Abstract
Purpose
The aim of the present work was to identify and characterize large rearrangements involving the USH2A gene in patients with Usher syndrome and nonsyndromic retinitis pigmentosa.
Methods
The multiplex ligation-dependent probe amplification (MLPA) technique combined with a customized array-based comparative genomic hybridization (aCGH) analysis was applied to 40 unrelated patients previously screened for point mutations in the USH2A gene in which none or only one pathologic mutation was identified.
Results
We detected six large deletions involving USH2A in six out of the 40 cases studied. Three of the patients were homozygous for the deletion, and the remaining three were compound heterozygous with a previously identified USH2A point mutation. In five of these cases, the patients displayed Usher type 2, and the remaining case displayed nonsyndromic retinitis pigmentosa. The exact breakpoint junctions of the deletions found in USH2A in four of these cases were characterized.
Conclusions
Our study highlights the need to develop improved efficient strategies of mutation screening based upon next generation sequencing (NGS) that reduce cost, time, and complexity and allow simultaneous identification of all types of disease-causing mutations in diagnostic procedures.
Introduction
Usher syndrome (USH) is a genetically and clinically heterogeneous autosomal recessive disorder that associates sensorineural hearing loss, retinitis pigmentosa (RP), and in some cases vestibular dysfunction. It accounts for over 50% of cases of hereditary forms combining deafness and blindness and has an estimated prevalence of 3 to 6.2 per 100,000 [1].
USH is divided into three clinical subtypes: Usher type 1(USH1), type 2 (USH2), and type 3 (USH3) [2]. USH1 displays severe–profound congenital deafness, absent vestibular function, and prepubertal onset of RP; in USH2 hearing loss is congenital, moderate to severe, with normal vestibular function, and pre- or postpubertal onset of RP; and in USH3, hearing loss may be pre- or postlingual but is progressive in course, with normal or abnormal vestibular function and often postpubertal onset of RP. Although this classification is generally deemed adequate, atypical clinical types have also been described [3].
To date, ten genes have been associated with this disease. For USH1, six genes have been identified: MYO7A (USH1B), USH1C (USH1C), CDH23 (USH1D), PCDH15 (USH1F), USH1G (USH1G) [4], and CIB2 (USH1J) [5]. Three genes have been found to cause USH2: USH2A (USH2A), GPR98 (USH2C), and DFNB31 (USH2D). Only the CLRN1 gene has been described for USH3. Most of these genes are also responsible for nonsyndromic hearing loss or isolated RP [4]. In addition to these ten genes, three other genes have been associated with USH. PDZD7 was proposed to contribute to digenic inheritance with GPR98 and also to have a role as a retinal disease modifier in USH2A patients [6]. Recently, a novel missense variant in HARS was identified in homozygosis in two patients with a phenotype compatible with USH3 [7]. Thus, this gene was proposed to be a novel gene causative for USH3 [4]. More recently, the CEP250 gene has been associated with atypical Usher syndrome [8]. Usher syndrome is included in a group of hereditary pathologies associated with defects in ciliary function known as ciliopathies [9] most USH1 and USH2 proteins are integrated in a protein network known as the “Usher-interactome” [10]. The central core of the interactome is formed by PDZD7, harmonin (USH1C), and whirlin (DFNB31), and the microtubule-associated protein SANS (USH1G). The remaining USH proteins are attached to this core [11]. This interacting network is mainly localized at the stereocilia or hair bundle of the inner ear hair cells and at the periciliary areas of the photoreceptors [12,13]. In the inner ear, Usher proteins play an essential role for correct development and cohesion of the hair bundle of hair cells in the cochlea and vestibular organ [14-18]. In the retina, the Usher protein network provides mechanical support to the membrane junction between the inner segment and the connecting cilium, participating in the control of vesicle docking and cargo handover in the periciliary ridge [19,20].
USH2 accounts for well over one-half of all Usher cases. Mutations in the USH2A gene are responsible for the majority of USH2 cases and are also responsible for atypical Usher syndrome [21,22] and recessive nonsyndromic RP [23,24]. Two main isoforms of the USH2A gene, the short isoform_a and the long isoform_b, have been described. The short isoform_a, which is reported to be 5 kb (exons 2–21; 1,547 amino acids [aa]), encodes a protein of 170 kDa [25,26]; the long isoform_b, which expands the whole length of the coding sequence to 15 kb (exons 2–72; 5,202 aa), encodes a large protein of 600 kDa [27]. The mutational spectrum of USH2A is extensive. Over 350 different point mutations, including nonsense, frameshift, missense, and splicing mutations (USH2A), have been identified by numerous mutation screenings.
Conventional USH2A mutation screenings have been performed by PCR amplification of coding exons followed by Sanger sequencing [28-30]. However, large duplications and large heterozygous deletions are not detectable if the breakpoints are located outside the amplified region. Recently, the multiplex ligation-dependent probe amplification (MLPA) and oligonucleotide array-based comparative genomic hybridization (aCGH) techniques have facilitated the detection of total and partial gene deletions and duplications that may escape conventional PCR-based screening methods [31,32]. Using these technologies, Steele-Stallard et al. [33] characterized five deletions and one duplication in heterozygosis in the USH2A gene in patients who had missing mutations (mono-allelic USH2A) or no mutations following Sanger sequencing.
Missing mutations can also lie in the promoter or deep intronic zones, which are usually not analyzed in conventional mutation screenings. In this regard, Vaché et al. [34] identified a deep intronic mutation (c.7595–2144A>G) after sequencing mRNA transcripts obtained from nasal epithelial cells of USH2 patients. Taking this into account, we screened our USH2 cohort of patients with unidentified mutations for c.7595–2144A>G and found it in five heterozygote and one homozygote cases.
In the present study, we used the MLPA technique to screen our cohort of USH2 patients for large deletions and duplications affecting the USH2A gene. Subsequently, we applied a customized oligonucleotide aCGH assay to confirm the presence of rearrangements and accurately determine the location of their breakpoints.
