of miRNAs that were differentially expressed in livers of vitellogenic compared to nonvitellogenic female zebrafish and their putative vtg target genes (derived from MicroCosm Targets). Also provided are locations of target sites in 3′UTRs and miRNA-target hybridization energy (ΔG, kcal/mol). Complementary sites in the 3′UTR sequences of putative target genes are represented by solid lines (Watson-Crick pairing) and dots (G:U wobbles).
Discussion
The results of this study show that estrogen exposure leads to widepread changes in miRNA expression profiles within the zebrafish liver. Different from the classical genomic mechanism, where ER directly regulates the transcription of target genes via DNA binding, the effect of E2 through miRNAs can represent an additional genomic pathway, where target genes are affected at the post-transcriptional level to fine tune gene expression. The observed changes can be the result of a direct effect of E2 on the transcription of miRNA genes, as was shown in the case of global miRNA repression by c-Myc.41 Another option is that E2 modulates key regulators or components of the miRNA biogenesis pathway and thereby regulates miRNA production and function. This regulation can occur at any stage of the miRNA maturation process, whether at the level of pri-miRNA processing, by affecting the microprocessor complex Drosha-DGCR8, or at the pre-miRNA processing level, by altering the activity of Dicer. One possible advantage for a global regulation effect of miRNA could be the possibility to affectively control a wide range of gene expression with a relatively low energetic cost by regulation of a single gene that acts upstream in the miRNA biogenesis pathway.
Strikingly, as shown above, E2 treatment leads to widespread miRNA repression in the zebrafish liver. Since regulation by miRNAs resulted in miRNA-mediated gene repression, it can be assumed that the regulation by repressed miRNAs is weakened and as a consequence, the mRNA stability of target genes is increased. Accordingly, our study shows that putative targets of miRNAs predominantly tend to be upregulated after E2 treatment. These anticorrelated trends are in line with the known role of miRNAs as negative regulators of gene expression and were also identified in other cellular and physiological systems. Global downregulation of miRNAs was observed in effector T cells compared to naïve cells.42 During an immune response, quiescent naïve T cells are known to proliferate enormously and develop into actively dividing effector T cells.42 Another study by Izzotti et al.43 shows miRNA downregulation in the lungs of rats exposed to environmental cigarette smoke, which also appears to be related to an induction of cell proliferation. A massive downregulation of miRNAs is also commonly observed in human cancers, where miRNAs show lower expression levels in tumors and cancer cell lines compared with normal tissues, suggesting that these miRNAs downregulate oncogenes.44 Furthermore, Maillot et al.,20 show that the expression of a broad set of miRNAs decreases following E2 treatment in breast cancer MCF-7 cell lines. They observed that 23 miRNAs were downregulated following E2 treatment; most of them were also downregulated during this study. Moreover, they show the involvement of several of the repressed miRNAs in E2-dependent cell growth and proliferation.20 This is further supported by the study of Yu et al.,45 which shows a set of E2-repressible miRNAs in MCF-7 cells that is associated with altered cell proliferation and demonstrates that those miRNAs are coordinately suppressed to upregulate their target genes, which are involved in cell cycle progression and survival, and play a role in controlling breast tumor growth. Together, these findings suggest that in response to E2 stimulation, global downregulation of miRNA contributes to cell growth and proliferation and cellular transformation.
