Abstract
Lyme disease, the most common tick-borne infection in Canada and much of the United States, is caused by the bacteria Borrelia burgdorferi. Peak incidence for Lyme disease is among children five to nine years of age and older adults (55 to 59 years of age). The bacteria are transmitted through the bite of infected black-legged ticks of the Ixodes species. The primary hosts of black-legged ticks are mice and other rodents, small mammals, birds (which are reservoirs for B burgdorferi) and white-tailed deer. Geographical distribution of Ixodes ticks is expanding in Canada and an increasing number of cases of Lyme disease are being reported. The present practice point reviews the epidemiology, clinical presentation, diagnosis, management and prevention of Lyme disease, with a focus on children.
Keywords: Black-legged tick, Borrelia burgdorferi, Erythema migrans, Post-treatment Lyme disease syndrome
Abstract
La maladie de Lyme, qui est l’infection à tiques la plus courante au Canada et dans une grande partie des États-Unis, est causée par la bactérie Borrelia burgdorferi. Elle touche surtout les enfants de cinq à neuf ans et les adultes d’âge mûr (de 55 à 59 ans). La bactérie est transmise par la piqûre de la tique occidentale à pattes noires de l’espèce Ixodes qui a été infectée, dont les hôtes primaires sont les souris et les autres rongeurs, les petits mammifères, les oiseaux (réservoirs du B burgdorferi) et les cerfs de Virginie. La répartition géographique des tiques Ixodes prend de l’expansion au Canada, et le nombre de cas de maladie de Lyme déclarés est en croissance. Le présent point de pratique porte sur l’épidémiologie, la présentation clinique, le diagnostic, la prise en charge et la prévention de la maladie de Lyme, particulièrement chez les enfants.
Français en page 384
Lyme disease (LD), a serious disease, is the most common tick-borne infection in Canada and the Northeastern to Midwestern United States, with cases also occurring (with less frequency) on the west coast. LD is caused by the bacteria spirochete, Borrelia burgdorferi, transmitted to humans through the bite of infected black-legged ticks: Ixodes scapularis in Eastern and Central Canada and Ixodes pacificus in British Columbia.(1)
The primary hosts (carriers) of black-legged ticks are mice and other small rodents, small mammals, birds (which are a reservoir for B burgdorferi) and white-tailed deer (Figure 1). Although dogs can contract LD and carry ticks into homes and yards, there is no evidence that they spread the infection directly to people.(2)
Figure 1).
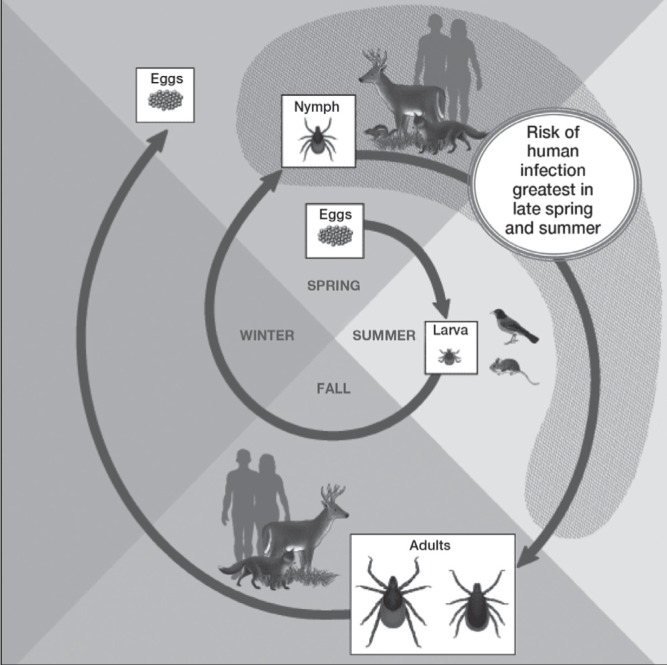
The life cycle of black-legged ticks and Lyme disease. Reproduced with permission from the United States Centers for Disease Control and Prevention (Atlanta, USA): www.cdc.gov/ticks/life_cycle_and_hosts.html
Peak incidence for LD is among children five to nine years of age and older adults (55 to 59 years of age), and many cases likely go unreported.(1) No relationship between treated maternal LD and abnormal pregnancies or disease in infants has been documented.(3) Although there is a theoretical risk, no case of infection has been linked to blood transfusion.(4)
Ticks cannot jump or fly. Instead, they climb and wait on tall grasses or shrubs for a potential host to brush against them. They then transfer to the host and seek an attachment site.(5) Immature ticks (nymphs) are responsible for most human LD infections because their very small size hinders detection.(1)
If a tick is found attached to or feeding on a child, remove it as soon as possible. Ticks can attach and feed for five days or longer (Figure 2). Removing a tick within 24 h to 36 h of its starting to feed is likely to prevent LD.(6)
Figure 2).