Methods
Patients
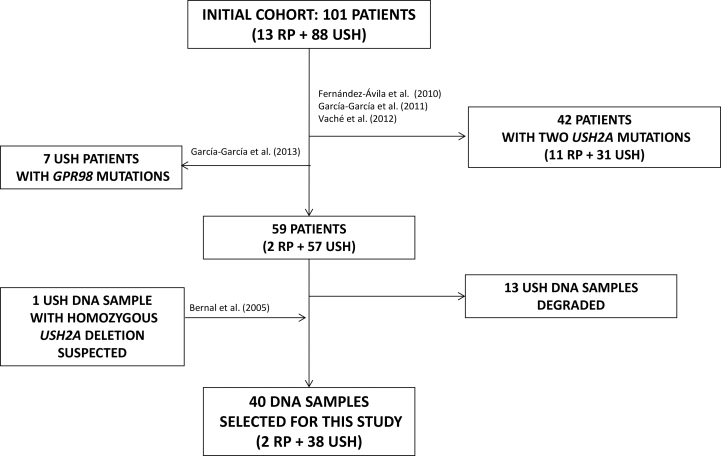
Thirteen nonsyndromic recessive RP patients [35] and 88 USH cases [30,34] were previously screened for point mutations in USH2A by Sanger sequencing [17,21,22]. In 42 of the patients (31 USH and 11 RP), point mutations in both USH2A alleles were identified. In a later study, seven USH patients were found to carry mutations in DFNB31 [36]. After these studies, only 39 out of the remaining 52 samples were useful for MLPA analysis to search for large deletions/duplications in the USH2A gene. A DNA sample of one USH patient carrying a homozygous USH2A deletion of exons 9–14 inferred from consistent PCR nonamplification [37] was included as a positive control (Figure 1).
Figure 1.
Diagram explaining the selection of patients for this study. Initially, 101 non-related patients, 13 diagnosed with retinitis pigmentosa (RP) and 88 diagnosed with Usher syndrome (USH) were screened for point mutations in USH2A by Sanger sequencing. Point mutations in both USH2A alleles were identified in 42 of them. Later one, seven USH patients were found to carry mutations in DFNB31. After all these previous studies, 13 USH DNA samples were degraded and only 39 samples were useful to perform the MLPA analysis to search for large deletions/duplications in the USH2A gene. Finally, an additional DNA sample from a patient carrying a homozygous USH2A deletion involving exons 9-14 was included in the study as a positive control for the MLPA analysis.
Thus, 40 unrelated patients (38 USH and two RP) were included in this study. Twelve of them (ten USH and two RP) carried one heterozygous USH2A point mutation, and one USH patient carried a suspected homozygous USH2A deletion.
Of the USH patients, 24 were classified as USH2 on the basis of ophthalmic studies that included visual acuity, visual field, fundus ophthalmoscopy, electroretinography, pure-tone and speech audiometry, and vestibular evaluation. Seven patients were classified as atypical USH, and detailed clinical data could not be obtained for another seven patients. For each patient, samples from parents as well as from siblings were obtained, when possible.
This research adhered to the tenets of the Declaration of Helsinki. The study was approved by the Hospital La Fe Ethics Committee, and consent for genetic testing was obtained from adult probands or the parents of the minors.
MLPA analysis
MLPA was used to determine the copy number of all USH2A exons (72) in two single multiplex PCR-based reactions. P361 and P362 SALSA MLPA kits (probemixes) were used (MRC Holland, Amsterdam, the Netherlands). P361 probemix contained 36 adjacent paired probes for odd exons, and P362 probemix contained another 36 paired probes for even exons. The MLPA analysis used 50 ng of DNA, diluted in 5 μl of Tris/EDTA buffer. During PCR, adjacent MLPA probes correctly ligated after hybridization to the sample DNA were amplified, and each MLPA reaction resulted in a set of PCR amplicons. One microliter of each reaction product was separated on POP-7 polymer by capillary electrophoresis with a DNA analyzer (model 3500XL; Applied Biosystems, Inc. [ABI], Foster City, CA). Freely available software provided by MRC Holland was used to analyze the MLPA data (Coffalyser; MRC Holland). Using this program, data generated was first normalized intra-sample by dividing the peak area of each probe’s amplification product by the total area. Second, inter-sample normalization was achieved by dividing the intra-normalized probe ratio in a sample by the average intra-normalized probe ratio of the reference sample. A probe dosage quotient value of 1±0.3 was considered normal; less than 0.7 was considered a deletion and greater than 1.3 a duplication.
Oligonucleotide aCGH
A custom aCGH chip (12×135 k) was used to compare control DNA (or reference sample) to DNA from the patient (test sample) that was labeled with two different dyes. Both DNAs were co-hybridized onto the chip containing 77,366 immobilized probes covering the genes MYO7A, CDH23, PCDH15, USH1C, USH1G, USH2A, GPR98, DFNB31, PDZD7, and USH3A and 10,000 nucleotides of 5′ and 3′ untranslated regions (UTRs) [32]. The average probe length was 60 bases, and the spacing between starts of probes covering exons and introns was 35 bp. Hybridization was performed in a high-resolution microarray platform (Roche NimbleGen, Inc., Basel, Switzerland). The slides were scanned using InnoScan900A (Inopsys, Toulouse, France) and analyzed using Deva1.2.1 software (Roche NimbleGen, Inc.). The signal intensity ratio between a test sample and a reference sample was normalized and converted to a log2 ratio to identify copy number changes, which were indicated by a deviation from the normal log2 ratio of zero. The predicted breakpoint location was defined by the positions of the last and first probes with the normal unaveraged value of the log2 ratio upstream and downstream from the corresponding aberration.
Identification of breakpoints
Specific primer pairs were designed to amplify patients’ genomic DNA regions where deletion breakpoints were indicated by the aCGH analysis (see Table 1). The primers were located 400–1,000 nt upstream and downstream of the last deleted probe in each deletion end. The PCR products were directly sequenced. The genomic USH2A reference sequence for deletions nomenclature using Mutalyzer 2.0. beta-29 program was NG_009497.1.
Table 1. Primers used to amplify and sequence breakpoint junctions of deletions characterized in the present study.
| Patient | Primer | Sequence 5′-3′ |
|---|---|---|
| RP-1622 |
IVS13-F |
TACCAGAGACTATGTTGGTG |
| IVS14-R |
GCTTCTCAGGGATAGGAGC |
|
| RP-838 |
IVS4-F1 |
CGAAACTGTCAATAATTCTGG |
| IVS13–2R |
GAGCTAATATTGGCTGACAG |
|
| RP-1638 |
IVS4-F |
GTATCAGGATGATGCTGGCC |
| IVS9-R |
GCATATTTACGGGCATGGTAG |
|
| RP-1678 |
IVS21–1D |
TAACCACAATCCACTAGCTTG |
| IVS29–3R | AATCATCTGGAGATGTGTTCAG |
Results
The present study led to the detection of six different partial USH2A gene deletions in six out of the 40 unrelated cases studied. Three of these deletions were homozygotes, and the remaining three were compound heterozygotes, having a previously USH2A point mutation identified. In five cases patients displayed Usher syndrome, and the remaining patient presented with nonsyndromic RP (summarized results in Table 2).
