Abstract
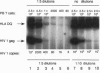
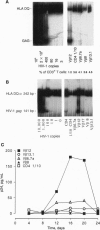
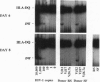
The human immunodeficiency virus 1 (HIV-1) replicates more efficiently in T-cell lines expressing T-cell receptors derived from certain V beta genes, V beta 12 in particular, suggesting the effects of a superantigen. The targeted V beta 12 subset was not deleted in HIV-1-infected patients. It was therefore possible that it might represent an in vivo viral reservoir. Viral load was assessed by quantitative PCR with gag primers and with an infectivity assay to measure competent virus. It was shown that the tiny V beta 12 subset (1-2% of T cells) often has a higher viral load than other V beta subsets in infected patients. Selective HIV-1 replication in V beta 12 cells was also observed 6-8 days after in vitro infection of peripheral blood lymphocytes from normal, HIV-1 negative donors. Viral replication in targeted V beta subsets may serve to promote a biologically relevant viral reservoir.
Full text
PDF

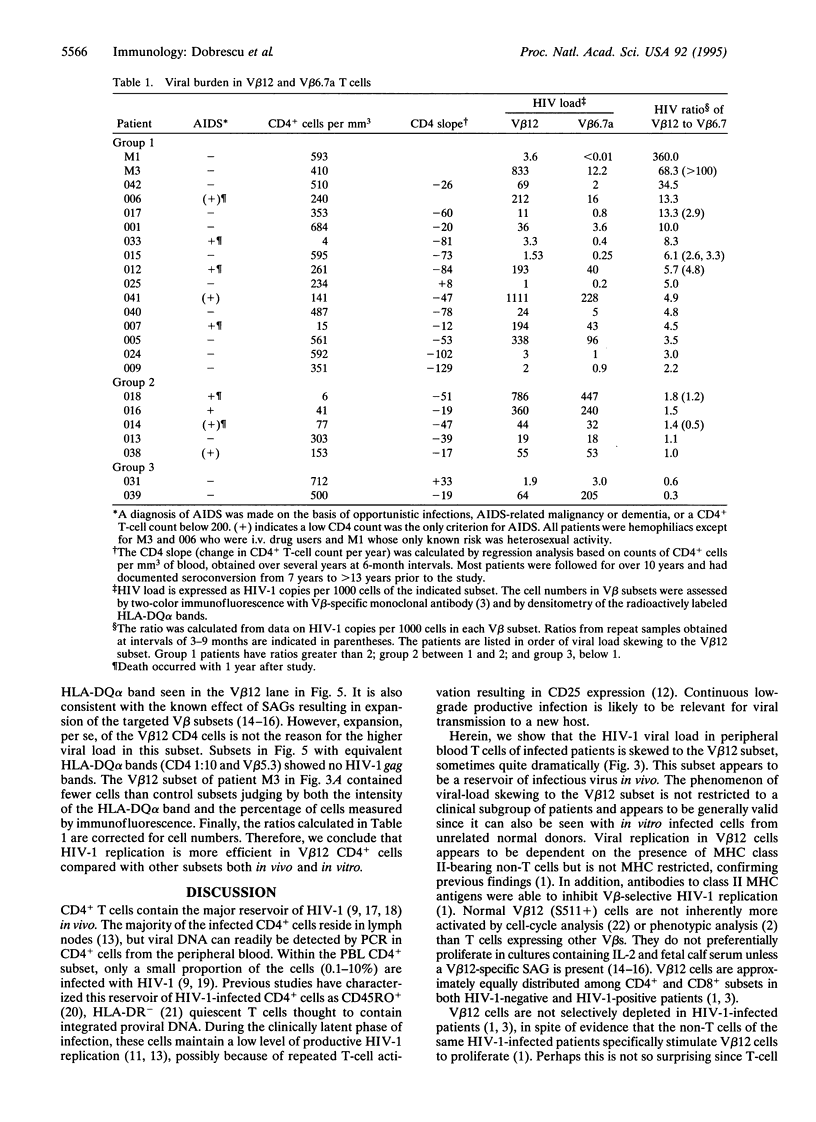
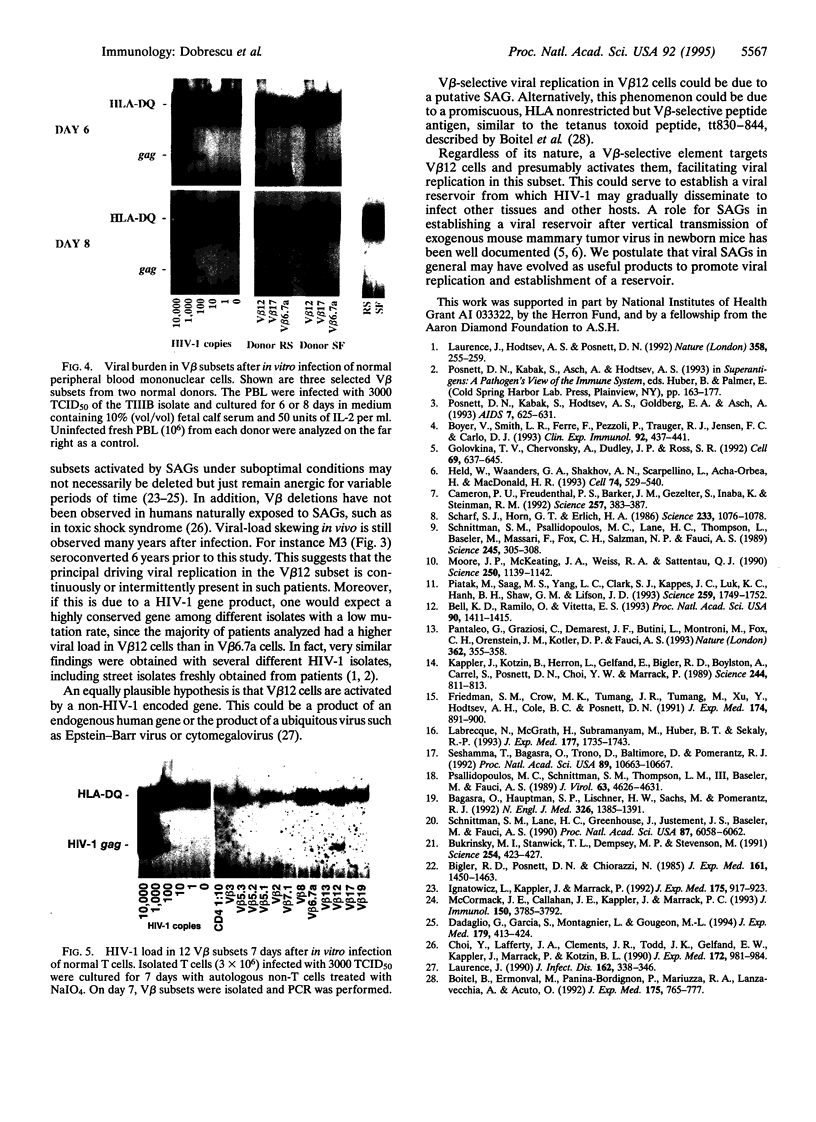
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagasra O., Hauptman S. P., Lischner H. W., Sachs M., Pomerantz R. J. Detection of human immunodeficiency virus type 1 provirus in mononuclear cells by in situ polymerase chain reaction. N Engl J Med. 1992 May 21;326(21):1385–1391. doi: 10.1056/NEJM199205213262103. [DOI] [PubMed] [Google Scholar]
- Bell K. D., Ramilo O., Vitetta E. S. Combined use of an immunotoxin and cyclosporine to prevent both activated and quiescent peripheral blood T cells from producing type 1 human immunodeficiency virus. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1411–1415. doi: 10.1073/pnas.90.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigler R. D., Posnett D. N., Chiorazzi N. Stimulation of a subset of normal resting T lymphocytes by a monoclonal antibody to a crossreactive determinant of the human T cell antigen receptor. J Exp Med. 1985 Jun 1;161(6):1450–1463. doi: 10.1084/jem.161.6.