Abstract
Emotional arousal, mediated by the amygdala, is known to modulate episodic memories stored by the hippocampus, a region involved in pattern separation (the process by which similar representations are independently stored). While emotional modulation and pattern separation have been examined independently, the current study attempts to link the two areas of research to propose an alternative account for how emotion modulates episodic memory. We utilized an emotional discrimination task designed to tax pattern separation of emotional information by concurrently varying valence and similarity of stimuli. To examine emotional modulation of memory at the level of hippocampal subfields, we used high-resolution fMRI (1.5 mm isotropic) of the medial temporal lobe. Consistent with prior reports, we observed engagement of the hippocampal dentate gyrus (DG) and CA3 during accurate discrimination of highly similar items (i.e. pattern separation). Furthermore, we observed an emotional modulation of this signal (negative > neutral) specific to trials on which participants accurately discriminated similar emotional items. The amygdala was also modulated by emotion, regardless of the accuracy of discrimination. Additionally, we found aberrant amygdala-hippocampal network activity in a sample of adults with depressive symptoms. In this sample, amygdala activation was enhanced and DG/CA3 activation was diminished during emotional discrimination compared to those without depressive symptoms. Depressive symptom severity was also negatively correlated with DG/CA3 activity. This study suggests a novel mechanistic account for how emotional information is processed by hippocampal subfields as well as how this network may be altered in mood disorders.
Keywords: dentate, ca3, high-resolution fMRI, depression, pattern separation, amygdala, hippocampus, emotion, arousal
INTRODUCTION
Emotional experiences reinforce learning so that these states can be remembered and utilized in future behaviors. The hippocampus is known to play a critical role in the encoding and storage of episodic memories (Squire et al., 2004), whereas the amygdala is thought to enhance episodic memory storage by signaling the emotional significance of events (McGaugh, 2004; LeDoux, 2007). While studies in rodents have demonstrated enhancing effects of arousal on memory (Gallagher et al., 1977; McGaugh, 2004), human studies have suggested an emotion-induced memory trade-off, where individuals remember the central emotional content (gist) of a stimulus but often forget the details (Buchanan and Adolphs, 2002; Kensinger, 2009). Overall, cross-species evidence strongly supports the notion that the amygdala influences hippocampal storage by providing information about the emotional context (Dolcos et al., 2004; McGaugh, 2004). However, the amygdala’s modulatory role in memory has not been investigated in light of computational models of hippocampal dynamics.
David Marr (1971) first suggested that the recurrent collaterals in CA3 enabled this region of the hippocampus to act as an auto-associative network capable of pattern completion (the process by which previously stored representations are retrieved when exposed to partial or degraded cues). In contrast, the dentate gyrus is thought to contribute to pattern separation – the process by which similar representations are stored in a distinct, non-overlapping fashion, which minimizes interference in mnemonic representations (McClelland et al., 1995; Yassa and Stark, 2011). The CA3 region of the hippocampus is also capable of performing pattern separation, depending on the similarity of the inputs to the system (Yassa and Stark, 2011), thus, in most high-resolution fMRI studies the DG/CA3 regions are combined. The computational descriptions of hippocampal function offer a potential mechanistic account by which information storage may be modulated (i.e. either by enhancing pattern separation or pattern completion).
The impact of emotional arousal on hippocampal pattern separation has not been examined in detail in humans. Furthermore, if this network is altered, dysfunctions in memory and the emotional modulation of memory could manifest leading to some of the symptoms common to mood disorders such as depression. Converging evidence from rodent and human studies of depression (chronic stress paradigms in rodents) find structural and functional changes in the hippocampus and amygdala as well as corresponding deficits in learning and memory, where depressed individuals are impaired in general episodic memory but show a bias towards remembering negative information (Watanabe et al., 1992a; McEwen and Magarinos, 2001; Sheline, 2011). Thus, gaining a clearer understanding of amygdala-hippocampal interactions in humans will advance our knowledge of the neurobiological mechanisms underlying such abnormalities.
In the current study, we utilized high-resolution fMRI to test hypotheses about specific subfields of the hippocampus and their involvement in the pattern separation of emotional stimuli. We hypothesized that the DG/CA3 subregion would show pattern separation signals during both negative and neutral item discrimination, but would be more strongly modulated by negative items (i.e. an emotional modulation effect). We also hypothesized that the amygdala would show emotional modulation effects. We additionally tested a small sample of individuals with depressive symptoms and examined amygdala-hippocampal network dysfunction during performance of this task to determine whether this paradigm may be sensitive to medial temporal lobe alterations in disease states. The current investigation offers an alternative conceptual framework by which to examine the impact of emotion on hippocampal computations.
MATERIALS AND METHODS
Participants
Eighteen healthy participants (N=18) were recruited from Johns Hopkins University and received $40 for their participation. Informed consent was obtained from all participants, with all procedures approved by the Johns Hopkins University Institutional Review Board. An additional ten participants experiencing symptoms of depression (DS group: N=10) were also recruited in the same manner. Demographic data are shown in Table 1.
Table 1.