Female black-legged ticks in various stages of feeding. Note the change in size. Reproduced from reference 1 © All rights reserved. With permission from the Minister of Health, 2014
How prevalent is LD in Canada?
Black-legged tick populations are well established in parts of British Columbia, Manitoba, Ontario, Quebec, New Brunswick and Nova Scotia, and may be expanding. Migratory birds can bring infected ticks into nonendemic areas, and people may also become infected while travelling to other endemic areas in North America and Europe.(6) In 2009, LD became a nationally reportable disease. The number of reported cases has increased from 128 in 2009 to an estimated ≥500 in 2013.(1,7)
What are the clinical manifestations of LD?
Clinical manifestations are divided into early localized, early disseminated and late disease.
Early localized disease:
Erythema migrans (EM) – a rash at the site of a recent tick bite – is the most common presentation in children and adults (Figures 3 and 4).(4,8) EM typically develops seven to 14 days (range three to 30 days) after a tick bite. EM begins as an erythematous macule or papule that rapidly expands centrifugally, sometimes with central clearing.(4) The lesions may be round or oval, flat or slightly raised, and are typically ≥5 cm in diameter. EM is usually painless and not pruritic. However, fever, malaise, headache, mild neck stiffness, myalgia and arthralgia often accompany EM.(1,4)
Figure 3).
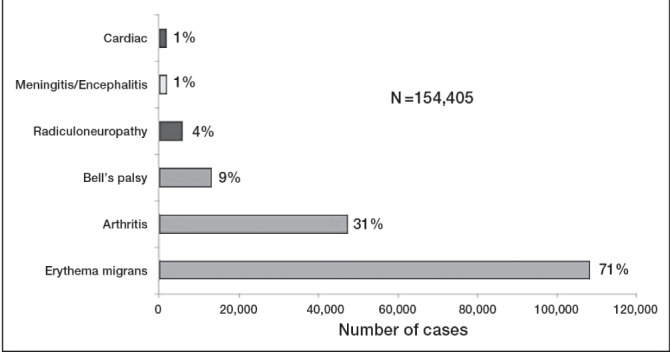
Clinical manifestations of confirmed Lyme disease cases (United States, 2001 to 2010). The most common presentation is the erythema migrans rash. Other symptoms are less common. Some individuals experienced >1 symptom. Reproduced from reference 8 with permission from the United States Centers for Disease Control and Prevention (Atlanta, USA)
Figure 4).
