Abstract
Background:
Sleep disordered breathing (SDB) is associated with significant cardiovascular sequelae and positive airway pressure (PAP) has been shown to improve heart failure and prevent the recurrence of atrial fibrillation in cardiac patients with sleep apnea. Patients who are hospitalized with cardiac conditions frequently have witnessed symptoms of SDB but often do not have a diagnosis of sleep apnea. We implemented a clinical paradigm to perform unattended sleep studies and initiate treatment with PAP in hospitalized cardiac patients with symptoms consistent with SDB. We hypothesized that PAP adherence in cardiac patients with SDB would reduce readmission rates 30 days after discharge.
Methods:
106 consecutive cardiac patients hospitalized for heart failure, arrhythmias, and myocardial infarction and who reported symptoms of SDB were evaluated. Patients underwent a type III portable sleep study and those patients diagnosed with sleep apnea were started on PAP. Demographic data, SDB type, PAP adherence, and data regarding 30-day hospital readmission/ED visits were collected.
Results:
Of 106 patients, 104 had conclusive diagnostic studies using portable monitoring systems. Seventy-eight percent of patients (81/104) had SDB (AHI ≥ 5 events/h). Eighty percent (65/81) had predominantly obstructive sleep apnea, and 20% (16/81) had predominantly central sleep apnea. None of 19 patients (0%) with adequate PAP adherence, 6 of 20 (30%) with partial PAP use, and 5 of 17 (29%) of patients who did not use PAP were readmitted to the hospital or visited the emergency department (ED) for a cardiac issue within 30 days from discharge (p = 0.025).
Conclusions:
Performing diagnostic unattended sleep studies and initiating PAP treatment in hospitalized cardiac patients was feasible and provided important clinical information. Our data indicate that hospital readmission and ED visits 30 days after discharge were significantly lower in patients with cardiac disease and SDB who adhered to PAP treatment than those who were not adherent.
Commentary:
A commentary on this article appears in this issue on page 1067.
Citation:
Kauta SR, Keenan BT, Goldberg L, Schwab RJ. Diagnosis and treatment of sleep disordered breathing in hospitalized cardiac patients: a reduction in 30-day hospital readmission rates. J Clin Sleep Med 2014;10(10):1051-1059.
Keywords: sleep disordered breathing, portable sleep studies, 30-day readmission rate, hospitalized patient testing
Sleep disordered breathing (SDB) is a common medical condition affecting up to 24% of men and 9% of women in the middle-aged population.1–3 There is growing evidence that untreated SDB is associated with a spectrum of adverse health outcomes, most notably those related to cardiovascular health.4,5 SDB is an independent risk factor for the development of systemic hypertension and is associated with congestive heart failure, coronary artery disease, atrial fibrillation, and stroke.6–8
Sleep apnea has significant adverse consequences on cardiac function. Obstructive apneas cause episodes of hypoxia, exaggerated negative intrathoracic pressure, and increased sympathetic activity. In response to these disruptions, there are abnormal elevations in blood pressure and heart rate during sleep that may worsen heart failure and promote arrhythmias.9,10 Heart failure further increases sympathetic activity and also can cause central sleep apnea.5,9 Thus sleep apnea can have a significant negative impact on heart failure.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Sleep disordered breathing (SDB) is known to be common in patients with underlying cardiovascular disease. We hypothesized that diagnosing and initiating treatment for SDB in hospitalized cardiac patients would improve the overall management of their underlying cardiac condition.
Study Impact: Our study identifies a model of care that can increase the diagnosis and treatment of SDB in hospitalized cardiac patients. Finding a reduced 30-day cardiac readmission rate in CPAP adherent patients is important for both improving patient care and hospital finances.
Heart failure is one of the leading causes of morbidity and mortality in the United States. According to the 2011 American Heart Association statistics, at age 40, the lifetime risk of developing heart failure is 1 in 5 for both men and women.11 Forty-three percent of these patients in the United States are hospitalized four or more times for heart failure over their lifetime, and 20% of patients admitted for heart failure will be re-hospitalized within 30 days.12,13 This is the highest nonsurgical cause for readmission among Medicare recipients and leads to a large economic burden on society.14,15 As a result, identifying and treating conditions that negatively affect heart failure, such as SDB, could potentially help reduce health care spending if readmission rates are decreased.
Sleep disordered breathing occurs in 50% to 80% of patients with heart failure.16 Symptoms of SDB, snoring, apneas, and hypoxia, are frequently observed in the inpatient setting. Unfortunately, many hospitalized patients with observed symptoms of SDB remain undiagnosed and untreated.17,18 One reason for this may be that SDB is primarily considered a disorder that is managed in an outpatient setting. However, performing outpatient sleep studies in hospitalized cardiac patients is limited by the wait time and cost for an outpatient polysomnogram (PSG).19 Moreover, many of these patients never follow up with their out-patient sleep study. Performing diagnostic studies and providing treatment for SDB in the hospital could address this issue.
Treating SDB with continuous positive airway pressure (CPAP) has been shown to improve serum markers of cardiac and vascular injury, decrease sympathetic activity during sleep, and increase plasma levels of nitric oxide derivatives, all which positively affect patients with cardiovascular disease.20,21 Patients using CPAP for SDB who undergo electrical cardioversion for atrial fibrillation have a 50% reduction in recurrence rate for atrial fibrillation after one year compared to those who are not treated.22 Moreover in heart failure patients, treating SDB with CPAP increases left ventricular ejection fraction by 2% to 8%.23–25 The 2013 report from the American College of Cardiac Foundation/American Heart Association Task Force on practice guidelines for the management of heart failure supports the use of PAP in patients with heart failure.26 This report indicated that PAP has been shown to increase ejection fraction and improve functional status in patients with heart failure and sleep apnea.26
In our study, we designed a new paradigm to diagnose and manage OSA in cardiac inpatients by performing sleep studies and positive airway pressure (PAP) initiation for hospitalized patients with observed SDB symptoms using portable sleep monitoring systems and auto-PAP. These patients were then monitored for PAP adherence using modem and data card tracking. We hypothesized that (1) this paradigm would effectively identify SDB patients in cardiac hospitalized patients with SDB related symptoms but no previous SBD diagnosis; and (2) PAP initiation would reduce 30-day hospital readmission rates in patients adherent to prescribed PAP treatment.