Participant Demographics and Neuropsychological Test Results
| Groups | NDS | DS | ||
|---|---|---|---|---|
| Sample Size | 18 | 10 | ||
| M : F | 9 : 9 | 3 : 7 | ||
| Variables | Mean | SEM | Mean | SEM |
| Age | 21.22 | 0.94 | 20.40 | 0.60 |
| Beck Depression Inventory-II | 3.22 | 0.60 | 23.22 | 2.78 |
| RAVLT* Immediate Recall | 12.44 | 0.49 | 13.40 | 0.58 |
| RAVLT Delayed Recall | 11.78 | 0.66 | 13.00 | 0.58 |
| Digit Span Forward | 11.94 | 0.45 | 12.30 | 0.63 |
| Digit Span Backward | 8.27 | 0.43 | 8.70 | 0.83 |
| Mini Mental State Exam | 29.11 | 0.27 | 29.10 | 0.38 |
| Trail Making Test A | 19.44 | 1.31 | 19.30 | 1.63 |
| Trail Making Test B | 46.89 | 2.33 | 40.10 | 4.49 |
Rey Auditory Verbal Learning Test
Inclusion/Exclusion criteria
All participants were screened against major medical or psychiatric morbidities, substance abuse history, as well with the additional exclusion criteria of MRI contraindications such as the presence of metal in the body. For participants in the DS group, a depression diagnosis was an inclusion criterion but lack of such a diagnosis was not used to exclude participants from the DS group. All healthy participants had a Beck Depression Inventory-II (BDI-II) score of 10 or below, while anyone in the DS group received a BDI-II score above 11. These cutoff criteria were based on the BDI-II symptom severity scale in which 1–10 is considered within normal limits. All participants included in the DS group were medication-free. All participants were right-handed with normal or corrected to normal vision.
Imaging data collection
Functional MRI data were collected using a 3-Tesla Philips scanner equipped with a SENSE head coil using both higher-order shims and SENSE imaging techniques. Functional images were collected using a high-speed EPI single-shot pulse sequence (1.5 mm isotropic resolution, 19 oblique axial slices parallel to the principal axis of the hippocampus, field of view = 96×96 mm, flip angle = 70°, SENSE parallel reduction factor = 2, TR/TE = 1500/30 ms, matrix size = 64×64).
We additionally collected a novel ultrahigh-resolution structural MPRAGE scan that we developed for accurate delineation of hippocampal subfields and high-resolution diffeomorphic alignment (0.55 mm isotropic resolution; 273 sagittal slices, field of view = 240×240 mm, flip angle = 9°, TR/TE = 13/5.9 ms, matrix size = 448×448, inversion pulse TI=1110 ms). SENSE parallel imaging was used in two directions (2×1.5). The SAR (<10%) and PNS (<75%) were within required limits based on the scanner-calculated values. Total scan time for the volume was 7m 51s. These scans were also used to create a group template, which was used as the standardized space for alignment of participant data before group analyses.
Neuropsychological battery
The battery was designed to examine memory function, as well as other aspects of general cognition. The assessment included the following: (1) Mini-Mental State Exam (MMSE) to assess global cognitive status, (2) Rey Auditory Verbal Learning Test (RAVLT) to assess verbal learning, immediate and delayed recall, and recognition, (3) Digit Span backwards and forwards to assess working memory, (4) Trail Making Tests A and B to assess attention, visual search, and mental processing speed, and (5) Beck Depression Inventory-II (BDI-II) to assess depressive symptoms. This battery was given on the day of the scan, 30 minutes prior to the scanning session. Results are shown in Table 1.
Emotional discrimination task
An Apple iMac equipped with MATLAB (RRID:nlx_153890, Version R2010a, Natick, MA) software and PsychToolbox version 3.0 was used to present the stimuli and record responses. Each trial consisted of 2 displays: an image display and a fixation display. During both study and test phases, images were presented on the center of the screen with a black background for 2500ms. Images were categorized a priori for emotional valence (negative, neutral, positive), arousal (very calming to very exciting), and similarity (median split of similarity ratings into “high” and “low”) (Leal et al., 2014). The fixation display consisted of a white fixation cross on the center of the screen with a black background for 500ms.
Participants underwent an incidental encoding phase where they were shown emotional and non-emotional images, presented in randomized order, and were asked to rate the images for emotional valence (negative, neutral, and positive). Participants were given a subsequent surprise test 5 minutes after the encoding phase, in which they saw another series of stimuli, some of which were seen once before in the incidental task (targets), some were similar to ones seen in the incidental task but not identical (lures), and some were new (foils) (Fig 1).
Figure 1. Emotional discrimination task.
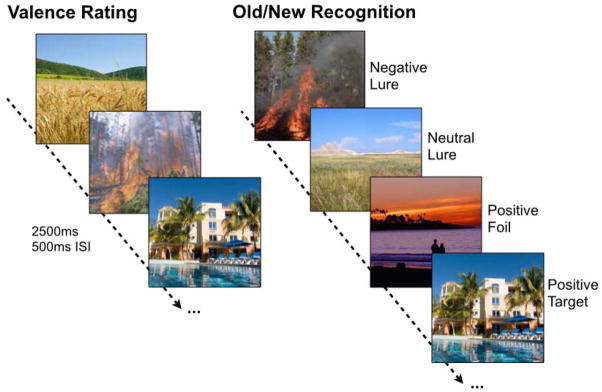
During encoding, participants rated images according to their emotional valence (using three buttons for negative, neutral, and positive). Each image was presented for 2500ms with a 500ms inter-stimulus-interval (ISI). After a 5-minute delay, participants underwent a surprise recognition test where they viewed negative, neutral, and positive targets (exact repetitions), foils (new items), and lures (similar items) and were asked to indicate whether items were “old” or “new”.