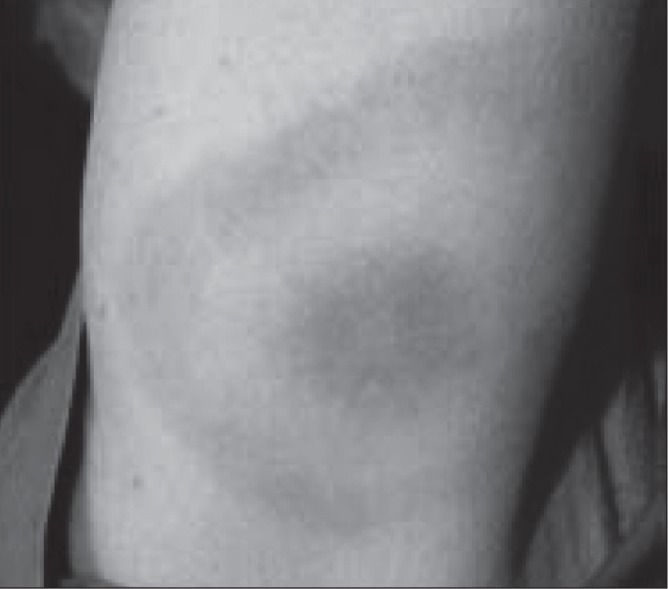
Erythema migrans rash showing the classic ‘bull’s eye’ form. Reproduced from reference 1 © All rights reserved. With permission from the Minister of Health, 2014
Early LD can occur without rash, and rash may not be detected by all patients in which it occurs. Without treatment, EM resolves spontaneously over a four-week period, on average.
Early disseminated disease:
Approximately 20% of children with LD first present to a health care provider with multiple, rather than single, EM lesions. This rash usually occurs several weeks after the tick bite and consists of secondary annular, erythematous lesions similar to but typically smaller than the primary lesion. These lesions reflect spirochetemia with cutaneous dissemination.(4)
Other manifestations of early disseminated disease (with or without rash) include acute neurological signs, such as facial nerve palsy, papilledema and lymphocytic meningitis.(4,9) Children with facial palsy should be assessed for meningitis, especially if neck stiffness or severe headache occurs.(9–11) Lyme carditis, resulting in heart block, is rare in children.(4)
Late disease:
Children treated with antimicrobial agents in the early stage of LD very rarely develop late disease.(4) The most common late-stage symptoms are pauciarticular arthritis affecting large joints, especially the knees, which may manifest weeks to months (mean four months) after the tick bite. Arthritis can occur without a history of earlier stages of illness. Peripheral neuropathy and central nervous system manifestations can also occur, although rarely during late disease in children.(4)
How is the diagnosis of LD made?
Early localized disease:
In general, the diagnosis of LD is principally clinical, supported by a history of potential tick exposure in an area where it is known or suspected that black-legged ticks have been established. However, because tick populations are expanding, it is possible that LD can be acquired outside of currently identified areas. Such a possibility should be considered when assessing patients. Patients with clear symptoms of early LD should be diagnosed and treated without laboratory confirmation,(1,4,10,11) because antibodies against B burgdorferi are often not detectable by serodiagnostic testing within the first four weeks after infection. (4,12,13) All clinical manifestations of possible LD, except EM, require laboratory confirmation.(13)
Early disseminated and late disease:
Two-tiered serological testing, including an ELISA screening test followed by a confirmatory Western blot test is used to supplement clinical suspicion of disseminated or late LD (Figure 5). Two-tiered testing is necessary because the ELISA may yield false-positive results from antibodies directed against other spirochetes, viral infections or auto-immune diseases.(1) Table 1 provides information related to the performance characteristics of serological assays in different clinical presentations of LD.(6,14)
Figure 5).
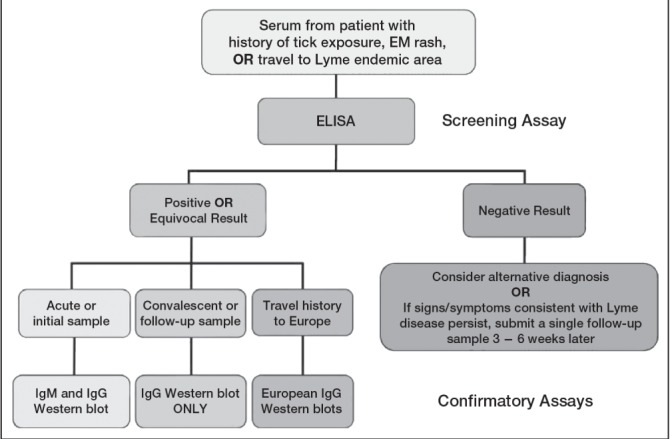
Two-tiered serological testing for Lyme disease. Source: Dr L Robbin Lindsay, Research Scientist, Field Studies (for the Public Health Agency of Canada’s Lyme Disease Surveillance Group). EM Erythema migrans; Ig Immunoglobulin
TABLE 1.