One of the genes most related to cell proliferation is c-myc.46 Estrogen can cause cellular growth, proliferation, and cancer by inducing proto-oncogenes such as c-myc.47 The transcription factor c-Myc physically interacts with ERα and is recruited to estrogen-responsive genes.48 A recent study shows that c-Myc amplifies the output of existing transcriptionally active genes in tumor cells and thus reduces rate-limiting constraints for growth and proliferation.49 Interestingly, a widespread miRNA repression by c-Myc was also reported and the observed substantial downregulation of miRNAs was correlated with enhanced cellular proliferation after c-Myc activation.41 Furthermore, c-Myc activates expression of a cluster of six miRNAs termed miR-17-92 cluster.50 miR-17-92 was shown to promote oncogenesis and enhance cell proliferation.51–53 Indeed, c-Myc is recruited to the miR-17-92 promoter in breast cancer cells upon E2 stimulation.54 miR-17-92 is a conserved miRNA cluster and contains six miRNA members that are processed from a single primary transcript: miR-17a, miR-18a, miR-19a, miR-19b, miR-20a, and miR-92a.55 According to miRNA microarray results shown here, the miR-17-92 cluster miRNA members were not among the majority of downregulated miRNAs after E2 treatment, although they were not upregulated either.
Other results that support the relation of E2 downregulated miRNAs with cell growth and proliferation are the three enriched pathways of putative targets: cell cycle, DNA replication, and proteasome. Among the miRNA putative targets are several cell cycle and DNA replication-related genes, which are also used as proliferation markers in cancer, such as pcna (proliferating cell nuclear antigen) and mcm genes.56 Other putative targets belong to the 26S proteasome complex; whether as subunits of the 20S core particle or its regulatory particles. The proteasome has a role in cell proliferation as negative regulators of cell cycle undergoing ubiquitin-mediated proteolysis to allow cell cycle progression.57
One approach that may assist in refining the prediction of miRNA target genes is to combine miRNA and gene expression profiles with computational prediction, for identification of miRNA-target associations.58–60 During this study, the combination of computational predictions with data obtained from miRNAs and gene expression microarrays, performed from the same biological samples, showed a significant bias toward upregulation of putative miRNA target genes after E2 treatment. Moreover, using this approach we show, to our knowledge for the first time, an association of miRNA expression with the process of vitellogenesis, in the liver of an egg-laying vertebrate, the zebrafish. The observation that miR-122 expression was lower in the liver of vitellogenic females was of special interest. Hepatic miR-122 expression was also decreased after E2 treatment in the male zebrafish. miR-122 is the most abundant miRNA in the liver of several organisms, including mammals, where it is expressed at high levels in hepatocytes, with 50,000–80,000 copies per cell.61 The existence of two different putative miR-122-binding sites in the 3′UTR sequence of the phosvitinless vtg3, an abundant and upregulated gene in the liver of vitellogenic females,14,27 may indicate a direct fine-tuning of its expression during vitellogenesis. It was previously shown that the presence of multiple miRNA-binding sites in a 3′UTR is likely to be an indicator of a strong miRNA regulation,58,62 especially when they are separated by 8–∼40 nt,63 while in the case of vtg3/miR-122, they are separated by 44 nt. It is widely accepted that miRNAs regulate gene expression by the association with 3′UTRs of their targets and several studies have shown that miRNAs can also target the coding sequence region and other parts of the target genes.62,64 Indeed, further analysis of the vtg3 coding sequence revealed two more putative miR-122-binding sites (Supplementary Fig. S1). Strikingly, these putative miR-122-binding sites are separated by just 8 nt. It was shown that expression of genes containing target sites in both coding regions and 3′UTRs are significantly more regulated than those containing target sites in 3′UTRs only.62
miR-122 has a role as the key regulator of lipid metabolism.59,65,66 Given the fact that lipids are crucial functional groups carried on the Vtg backbone,11 it is reasonable to suggest that miR-122 can also indirectly affect synthesis of lipids carried by plasma Vtgs and other lipoproteins to the ovary. Furthermore, vtg3 and vtg7 are also potential target genes for miR-107, a downregulated miRNA in vitellogenic females that was recently predicted to act on genes in lipid metabolic pathways.67
Another additional interesting result is the higher expression of miR-17-92 cluster members in livers of vitellogenic females compared to nonvitellogenic females. Notably, the miR-17-92 cluster also increased during oogenesis in growing oocytes of developing mice.68 Five of the miR-17-92 miRNA members were highly abundant in the zebrafish liver and were also upregulated in vitellogenic females, but miR-18a was barely detected in any of the liver samples. This pattern of expression is reminiscent of observations previously reported, where four members of the miR-17-92 cluster were ubiquitously expressed during early zebrafish embryonic development, except for miR-18a.69 Several functions have been documented so far for the miR-17-92 cluster, including recent reports that also describe its role in the development of normal B cells in mice.70,71 Ventura et al.71 showed that depletion of miR-17-92 leads to apoptosis in specific regions of the liver. Due to the known effects of these miRNAs as promoters of cellular proliferation and suppressors of apoptosis,72 this raises the possibility that higher expression of miR-17-92 may enhance proliferation and inhibit apoptosis in the liver during vitellogenesis and in consequence, indirectly facilitate Vtgs production and synthesis of other proteins.
