Abstract
Therapeutic efforts in neurodegenerative diseases have been very challenging, particularly due to a lack of validated and mechanism-based therapeutic targets and biomarkers. The basic idea underlying the novel therapeutic approaches reviewed here is that by exploring the molecular basis of neurodegeneration in a rare lysosomal disease such as Gaucher’s disease (GD), new molecular targets will be identified for therapeutic development in common synucleinopathies. Accumulation of α-synuclein plays a key role in the pathogenesis of Parkinson’s disease (PD) and other synucleinopathies, suggesting that improved clearance of α-synuclein may be of therapeutic benefit. To achieve this goal, it is important to identify specific mechanisms and targets involved in the clearance of α-synuclein. Recent discovery of clinical, genetic, and pathological linkage between GD and PD offers a unique opportunity to examine lysosomal glucocerebrosidase, an enzyme mutated in GD, for development of targeted therapies in synucleinopathies. While modulation of glucocerebrosidase and glycolipid metabolism offers a viable approach to treating disorders associated with synuclein accumulation, the compounds described to date either lack the ability to penetrate the CNS or have off-target effects that may counteract or limit their capabilities to mediate the desired pharmacological action. However, recent emergence of selective inhibitors of glycosphingolipid biosynthesis and noninhibitory pharmacological chaperones of glycosphingolipid processing enzymes that gain access to the CNS provide a novel approach that may overcome some of the limitations of compounds reported to date. These new strategies may allow for development of targeted treatments for synucleinopathies that affect both children and adults.
Keywords: glucocerebrosidase, lysosomes, glycosphingolipids
A LINK BETWEEN GAUCHER’S AND PARKINSON’S DISEASE
The autophagy-lysosomal pathway plays an important role in maintaining cellular homeostasis by degrading bulky cytoplasmic material, including damaged organelles and misfolded and accumulated proteins (1). This degradation pathway appears crucial for clearance of aggregated proteins that represent a pathologic hallmark of several neurodegenerative disorders, such as Parkinson’s disease (PD), Huntington’s disease, and Alzheimer’s disease (2). The observation that mutant proteins accumulate and aggregate in different neurodegenerative disorders indicates the possibility of a shared pathogenic mechanism. Recent data suggest that elimination of mutant protein accumulation can lead not only to a halt of symptomatic progression but also to regression of the disease. This is best demonstrated through conditional mouse models, where elimination of expression of disease-linked proteins, such as mutant huntingtin, ataxin1, and α-synuclein, resulted in reversal of the pathological phenotype (3–5).
In PD and related synucleinopathies, accumulation of α-synuclein plays a key role in disease pathogenesis. This is especially evident in some forms of familial PD, most notably in α-synuclein locus triplications and duplications where the expression levels of α-synuclein closely correlate with clinical phenotypes (6). However, one of the main challenges is to identify specific mechanisms and targets involved in the clearance of α-synuclein in order to develop specific therapeutics. To tackle this challenge, it is particularly informative to examine disorders that are caused by mutations in lysosomal proteins such as rare lysosomal storage disorders (LSDs) that commonly exhibit neurodegeneration. More than 50 LSDs are known and are usually classified according to the nature of the accumulating substrate. Pathology in the CNS is a common feature of most LSDs (7, 8). While the best example of this is the link between Gaucher’s disease (GD) and parkinsonism, the link between other LSDs and PD is suggested, but less well documented due to the small number of patients (8).
GD is a rare LSD caused by mutations in the GBA1 gene and is characterized by the accumulation of glucosylceramide (GlcCer). It was first noted in 1980 that some patients with GD also exhibit parkinsonism (9). Several other papers have since confirmed that patients with adult onset GD have up to a 20-fold higher chance of developing parkinsonism or diffuse Lewy body disease (10–12). More recently in 2004, it was noted that patients with GD and parkinsonism frequently had relatives with parkinsonism that were heterozygous for GBA1 mutations (13). Neuropathological analysis revealed the presence of Lewy bodies in the brains of these GD patients similar to those found in idiopathic PD or diffuse Lewy body disease (14, 15). Additionally, genotyping studies using large patient cohorts have identified mutations in the GBA1 gene as the highest risk factor (genetic or environmental) for developing idiopathic PD to date, with a 5-fold increase (16). Therefore, the clinical and genetic link between GD and parkinsonism has been established in “both directions” patients with GD and their relatives have increased incidence of parkinsonism, and patients with idiopathic parkinsonism have increased incidence of mutations in the gene glucocerebrosidase (GCase) that causes GD. However, the molecular mechanism that would explain how this rare LSD is linked to adult onset synucleinopathies and neurodegeneration is just emerging. Recent evidence establishes a link between GlcCer metabolism and α-synuclein (a-syn) accumulation (17). Specifically, inactive glucocerebrosidase leads to accumulation of the sphingolipid GlcCer in neurons. This accumulation of GlcCer leads to stabilization of toxic a-syn oligomers. While general lysosomal inhibition does not have an effect on formation of a-syn oligomers, it is specific inhibition of glucocerebrosidase that is required for the effect. Importantly, accumulation of a-syn also affects the lysosomal maturation and activity of normal glucocerebrosidase in neurons and human brain, suggesting that GlcCer accumulation also plays a role in sporadic PD and other synucleinopathies.
Recent studies have revealed a significant decrease of GCase activity in PD brains with GBA mutations, most prominent in the substantia nigra, leading to mitochondrial dysfunction and decreased microautophagy. Wild-type GCase protein expression changes in vitro could contribute to the GCase deficiency observed in sporadic PD (18, 19). Loss of GCase activity did not immediately raise α-synuclein concentrations, but first led to neuronal ubiquitinopathy and axonal spheroids (20).