Performance characteristics of serological assays in Lyme disease
| Test |
Percent reactivity in patients with
|
|||
|---|---|---|---|---|
| EM, acute | EM, convalescent* | Neurological involvement | Arthritis | |
| Whole-cell ELISA | 33–49 | 75–86 | 79 (IgG only) | 100 (IgG only) |
| IgM WB | 43–44 | 75–84 | 80 | 16 |
| IgG WB | 0–13 | 15–21 | 64–72 | 96–100 |
| Two-tier testing | 29–40 | 29–78 | 87 | 97 |
Reproduced from reference 14 with permission from the American Society for Microbiology.
Sera obtained after antibiotic treatment; percent reactivity refers to the frequency that the different serological assays will be positive depending of the stage of the Lyme disease infection. EM Erythema migrans; Ig Immunoglobulin; WB Western blot
Supplemental tests can detect Borrelia species that cause LD outside of North America. Therefore, travel history should be documented.(1)
Some individuals treated with antimicrobials for early LD never develop antibodies against B burgdorferi. They are cured.(4,13)
Most individuals with early disseminated disease and almost all individuals with late disease have antibodies against B burgdorferi. Once such antibodies develop, they persist for years. A decline in antibody levels is not useful to assess treatment response.(1,3) Serological test results for LD should be interpreted along with careful consideration of the clinical setting and quality of the testing laboratory.(1,15) Tests of joint fluid for antibody to B burgdorferi and urinary antigen detection have no role in diagnosis.(3) In suspected Lyme meningitis, testing for intrathecal immunoglobulin M or immunoglobulin G antibodies may be helpful.(1,5,14)
How is LD treated?
Treatment of LD should follow the clinical practice guidelines by the Infectious Diseases Society of America(1,10,11,16) and the American Academy of Pediatrics (Tables 2 and 3).(4)
TABLE 2.
Antibiotic therapy for children and youth with Lyme disease
| Drug, route | Dosage | Maximum per day |
|---|---|---|
| Oral | ||
| Doxycycline (≥8 years of age) | 4 mg/kg per day, in two divided doses | 200 mg |
| Amoxicillin (<8 years OR unable to tolerate doxycycline) | 50 mg/kg/day, in three divided doses | 1.5 g |
| Cefuroxime | 30 mg/kg per day, in two divided doses | 1 g |
| Intravenous | ||
| Ceftriaxone | 50–75 mg/kg once daily | 2 g |
| Penicillin G | 200,000–400,000 units/kg/day in divided doses every 4 h | 18–24 million units |
Data adapted from references 4 and 10. For patients allergic to penicillin, the alternative drug is cefuroxime. Macrolides (azithromycin, clarithromycin and erythromycin) have lower efficacy. Patients treated with macrolides should be closely observed to ensure resolution of clinical manifestations.(1,10,11)
TABLE 3.