Participants were asked to indicate whether items were “old” or “new” via button press. Participants were explicitly told that in order for an image to be called “old,” it had to be the exact same image they saw before. The experiment consisted of 149 images during the study phase and 291 images during the test phase. Targets, lures, and foils were evenly distributed across emotion and similarity level.
Image analysis
All data analyses were conducted using Analysis of Functional NeuroImages (AFNI – RRID:nif-0000-00259) (Cox, 1996). Images were corrected for slice timing and subject motion. Time points in which significant motion events occurred (movement exceeded 3 degrees of rotation or 2 mm of translation in any direction relative to prior acquisition ± 1 time point) were censored from further analyses. Functional images were then co-registered to the structural scans acquired in the same session using AFNI’s 3dAllineate algorithm. Structural scans were aligned to a common template based on the entire sample using Advanced Normalization Tools (ANTs – RRID:nlx_75959) (Avants et al., 2011) which uses a powerful diffeomorphic algorithm (SyN) (Klein et al., 2009) to warp individual participants into the template space. The transformation parameters were then applied to the coplanar functional data.
Behavioral vectors based on trial type (classified according to emotion, similarity, and behavioral decision) were used to model the data using a deconvolution approach based on multiple linear regression. The resultant fit coefficients (betas) estimated activity versus an implicit baseline (novel foils) for a given time point and trial type in a voxel. The sum of the fitcoefficients over the expected hemodynamic response (3–12 s after trial onset) was taken as the model’s estimate of the response to each trial type (relative to baseline).
Extracting region of interest (ROI) voxels
We selected voxels for subsequent analyses based on combining the voxels that changed with any of the task conditions in the healthy sample with anatomical ROIs. Active voxels were selected based on the overall F, agnostic to specific condition or contrast so as not to bias subsequent analyses and remove concerns regarding circularity and double-dipping (Kriegeskorte et al., 2009). This served to remove voxels that did not respond to any of the task conditions so that the analyses can be more sensitive to subtle changes across conditions.
This voxel mask was then combined with anatomical ROI masks that were based on manual delineations of the subfields and regions of interest on the common template. Amygdala segmentation was based on our prior published protocol (Yassa et al., 2012). Briefly, we segmented the amygdala on axial slices using the hippocampal uncus and temporal horn of the lateral ventricle as the posterior boundary in superior slices and using the hippocampus itself as the posterior boundary in inferior slices. The lateral boundary was defined by an arbitrary line drawn from the most medial white matter to the lateral fissure excluding gray matter medial to this line. The medial boundary was set by the hippocampal uncus in anterior slices and by white matter in the posterior slices.
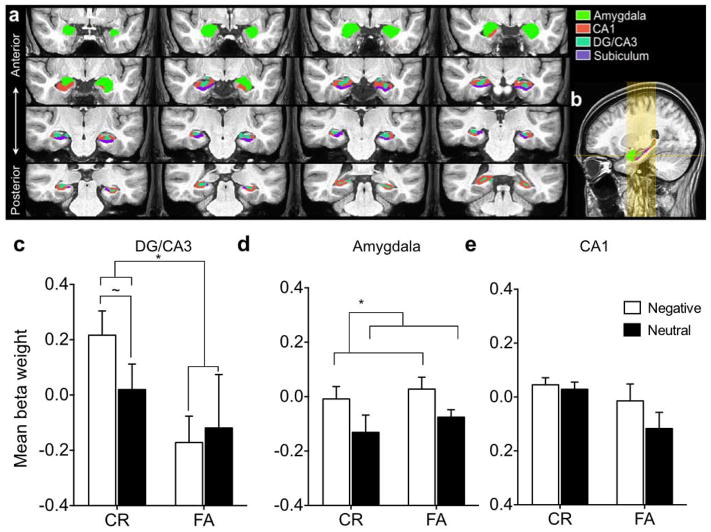
Hippocampal subfield segmentation was also based on prior work (Yassa et al., 2010b), which is defined according to the atlas of Duvernoy (Duvernoy, 1998). The subfields are defined on eight coronal slices along the anterior–posterior axis of the hippocampus. Representative slices in each hippocampus that best (closest) resembled the slices described were chosen and segmented according to the atlas description. The segmentation then proceeded from these slices in both directions slice by slice to ensure a smooth transition across slices. The ROI masks for both the amygdala and hippocampal subfields are shown in Fig 2a, b for reference. Voxel betas from the resulting hybrid functional/structural ROIs were averaged and all subsequent statistical analyses were conducted on these averages.