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boitel B., Ermonval M., Panina-Bordignon P., Mariuzza R. A., Lanzavecchia A., Acuto O. Preferential V beta gene usage and lack of junctional sequence conservation among human T cell receptors specific for a tetanus toxin-derived peptide: evidence for a dominant role of a germline-encoded V region in antigen/major histocompatibility complex recognition. J Exp Med. 1992 Mar 1;175(3):765–777. doi: 10.1084/jem.175.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer V., Smith L. R., Ferre F., Pezzoli P., Trauger R. J., Jensen F. C., Carlo D. J. T cell receptor V beta repertoire in HIV-infection individuals: lack of evidence for selective V beta deletion. Clin Exp Immunol. 1993 Jun;92(3):437–441. doi: 10.1111/j.1365-2249.1993.tb03417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukrinsky M. I., Stanwick T. L., Dempsey M. P., Stevenson M. Quiescent T lymphocytes as an inducible virus reservoir in HIV-1 infection. Science. 1991 Oct 18;254(5030):423–427. doi: 10.1126/science.1925601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron P. U., Freudenthal P. S., Barker J. M., Gezelter S., Inaba K., Steinman R. M. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science. 1992 Jul 17;257(5068):383–387. doi: 10.1126/science.1352913. [DOI] [PubMed] [Google Scholar]
- Choi Y., Lafferty J. A., Clements J. R., Todd J. K., Gelfand E. W., Kappler J., Marrack P., Kotzin B. L. Selective expansion of T cells expressing V beta 2 in toxic shock syndrome. J Exp Med. 1990 Sep 1;172(3):981–984. doi: 10.1084/jem.172.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadaglio G., Garcia S., Montagnier L., Gougeon M. L. Selective anergy of V beta 8+ T cells in human immunodeficiency virus-infected individuals. J Exp Med. 1994 Feb 1;179(2):413–424. doi: 10.1084/jem.179.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S. M., Crow M. K., Tumang J. R., Tumang M., Xu Y. Q., Hodtsev A. S., Cole B. C., Posnett D. N. Characterization of human T cells reactive with the Mycoplasma arthritidis-derived superantigen (MAM): generation of a monoclonal antibody against V beta 17, the T cell receptor gene product expressed by a large fraction of MAM-reactive human T cells. J Exp Med. 1991 Oct 1;174(4):891–900. doi: 10.1084/jem.174.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovkina T. V., Chervonsky A., Dudley J. P., Ross S. R. Transgenic mouse mammary tumor virus superantigen expression prevents viral infection. Cell. 1992 May 15;69(4):637–645. doi: 10.1016/0092-8674(92)90227-4. [DOI] [PubMed] [Google Scholar]