Table 2. Summary of USH2A deletions identified in our cohort of patients.
| Family | Patient | Allele 1 | Allele 2 | Daignosis | References |
|---|---|---|---|---|---|
| FRP-429§ |
RP-1696/RP-1697 |
del ex 9–14* |
del ex 9–14* |
USH2 |
*Bernal et al. (2005) [37] |
| FRP-404 |
RP-1622 |
del ex 14 |
del ex 14 |
USH2 |
|
| FRP-298 |
RP-838 |
del ex 5–13 |
del ex 5–13 |
USH2 |
|
| FRP-323 |
RP-1397 |
c.8167C>T/p.R2723X |
del ex 1–4 |
USH2 |
|
| FRP-413 |
RP-1637/RP-1638 |
c.5540dupA/p.N1848EfsX20* |
del ex 5–9 |
USH2 |
*García-García et al. (2011) [30] |
| FRP-423# | RP-1678/RP-1679 | c.2276G>T/p.C759F* | del ex 22–29 | ARRP | *Avila-Fernandez et al. (2010) [35] |
MLPA and segregation analysis
Homozygous cases
In patients RP-1696 and RP-1697, which belong to the same family (FRP-429), a homozygous deletion involving exons 9–14 had already been inferred from consistent PCR nonamplification (family Ush-148 in [37]). Thus, these patients were used as positive controls.
Patient RP-1622 showed no point mutations after USH2A screening by direct sequencing. All polymorphisms detected were in homozygosis, and exon 14 was impossible to amplify, strongly suggesting the existence of one deletion in this region.
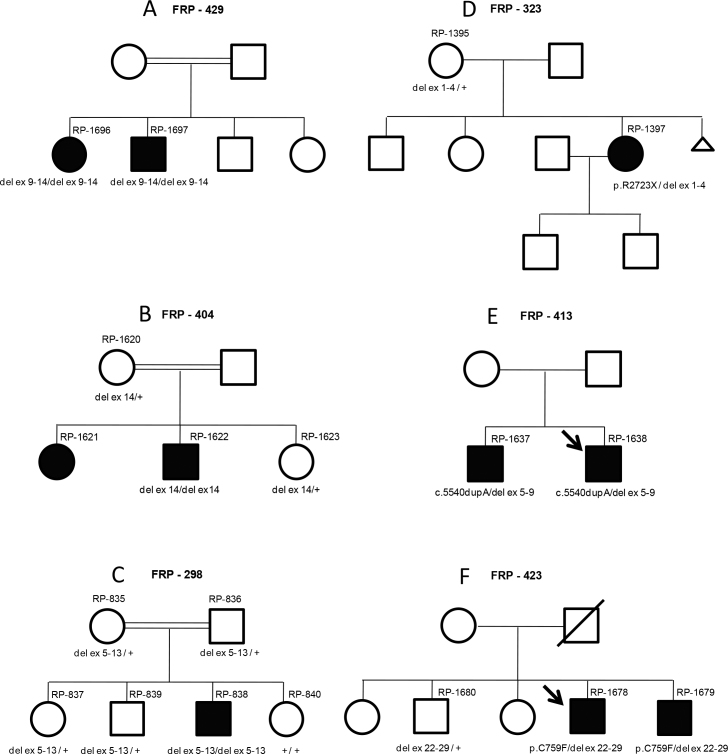
In patient RP-838, amplification of exons 5–13 failed, suggesting the existence of a rearrangement within this area. MLPA analysis detected homozygous deletions in three cases: a deletion of exons 9–14 in patients RP-1696 and RP-1697 (FRP-429); a deletion of exon 14 in patient RP-1622 (FRP-404), and a deletion involving exons 5–13 in patient RP-838 (FRP-298). Three deletions co-segregated with the disease in three families. All three referred consanguinity (Figure 2A-C).
Figure 2.
Family pedigrees showing segregation analysis of USH2A deletions identified in this study. A: Segregation analysis of the USH2A deletion involving exons 9-14 (del ex 9-14) performed in available DNA samples from family FRP-429. B: Segregation analysis of exon 14 USH2A deletion (del ex 14) performed in available DNA samples from family FRP-404. C: Segregation analysis of deletion involving exons 5-13 (del ex 5-13) of USH2A in family FRP-298. D: Segregation analysis of the USH2A mutations p.R2723X and deletion of exons 1-4 (del ex 1-4), performed in available samples from family FRP-323. E: Segregation analysis of the USH2A mutations p.5540dupA and deletion of exons 5-9 (del ex 5-9), performed in both affected patients from family FRP-413. F: Segregation analysis of the USH2A mutations p.C759F and deletion of exons 22-29 (del ex 22-29), performed in available DNA samples from family FRP-423.
In patients RP-1696 and RP-1697 (homozygous for exons 9–14 deletion), RP started when the patients were around 30-years old. At the age of 50, electroretinogram (ERG) responses ceased in both patients, who presented typical RP eye-fundus examination results. Vestibular function was normal in both cases. Hearing loss was prelingual and severe. In patient RP-1696, the loss of hearing was progressive, whereas in patient RP-1697, it was nonprogressive [37].
Patient RP-1622 (homozygous for exon 14 deletion) displayed typical USH2 clinical manifestations: RP started when the patient was around 23-years old; hearing loss was congenital, stable, and moderate to severe. Vestibular function in this patient was normal.
Patient RP-808 (homozygous for exons 5–13 deletion) was referred first with RP symptoms at the age of 12 but was diagnosed with USH2 at the age of 14. At that time, he presented hearing loss, ERG responses ceased, visual acuity was 0.08, and visual field showed a concentric reduction, preserving only 5 central degrees.