Route/duration of antibiotic therapy for Lyme disease
| Clinical stage | Route | Duration, days |
|---|---|---|
| Early disease | ||
| Erythema migrans | Oral | 14–21 |
| Early disseminated and late disease | ||
| Isolated facial palsy (Bell’s palsy) | Oral | 14–21 |
| Multiple erythema migrans | Oral | 21 |
| Arthritis | Oral | 28 |
| Recurrent or persistent arthritis | Oral or IV | 28 |
| Heart block or carditis | IV | 14–21 |
| Meningitis | IV | 14 (range 10–28) |
| Encephalitis/late neurological disease (encephalopathy, peripheral neuropathy) | IV | 14–28 |
Data adapted from reference 4. IV intravenous
Arthritis frequency has decreased in the United States, probably because of improved recognition and earlier treatment of patients with early LD. Up to one-third of LD patients with arthritis experience residual synovitis and joint swelling, which almost always resolve without repeating the antibiotic course. For patients who have persistent or recurrent joint swelling after a recommended course of oral antibiotic therapy, some experts recommend retreatment with another four-week course of oral antibiotics or with a course of parenteral ceftriaxone.(10) For cases with ongoing arthritis, consultation with an expert is recommended.(4) Consider hospitalization and constant monitoring for a child with heart block and syncope that may rapidly worsen enough to require a pacemaker.(10)
The Jarisch-Herxheimer reaction (fever, headache, myalgia and an aggravated clinical picture lasting <24 h) can occur when therapy is initiated. Nonsteroidal anti-inflammatory agents should be started and the antimicrobial agent continued.(4)
Approximately 10% to 20% of cases experience lingering symptoms of fatigue and joint and muscle aching that last longer than six months. The clinical term for this condition is ‘post-treatment Lyme disease syndrome’ (PTLDS).(17) The exact cause of PTLDS is not yet known. Most medical experts believe that lingering symptoms are the result of residual damage to tissues and the immune system.(17,18) Recent evidence suggests that persistent infection with B burgdorferi occurs only rarely after appropriate treatment.(19) Long-course antibiotic treatments do not provide long-term improvement in PTLDS cases.(10,13,17)
How to remove a tick
Use fine-tipped tweezers to grasp the tick close to the skin surface (Figure 6A).
Pull upward with steady, even pressure (Figure 6B). Try not to twist or jerk, which can cause the mouthpart of the tick to break off and remain in the skin. If this happens and you are unable to remove the mouthpart easily with clean tweezers, leave it alone and let the skin heal.
Clean the bite area and your hands with rubbing alcohol, an iodine scrub, or soap and water.(21)
Figure 6).
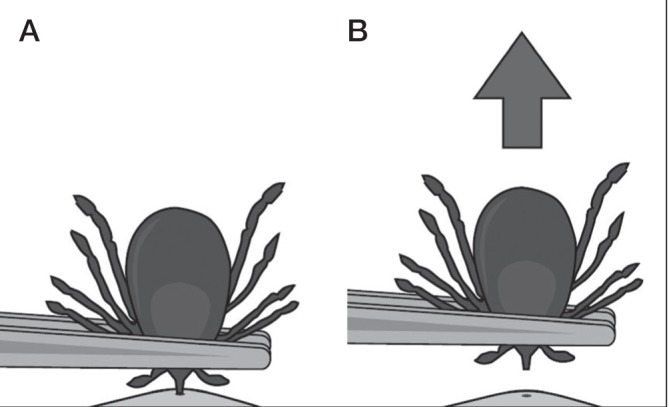
How to remove a tick. Reproduced with permission from the United States Centers for Disease Control and Prevention (Atlanta, USA)
The Public Health Agency of Canada advises people to:
Keep any ticks they remove themselves in a resealable plastic bag or pill vial and note the location and date of the bite.
Watch for symptoms and see a health care professional immediately should symptoms appear.
Take the tick with them to their medical appointment, to verify species and test as needed.(1)
How can LD be prevented?
Physicians should be aware of the epidemiology of tick-borne LD in their area,(1,2,7) and recommend some basic precautions for families living, hiking or camping in rural or wooded areas where they may be exposed to ticks.(1,2,3)
Where play spaces adjoin wooded areas, landscaping can reduce contact with ticks.(3) A pictogram from the Centers for Disease Control and Prevention is available at: www.cdc.gov/lyme/prev/in_the_yard.html.
Apply 20% to 30% DEET or icaridin repellents. Repellents can be applied to clothing as well as to exposed skin. Always read and follow label directions.(1,20)
Do a ‘full body’ check every day for ticks. Promptly remove ticks found on yourself, children and pets. Shower or bathe within two hours of being outdoors to wash off unattached ticks.(1)
For more information on how to prevent tick bites, refer to a recent practice point from the Canadian Paediatric Society at: www.cps.ca/en/documents/position/preventing-mosquito-and-tick-bites.