- Held W., Waanders G. A., Shakhov A. N., Scarpellino L., Acha-Orbea H., MacDonald H. R. Superantigen-induced immune stimulation amplifies mouse mammary tumor virus infection and allows virus transmission. Cell. 1993 Aug 13;74(3):529–540. doi: 10.1016/0092-8674(93)80054-i. [DOI] [PubMed] [Google Scholar]
- Ignatowicz L., Kappler J., Marrack P. The effects of chronic infection with a superantigen-producing virus. J Exp Med. 1992 Apr 1;175(4):917–923. doi: 10.1084/jem.175.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J., Kotzin B., Herron L., Gelfand E. W., Bigler R. D., Boylston A., Carrel S., Posnett D. N., Choi Y., Marrack P. V beta-specific stimulation of human T cells by staphylococcal toxins. Science. 1989 May 19;244(4906):811–813. doi: 10.1126/science.2524876. [DOI] [PubMed] [Google Scholar]
- Labrecque N., McGrath H., Subramanyam M., Huber B. T., Sékaly R. P. Human T cells respond to mouse mammary tumor virus-encoded superantigen: V beta restriction and conserved evolutionary features. J Exp Med. 1993 Jun 1;177(6):1735–1743. doi: 10.1084/jem.177.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurence J., Hodtsev A. S., Posnett D. N. Superantigen implicated in dependence of HIV-1 replication in T cells on TCR V beta expression. Nature. 1992 Jul 16;358(6383):255–259. doi: 10.1038/358255a0. [DOI] [PubMed] [Google Scholar]
- Laurence J. Molecular interactions among herpesviruses and human immunodeficiency viruses. J Infect Dis. 1990 Aug;162(2):338–346. doi: 10.1093/infdis/162.2.338. [DOI] [PubMed] [Google Scholar]
- McCormack J. E., Callahan J. E., Kappler J., Marrack P. C. Profound deletion of mature T cells in vivo by chronic exposure to exogenous superantigen. J Immunol. 1993 May 1;150(9):3785–3792. [PubMed] [Google Scholar]
- Moore J. P., McKeating J. A., Weiss R. A., Sattentau Q. J. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science. 1990 Nov 23;250(4984):1139–1142. doi: 10.1126/science.2251501. [DOI] [PubMed] [Google Scholar]