METHODS
Patients
Between January 2012 and March 2013, all patients who were hospitalized at the Hospital of the University of Pennsylvania (HUP) for a cardiac condition in the cardiac intermediate care unit (CICU) and underwent a sleep study were approached for this study. Cardiac conditions included heart failure, arrhythmias, and myocardial infarction. Not all patients admitted for cardiovascular conditions were tested. However, all cardiac patients who underwent an inpatient sleep study were approached to be in the study. Sleep studies were ordered (after the subjects were evaluated by the sleep consult team) if the subject had a clinical presentation consistent with sleep apnea. The study was approved by the Institutional Review Board (IRB) at the University of Pennsylvania (#816517). Informed consent was waived for retrospective review of patients who were evaluated and treated prior to the IRB approval. The clinical protocol to perform in-hospital sleep studies on cardiac patients was established before IRB submission and is still ongoing.
Study Protocol
Routine clinical care at the Hospital of the University of Pennsylvania (HUP) included sleep studies performed on hemodynamically stable patients who were identified by the cardiology and sleep medicine team as likely to have SDB by clinical history (loud snoring, excessive daytime sleepiness, or witnessed apneas), physical examination (body habitus—obesity, large neck size, crowded pharyngeal airway, retrognathia) and/or nocturnal hypoxia. All patients undergoing an inpatient sleep study were screened for the research study by one of the investigators (SK). Patients with SDB, defined by an apnea-hypopnea index (AHI) ≥ 5 events/h, in whom PAP was prescribed, were started on auto-titrating PAP (5-20 cm of water) as an inpatient and discharged from the hospital with their own auto-PAP. The decision to prescribe PAP was ultimately at the discretion of the attending physician on the sleep medicine consult service. Patients were excluded from the study if they had a preexistent diagnosis of SDB and were on PAP prior to the hospital admission.
Medical records were reviewed to obtain baseline demographic data, admission diagnoses, comorbid illnesses, echocardiogram results, sleep study results, decision for treatment, and readmission data. Patients who were discharged with PAP were monitored for adherence data using modem or data card technology.
Procedures
In-Hospital Sleep Study and CPAP/Bilevel Treatment
In-hospital sleep studies were performed with a type III sleep study, which is classified according to the American Academy of Sleep Medicine.27 This unattended cardiorespiratory monitor (Embletta Gold) measures nasal pressure, respiratory effort, oxygen saturation, electrocardiogram, and body position. The sleep study was set up by a previously trained sleep technician who explained the study to the patient. The next morning, data on the time the patient fell asleep and awoke were collected by the sleep technician and the study recordings were downloaded and transmitted via the hospital network for interpretation by a sleep specialist.
Based on the results of the study, if the patient had SDB and the sleep specialist believed the best treatment was PAP, patients were offered a trial of therapy. If they agreed, they were treated with auto-CPAP or auto-bilevel (Phillips Respironics REMstar Auto 550P A-Flex and BiPAP Auto 750P Bi-Flex), monitored by overnight oximetry (Embletta Gold), and fitted for a mask by the sleep technician. The next morning, treatment data were collected using the data card from the PAP unit. If adequately treated, an auto-PAP unit and mask were ordered for the patient to be delivered in the hospital or at home depending on their date of discharge.
Interpretation of Sleep Studies
SDB was defined as AHI ≥ 5 events/h. Apnea was scored when there was ≥ 90% cessation of airflow detected through the nasal pressure sensor. Hypopnea was scored when there was ≥ 50% reduction in airflow with an associated ≥ 3% oxy-hemoglobin desaturation. Central apnea was scored when there was ≥ 90% cessation of airflow detected through the nasal pressure sensor and no effort in the thorax and abdomen. Each of these events needed ≥ 10 s duration to be scored.27 An AHI cutoff ≥ 5 events/h was selected to account for the potential overestimation of sleep time in the inpatient setting compared to a conventional in laboratory sleep study where EEG is obtained, which would lead to an underestimation of the AHI. If > 50% of the apneas were central, the SDB was classified as central sleep apnea (CSA). If > 50% of the apneas were obstructive in nature, it was considered obstructive sleep apnea (OSA).
PAP Compliance Data
PAP compliance data were collected via modem or data card. If a modem was not supplied by the durable medical supply company or the modem was not working, data were collected from the data card in the PAP unit. Based on the data available, patients were classified as non-users, partial users, or full users. Full users were classified based on Centers of Medicare and Medicaid Services (CMS) guidelines defined as use of PAP ≥ 4 h/night on 70% of nights during 30 consecutive days in the first 90 days of PAP treatment.28 Partial users were those with some usage who did not meet the criteria for full usage, and non-users were those with no PAP usage recorded or who could have been prescribed PAP treatment but refused or were not discharged with it. As a sensitivity analysis, we also used additional methods for calculating PAP usage group for patients who were readmitted during the first 30 days. First, 30-day PAP use was recalculated after removing the days in which the patients were in the hospital. Second, we calculated their PAP usage group using only the days before readmission. In both cases, the PAP usage group remained the same for all patients.
Determining 30-Day Hospital Readmission
Thirty-day hospital readmission was defined as a hospitalization or visit to the emergency department (ED) for a cardiac cause > 48 h after discharge. Hospitalizations and ED visits at the primary hospital and other hospitals in the tri-state region (Pennsylvania, New Jersey, and Delaware) were included. Data were collected from clinical records or by speaking with the patient if records did not clearly reference readmissions. Elective admissions were not included in the definition of readmission. However, only one patient studied had an elective admission, and this was for a heart transplant as a heart became available during follow-up. The 30-day readmission rate was specifically examined in light of the CMS regulations which withhold hospital reimbursement for the care of patients readmitted within 30 days after hospital discharge.29 Readmission rates were compared between the 3 different PAP user groups (non-users, partial users, and full users). Eleven patients who had refused PAP, and 3 CSA patients who could have been given PAP but were not, were included in the non-user group for further analysis of the impact of PAP use on hospital 30-day readmission.