Postexposure antibiotic therapy
Consensus on postexposure prophylaxis for LD is lacking at this time. Some experts recommend giving doxycycline as a single dose of 200 mg for children and youth ≥8 years of age after a tick bite (for individuals weighing <45 kg, 4 mg/kg to a maximum of 200 mg). Prophylaxis can be started within 72 h of removing a tick, even if it has been attached for ≥36 h.(1,4,7,10) Data are insufficient to recommend amoxicillin prophylaxis in younger children.(1,4,10,11)
In Canada, such prophylaxis should be considered in ‘known endemic areas’ (see Table 1 and Figure 1 in reference 1). Physicians should bear in mind that the true prevalence of B burgdorferi is often unknown and that the geographical range of infected ticks is expanding in some areas.(1) The Public Health Agency of Canada continues to monitor the distribution and prevalence of infected ticks as well as cases of LD.(1,7)
A vaccine to prevent LD in humans is not available at the present time.(1,4)
Selected resource:
Government of Canada. Lyme disease (video): http://healthycanadians.gc.ca/video/lyme-eng.php (Accessed July 22, 2014).
Acknowledgments
This position statement was reviewed by the Acute Care and Community Paediatrics Committees of the CPS. Special thanks are due to Drs Nicholas Ogden and Michel Deilgat, with the Centre for Food-borne, Environmental and Zoonotic Infections Diseases, and Dr L Robbin Lindsay, Research Scientist, Field Studies, for the Public Health Agency of Canada’s Lyme Disease Surveillance Group.
Footnotes
CPS INFECTIOUS DISEASES AND IMMUNIZATION COMMITTEE
Members: Natalie A Bridger MD; Jane C Finlay MD (past member); Susanna Martin MD (Board Representative); Jane C McDonald MD; Heather Onyett MD; Joan L Robinson MD (Chair); Marina I Salvadori MD (past member); Otto G Vanderkooi MD
Liaisons: Upton D Allen MBBS, Canadian Paediatric AIDS Research Group; Michael Brady MD, Committee on Infectious Diseases, American Academy of Pediatrics; Charles PS Hui MD, Committee to Advise on Tropical Medicine and Travel (CATMAT), Public Health Agency of Canada; Nicole Le Saux MD, Immunization Monitoring Program, ACTive (IMPACT); Dorothy L Moore MD, National Advisory Committee on Immunization (NACI); Nancy Scott-Thomas MD, College of Family Physicians of Canada; John S Spika MD, Public Health Agency of Canada
Consultant: Noni E MacDonald MD
Principal author: Heather Onyett MD
The recommendations in this document do not indicate an exclusive course of treatment or procedure to be followed. Variations, taking into account individual circumstances, may be appropriate. All Canadian Paediatric Society position statements and practice points are reviewed on a regular basis. Retired statements are removed from the website. Please consult the Position Statements section of the CPS website (www.cps.ca) for the full-text, current version.
REFERENCES
- 1.Public Health Agency of Canada Lyme disease and other tick-borne diseases: Information for healthcare professionals: www.phacaspc.gc.ca/id-mi/tickinfo-eng.php (Accessed July 14, 2014).
- 2.Public Health Agency of Canada Lyme Disease: www.phac-aspc.gc.ca/id-mi/lyme-fs-eng.php (Accessed July 14, 2014).
- 3.Centers for Disease Control and Prevention Lyme disease: www.cdc.gov/Lyme (Accessed July 17, 2014).
- 4.American Academy of Pediatrics . Lyme disease (Lyme borreliosis, Borrelia burgdorferi infection) In: Pickering LK, Baker CJ, Kimberlin DW, Long SS, editors. Red Book: 2012 Report of the Committee on Infectious Diseases. 29th edn. Elk Grove Village: American Academy of Pediatrics; 2012. pp. 474–9. [Google Scholar]
- 5.U.S. Department of Health and Human Services, Centres for Disease Control and Prevention Tickborne diseases of the United States: A reference manual for health care providers. 2013. www.cdc.gov/lyme/resources/TickborneDiseases.pdf (Accessed July 14, 2014).