Heterozygous cases
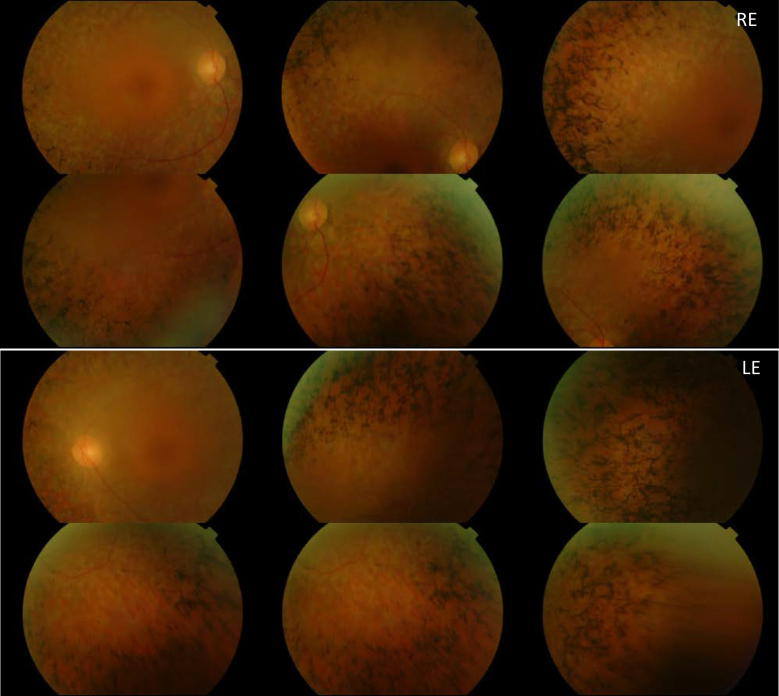
A heterozygous deletion affecting exons 1–4 was detected in patient RP-1397. This patient was already found to carry the mutation c.8167C>T/p.Arg2723* (unpublished results). Segregation analysis showed that the deletion was inherited from the healthy mother (Figure 2D). Sensorineural hearing loss was first suspected in this patient at the age of 7. Results from audiograms performed when the patient was 19, 24, 30, and 35 years showed a stable moderate–severe hearing loss, which was accentuated at higher frequencies. RP started when the patient was around 30-years old. Ophthalmologic evaluations at the age of 39 showed abolished ERG responses, visual acuity was 0.6, and visual field was tubular with islets. An eye fundus examination (Figure 3) showed bone spicule deposits, attenuation of vessels, and a waxy pallor of the optic nerve head. The macula of this patient was normal.
Figure 3.
Eye fundus images obtained after examination of patient RP-1397 show bone spicule deposits, attenuation of vessels and waxy pallor of the optic nerve head in both eyes.
In patient RP-1638, MLPA analysis indicated the presence of a deletion involving exons 5–9. This patient was previously found to carry the c.5540dupA mutation [30]. His affected brother (RP-1637) also carried both mutations (Figure 2E). These patients were referred to our laboratory as USH2. Unfortunately, it has not been possible to obtain more detailed clinical data.
Finally, a heterozygous deletion of exons 22–29 was detected in a patient (RP-1678) who carried the c.2276G>T/p.Cys759Phe mutation [35]. Segregation analysis in this family showed that the affected brother (RP-1679) carried both mutations, whereas the healthy brother only carried the partial USH2A deletion (Figure 2F). Detailed clinical data could not be obtained for RP-1678. In his affected brother, RP-1679, RP was diagnosed when he was 35-years old. At the age of 38, ERG responses were abolished, visual acuity was good (1), but visual field was concentrically reduced to only 10 central degrees. Eye fundus examination of this patient revealed bone spicule deposits in the peripheral retina, attenuation of vessels, and a waxy pallor of the optic nerve head. Currently (48-years old), this patient has noted a slight degree of hearing loss. Unfortunately, it has not been possible to confirm this by audiological evaluation.
Oligonucleotide aCGH analysis and identification of breakpoint positions
An aCGH analysis was performed to confirm the deletions detected by MLPA and to precisely define the breakpoint positions. Unfortunately, the amount and quality of the DNA from patients RP-1696/RP-1697 DNA was not sufficient to perform the aCGH analysis.
RP-1622
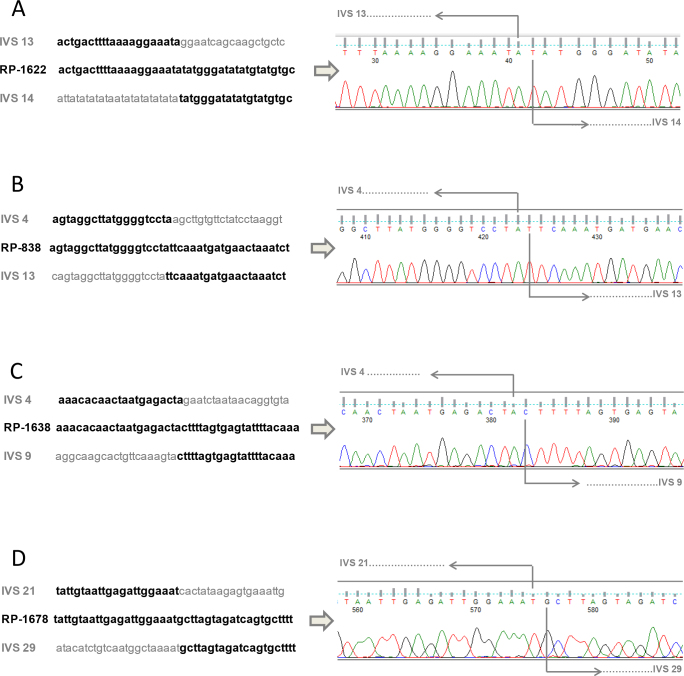
The results from aCGH analysis indicated that the deletion expanded from approximately chromosome 1 positions 216,404,500 to 216,409,000. A specific primer pair (Table 1) was designed to amplify and sequence this region. Sequencing of the PCR product obtained using these primers identified the exact breakpoints from nucleotides g.192,143 (intron 13) to g.197,160 (intron 14); (Figure 4A), confirming the presence of a deletion of over 5 kb (c.2810–4121_2993+718del). The deletion of exon 14 is expected to generate a premature stop codon, translated into a truncated protein: p.Gly937Aspfs*13.
Figure 4.
Breakpoint junctions of USH2A deletions characterized by PCR amplification and sequencing. A: Exact breakpoints of deletion involving exon 14 of USH2A in patient RP-1622. B: Exact breakpoints of deletion involving exons 5-13 of USH2A in patient RP-838. C: Exact breakpoints of deletion involving exons 5-9 of USH2A in patient RP-1638. D: Exact breakpoints of deletion involving exons 22-29 of USH2A in patient RP-1678. IVS: Intervening sequence.