Figure 2. Region of interest activity profiles during highly similar lure trials.
a) Region of interest (ROI’s) segmentations of the amygdala, DG/CA3, CA1, and subiculum on a custom group template, (b) ROI locations shown along the longitudinal axis of the medial temporal lobe, c) Mean activity beta weights during correct rejection (CR) and false alarms (FA) of highly similar lures in hippocampal DG/CA3, which shows an emotional pattern separation signal (CR>FA and Negative>Neutral in CR), d) Mean activity beta weights during CR and FA of highly similar lures in the amygdala, which show only an emotional modulation signal (Negative>Neutral), e) Mean activity beta weights during CR and FA of highly similar lures in hippocampal CA1, which shows only a marginal pattern separation signal. * indicates significance at p<.05, ~ indicates a trend at p<.1. Error bars are ± S.E.M.
Statistical analyses
All statistical analyses of behavioral variables and ROI activation means were conducted in SPSS v. 20.0 (IBM Corp., released 2011, Armonk, NY). Planned comparisons were conducted using additional F-tests or t-tests. Post hoc statistical tests were corrected for multiple comparisons using Scheffé’s correction, with critical F values indicated in the text corresponding to the degrees of freedom (df) of the F-test (mentioned only once for each pair of df’s). All tests used the General Linear Model (ANOVA and correlations). Normality assumptions were investigated using Kolmogorov-Smirnov tests and all distributions investigated did not significantly deviate from the normal distribution. Repeated measures tests were corrected for error nonsphericity using Greenhouse-Geisser correction where appropriate. Statistical values were considered significant at a final corrected alpha level of .05, which appropriately controlled for Type I error.
RESULTS
High-resolution fMRI of emotional pattern separation
We analyzed behavioral performance in healthy participants and found a significant effect of emotion [F(2,34) = 4.30, P = .024] during correct rejections of highly similar lures. There were fewer correct rejections of negative and positive lures compared to neutral [F(1,34) = 10.45, P < .01, critical Scheffé = 6.54]. This replicates our prior behavioral work (Leal et al., 2014). Raw performance data (accuracy and reaction times) are shown in Table 2 and Table S1, respectively. There were no gender differences in behavioral performance or BOLD fMRI activation.
Table 2.
Accuracy (Proportion Correct) on Emotional Discrimination Task
| Measures | NDS | DS | ||
|---|---|---|---|---|
| Mean | SEM | Mean | SEM | |
| Negative Lure CR | 0.60 | 0.05 | 0.70 | 0.06 |
| Neutral Lure CR | 0.73 | 0.03 | 0.68 | 0.04 |
| Positive Lure CR | 0.58 | 0.05 | 0.75 | 0.05 |
| Negative Target Hits | 0.93 | 0.02 | 0.96 | 0.02 |
| Neutral Target Hits | 0.91 | 0.02 | 0.95 | 0.02 |
| Positive Target Hits | 0.93 | 0.02 | 0.96 | 0.02 |
| Negative Foil CR | 0.97 | 0.02 | 0.98 | 0.01 |
| Neutral Foil CR | 0.98 | 0.01 | 0.97 | 0.02 |
| Positive Foil CR | 0.97 | 0.01 | 0.98 | 0.01 |
CR: Correct rejections
Next, we utilized high-resolution fMRI to test hypotheses about specific subfields of the hippocampus and their involvement in the pattern separation of emotional stimuli. We focused our analysis on the specific conditions where we could test hypotheses about emotional pattern separation. Thus, our analyses compared the following conditions: 1) retrieval trials where highly similar lures were presented, since these trials were hypothesized to maximize interference and 2) comparison of negative and neutral items (excluding positive items, as these items were not matched to negative items for arousal (Leal et al., 2014). We analyzed ROI activity in hippocampal DG/CA3, CA1, subiculum, and the amygdala (Fig 2a). All data across all ROI’s, conditions, and groups are in Fig S1 and S2.
We operationally define “emotional pattern separation” signals in the context of this experiment as meeting two directional criteria: (1) activity in a particular ROI was higher during lure correct rejections (CR) than lure false alarms (FA), i.e. showing a pattern separation signal (Yassa et al., 2010a, 2010b); and (2) activity during lure CR’s was higher for negative compared to neutral stimuli (i.e. showing an emotional modulation signal). Thus, in order to determine if specific ROI’s met these criteria, we ran a 2×2 ANOVA with memory (lure CR and lure FA) and emotion (negative and neutral) as factors in each ROI. If the region showed a pattern separation signal, we then performed paired comparisons within memory condition to determine if an emotional modulation signal exists (second criterion of emotional modulation signals). The baseline condition consisted of all novel items (i.e. foils).
Pattern separation of emotional information in DG/CA3
In hippocampal DG/CA3 we observed a significant main effect of memory (lure CR > lure FA) [F(1,17) = 5.93, P = .026] (Fig 2c), which met our criterion for a pattern separation signal. There was greater activity when correctly rejecting a lure compared to falsely recognizing a lure. Furthermore, on trials where lures were correctly rejected, we observed a trend towards an emotional modulation signal, where there was greater activity when negative items were correctly rejected compared to neutral items [t(17) = 1.76, P = .09]. While we focused our analysis on highly similar items here, low similarity lures showed the same pattern (Fig S1). Thus, we ran a second ANOVA including all trials (collapsing across similarity bins) to increase power and found a significant main effect of memory [F(1,17) = 12.33, P = .003] as well as a marginal effect of emotion [F(1,17) = 4.03, P = .06]. The planned post-hoc comparison within memory condition revealed an emotional modulation effect specific to lure CR’s [t(17) = 2.62, P = .018]. Hippocampal DG/CA3 showed a pattern separation signal (lure CR > lure FA) and an emotional modulation effect specific to lure CR’s across both high and low similarity items. Together, these data suggest that DG/CA3 is involved in pattern separation of emotional stimuli and is involved in resolving emotional interference irrespective of stimulus similarity level. This is consistent with prior work (Lacy et al., 2011) showing that DG/CA3 activity is similar across low and high similarity bins suggesting that it is sensitive to even minor distortions in input (i.e. high interference).