Statistical Analysis
Demographic and clinical characteristics were summarized using means and standard deviations for continuous variables and frequencies and percentages for categorical variables. Continuous variables were compared among groups using t-tests or analysis of variance (ANOVA), where appropriate, and categorical variables using χ2 or Fisher exact tests. To further examine the relationship between 30-day readmission and PAP usage, we performed a Kaplan-Meier survival analysis, comparing the resulting survival curves among the PAP groups using a log-rank test. Statistical analyses were performed using Stata Version 12 (StataCorp, College Station, TX) or SAS Software, Version 9.3 (SAS Institute, Cary, NC).
RESULTS
Patient Characteristics
During the study period (January 2012 through March 2013), 106 cardiac patients were identified as patients currently not treated with PAP at home who underwent evaluation with a type III sleep study while hospitalized. One hundred four (98.1%) patients had conclusive diagnostic studies. The 2 patients with inconclusive studies were secondary to multiple channel failure on the recording and insufficient time during the patient's hospital stay to repeat the study. The mean ± standard deviation (SD) length of stay for all patients tested was 12.8 ± 8.7 days, and the mean ± SD percent of the stay that was completed at the time of the sleep study was 53.9% ± 22.5%.
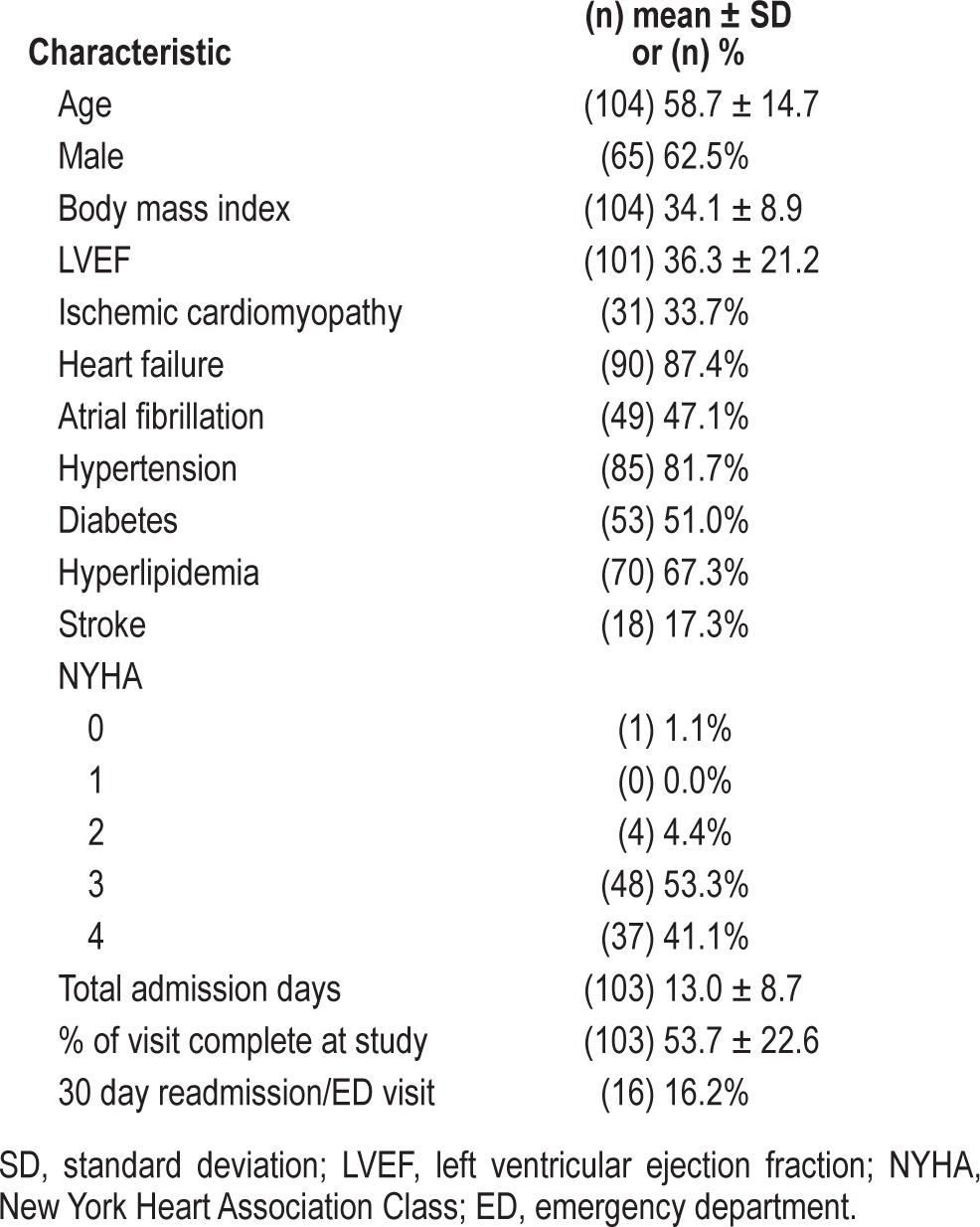
Clinical characteristics of the patients with conclusive diagnostic studies (n = 104) are shown in Table 1. Sixty-five (62.5%) of the patients were male; they had a mean ± SD age of 58.7 ± 14.7 years and body mass index (BMI) of 34.1 ± 8.9 kg/m2. The majority of the patients (87.4%) in the study carried the diagnosis of heart failure (based on medical record data) with a mean ± SD left ventricular ejection fraction (LVEF) of 32.7 ± 19.8. Forty-nine (47.1%) patients had atrial fibrillation and 44 (42.3%) of these patients also had heart failure. There were only 8 (7.7%) patients who were not admitted for either of these causes; they were primarily admitted for chest pain and myocardial infarction.
Table 1.
Clinical characteristics of cohort.