- Pantaleo G., Graziosi C., Demarest J. F., Butini L., Montroni M., Fox C. H., Orenstein J. M., Kotler D. P., Fauci A. S. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993 Mar 25;362(6418):355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- Piatak M., Jr, Saag M. S., Yang L. C., Clark S. J., Kappes J. C., Luk K. C., Hahn B. H., Shaw G. M., Lifson J. D. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science. 1993 Mar 19;259(5102):1749–1754. doi: 10.1126/science.8096089. [DOI] [PubMed] [Google Scholar]
- Posnett D. N., Kabak S., Hodtsev A. S., Goldberg E. A., Asch A. T-cell antigen receptor V beta subsets are not preferentially deleted in AIDS. AIDS. 1993 May;7(5):625–631. doi: 10.1097/00002030-199305000-00003. [DOI] [PubMed] [Google Scholar]
- Psallidopoulos M. C., Schnittman S. M., Thompson L. M., 3rd, Baseler M., Fauci A. S., Lane H. C., Salzman N. P. Integrated proviral human immunodeficiency virus type 1 is present in CD4+ peripheral blood lymphocytes in healthy seropositive individuals. J Virol. 1989 Nov;63(11):4626–4631. doi: 10.1128/jvi.63.11.4626-4631.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf S. J., Horn G. T., Erlich H. A. Direct cloning and sequence analysis of enzymatically amplified genomic sequences. Science. 1986 Sep 5;233(4768):1076–1078. doi: 10.1126/science.3461561. [DOI] [PubMed] [Google Scholar]
- Schnittman S. M., Lane H. C., Greenhouse J., Justement J. S., Baseler M., Fauci A. S. Preferential infection of CD4+ memory T cells by human immunodeficiency virus type 1: evidence for a role in the selective T-cell functional defects observed in infected individuals. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6058–6062. doi: 10.1073/pnas.87.16.6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnittman S. M., Psallidopoulos M. C., Lane H. C., Thompson L., Baseler M., Massari F., Fox C. H., Salzman N. P., Fauci A. S. The reservoir for HIV-1 in human peripheral blood is a T cell that maintains expression of CD4. Science. 1989 Jul 21;245(4915):305–308. doi: 10.1126/science.2665081. [DOI] [PubMed] [Google Scholar]
- Seshamma T., Bagasra O., Trono D., Baltimore D., Pomerantz R. J. Blocked early-stage latency in the peripheral blood cells of certain individuals infected with human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10663–10667. doi: 10.1073/pnas.89.22.10663. [DOI] [PMC free article] [PubMed] [Google Scholar]