Emotional modulation in amygdala regardless of memory performance
We performed the same analysis in the amygdala, where we observed a significant main effect of emotion (negative > neutral) [F(1,17) = 5.08, P = .038] (Fig 2d), thus meeting our criterion for an emotional modulation signal. There was greater activity for negative items compared to neutral. There was no main effect of memory and no interaction between emotion and memory (P > .05). This suggests that the amygdala is involved in emotional modulation, regardless of memory performance.
In the CA1 subregion of the hippocampus, we observed a marginal effect of memory (lure CR > lure FA) [F(1,17) = 4.35, P = .052] (Fig 2e), suggesting the CA1 subregion may also show pattern separation signals. There was no main effect of emotion and no interaction between emotion and memory (P > .05). Thus, CA1 does not seem to be modulated by emotion but may reflect a downstream pattern separation signal from DG/CA3.
Emotional pattern separation alterations in a mood disturbance model
We tested a small sample of individuals with depressive symptoms (DS group, N=10) to test the power of the emotional pattern separation approach. The DS group showed moderate to severe depressive symptoms according to the BDI-II (score >10; average BDI-II = 23 ± 8.8 SD, see Materials and Methods for details). We used participants from our first experiment as controls with little to no depressive symptoms (NDS: average BDI-II = 3.22 ± 2.56 SD). Participant characteristics and results of neuropsychological testing are shown in Table 2. We used a 2×3 ANOVA with group (DS and NDS) and emotion (negative, positive, and neutral) to assess behavioral differences. Consistent with our prior work (Leal et al. 2014), there was a significant interaction [F(2,52) = 3.28, P = .046] between emotion and group. A post hoc contrast showed that DS were better than NDS at discriminating emotional lures [F(1,52) = 5.36, P < .05, critical Scheffé = 4.03]. Raw performance data are shown in Table 2 and Table S1.
Next, we analyzed negative and neutral retrieval trials where highly similar lures were presented and correctly rejected for each ROI. We conducted an ANOVA of DG/CA3 regional activation during lure CR’s and found an emotion x group interaction [F(1,26) = 6.35, P = .018; Fig 3a] but no significant main effect of emotion or group (P > .05). Post-hoc contrasts confirmed the interaction was driven by a reversal of the emotional modulation signal in DG/CA3, where NDS individuals showed increased activity on negative versus neutral lure discrimination trials while DS individuals showed increased activity on neutral compared to negative lure discrimination trials [F(1,26) = 8.10, P < .05; critical Scheffé = 4.23]. We conducted an ANOVA of amygdala regional activation during lure CR’s, which revealed a significant main effect of emotion [F(1,26) = 6.16, P = .02; Fig 3b], but no main effect of group or interaction between emotion and group (P > .05). Thus, both DS and NDS groups showed an emotional modulation signal in the amygdala. We conducted an ANOVA on CA1 regional activation during lure CR’s and found no significant effects (all P’s > .05; Fig S1 and S2).
Figure 3. Amygdala-hippocampal network alterations with depressive symptoms.
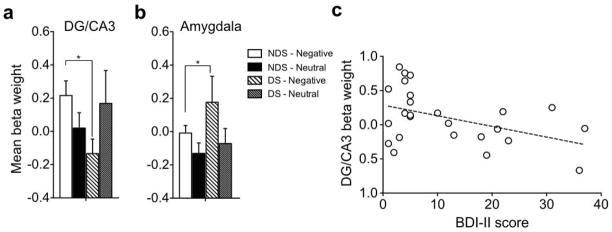
a) Mean activity beta weights during correct rejection of highly similar lures in hippocampal DG/CA3, which shows decreased DG/CA3 activity in DS compared to NDS during negative lures, b) Mean activity beta weights during correct rejection of highly similar lures in the amygdala, which shows increased activity in the DS group during negative lure CR’s, c) Negative correlation between depressive symptom severity and DG/CA3 beta weight during negative lure CR’s. Note that other significant comparisons (e.g. within NDS) are duplicated from Fig 2 and are not indicated here as significant. * indicates significance at p<.05, Error bars are ± S.E.M.
To examine the relationship between the amygdala and hippocampal DG/CA3 during correct lure discrimination, we conducted a 3-way ANOVA with emotion (negative and neutral), group (DS and NDS), and region (amygdala and DG/CA3) during lure CR’s. Here, we observed a significant emotion x region interaction [F(1,26) = 4.59, P = .04] as well as significant emotion x region x group interaction [F(1,26) = 7.87, P = .009] (Fig 3a, b). The interaction between emotion and region was driven by the emotional modulation effect in the amygdala, where negative activity was greater than neutral activity across groups [F(1,26) = 6.7, P < .05]. A post hoc contrast showed that the three-way interaction was driven by the reversal in DG/CA3 where NDS participants showed greater activity for negative compared to neutral items and DS participants showed greater activity for neutral compared to negative items, while the amygdala showed greater activity for negative compared to neutral items across groups [F(1,26) = 12.27, P < .05].