A decrease in the expression of miRNAs belonging to the miR-200 superfamily was also observed in the liver of vitellogenic females. Similar miRNA expression patterns were revealed in primordial germ cells (PGCs) of developing mice.73 Hayashi et al.73 show that the miR-17-92 cluster was highly expressed, while miRNAs belonging to the miR-200 superfamily decreased gradually in developing PGCs, suggesting that these two groups of miRNAs may have synergistic functions.
Intriguingly, seven of the differentially expressed miRNAs during vitellogenesis are miRNA specific to oviparous animals (ZF_nl_249, ZF_nl_302, miR-456, miR-457a, miR-458, miR-462, and miR-738) (miRBase database release 19). Kloosterman et al.32 suggest that these miRNAs are generally expressed at low levels. However, the results of this study indicate that most of these miRNAs (ZF_nl_249, miR-456, miR-457a, miR-458, and miR-738) show relatively high expression levels in vitellogenic females and presumably can be specifically expressed during the process of vitellogenesis.
Identification of miRNA target genes relies mainly on bioinformatics predictive tools, and while experimental data are increasingly available on mammalian cells and embryos, there are limited experimental data on adult animals, including zebrafish. The results presented here provide additional important information, mostly relevant to genes associated with vitellogenesis and oocyte development. These include the vtg3 (a putative target of miR-122 and miR-107) and esr1 (a putative target of miR-214). Additionally, two hydroxysteroid dehydrogenases that serve as rate-limiting enzymes in steroidogenesis and participate in gonad differentiation and development of teleost fish74,75 were identified, hsd11b3 (a putative target of miR-148) and hsd3b7 (a putative target of miR-20a).
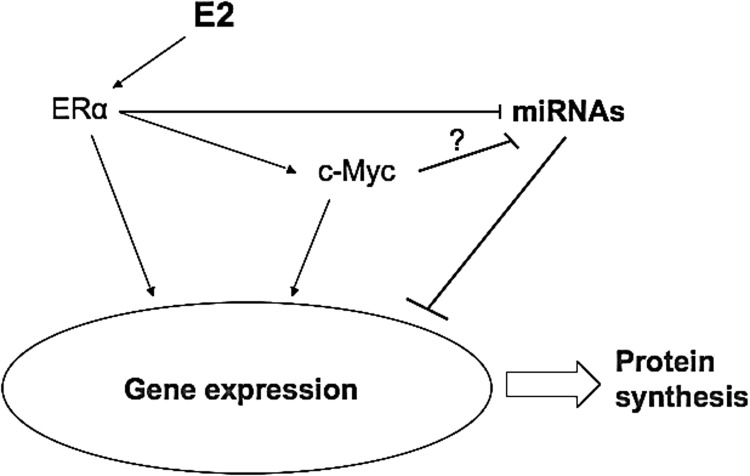
Altogether, the data presented suggest a model (Fig. 5) in which, E2, ERα, and possibly c-Myc, cause widespread miRNA repression within the zebrafish liver, consequently affecting the expression of miRNA targets, and suggest a role for miRNAs in estrogen-regulated pathways. This study shows for the first time the association of miRNA expression with vitellogenesis witin the zebrafish liver. It improves our knowledge on the process of oocyte growth in oviparous animals and because of the conserved nature of miRNA; it can be used for studies on other oviparous vertebrates and fish species with yet unknown genomes. It can also be relevant to other research areas, such as on the effects of xenoestrogens, which pollute our environment, or for cancer research, as molecular hallmarks are known to be conserved between zebrafish and human liver tumors.76
FIG. 5.