RP-838
aCGH analysis showed the presence of a deletion expanding around chromosome 1 positions 216,420,000 to 216,520,000. After PCR amplification and sequencing using a specific primer pair (Table 1), exact breakpoint positions were identified, showing a deletion expanding from g.82,673 (intron 4) to g.182,184 (intron 13); (Figure 4B). This deletion (c.785–18070_2809+372del) is expected to be in-frame, resulting in a protein lacking 675 aa (p.Leu263_Gly937del).
RP-1397
Results of aCGH analysis from this patient indicated the existence of a deletion expanding from approximately chromosome 1 positions 216,511,000 to 216,606,000. Three different pairs of primers were designed to amplify the breakpoint deletions in this patient. Unfortunately, no PCR products were obtained using RP-1397 and RP-1395 DNAs.
RP-1638
In this case, a deletion was found around chromosome 1 positions 259,216,460,000 to 216,510,000. Specific PCR amplification and sequencing showed that the deletion affected nucleotides g.79,628 (intron 4) to g.135,435 (intron 9) spanning 55.8 kb (c.784+16184_1645–592del; Figure 4C). The loss of exons 5 to 9 is expected to generate a truncated protein p.(Gly262Valfs*2).
RP-1678
aCGH results indicated a deletion spanning chromosome 1 positions 216,246,000 to 216,309,000. Sequencing after PCR amplification showed a deletion of 62.7 kb from g.292,921 (intron 21) to g.355,667 (intron 29), (Figure 4D). This deletion (c.4628–38263_5857+159del) would produce the loss of the in-frame exons 22–29; generating a protein lacking amino acids 1,543–1,952 (p.Gly1543_Ala1952del).
Discussion
In this study we detected six large deletions in six unrelated patients affecting the USH2A gene (five of them not previously described), using the MLPA technique. In three cases, the deletions were detected to be in a homozygous state. In the remaining three probands, deletions were found to be in trans with a USH2A point mutation previously identified.
From an initial cohort of 101 patients, a USH2A mutation screening by Sanger sequencing allowed the identification of mutations in both USH2A alleles responsible for the disease in 42 cases [30,34,35]. The present study, searching for large rearrangements, allowed the identification of both USH2A mutated alleles in six additional cases (three heterozygous and three homozygous). It is difficult to estimate the contribution of large deletions to the total mutational spectrum of USH2A based on our studies since our data are biased (see Figure 1). However, if we used these data (all patients with both USH2A mutated alleles detected: 48 patients, 96 alleles) as an approximation of the real population, we might predict that large deletions would represent 9.4% (nine out of 96) of all USH2A mutations.
Interestingly, one of the cases with a novel USH2A deletion identified in this study was a nonsyndromic RP patient. RP-1678 was found to carry a heterozygous in-frame deletion of exons 22–29 in trans with the c.2276G>T/p.Cys759Phe point mutation. As expected, the affected brother (RP-1679) also carried both mutations. Both patients were initially diagnosed with nonsyndromic RP, but RP-1679 (now 48-years old) presents with a slight degree of hearing loss. Heterogeneous phenotypes have been correlated with the p.Cys759Phe mutation in this and other previous studies (USH2A). Thus, whereas those patients who are compound heterozygous for p.Cys759Phe and another USH2A mutation can display RP [23,24,35,38,39], atypical USH [21,22], or USH2 [25-30], patients homozygous for the p.Cys759Phe mutation are correlated with nonsyndromic RP [22,23,35,38-40].
MLPA is the most rapid and cost-efficient method to search for large deletions/duplications involving the USH2A gene. It allows determination of the copy number of all USH2A coding exons (72) in only two multiplex PCR-based reactions. Nevertheless, it is recommended that the presence of rearrangements detected by MLPA be confirmed by other techniques, like aCGH analysis, real-time quantitative PCR, or reverse-transcription (RT)–PCR, on RNA obtained from nasal epithelial cells [34], fibroblasts [33], or hair roots [41]. In the present work, a customized aCGH, including intron USH2A gene sequences, was applied to confirm the presence of deletions detected by MLPA and to accurately determine their breakpoint positions. Unfortunately, breakpoints could not be successfully determined in two cases. In family FRP-429, the amount and quality of the DNA samples were not sufficient to perform the aCGH analysis.
In family FRP-323, aCGH was successfully performed, pointing out the location of breakpoints around chromosone 1 positions: 216,511,000 (5′ UTR region) and 216,606,000 (intron 4). This result was in agreement with MLPA analysis that showed a deletion involving exons 1–4. However, the breakpoints in that DNA region could not be amplified by PCR, suggesting that the rearrangement may be more complex than suspected. In this regard it is worth noting that Le Guédard et al. [42] also failed to determine the exact breakpoint junctions for two large deletions affecting the PCDH15 gene. These results together with ours point to the existence of large complex rearrangements involving USH genes and highlight the difficulty in characterizing them.
To date, only 12 different large deletions within the USH2A gene have been reported (USH2A). None of these coincide with the five novel deletions detected in our patients except the deletion involving exon 14. Recently, Glöckle et al. [40] identified a deletion also involving this exon; but exact breakpoints have not been described. Consequently, we are unable to compare, and therefore know, if this deletion is the same as that found in our patient RP-1622.
It has been proven by this work and several other studies that an exhaustive molecular screening of USH genes must include approaches (such as MLPA or aCGH analysis) other than sequencing. These screenings based upon conventional techniques are time intensive and expensive and therefore are thought to be replaced by next-generation sequencing (NGS) approaches. Whole exome capture and targeted USH exome sequencing have specifically been applied for Usher syndrome [43-45]. These studies confirm the urgency to improve base calling and alignment software to accurately identify point mutations and specific bioinformatics’ tools to detect copy number variations (CNVs) using NGS methodologies. The detection of CNVs (large deletions and duplications) in concert with other variants in a single experiment will help to reduce total cost, time, and complexity and will allow researchers to gain a broader insight into disease from a limited amount of DNA sample. Deep intronic mutations (such as c.7595–2144A>G) recently identified in USH2A by mRNA studies [34] may be involved in some cases. Future approaches of targeted re-sequencing of USH genes, including sequences from noncoding regions (UTRs and introns), are needed to further identify this kind of mutation.