Furthermore, to investigate the relationship between the amygdala and DG/CA3 specifically during correct negative lure discrimination, we conducted an ANOVA with group (NDS and DS) and region (DG/CA3 and amygdala) and found a significant group x region interaction [F(1,26) = 7.49, P = .011], where a post hoc contrast showed that NDS individuals had increased DG/CA3 activity relative to DS, while DS individuals had increased amygdala activity compared to NDS [F(1,26) = 8.08, P < .05]. We did not observe any significant effects for neutral lure discrimination (all P’s > .05).
We next examined whether activity patterns in the DG/CA3 were related to depressive symptom severity. We included all individuals with BDI-II scores of above zero (N = 25). We observed a significant negative correlation between activity in the DG/CA3 region during negative lure correct rejections and BDI-II scores [Pearson r = −0.44, P = .026; Fig 3c], suggesting that depressive symptom severity was linearly associated with decreased activity in this hippocampal subfield. There was no correlation between amygdala activity and depressive symptoms.
DISCUSSION
Past fMRI studies in humans have shown that BOLD activity in the amygdala and the hippocampus is correlated during encoding (Kensinger and Corkin, 2004) and retrieval (Kensinger and Schacter, 2007) of emotional information. Furthermore, amygdala-hippocampal functional connectivity predicts enhanced later recall of emotional memories (St Jacques et al., 2009). The amygdala is promiscuous in influencing the consolidation of memory for many different kinds of motivationally arousing training experiences (McGaugh, 2002, 2004). This is thought to occur through the amygdala’s ability to modulate hippocampal representations. The basolateral amygdala (BLA) projects both directly and indirectly to different subregions of the hippocampus (Pitkanen, 2000; Petrovich et al., 2001; Aggleton, 2000), which may allow specific influences of the amygdala on particular hippocampal subregions.
Neuroimaging studies on amygdala-hippocampal dynamics have been unable to determine differential contributions of the hippocampal subregions in processing emotional information. More recent studies have used high-resolution functional neuroimaging and found similar pattern separation signals using neutral objects (Bakker et al., 2008). The current study is the first to show that the DG/CA3 regions of the hippocampus are involved in pattern separation of emotional stimuli. Using an emotional discrimination task, we were able to manipulate emotional valence and similarity of stimuli to investigate the amygdala-hippocampal network’s involvement in the pattern separation of emotional information at high-resolution. We observed engagement of hippocampal DG/CA3 during accurate discrimination of similar items (i.e. pattern separation signal). Furthermore, we observed an emotional modulation of this signal (negative > neutral) specific to trials on which participants accurately discriminated similar emotional items. Although the data from high similarity items alone showed a trend towards an emotional modulation effect, increasing our power by analyzing all similar items (both high and low) resulted in a significant emotional modulation signal specific to lure correct rejections. This suggests that the DG/CA3 is using emotional information in order to discriminate similar experiences and is capable of resolving emotional interference irrespective of stimulus similarity level. The amygdala was also modulated by emotion; however, this signal manifested regardless of the accuracy of discrimination. While the amygdala generally responds to highly similar emotional stimuli, the DG/CA3 selectively exhibits emotional modulation when attempting to differentiate interfering information. Absent the accurate discrimination response, the DG/CA3 does not reflect such emotional modulation, suggesting that the amygdala modulation may be an essential signal when faced with remembering highly interfering emotional experiences.
The underlying mechanism for these effects is still unknown. We do not know how emotional information is processed preferentially by one region versus another in addition to why we see emotion-induced memory trade-offs where emotional gist information is enhanced and detail information is impaired. This finding replicates what we recently reported in Leal et al. (2014) using the same task in an orthogonal sample. Discrimination memory for emotional details is impaired relative to neutral information. This may be specific to a discrimination design, which specifically taxes memory for details. We have shown recently that discrimination and generalization are not synonymous with recollection and familiarity (Kim and Yassa, 2013) and that the two dimensions can be orthogonalized. In addition, the current study does not speak to how emotional memories change over time, in which studies have shown the largest effects of emotion’s influence on memory after a longer delay (Kensinger, 2009). Consolidation of emotional memories after sleep has been shown to increase the likelihood that certain pieces of an experience are stabilized in memory (Payne and Kensinger, 2010), which may play a role in remembering emotional versus neutral items.
Future studies investigating the functional connectivity between the amygdala and hippocampal subregions will be important to determine if connectivity between these regions changes as a function of the task. The computational descriptions of hippocampal function offer a potential mechanistic account by which information storage may be modulated (i.e. either by enhancing pattern separation or pattern completion). Paradigms that tax pattern separation offer a robust empirical framework by which hippocampal function can be assessed. Here, we propose that this framework can be used to investigate the impact of emotional modulation on hippocampal memory and may allow insight into alterations of this modulation in disorders of mood and memory, such as depression.