A model summarizing the relationship between key players revealed during this study. Estrogen causes widespread miRNA repression within the zebrafish liver and affects the expression of miRNA putative target genes. The transcription factor c-Myc may act as an intermediate regulator of miRNAs in this model. Arrows represent activation and blocked arrows indicate repression. The indicated “?” denotes a possible regulation by c-Myc.
Supplementary Material
Acknowledgments
Part of this study was conducted in Israel Oceanographic and Limnological Research, Haifa, Israel. We thank Dr. Liraz Levi and Dr. Esther Lubzens for the RNA samples of female zebrafish and Irena Pekarsky for technical assistance. This work was supported by the Israel Science Foundation Grant (ISF #1195/07).
Disclosure Statement
No competing financial interests exist.
References
- 1.Korach KS. Insights from the study of animals lacking functional estrogen receptor. Science 1994;266:1524–1527 [DOI] [PubMed] [Google Scholar]
- 2.McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev 1999;20:279–307 [DOI] [PubMed] [Google Scholar]
- 3.Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest 2006;116:561–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, et al. . The nuclear receptor superfamily: the second decade. Cell 1995;83:835–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem 2001;276:36869–36872 [DOI] [PubMed] [Google Scholar]
- 6.Gruber CJ, Gruber DM, Gruber IM, Wieser F, Huber JC. Anatomy of the estrogen response element. Trends Endocrinol Metab 2004;15:73–78 [DOI] [PubMed] [Google Scholar]
- 7.Schultz JR, Petz LN, Nardulli AM. Cell- and ligand-specific regulation of promoters containing activator protein-1 and Sp1 sites by estrogen receptors alpha and beta. J Biol Chem 2005;280:347–354 [DOI] [PubMed] [Google Scholar]
- 8.Manavathi B, Kumar R. Steering estrogen signals from the plasma membrane to the nucleus: two sides of the coin. J Cell Physiol 2006;207:594–604 [DOI] [PubMed] [Google Scholar]
- 9.Nagahama Y. Endocrine regulation of gametogenesis in fish. Int J Dev Biol 1994;38:217–229 [PubMed] [Google Scholar]
- 10.Peter RE, Yu KL. Neuroendocrine regulation of ovulation in fishes: basic and applied aspects. Rev Fish Biol Fisher 1997;7:173–197 [Google Scholar]
- 11.Arukwe A, Goksoyr A. Eggshell and egg yolk proteins in fish: hepatic proteins for the next generation: oogenetic, population, and evolutionary implications of endocrine disruption. Comp Hepatol 2003;2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tyler CR, Sumpter JP, Witthames PR. The dynamics of oocyte growth during vitellogenesis in the rainbow trout (Oncorhynchus mykiss). Biol Reprod 1990;43:202–209 [DOI] [PubMed] [Google Scholar]
- 13.Jalabert B. Particularities of reproduction and oogenesis in teleost fish compared to mammals. Reprod Nutr Dev 2005;45:261–279 [DOI] [PubMed] [Google Scholar]
- 14.Wang H, Tan JT, Emelyanov A, Korzh V, Gong Z. Hepatic and extrahepatic expression of vitellogenin genes in the zebrafish, Danio rerio. Gene 2005;356:91–100 [DOI] [PubMed] [Google Scholar]
- 15.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004;116:281–297 [DOI] [PubMed] [Google Scholar]
- 16.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009;116:215–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuellar TL, McManus MT. MicroRNAs and endocrine biology. J Endocrinol 2005;187:327–332 [DOI] [PubMed] [Google Scholar]
- 18.Cochrane DR, Cittelly DM, Richer JK. Steroid receptors and microRNAs: relationships revealed. Steroids 2011;76:1–10 [DOI] [PubMed] [Google Scholar]
- 19.Cohen A, Shmoish M, Levi L, Cheruti U, Levavi-Sivan B, Lubzens E. Alterations in micro-ribonucleic acid expression profiles reveal a novel pathway for estrogen regulation. Endocrinology 2008;149:1687–1696 [DOI] [PubMed] [Google Scholar]