The development of efficient and cost-effective strategies to identify disease-causing mutations is mandatory. Determination of the underlying genetic defect in a patient is a prerequisite for gene or mutation-specific therapy. In this sense, preliminary results in gene therapeutic approaches for Usher syndrome [46-48] are promising.
Acknowledgments
We are grateful to Almudena Ávila-Fernández for its help and cooperation and also to the FARPE association and all the patients involved in this study. This work was supported by grant PI10/01825 from the Fondo de Investigaciones Sanitarias (FIS) from the Spanish Government. CIBERER (CB/06/07/1030) is an initiative of the Institute of Health Carlos III from the Spanish Government. The bioanalyzer ABI3500×l was purchased with the grant PROMIS (II12/00023) from the Instituto de Salud Carlos III. GGG and MJA were recipient of a fellowship from the Ministerio de Educación (REFs: AP2099–3344 and AP2008–02760, respectively).
References
- 1.Keats BJ, Corey DP. The Usher syndromes. Am J Med Genet. 1999;89:158–66. Review. [PubMed] [Google Scholar]
- 2.Davenport SLH, Omenn GS. The heterogeneity of Usher syndrome. Vth Int. Conf. Birth Defects, Montreal; 1977. [Google Scholar]
- 3.Otterstedde CR, Spandau U, Blankenagel A, Kimberling WJ, Reisser C. A new clinical classification for Usher’s syndrome based on a new subtype of Usher’s syndrome type I. Laryngoscope. 2001;111:84–6. doi: 10.1097/00005537-200101000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Millán JM, Aller E, Jaijo T, Blanco-Kelly F, Gimenez-Pardo A, Ayuso C. An update on the genetics of usher syndrome. J Ophthalmol. 2011;2011:417217. doi: 10.1155/2011/417217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riazuddin S, Belyantseva IA, Giese AP, Lee K, Indzhykulian AA, Nandamuri SP, Yousaf R, Sinha GP, Lee S, Terrell D, Hegde RS, Ali RA, Anwar S, Andrade-Elizondo PB, Sirmaci A, Parise LV, Basit S, Wali A, Ayub M, Ansar M, Ahmad W, Khan SN, Akram J, Tekin M, Riazuddin S, Cook T, Buschbeck EK, Frolenkov GI, Leal SM, Friedman TB, Ahmed ZM. Alterations of the CIB2 calcium- and integrin-binding protein cause Usher syndrome type 1J and nonsyndromic deafness DFNB48. Nat Genet. 2012;44:1265–71. doi: 10.1038/ng.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ebermann I, Phillips JB, Liebau MC, Koenekoop RK, Schermer B, Lopez I, Schäfer E, Roux AF, Dafinger C, Bernd A, Zrenner E, Claustres M, Blanco B, Nürnberg G, Nürnberg P, Ruland R, Westerfield M, Benzing T, Bolz HJ. PDZD7 is a modifier of retinal disease and a contributor to digenic Usher syndrome. J Clin Invest. 2010;120:1812–23. doi: 10.1172/JCI39715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puffenberger EG, Jinks RN, Sougnez C, Cibulskis K, Willert RA, Achilly NP, Cassidy RP, Fiorentini CJ, Heiken KF, Lawrence JJ, Mahoney MH, Miller CJ, Nair DT, Politi KA, Worcester KN, Setton RA, Dipiazza R, Sherman EA, Eastman JT, Francklyn C, Robey-Bond S, Rider NL, Gabriel S, Morton DH, Strauss KA. Genetic mapping and exome sequencing identify variants associated with five novel diseases. PLoS ONE. 2012;7:e28936. doi: 10.1371/journal.pone.0028936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khateb S, Zelinger L, Mizrahi-Meissonnier L, Ayuso C, Koenekoop RK, Laxer U, Gross M, Banin E, Sharon D. A homozygous nonsense CEP250 mutation combined with a heterozygous nonsense C2orf71 mutation is associated with atypical Usher syndrome. J Med Genet. 2014;51:460–9. doi: 10.1136/jmedgenet-2014-102287. [DOI] [PubMed] [Google Scholar]
- 9.van Reeuwijk J, Arts HH, Roepman R. Scrutinizing ciliopathies by unraveling ciliary interaction networks. Hum Mol Genet. 2011;20(R2):R149–57. doi: 10.1093/hmg/ddr354. [DOI] [PubMed] [Google Scholar]
- 10.Kremer H, van Wijk E, Marker E, Wolfrum U, Roepman R. Usher syndrome: molecular links of pathogenesis, proteins and pathways. Hum Mol Genet. 2006;15:R262–70. doi: 10.1093/hmg/ddl205. [DOI] [PubMed] [Google Scholar]
- 11.Reiners J, Nagel-Wolfrum K, Jürgens K, Märker T, Wolfrum U. Molecular basis of human Usher syndrome: deciphering the meshes of the Usher protein network provides insights into the pathomechanisms of the Usher disease. Exp Eye Res. 2006;83:97–119. doi: 10.1016/j.exer.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Liu X, Bulgakov OV, Darrow KN, Pawlyk B, Adamian M, Liberman MC, Li T. Usherin is required for maintenance of retinal photoreceptors and normal development of cochlear hair cells. Proc Natl Acad Sci USA. 2007;104:4413–8. doi: 10.1073/pnas.0610950104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang J, Liu X, Zhao Y, Adamian M, Pawlyk B, Sun X, McMillan DR, Liberman MC, Li T. Ablation of whirlin long isoform disrupts the USH2 protein complex and causes vision and hearing loss. PLoS Genet. 2010;6:e1000955. doi: 10.1371/journal.pgen.1000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adato A, Lefèvre G, Delprat B, Michel V, Michalski N, Chardenoux S, Weil D, El-Amraoui A, Petit C. Usherin, the defective protein in Usher syndrome type IIA, is likely to be a component of interstereocilia ankle links in the inner ear sensory cells. Hum Mol Genet. 2005;14:3921–32. doi: 10.1093/hmg/ddi416. [DOI] [PubMed] [Google Scholar]
- 15.El-Amraoui A, Petit C. Usher I syndrome: unravelling the mechanisms that underlie the cohesion of the growing hair bundle in inner ear sensory cells. J Cell Sci. 2005;118:4593–4603. doi: 10.1242/jcs.02636. [DOI] [PubMed] [Google Scholar]