Clinical disorders such as depression, anxiety, and Alzheimer’s disease all display alterations in mood and memory, in which a subset of their cognitive and behavioral symptoms are produced by alterations in the medial temporal lobes. Many studies of major depressive disorder have found general episodic memory deficits in depressed individuals (Airaksinen et al., 2007; Dere et al., 2010) in addition to a negativity bias where negative information is preferentially remembered (Watkins et al., 1996, 2000; Hasler et al., 2004; Haas and Canli, 2008). Furthermore, recent work suggests that individuals with depressive symptoms have a diminished capacity for discrimination of highly similar object stimuli (Leal et al., 2014; Shelton and Kirwan, 2013).
To determine if our emotional discrimination task may provide a sensitive measure of medial temporal lobe changes in disorders of mood and memory, we tested a small sample of individuals experiencing depressive symptoms on the emotional discrimination task. We have previously found behavioral alterations in the emotional discrimination task in an orthogonal sample of individuals with depressive symptoms (Leal et al., 2014). We found the emotional discrimination task to be sensitive to subtle neurobiological changes occurring in the human medial temporal lobe, where amygdala and hippocampal DG/CA3 activity tends to decouple in individuals experiencing depressive symptoms. The magnitude of DG/CA3 activity during correct rejections of negative lures was inversely correlated with depressive symptoms. This suggests that enhanced processing of negative information may be due to a network imbalance between the amygdala and the DG/CA3 where the influence of the amygdala is increased and the influence of the DG/CA3 is decreased. Given the behavioral results (increased correct rejections for emotional items compared to healthy individuals), the most parsimonious explanation of the DG/CA3 diminished signal and the heightened amygdala activity is that the amygdala’s activity may have enhanced processing of negative information that facilitated discrimination in the absence of the normal DG/CA3 response. Thus, we surmise that a discrimination behavioral response can be the result of either effective pattern separation in the DG/CA3 or enhanced processing by the amygdala. Whether this effect is driven by impairment in the amygdala, DG, CA3, or all of the regions is still unknown, although data from animal studies suggest that structural changes in the CA3 may be critical (Watanabe et al., 1992b; Conrad et al., 1996, 1999; Vyas et al., 2002). Reductions in DG neurogenesis may also contribute to this effect (Dranovsky and Hen, 2006). Also, we should note that our analyses used diffeomorphically-aligned images, which control for any structural differences between groups, thus our results are unlikely to arise from volumetric differences. Our results demonstrate the power of the emotional pattern separation framework and its utility for examining memory and mood disturbances.
This study had several limitations that should be noted. While the amygdala responds to both positive and negative valence, arousal is a major component of amygdala processing. Since our positive stimuli were not as arousing as negative stimuli, we opted not to include positive trials in our fMRI analyses. Emotional processing of positive stimuli is an important facet in the modulation of memory (Hamann et al., 1999) that, if better understood, may be helpful in reversing the negativity bias found in patient populations with mood disorders. We did not examine this in detail in our study, but future experiments should address the role of positive emotions in more detail. In addition, we limited our analyses to high similarity lure items since these are expected to rely on pattern separation. Analysis of low similarity stimuli could be interesting as well, although different neural mechanisms may be at play when interference is not maximized. One other potential limitation is the correlational nature of fMRI studies, which limits our interpretations in terms of pattern separation computations. Our approach in this study was to come up with a set of criteria for an operational definition for what we termed “emotional pattern separation” but we should caution against strong interpretations of this concept. Convergent evidence in animal studies using neurophysiological recordings from hippocampal cells in DG and CA3 during tasks that manipulate valence will be necessary.
Although we found no significant gender differences in either behavioral performance or BOLD fMRI activation, our sample sizes were too small to fully appreciate gender differences. This absence of evidence should not be taken as evidence of absence and we realize that there are likely gender differences here that need to be considered in future experiments. Additionally, we should note that while our sample of individuals with depressive symptoms was small, we were clearly powered to detect effects both in the DG/CA3 and the amygdala, further demonstrating the capabilities of our high-resolution imaging approach coupled with targeted task design. In addition, using a continuous measure of depressive symptoms resulted in an increased sample size for our correlational analyses. While our data in those with depressive symptoms are suggestive, future studies with larger samples including depression diagnosis, information regarding the history of depression, number of depressive episodes, and treatment status are required to bridge this work to translational applications.
CONCLUSIONS
Emotional arousal influences the fidelity by which memories are stored. The experiments reported here provide a novel account of how emotional modulation of memory may occur in the context of resolving interference. We propose that the amygdala and hippocampal DG/CA3 are key players in this process and that aberrations in this network might manifest in the context of mood disorders. These experiments highlight the complexities of emotional modulation of memory and help to tease apart the nuances underlying the neural mechanisms of amygdala-hippocampal interactions.
Supplementary Material
Acknowledgments
We thank Liz Murray and Ayobami Ward for help with participant recruitment and testing. We also acknowledge Clare King and the Johns Hopkins Student Counseling Center for help with participant recruitment, and the staff of the F.M. Kirby Research Center for Functional Brain Imaging at the Kennedy Krieger Institute for help with participant MRI scanning. This research is supported by NIA grants P50 AG05146 and R01 AG034613.