- 20.Maillot G, Lacroix-Triki M, Pierredon S, Gratadou L, Schmidt S, Benes V, et al. . Widespread estrogen-dependent repression of microRNAs involved in breast tumor cell growth. Cancer Res 2009;69:8332–8340 [DOI] [PubMed] [Google Scholar]
- 21.Bhat-Nakshatri P, Wang G, Collins NR, Thomson MJ, Geistlinger TR, Carroll JS, et al. . Estradiol-regulated microRNAs control estradiol response in breast cancer cells. Nucleic Acids Res 2009;37:4850–4861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamagata K, Fujiyama S, Ito S, Ueda T, Murata T, Naitou M, et al. . Maturation of microRNA is hormonally regulated by a nuclear receptor. Cell 2009;36:340–347 [DOI] [PubMed] [Google Scholar]
- 23.Cicatiello L, Mutarelli M, Grober OM, Paris O, Ferraro L, Ravo M, et al. . Estrogen receptor alpha controls a gene network in luminal-like breast cancer cells comprising multiple transcription factors and microRNAs. Am J Pathol 2010;176:2113–2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferraro L, Ravo M, Nassa G, Tarallo R, De Filippo MR, Giurato G, et al. . Effects of oestrogen on microRNA expression in hormone-responsive breast cancer cells. Horm Cancer 2012;3:65–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Brachydanio rerio). Eugene, OR: University of Oregon Press, 1995 [Google Scholar]
- 26.Tong Y, Shan T, Poh YK, Yan T, Wang H, Lam SH, et al. . Molecular cloning of zebrafish and medaka vitellogenin genes and comparison of their expression in response to 17beta-estradiol. Gene 2004;328:25–36 [DOI] [PubMed] [Google Scholar]
- 27.Levi L, Pekarski I, Gutman E, Fortina P, Hyslop T, Biran J, et al. . Revealing genes associated with vitellogenesis in the liver of the zebrafish (Danio rerio) by transcriptome profiling. BMC Genomics 2009;10:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi R, Chiang VL. Facile means for quantifying microRNA expression by real-time PCR. Biotechniques 2005;39:519–525 [DOI] [PubMed] [Google Scholar]
- 29.Olsvik PA, Lie KK, Jordal AE, Nilsen TO, Hordvik I. Evaluation of potential reference genes in real-time RT-PCR studies of Atlantic salmon. BMC Mol Biol 2005;6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. . Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 2002;3:research0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 2002;30:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kloosterman WP, Steiner FA, Berezikov E, de Bruijn E, van de Belt J, Verheul M, et al. . Cloning and expression of new microRNAs from zebrafish. Nucleic acids Res 2006;34:2558–2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang YH, Dudoit S, Luu P, Lin DM, Peng V, Ngai J, et al. . Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res 2002;30:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic acids Res 2006;34:D140–D144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sprague J, Bayraktaroglu L, Clements D, Conlin T, Fashena D, Frazer K, et al. . The Zebrafish Information Network: the zebrafish model organism database. Nucleic acids Res 2006;34:D581–D585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 2009;4:44–57 [DOI] [PubMed] [Google Scholar]
- 37.Karolchik D, Baertsch R, Diekhans M, Furey TS, Hinrichs A, Lu YT, et al. . The UCSC Genome Browser Database. Nucleic acids Res 2003;31:51–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, et al. . Clustal W and Clustal X version 2.0. Bioinformatics 2007;23:2947–2948 [DOI] [PubMed] [Google Scholar]