Classification and Characteristics of SDB
Eighty-one (77.9%) patients studied had SDB (AHI ≥ 5 events/h). Of those with SDB, 65 (80.2%) were classified having as obstructive sleep apnea (OSA) and 16 (19.8%) with central sleep apnea (CSA). Many of these patients had a combination of both obstructive and central sleep apnea. Of the 16 patients with CSA, 9 had 30% to 49% obstructive events. Twenty-three (22.1%) patients did not have evidence of SDB on their in-hospital sleep study.
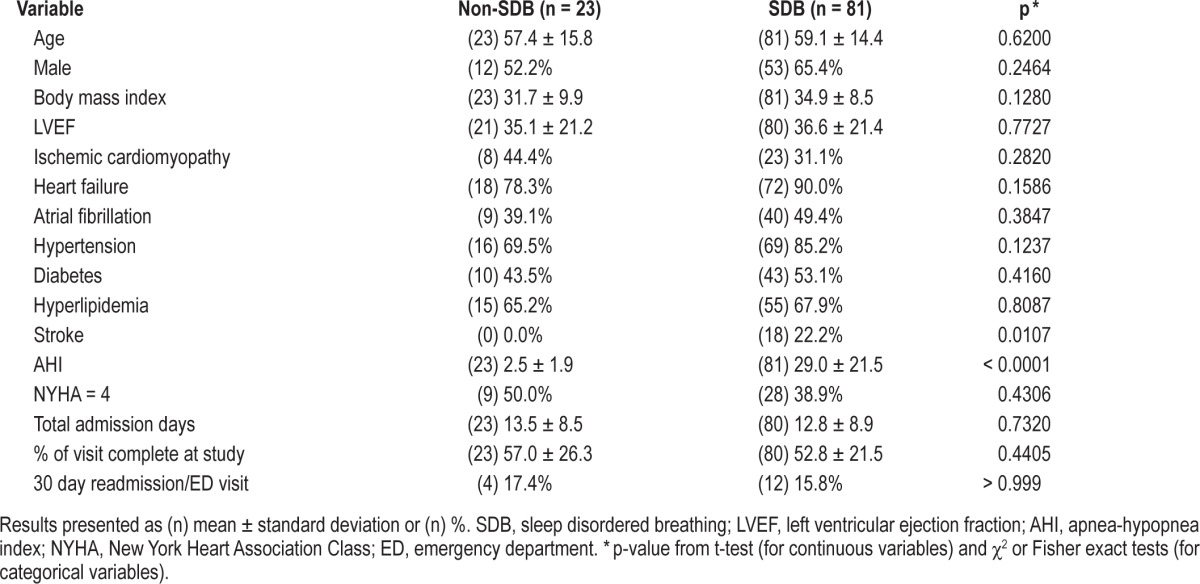
When patients with SDB were compared to those without SDB in this population (Table 2), there was no difference in age, gender, BMI, 30-day hospital readmission/emergency department visit, or left ventricular ejection fraction. The only a difference noted was a history of a prior stroke, which was more common in patients with SDB (p = 0.011).
Table 2.
Comparison of demographic and clinical characteristics between SDB and non-SDB patients.
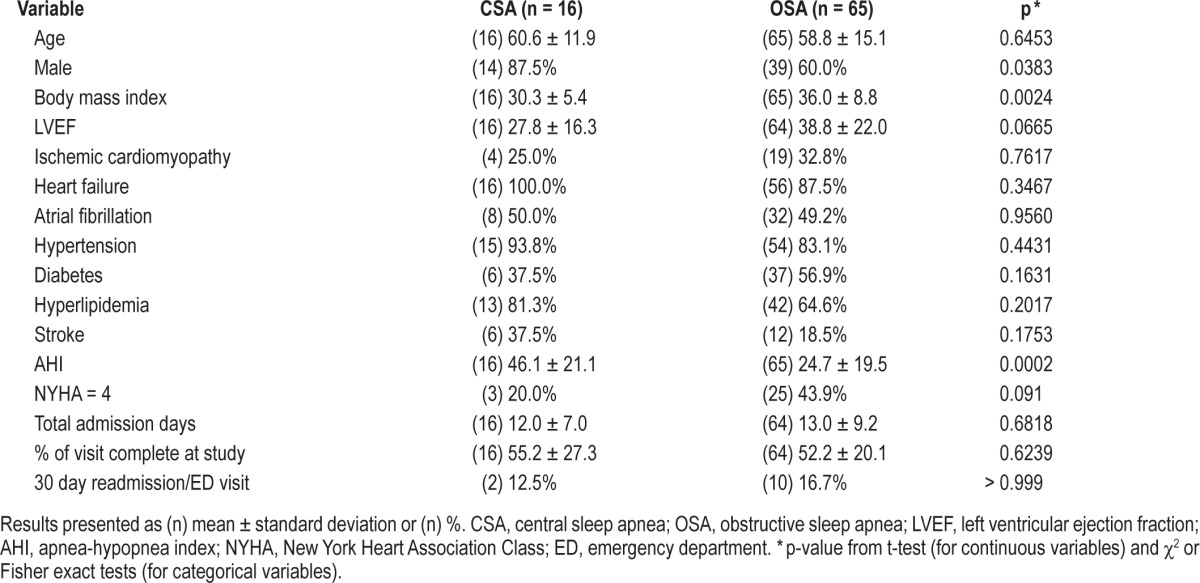
In the comparison of OSA patients to CSA patients (Table 3), patients with CSA were more likely to be male, have a lower LVEF, and have a higher AHI than OSA patients. OSA patients had a higher BMI than CSA patients.
Table 3.
Comparison of demographic and clinical characteristics between CSA and OSA patients.