- 16.Michalski N Michel V, Bahloul A, Lefèvre G, Barral J, Yagi H, Chardenoux S, Weil D, Martin P, Hardelin JP, Sato M, Petit C. Molecular characterization of the ankle-link complex in cochlear hair cells and its role in the hair bundle functioning. J Neurosci. 2007;27:6478–88. doi: 10.1523/JNEUROSCI.0342-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kazmierczak P, Müller U. Sensing sound: molecules that orchestrate mechanotransduction by hair cells. Trends Neurosci. 2012;35:220–9. doi: 10.1016/j.tins.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zou J Zheng T, Ren C, Askew C, Liu XP, Pan B, Holt JR, Wang Y, Yang J. Deletion of PDZD7 disrupts the Usher syndrome type 2 protein complex in cochlear hair cells and causes hearing loss in mice. Hum Mol Genet. 2014;23:2374–90. doi: 10.1093/hmg/ddt629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maerker T, van Wijk E, Overlack N, Kersten FF, McGee J, Goldmann T, Sehn E, Roepman R, Walsh EJ, Kremer H, Wolfrum U. A novel Usher protein network at the periciliary reloading point between molecular transport machineries in vertebrate photoreceptor cells. Hum Mol Genet. 2008;17:71–86. doi: 10.1093/hmg/ddm285. [DOI] [PubMed] [Google Scholar]
- 20.Jacobson SG, Cideciyan AV, Aleman TS, Sumaroka A, Roman AJ, Gardner LM, Prosser HM, Mishra M, Bech-Hansen NT, Herrera W, Schwartz SB, Liu XZ, Kimberling WJ, Steel KP, Williams DS. Usher syndromes due to MYO7A, PCDH15, USH2A or GPR98 mutations share retinal disease mechanism. Hum Mol Genet. 2008;17:2405–15. doi: 10.1093/hmg/ddn140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X-Z, Hope C, Liang CY, Zou JM, Xu LR, Cole T, Mueller RF, Bundey S, Nance W, Steel KP, Brown SDM. A mutation (2314delG) in the Usher syndrome type IIA gene: high prevalence and phenotypic variation. Am J Hum Genet. 1999;64:1221–5. doi: 10.1086/302332. Letter. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aller E, Nájera C, Millán JM, Oltra JS, Pérez-Garrigues H, Vilela C, Navea A, Beneyto M. Genetic analysis of 2299delG and C759F mutations (USH2A) in patients with visual and/or auditory impairments. Eur J Hum Genet. 2004;12:407–10. doi: 10.1038/sj.ejhg.5201138. [DOI] [PubMed] [Google Scholar]
- 23.Rivolta C, Sweklo EA, Berson EL, Dryja TP. Missense mutation in the USH2A gene: association with recessive retinitis pigmentosa without hearing loss. Am J Hum Genet. 2000;66:1975–8. doi: 10.1086/302926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Méndez-Vidal C, González-Del Pozo M, Vela-Boza A, Santoyo-López J, López-Domingo FJ, Vázquez-Marouschek C, Dopazo J, Borrego S, Antiñolo G. Whole-exome sequencing identifies novel compound heterozygous mutations in USH2A in Spanish patients with autosomal recessive retinitis pigmentosa. Mol Vis. 2013;19:2187–95. [PMC free article] [PubMed] [Google Scholar]
- 25.Eudy JD, Weston MD, Yao S, Hoover DM, Rehm HL, Ma-Edmonds M, Yan D, Ahmad I, Cheng JJ, Ayuso C, Cremers C, Davenport S, Moller C, Talmadge CB, Beisel KW, Tamayo M, Morton CC, Swaroop A, Kimberling WJ, Sumegi J. Mutation of a gene encoding a protein with extracellular matrix motifs in Usher syndrome type IIa. Science. 1998;280:1753–7. doi: 10.1126/science.280.5370.1753. [DOI] [PubMed] [Google Scholar]
- 26.Weston MD, Eudy JD, Fujita S, Yao S, Usami S, Cremers C, Greenberg J, Ramesar R, Martini A, Moller C, Smith RJ, Sumegi J, Kimberling WJ. Genomic structure and identification of novel mutations in usherin, the gene responsible for Usher syndrome type IIa. Am J Hum Genet. 2000;66:1199–210. doi: 10.1086/302855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Wijk E, Pennings RJ, te Brinke H, Claassen A, Yntema HG, Hoefsloot LH, Cremers FP, Cremers CW, Kremer H. Identification of 51 novel exons of the Usher syndrome type 2A (USH2A) gene that encode multiple conserved functional domains and that are mutated in patients with Usher syndrome type II. Am J Hum Genet. 2004;74:738–44. doi: 10.1086/383096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baux D, Larrieu L, Blanchet C, Hamel C, Ben Salah S, Vielle A, Gilbert-Dussardier B, Holder M, Calvas P, Philip N, Edery P, Bonneau D, Claustres M, Malcolm S, Roux AF. Molecular and in silico analyses of the full-length isoform of usherin identify new pathogenic alleles in Usher type II patients. Hum Mutat. 2007;28:781–9. doi: 10.1002/humu.20513. [DOI] [PubMed] [Google Scholar]
- 29.Dreyer B, Brox V, Tranebjaerg L, Rosenberg T, Sadeghi AM, Möller C, Nilssen O. Spectrum of USH2A mutations in Scandinavian patients with Usher syndrome type II. Hum Mutat. 2008;29:451. doi: 10.1002/humu.9524. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Garcia G, Aparisi MJ, Jaijo T, Rodrigo R, Leon AM, Avila-Fernandez A, Blanco-Kelly F, Bernal S, Navarro R, Diaz-Llopis M, Baiget M, Ayuso C, Millan JM, Aller E. Mutational screening of the USH2A gene in Spanish USH patients reveals 23 novel pathogenic mutations. Orphanet J Rare Dis. 2011;6:65. doi: 10.1186/1750-1172-6-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aller E, Jaijo T, García-García G, Aparisi MJ, Blesa D, Díaz-Llopis M, Ayuso C, Millán JM. Identification of large rearrangements of the PCDH15 gene by combined MLPA and a CGH: large duplications are responsible for Usher syndrome. Invest Ophthalmol Vis Sci. 2010;51:5480–5. doi: 10.1167/iovs.10-5359. [DOI] [PubMed] [Google Scholar]