- 39.Enright AJ, John B, Gaul U, Tuschl T, Sander C, Marks DS. MicroRNA targets in Drosophila. Genome Biol 2003;5:r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Creighton CJ, Nagaraja AK, Hanash SM, Matzuk MM, Gunaratne PH. A bioinformatics tool for linking gene expression profiling results with public databases of microRNA target predictions. RNA 2008;14:2290–2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang TC, Yu D, Lee YS, Wentzel EA, Arking DE, West KM, et al. . Widespread microRNA repression by Myc contributes to tumorigenesis. Nat Genet 2008;40:43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu H, Neilson JR, Kumar P, Manocha M, Shankar P, Sharp PA, et al. . miRNA profiling of naïve, effector and memory CD8 T cells. PLoS One 2007;2:e1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Izzotti A, Calin GA, Arrigo P, Steele VE, Croce CM, De Flora S. Downregulation of microRNA expression in the lungs of rats exposed to cigarette smoke. FASEB J 2009;23:806–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, et al. . MicroRNA expression profiles classify human cancers. Nature 2005;435:834–838 [DOI] [PubMed] [Google Scholar]
- 45.Yu X, Zhang X, Dhakal IB, Beggs M, Kadlubar S, Luo D. Induction of cell proliferation and survival genes by estradiol-repressed microRNAs in breast cancer cells. BMC Cancer 2012;12:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bouchard C, Staller P, Eilers M. Control of cell proliferation by Myc. Trends Cell Biol 1998;8:202–206 [DOI] [PubMed] [Google Scholar]
- 47.Musgrove EA, Sergio CM, Loi S, Inman CK, Anderson LR, Alles MC, et al. . Identification of functional networks of estrogen- and c-Myc-responsive genes and their relationship to response to tamoxifen therapy in breast cancer. PLoS One 2008;3:e2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng AS, Jin VX, Fan M, Smith LT, Liyanarachchi S, Yan PS, et al. . Combinatorial analysis of transcription factor partners reveals recruitment of c-MYC to estrogen receptor-alpha responsive promoters. Mol Cell 2006;21:393–404 [DOI] [PubMed] [Google Scholar]
- 49.Lin CY, Loven J, Rahl PB, Paranal RM, Burge CB, Bradner JE, et al. . Transcriptional amplification in tumor cells with elevated c-Myc. Cell 2012;151:56–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O'Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature 2005;435:839–843 [DOI] [PubMed] [Google Scholar]
- 51.Meltzer PS. Small RNAs with big impacts. Nature 2005;435:745–746 [DOI] [PubMed] [Google Scholar]
- 52.He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, et al. . A microRNA polycistron as a potential human oncogene. Nature 2005;435:828–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hayashita Y, Osada H, Tatematsu Y, Yamada H, Yanagisawa K, Tomida S, et al. . A polycistronic microRNA cluster, miR-17-92, is overexpressed in human lung cancers and enhances cell proliferation. Cancer Res 2005;65:9628–9632 [DOI] [PubMed] [Google Scholar]
- 54.Castellano L, Giamas G, Jacob J, Coombes RC, Lucchesi W, Thiruchelvam P, et al. . The estrogen receptor-alpha-induced microRNA signature regulates itself and its transcriptional response. Proc Natl Acad Sci U S A 2009;106:15732–15737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tanzer A, Stadler PF. Molecular evolution of a microRNA cluster. J Mol Biol 2004;339:327–335 [DOI] [PubMed] [Google Scholar]
- 56.Padmanabhan V, Callas P, Philips G, Trainer TD, Beatty BG. DNA replication regulation protein Mcm7 as a marker of proliferation in prostate cancer. J Clin Pathol 2004;57:1057–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Naujokat C, Hoffmann S. Role and function of the 26S proteasome in proliferation and apoptosis. Lab Invest 2002;82:965–980 [DOI] [PubMed] [Google Scholar]