- 6.Sider D, Patel S, Russell C, Jain-Sheehan N, Moore S. Technical report: Update on Lyme disease prevention and control. Mar, 2012. Public Health Ontario: www.publichealthontario.ca/en/eRepository/PHO%20Technical%20Report%20-%20Update%20on%20Lyme%20Disease%20Prevention%20and%20Control%20Final%20030212.pdf (Accessed July 14, 2014).
- 7. Public Health Agency of Canada, Canada Communicable Disease Report. Lyme disease. Vol. 40–5 (March 6, 2014): www.phac-aspc.gc.ca/publicat/ccdr-rmtc/14vol40/dr-rm40-05/index-eng.php (Accessed July 14, 2014).
- 8.Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases, Division of Vector-Borne Diseases Clinical manifestations of confirmed Lyme disease cases--United States, 2001–2010: www.cdc.gov/lyme/stats/chartstables/casesbysymptom.html (Accessed July 14, 2014).
- 9.Cherry J, Demmler-Harrison G, Kaplan S, Steinbach WJ, Hotez P. Feigin and Cherry’s Textbook of Pediatric Infectious Diseases. 7th edn. Toronto: Elsevier-Saunders; 2014. Lyme disease; pp. 1729–39. [Google Scholar]
- 10.Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: Clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43(9):1089–1134. doi: 10.1086/508667. (Erratum in 2007;45:941): http://cid.oxfordjournals.org/content/43/9/1089.long (Accessed July 14, 2014). [DOI] [PubMed] [Google Scholar]
- 11.Lantos PM, Charini WA, Medoff G, et al. Final report of the Lyme disease review panel of the Infectious Diseases Society of America. Clin Infect Dis. 2010;51(1):1–5. doi: 10.1086/654809. [DOI] [PubMed] [Google Scholar]
- 12.Henry B, Crabtree A, Roth D, Blackman D, Morshed M. Lyme disease: Knowledge, beliefs, and practices of physicians in a low-endemic area. Can Fam Physician. 2012;58(5):e289–95. [PMC free article] [PubMed] [Google Scholar]
- 13.Halperin JJ, Baker P, Wormser GP. Common misconceptions about Lyme disease. Am J Med. 2013;126(3):264.el–7. doi: 10.1016/j.amjmed.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Aguero-Rosenfeld ME, Wang G, Schwartz I, Wormser GP. Diagnosis of Lyme borreliosis. Clin Microbiol Rev. 2005;18(3):484–509. doi: 10.1128/CMR.18.3.484-509.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease and Prevention Notice to readers: Caution regarding testing for Lyme disease. MMWR. 2005;54(05):125. www.cdc.gov/mmwr/preview/mmwrhtml/mm5405a6.htm (Accessed July 14, 2014). [Google Scholar]
- 16.Infectious Disease Society of America Lyme Disease Case Study Course, May 2012–May 2015: http://lymecourse.idsociety.org (Accessed July 14, 2014).
- 17.Centers for Disease Control and Prevention Post-treatment Lyme disease syndrome: www.cdc.gov/lyme/postLDS/index.html (Accessed July 14, 2014).
- 18.Feder HM, Jr, Johnson BJ, O’Connell S, et al. A critical appraisal of “chronic Lyme disease”. N Engl J Med. 2007;357(14):1422–30. doi: 10.1056/NEJMra072023. [DOI] [PubMed] [Google Scholar]
- 19.Marques A, Telford SR, Turk SP, et al. Xenodiagnosis to detect Borrelia burgdorferi infection: A first-in-human study. Clin Infect Dis. 2014;58(7):937–45. doi: 10.1093/cid/cit939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canadian Paediatric Society Preventing mosquito and tick bites: A Canadian update. Paediatr Child Health. 2014;19(6):326–8. [PMC free article] [PubMed] [Google Scholar]
- 21.U.S. Centers for Disease Control and Prevention Tick removal: www.cdc.gov/ticks/removing_a_tick.html (Accessed May 6, 2014).