References
- Airaksinen E, Wahlin A, Forsell Y, Larsson M. Low episodic memory performance as a premorbid marker of depression: evidence from a 3-year follow-up. Acta Psychiatr Scand. 2007;115:458–465. doi: 10.1111/j.1600-0447.2006.00932.x. [DOI] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54:2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan TW, Adolphs R. The role of the human amygdala in emotional modulation of long-term declarative memory. Emotional cognition From brain to behaviour. 2002:9–34. [Google Scholar]
- Conrad CD, Galea La, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- Conrad CD, LeDoux JE, Magariñosa M, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dere E, Pause BM, Pietrowsky R. Emotion and episodic memory in neuropsychiatric disorders. Behav Brain Res. 2010;215:162–171. doi: 10.1016/j.bbr.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004;42:855–863. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- Dranovsky A, Hen R. Hippocampal neurogenesis: regulation by stress and antidepressants. Biol Psychiatry. 2006;59:1136–1143. doi: 10.1016/j.biopsych.2006.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy H. The Human Hippocampus: Functional Anatomy, Vascularization, and Serial Sections with MRI. Berlin: Springer; 1998. [Google Scholar]
- Gallagher M, Kapp BS, Musty RE, Driscoll PA. Memory formation: evidence for a specific neurochemical system in the amygdala. Science (80-) 1977;198:423–425. doi: 10.1126/science.20664. [DOI] [PubMed] [Google Scholar]
- Haas BW, Canli T. Emotional memory function, personality structure and psychopathology: a neural system approach to the identification of vulnerability markers. Brain Res Rev. 2008;58:71–84. doi: 10.1016/j.brainresrev.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann SB, Ely TD, Grafton ST, Kilts CD. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nat Neurosci. 1999;2:289–293. doi: 10.1038/6404. [DOI] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29:1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- Kensinger Ea, Schacter DL. Remembering the specific visual details of presented objects: neuroimaging evidence for effects of emotion. Neuropsychologia. 2007;45:2951–2962. doi: 10.1016/j.neuropsychologia.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Kensinger EA. Remembering the Details: Effects of Emotion. 2009;1:99–113. doi: 10.1177/1754073908100432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Corkin S. Two routes to emotional memory: Distinct neural processes for valence and arousal. 2004 doi: 10.1073/pnas.0306408101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang M-C, Christensen GE, Collins DL, Gee J, Hellier P, Song JH, Jenkinson M, Lepage C, Rueckert D, Thompson P, Vercauteren T, Woods RP, Mann JJ, Parsey RV. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PSF, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal SL, Tighe SK, Yassa MA. Asymmetric effects of emotion on mnemonic interference. Neurobiol Learn Mem. 2014;111:41–48. doi: 10.1016/j.nlm.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. The amygdala. Curr Biol. 2007;17:868–874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Marr D. Simple memory: a theory for archicortex. Philos Trans R Soc London Ser B Biol Sci. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O’Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Magarinos AM. Stress and hippocampal plasticity: implications for the pathophysiology of affective disorders. Hum Psychopharmacol. 2001;16:S7–S19. doi: 10.1002/hup.266. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Payne JD, Kensinger EA. Sleep’s Role in the Consolidation of Emotional Episodic Memories. Curr Dir Psychol Sci. 2010;19:290–295. [Google Scholar]
- Sheline YI. Depression and the hippocampus: cause or effect? Biol Psychiatry. 2011;70:308–309. doi: 10.1016/j.biopsych.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Shelton DJ, Kirwan CB. A possible negative influence of depression on the ability to overcome memory interference. Behav Brain Res. 2013;256:20–26. doi: 10.1016/j.bbr.2013.08.016. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CEL, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- St Jacques PL, Bessette-Symons B, Cabeza R. Functional neuroimaging studies of aging and emotion: fronto-amygdalar differences during emotional perception and episodic memory. J Int Neuropsychol Soc JINS. 2009;15:819–825. doi: 10.1017/S1355617709990439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992a;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992b;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Watkins PC, Martin CK, Stern LD. Unconscious memory bias in depression: Perceptual and conceptual processes. J Abnorm Psychol. 2000;109:282–289. [PubMed] [Google Scholar]
- Watkins PC, Vache K, Verney SP, Muller S, Mathews A. Unconscious mood-congruent memory bias in depression. 1996 doi: 10.1037//0021-843x.105.1.34. [DOI] [PubMed] [Google Scholar]
- Yassa Ma, Stark CEL. Pattern separation in the hippocampus. Trends Neurosci. 2011;34:515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Hazlett RL, Stark CEL, Hoehn-Saric R. Functional MRI of the amygdala and bed nucleus of the stria terminalis during conditions of uncertainty in generalized anxiety disorder. J Psychiatr Res. 2012;46:4–11. doi: 10.1016/j.jpsychires.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Lacy JW, Stark SM, Albert MS, Gallagher M, Stark CEL. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2010a;000:968–979. doi: 10.1002/hipo.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark SM, Bakker A, Albert MS, Gallagher M, Stark CEL. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic Mild Cognitive Impairment. Neuroimage. 2010b;51:1242–1252. doi: 10.1016/j.neuroimage.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.