- 58.Wang X, Wang X. Systematic identification of microRNA functions by combining target prediction and expression profiling. Nucleic Acids Res 2006;34:1646–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Elmén J, Lindow M, Silahtaroglu A, Bak M, Christensen M, Lind-Thomsen A, et al. . Antagonism of microRNA-122 in mice by systemically administered LNA-antimiR leads to up-regulation of a large set of predicted target mRNAs in the liver. Nucleic Acids Res 2008;36:1153–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheng C, Li LM. Inferring microRNA activities by combining gene expression with microRNA target prediction. PLoS One 2008;3:e1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chang J, Nicolas E, Marks D, Sander C, Lerro A, Buendia MA, et al. . miR-122, a mammalian liver-specific microRNA, is processed from hcr mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1. RNA Biol 2004;1:106–113 [DOI] [PubMed] [Google Scholar]
- 62.Fang Z, Rajewsky N. The impact of miRNA target sites in coding sequences and in 3′UTRs. PLoS One 2011;6:e18067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell 2007;27:91–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tay Y, Zhang J, Thomson AM, Lim B, Rigoutsos I. MicroRNAs to Nanog, Oct4 and Sox2 coding regions modulate embryonic stem cell differentiation. Nature 2008;455:1124–1128 [DOI] [PubMed] [Google Scholar]
- 65.Krützfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, et al. . Silencing of microRNAs in vivo with ‘antagomirs'. Nature 2005;438:685–689 [DOI] [PubMed] [Google Scholar]
- 66.Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, et al. . miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab 2006;3:87–98 [DOI] [PubMed] [Google Scholar]
- 67.Wilfred BR, Wang WX, Nelson PT. Energizing miRNA research: a review of the role of miRNAs in lipid metabolism, with a prediction that miR-103/107 regulates human metabolic pathways. Mol Genet Metab 2007;91:209–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tang F, Kaneda M, O'Carroll D, Hajkova P, Barton SC, Sun YA, et al. . Maternal microRNAs are essential for mouse zygotic development. Genes Dev 2007;21:644–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ason B, Darnell DK, Wittbrodt B, Berezikov E, Kloosterman WP, Wittbrodt J, et al. . Differences in vertebrate microRNA expression. Proc Natl Acad Sci U S A 2006;103:14385–14389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koralov SB, Muljo SA, Galler GR, Krek A, Chakraborty T, Kanellopoulou C, et al. . Dicer ablation affects antibody diversity and cell survival in the B lymphocyte lineage. Cell 2008;132:860–874 [DOI] [PubMed] [Google Scholar]
- 71.Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ, et al. . Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell 2008;132:875–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mendell JT. miRiad roles for the miR-17-92 cluster in development and disease. Cell 2008;133:217–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hayashi K, Chuva de Sousa Lopes SM, Kaneda M, Tang F, Hajkova P, Lao K, et al. . MicroRNA biogenesis is required for mouse primordial germ cell development and spermatogenesis. PLoS One 2008;3:e1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baron D, Houlgatte R, Fostier A, Guiguen Y. Large-scale temporal gene expression profiling during gonadal differentiation and early gametogenesis in rainbow trout. Biol Reprod 2005;73:959–966 [DOI] [PubMed] [Google Scholar]
- 75.Ijiri S, Kaneko H, Kobayashi T, Wang DS, Sakai F, Paul-Prasanth B, et al. . Sexual dimorphic expression of genes in gonads during early differentiation of a teleost fish, the Nile tilapia Oreochromis niloticus. Biol Reprod 2008;78:333–341 [DOI] [PubMed] [Google Scholar]
- 76.Lam SH, Wu YL, Vega VB, Miller LD, Spitsbergen J, Tong Y, et al. . Conservation of gene expression signatures between zebrafish and human liver tumors and tumor progression. Nat Biotechnol 2006;24:73–75 gb-2003-5-1-r1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.