Inpatient Model for Diagnosis and Treatment of SDB: Classification and Characteristics Based on PAP Use
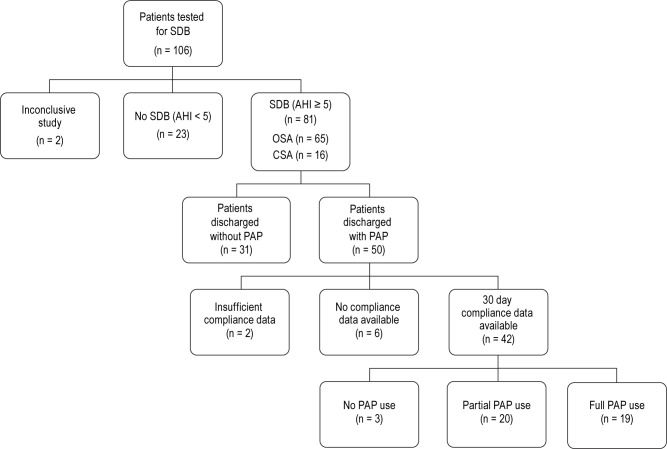
For the majority of patients, data from the type III sleep study and auto-PAP trial provided sufficient information to prescribe treatment. Fifty (61.7%) patients with SDB were prescribed PAP at the time of discharge (Figure 1). Five of these 50 patients had CSA. Patients with SDB who were not discharged with PAP included those who refused treatment (n = 11 [13.6%]), had CSA/Cheyne Stokes Respirations in the setting of incomplete heart failure management optimization (n = 3 [3.7%]), died while hospitalized (n = 1 [1.2%]) or needed further testing with an in-laboratory sleep study to determine the best treatment (n = 16 [17.8%]).
Figure 1. Disposition of the 106 consecutive cardiac patients who underwent an in-hospital sleep study between January 2012 and March 2013.
There were 31 patients who were discharged without PAP due to patient refusal of treatment, further in-lab testing, heart failure optimization required for treatment prescription, or death before discharge. Insufficient compliance data were secondary to 1 death and 1 patient requiring a tracheostomy within 30 days from discharge. Full PAP use was defined as use of PAP ≥ 4 h per night on 70% of nights during a consecutive 30-day period in the first 90 days of PAP treatment. SDB, sleep disordered breathing; OSA, obstructive sleep apnea; CSA, central sleep apnea; PAP, positive airway pressure.
PAP usage data from either a modem or data card were available for 42 (84.0%) patients discharged with a PAP unit. Nineteen (45.2%) patients were classified as full PAP users. On average, these patients used PAP on 94.9% ± 6.9% of nights in a 30 day period for 6.4 ± 1.4 h/night used. In the partial use group (n = 20 [47.6%]), the mean ± SD percent of days used was 59.9% ± 28.7% and the mean ± SD number of treatment hours on the days used was 5.3 ± 3.2 (Table 4). Three (7.1%) patients had no PAP use recorded over the first 90 days of treatment. Of the 5 CSA patients prescribed PAP at the time of discharge, 4 had usage data. Three of these patients were classified as full PAP users.
Table 4.
Positive airway pressure (PAP) usage and OSA variables for PAP users only.
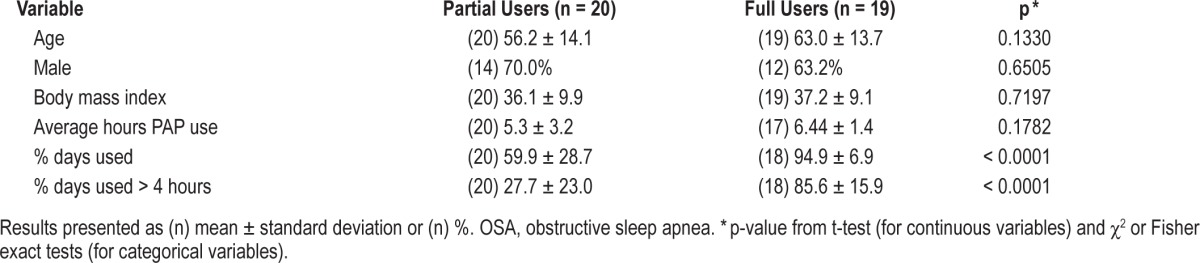
Adequate PAP Use Decreases 30-Day Hospital Readmission Rate
In the comparison of the 3 PAP usage groups (Table 5), the most notable finding was a difference in the 30-day readmission among the PAP groups (p = 0.025). Specifically, PAP compliant patients (full-users) were significantly less likely to be readmitted within 30 days from discharge when compared to both partial (p = 0.045) and non-users (p = 0.022). Partial PAP use did not affect the readmission rate, as they were not significantly different from non-users. None of the full users were readmitted within 30 days. Six (30%) of the partial users and 5 (29.4%) of the non-users were readmitted. This is also seen in our Kaplan-Meier analysis, where compliant patients performed better than those who were not fully compliant (Figure 2, p = 0.048). None of the CSA patients discharged with PAP were readmitted within 30 days.
Table 5.
Comparison of demographic and clinical characteristics between various positive airway pressure (PAP) usage groups.
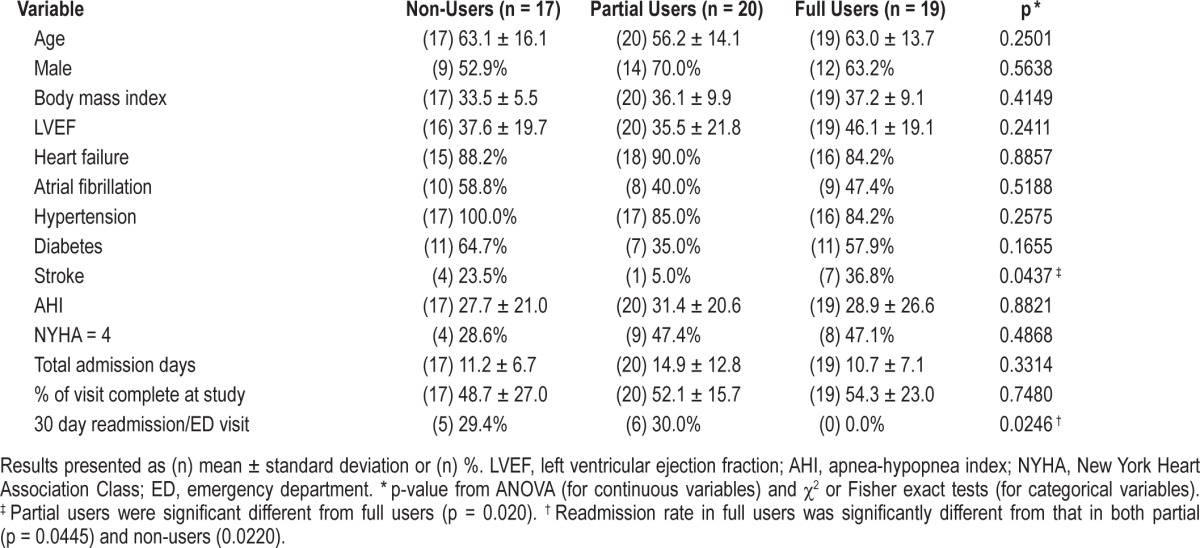
Figure 2. Kaplan-Meier analysis and curves for readmission demonstrating the difference in readmission rates between the 3 PAP usage groups.