- 32.Roux AF, Faugère V, Vaché C, Baux D, Besnard T, Léonard S, Blanchet C, Hamel C, Mondain M, Gilbert-Dussardier B, Edery P, Lacombe D, Bonneau D, Holder-Espinasse M, Ambrosetti U, Journel H, David A, Lina-Granade G, Malcolm S, Claustres M. Four-year follow-up of diagnostic service in USH1 patients. Invest Ophthalmol Vis Sci. 2011;52:4063–71. doi: 10.1167/iovs.10-6869. [DOI] [PubMed] [Google Scholar]
- 33.Steele-Stallard HB, Le Quesne Stabej P, Lenassi E, Luxon LM, Claustres M, Roux AF, Webster AR, Bitner-Glindzicz M. Screening for duplications, deletions and a common intronic mutation detects 35% of second mutations in patients with USH2A monoallelic mutations on Sanger sequencing. Orphanet J Rare Dis. 2013;8:122. doi: 10.1186/1750-1172-8-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vaché C, Besnard T, le Berre P, García-García G, Baux D, Larrieu L, Abadie C, Blanchet C, Bolz HJ, Millan J, Hamel C, Malcolm S, Claustres M, Roux AF. Usher syndrome type 2 caused by activation of an USH2A pseudoexon: implications for diagnosis and therapy. Hum Mutat. 2012;33:104–8. doi: 10.1002/humu.21634. [DOI] [PubMed] [Google Scholar]
- 35.Ávila-Fernández A, Cantalapiedra D, Aller E, Vallespín E, Aguirre-Lambán J, Blanco-Kelly F, Corton M, Riveiro-Álvarez R, Allikmets R, Trujillo-Tiebas MJ, Millán JM, Cremers FP, Ayuso C. Mutation analysis of 272 Spanish families affected by autosomal recessive retinitis pigmentosa using a genotyping microarray. Mol Vis. 2010;16:2550–8. [PMC free article] [PubMed] [Google Scholar]
- 36.García-García G, Besnard T, Baux D, Vaché C, Aller E, Malcolm S, Claustres M, Millan JM, Roux AF. The contribution of GPR98 and DFNB31 genes to a Spanish Usher syndrome type 2 cohort. Mol Vis. 2013;19:367–73. [PMC free article] [PubMed] [Google Scholar]
- 37.Bernal S, Medà C, Solans T, Ayuso C, Garcia-Sandoval B, Valverde D, Del Rio E, Baiget M. Clinical and genetic studies in Spanish patients with Usher syndrome type II: description of new mutations and evidence for a lack of genotype-phenotype correlation. Clin Genet. 2005;68:204–14. doi: 10.1111/j.1399-0004.2005.00481.x. [DOI] [PubMed] [Google Scholar]
- 38.Bernal S, Ayuso C, Antiñolo G, Gimenez A, Borrego S, Trujillo MJ, Marcos I, Calaf M, Del Rio E, Baiget M. Mutations in USH2A in Spanish patients with autosomal recessive retinitis pigmentosa: high prevalence and phenotypic variation. J Med Genet. 2003;40:e8. doi: 10.1136/jmg.40.1.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seyedahmadi BJ, Rivolta C, Keene JA, Berson EL, Dryja TP. Comprehensive screening of the USH2A gene in Usher syndrome type II and non-syndromic recessive retinitis pigmentosa. Exp Eye Res. 2004;79:167–73. doi: 10.1016/j.exer.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 40.Glöckle N, Kohl S, Mohr J, Scheurenbrand T, Sprecher A, Weisschuh N, Bernd A, Rudolph G, Schubach M, Poloschek C, Zrenner E, Biskup S, Berger W, Wissinger B, Neidhardt J. Panel-based next generation sequencing as a reliable and efficient technique to detect mutations in unselected patients with retinal dystrophies. Eur J Hum Genet. 2014;22:99–104. doi: 10.1038/ejhg.2013.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakanishi H, Ohtsubo M, Iwasaki S, Hotta Y, Mizuta K, Mineta H, Minoshima S. Hair roots as an mRNA source for mutation analysis of Usher syndrome-causing genes. J Hum Genet. 2010;55:701–3. doi: 10.1038/jhg.2010.83. [DOI] [PubMed] [Google Scholar]
- 42.Le Guédard S, Faugère V, Malcolm S, Claustres M, Roux AF. Large genomic rearrangements within the PCDH15 gene are a significant cause of USH1F syndrome. Mol Vis. 2007;13:102–7. [PMC free article] [PubMed] [Google Scholar]
- 43.Licastro D, Mutarelli M, Peluso I, Neveling K, Wieskamp N, Rispoli R, Vozzi D, Athanasakis E, D'Eustacchio A, Pizzo M, D'Amico F, Ziviello C, Simonelli F, Fabretto A, Scheffer H, Gasparini P, Banfi S, Nigro V. Molecular diagnosis of Usher syndrome: application of two different next generation sequencing-based procedures. PLoS ONE. 2012;7:e43799. doi: 10.1371/journal.pone.0043799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang XF, Xiang P, Chen J, Xing DJ, Huang N, Min Q, Gu F, Tong Y, Pang CP, Qu J, Jin ZB. Targeted exome sequencing identified novel USH2A mutations in Usher syndrome families. PLoS ONE. 2013;8:e63832. doi: 10.1371/journal.pone.0063832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Besnard T, García-García G, Baux D, Vaché C, Faugère V, Larrieu L, Léonard S, Millan JM, Malcolm S, Claustres M, Roux AF. Experience of targeted Usher exome sequencing as a clinical test. Mol Genet Genomic Med. 2014;2:30–43. doi: 10.1002/mgg3.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zou J, Luo L, Shen Z, Chiodo VA, Ambati BK, Hauswirth WW, Yang J. Whirlin replacement restores the formation of the USH2 protein complex in whirlin knockout photoreceptors. Invest Ophthalmol Vis Sci. 2011;52:2343–51. doi: 10.1167/iovs.10-6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goldmann T, Overlack N, Möller F, Belakhov V, van Wyk M, Baasov T, Wolfrum U, Nagel-Wolfrum K. A comparative evaluation of NB30, NB54 and PTC124 in translational read-through efficacy for treatment of an USH1C nonsense mutation. EMBO Mol Med. 2012;4:1186–99. doi: 10.1002/emmm.201201438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Overlack N, Goldmann T, Wolfrum U, Nagel-Wolfrum K. Gene repair of an Usher syndrome causing mutation by zinc-finger nuclease mediated homologous recombination. Invest Ophthalmol Vis Sci. 2012;53:4140–6. doi: 10.1167/iovs.12-9812. [DOI] [PubMed] [Google Scholar]