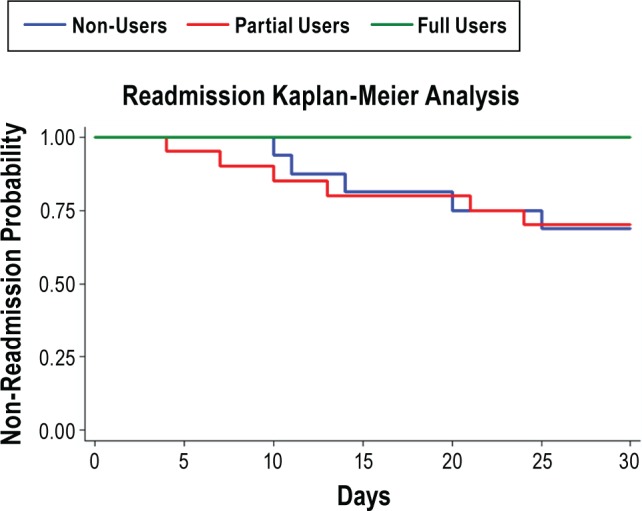
Full users were defined as patients with use of PAP ≥ 4 h per night on 70% of nights during a consecutive 30-day period in the first 90 days of PAP treatment. Readmission was defined as hospitalization or visit to the emergency department from 48 h to 30 days after discharge. PAP, positive airway pressure.
As a sensitivity analysis, we explored additional methods for classifying PAP use relative to hospital readmission. We reanalyzed our data for the n = 6 patients with partial PAP use in 2 ways: (1) excluding days they were admitted to the hospital; and (2) using only the days prior to hospital admission or ED visit to classify their PAP usage group. All 6 patients who were readmitted remained in the partial PAP use classification based on either of these methods. Therefore, the association between hospital readmission and PAP usage remains the same for each potential classification method.
To further evaluate the various factors affecting readmission, the association between readmission and several variables (age, BMI, LVEF, New York Heart Association Classification [NYHA], AHI) were studied in bivariate analyses (Table 6). These analyses only identified LVEF as a predictor of readmission (p = 0.037). Patients with a lower LVEF during the initial hospital admission (before the sleep study was performed) were more likely to be readmitted within 30 days from discharge. Fifteen (93.8%) of 16 patients who were readmitted had heart failure.
Table 6.
Comparison of demographic and clinical characteristics between patients not readmitted and those readmitted within 30 days.
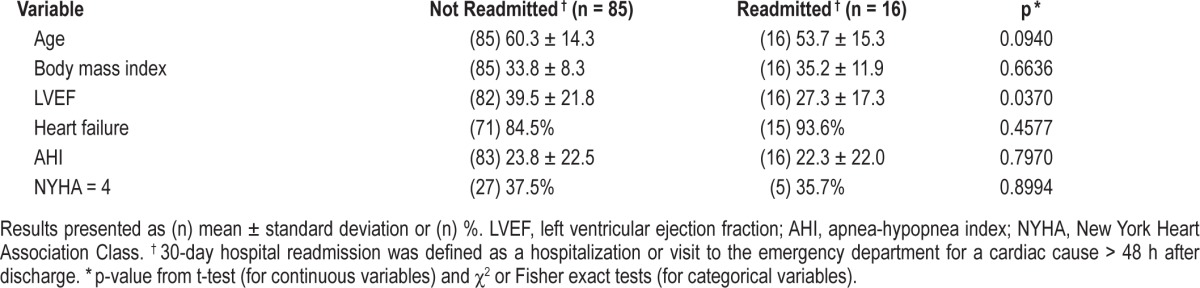
Given that LVEF and CPAP adherence were the only variables significantly (p < 0.05) associated with readmission, we performed analyses including both variables in a single model within the subset (N = 53) of patients with both CPAP adherence and LVEF data. In this analysis, CPAP adherence remained significantly associated with readmission (p = 0.048), while LVEF was no longer significant (p = 0.295). We note that in bivariate analyses restricted to this sample of patients with CPAP adherence, LVEF was not significantly associated with readmission (p = 0.182), as it was in the overall sample.
DISCUSSION
Using a new paradigm, we have shown that approximately 80% of hospitalized cardiac patients with sleep disordered breathing related symptoms, but no previous SDB diagnosis, have OSA or CSA. Moreover, our data indicate that PAP adherence for SDB in these cardiac patients leads to a reduction in 30-day readmission rates. We were able to demonstrate this by implementing a protocol to diagnose and treat SDB in hospitalized patients using unattended studies and PAP units with modems and data cards for monitoring treatment adherence.
Although the impact of SDB on underlying cardiac disease5,8,9 and the benefits of PAP treatment22,23,25,30 have been well documented in prior studies, to our knowledge, our study is the first to investigate the impact of PAP treatment on 30-day hospital readmission rates. None of the patients (n = 19) who were compliant with CPAP based on CMS guidelines28 were readmitted to the hospital or had an ED visit within 48 hours to 30 days after discharge. In comparison, patients with no PAP use or partial use had readmission rates of 29.4% and 30.0%, respectively. Of note, this is a higher readmission rate than the general readmission rate for patients admitted to our institution's cardiac intermediate care unit, which ranges from 12% to 18%. This variation in the rates may be due to a number of factors, including patients with cardiac disease and SDB being more likely to be readmitted to the hospital than cardiac patients without SDB.31 Furthermore, in order to be comprehensive, our study included emergency department visits as well as admissions to other institutions.
The difference in 30-day readmission rates between the PAP usage groups is noteworthy, as it can affect hospital reimbursement based on the current Medicare guidelines, which both withhold payment and charge additional fees to hospitals when patients are readmitted within 30 days from discharge.29 Our data support prior findings of Kasai et al., which showed heart failure patients with untreated or inadequately treated SDB are more likely to die or be hospitalized than patients who were compliant (average PAP use of 6 h/night).32 In a larger scale study using Medicare claims, Javaheri et al. found that patients with newly diagnosed heart failure and SDB who were started on PAP treatment had a better 2-year survival rate that those who were not treated.33 However, this study was not able to document the effect of PAP compliance on survival or hospital readmission.
The average total cost per heart failure hospitalization ranges from $13,000 to $18,000.34,35 The cost of running an inpatient sleep consult program that provides a sleep physician consult, sleep study set up by sleep technician, and trial of auto-PAP is variable, based on staffing and the number of patients tested. However, using our data, we estimate the cost to range from $40,000-$60,000 annually. Based on these numbers, decreasing 30-day hospital readmission rates by 3-5 patients per year would offset the cost of funding an inpatient sleep consult service. Our preliminary data suggest that this is possible; however, more data and a closer financial analysis on the cost of hospital readmissions are necessary.
Studies performed by Khayat et al.36 introduced the concept of inpatient sleep studies as a method of diagnosing OSA. They performed type III attended studies on hospitalized patients with heart failure and were able to validate the results of OSA in a subset of 62 patients who had a follow-up in-lab polysomnography 6-8 weeks after discharge. We did not repeat sleep studies in the outpatient setting, but we used a similar model of inpatient evaluation and found that 78% of the patients studied had SDB. The majority of these patients had primarily obstructive apnea (80%) and a smaller percentage of patients (20%) had primarily central apnea. These results were consistent with the findings of Khayat and those from a study performed by Egea et al.25
PAP compliance data were available for 42 of the 50 (84%) patients who were discharged from the hospital with PAP. Studies have shown a variety of results for PAP compliance in the out-patient setting, ranging from 29% to 85%.37–40 Nineteen (45%) of the patients in our study with available compliance data showed adequate use; however, for this population of patients with significant cardiac disease we had a higher goal for adherence. Sin et al. found a compliance rate > 85% when patients with moderate to severe SDB were closely followed after initial PAP treatment. This included a daily telephone call during the first week of treatment followed by clinic visits at 2 weeks, 4 weeks, 3 months, and 6 months.39 This model may be difficult to follow due to the staff needed to maintain such close monitoring; however, with modem tracking technology a modified version of this model could be established. Another option includes incorporating SDB follow-up into other routine visits. Many patients are closely followed by their cardiology team specifically for heart failure after discharge from the hospital. Thus the cardiologist could address PAP use to improve compliance.
Limitations to our study include the potential that reduced re-admission rates may be affected by the fact that patients who are adherent to their other medical regimens may also be compliant with PAP and as a result reduce their risk for readmission. However, no data were collected on medication history and lifestyle changes, and as a result these factors were not controlled for as possible confounders. Another limitation was there were too few patients with follow-up echocardiogram results to evaluate the potential benefits of PAP use specifically on cardiac function. Due to limitations in PAP usage data and variations in patient PAP use, 4 patients were classified on PAP usage based on their use in the 30-90 day period. When the data were reanalyzed after excluding these patients, the differences in 30-day readmission among the PAP groups remained significant (p = 0.033). While our results suggest a relationship between CPAP use in cardiac patients with SDB and a reduction in 30-day readmissions, given the relatively small sample size and observational nature of the study, results should be replicated in future randomized trials. Moreover, given the relatively small sample size in this study, negative results (for outcomes other than CPAP adherence and readmission rates) should be interpreted with caution, as there is limited power to observe small effect sizes. Furthermore, in general, it is not a recommended practice to prescribe auto-CPAP to patients with CSA. However, there are not a lot of data to support this recommendation. Moreover, studies using auto-CPAP have shown that it improves left ventricular ejection fraction in patients with heart failure.24 In addition, most of the subjects in this study had obstructive sleep apnea, and auto-CPAP is indicated in those patients.
In conclusion, our study showed that (1) SDB is common in hospitalized cardiac patients with symptoms but no previous SDB diagnosis, and (2) that cardiac patients with SDB who are adherent to treatment with PAP therapy have a lower 30-day hospital readmission rate than similar subjects who are not adherent to PAP. This potential benefit is important in the setting of new Medicare regulations for withholding payment and penalties for 30-day hospital readmissions for heart failure and myocardial infarction. We believe there are a number of cardiac patients with SDB who are hospitalized all over the world and who remain undiagnosed and untreated. Identifying these patients and promoting compliance with prescribed PAP treatment appear to be critical elements in their overall medical management.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Goldberg ‘s cardiology department has received research grants from Medtronic, Gambro, and Respicardia. He has received honoraria from Medtronic and Thoratec for consulting activities. The authors have indicated no financial conflicts of interest. The work was performed at the Center for Sleep and Circadian Neurobiology, University of Pennsylvania, Philadelphia, PA.
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136–43. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kapur V, Strohl KP, Redline S, Iber C, O'Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath. 2002;6:49–54. doi: 10.1007/s11325-002-0049-5. [DOI] [PubMed] [Google Scholar]
- 4.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 5.Selim B, Won C, Yaggi HK. Cardiovascular consequences of sleep apnea. Clin Chest Med. 2010;31:203–20. doi: 10.1016/j.ccm.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 7.Munoz R, Duran-Cantolla J, Martinez-Vila E, et al. Severe sleep apnea and risk of ischemic stroke in the elderly. Stroke. 2006;37:2317–21. doi: 10.1161/01.STR.0000236560.15735.0f. [DOI] [PubMed] [Google Scholar]
- 8.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 9.Leung RS, Bradley TD. Sleep apnea and cardiovascular disease. Am J Respir Crit Care Med. 2001;164:2147–65. doi: 10.1164/ajrccm.164.12.2107045. [DOI] [PubMed] [Google Scholar]
- 10.Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173:910–6. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunlay SM, Redfield MM, Weston SA, et al. Hospitalizations after heart failure diagnosis a community perspective. J Am Coll Cardiol. 2009;54:1695–702. doi: 10.1016/j.jacc.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bueno H, Ross JS, Wang Y, et al. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993-2006. JAMA. 2010;303:2141–7. doi: 10.1001/jama.2010.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–28. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 15.Lee WC, Chavez YE, Baker T, Luce BR. Economic burden of heart failure: a summary of recent literature. Heart Lung. 2004;33:362–71. doi: 10.1016/j.hrtlng.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Khayat R, Small R, Rathman L, et al. Sleep-disordered breathing in heart failure: identifying and treating an important but often unrecognized comorbidity in heart failure patients. J Card Fail. 2013;19:431–44. doi: 10.1016/j.cardfail.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20:705–6. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- 18.Spurr KF, Graven MA, Gilbert RW. Prevalence of unspecified sleep apnea and the use of continuous positive airway pressure in hospitalized patients, 2004 National Hospital Discharge Survey. Sleep Breath. 2008;12:229–34. doi: 10.1007/s11325-007-0166-2. [DOI] [PubMed] [Google Scholar]
- 19.Flemons WW, Douglas NJ, Kuna ST, Rodenstein DO, Wheatley J. Access to diagnosis and treatment of patients with suspected sleep apnea. Am J Respir Crit Care Med. 2004;169:668–72. doi: 10.1164/rccm.200308-1124PP. [DOI] [PubMed] [Google Scholar]
- 20.Narkiewicz K, Kato M, Phillips BG, Pesek CA, Davison DE, Somers VK. Nocturnal continuous positive airway pressure decreases daytime sympathetic traffic in obstructive sleep apnea. Circulation. 1999;100:2332–5. doi: 10.1161/01.cir.100.23.2332. [DOI] [PubMed] [Google Scholar]
- 21.Luthje L, Andreas S. Obstructive sleep apnea and coronary artery disease. Sleep Med Rev. 2008;12:19–31. doi: 10.1016/j.smrv.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107:2589–94. doi: 10.1161/01.CIR.0000068337.25994.21. [DOI] [PubMed] [Google Scholar]
- 23.Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348:1233–41. doi: 10.1056/NEJMoa022479. [DOI] [PubMed] [Google Scholar]
- 24.Khayat RN, Abraham WT, Patt B, Pu M, Jarjoura D. In-hospital treatment of obstructive sleep apnea during decompensation of heart failure. Chest. 2009;136:991–7. doi: 10.1378/chest.09-0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Egea CJ, Aizpuru F, Pinto JA, et al. Cardiac function after CPAP therapy in patients with chronic heart failure and sleep apnea: a multicenter study. Sleep Med. 2008;9:660–6. doi: 10.1016/j.sleep.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 26.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–52. doi: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 27.ATS/ACCP/AASM Taskforce Steering Committee. Executive summary on the systematic review and practice parameters for portable monitoring in the investigation of suspected sleep apnea in adults. Am J Respir Crit Care Med. 2004;169:1160–3. doi: 10.1164/rccm.169.1160. [DOI] [PubMed] [Google Scholar]
- 28. Centers for Medicare & Medicaid Services, PAP Devices for the Treatment of OSA (L11528, L11528, L11518, L171), U.S. Department of Health and Human Services (revision effective date 10/1/2011)
- 29. Centers for Medicare & Medicaid Services, “Readmissions Reduction Program,” U.S. Department of Health and Human Services.
- 30.Mansfield DR, Gollogly NC, Kaye DM, Richardson M, Bergin P, Naughton MT. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med. 2004;169:361–6. doi: 10.1164/rccm.200306-752OC. [DOI] [PubMed] [Google Scholar]
- 31.Khayat R, Abraham W, Patt B, et al. Central sleep apnea is a predictor of cardiac readmission in hospitalized patients with systolic heart failure. J Card Fail. 2012;18:534–40. doi: 10.1016/j.cardfail.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kasai T, Narui K, Dohi T, et al. Prognosis of patients with heart failure and obstructive sleep apnea treated with continuous positive airway pressure. Chest. 2008;133:690–6. doi: 10.1378/chest.07-1901. [DOI] [PubMed] [Google Scholar]
- 33.Javaheri S, Caref EB, Chen E, Tong KB, Abraham WT. Sleep apnea testing and outcomes in a large cohort of Medicare beneficiaries with newly diagnosed heart failure. Am J Respir Crit Care Med. 2011;183:539–46. doi: 10.1164/rccm.201003-0406OC. [DOI] [PubMed] [Google Scholar]
- 34.Agency for Health Care Research and Quality. National healthcare quality report-chapter 6 care coordination. U.S. Department of Health and Human Services. 2010 [Google Scholar]
- 35.Titler MG, Jensen GA, Dochterman JM, et al. Cost of hospital care for older adults with heart failure: medical, pharmaceutical, and nursing costs. Health Serv Res. 2008;43:635–55. doi: 10.1111/j.1475-6773.2007.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khayat RN, Jarjoura D, Patt B, Yamokoski T, Abraham WT. In-hospital testing for sleep-disordered breathing in hospitalized patients with decompensated heart failure: report of prevalence and patient characteristics. J Card Fail. 2009;15:739–46. doi: 10.1016/j.cardfail.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weaver TE, Kribbs NB, Pack AI, et al. Night-to-night variability in CPAP use over the first three months of treatment. Sleep. 1997;20:278–83. doi: 10.1093/sleep/20.4.278. [DOI] [PubMed] [Google Scholar]
- 38.Lindberg E, Berne C, Elmasry A, Hedner J, Janson C. CPAP treatment of a population-based sample--what are the benefits and the treatment compliance? Sleep Med. 2006;7:553–60. doi: 10.1016/j.sleep.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 39.Sin DD, Mayers I, Man GC, Pawluk L. Long-term compliance rates to continuous positive airway pressure in obstructive sleep apnea: a population-based study. Chest. 2002;121:430–5. doi: 10.1378/chest.121.2.430. [DOI] [PubMed] [Google Scholar]
- 40.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5:173–8. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]