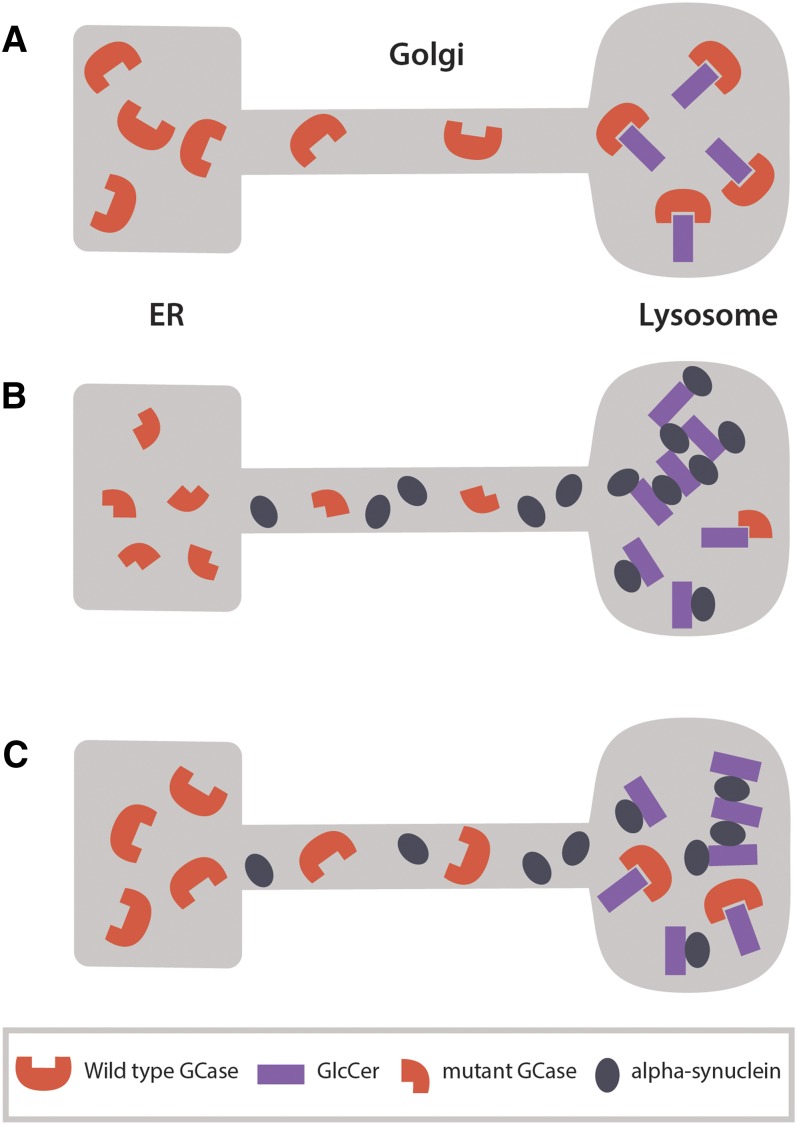
These findings suggest that this molecular pathway applies not only to patients with GD or patients with PD and GBA mutation, but also to patients with idiopathic PD or other synucleinopathies who have a normal glucocerebrosidase gene. The bidirectional effects of a-syn and glucocerebrosidase form a positive feedback loop that, after a threshold, leads to self propagating disease (17) (Fig. 1).
Fig. 1.
Bidirectional effect of α-synuclein and glucocerebrosidase (GCase) forms a positive feedback loop that may lead to a self-propagating disease. A: In healthy neurons, wild-type GCase translocates from the ER to the lysosome to degrade its substrate GlcCer. B: Mutant GCase is misfolded and partially degraded in the ER. This results in deficient GCase activity in the lysosome and accumulation of GlcCer that in turn accelerates and stabilizes soluble α-synuclein oligomers. Accumulation of α-synuclein interferes with ER-Golgi trafficking of GCase resulting in a positive feedback loop. C: Accumulation of α-synuclein also interferes with the trafficking of wild-type GCase resulting in decreased activity of GCase in the lysosome. This further amplifies GlcCer accumulation and stabilization of soluble α-synuclein oligomers, and results in a stronger inhibition of GCase ER-Golgi trafficking with each pathogenic cycle.
Together, these findings place lysosomal glucocerebrosidase at the center of a-syn biology, and suggest a general role of this pathway in idiopathic PD and other synucleinopathies. Therefore, therapeutic targeting of mutated or normal glucocerebrosidase to lysosomes is expected to prevent or diminish formation of toxic a-syn oligomers and break the vicious cycle of a-syn aggregation and toxicity in any disease that is characterized by accumulation of α-synuclein.
APPROACHES TO THERAPEUTIC INTERVENTION
The insights into the connections between glycosphingolipids, α-synuclein, and lysosomal function provide a basis for novel approaches to therapeutic intervention in neurological disorders associated with synucleinopathy. Figure 2 illustrates pathways of glycosphingolipid biosynthesis and catabolism and identifies several enzymes that are potential targets of intervention. To date, no therapeutic intervention has been demonstrated to be clinically effective in reversing or treating a disease associated with synuclein accumulation. In the LSDs, enzyme replacement therapy has not provided a viable means to combat the neurological manifestations, given the lack of adequate CNS exposure to protein following systemic administration. The use of glycosphingolipid biosynthesis inhibitors has also met with limited success. Eliglustat has been demonstrated to be highly effective in reducing signs and symptoms of peripheral GlcCer accumulation in type 1 GD (21, 22). However, this compound does not penetrate the CNS and is unlikely to impact neurological manifestations in either type 1 GD patients or in the neuronopathic type 2 and 3 forms of the disease (23, 24). Miglustat, another biosynthesis inhibitor, has also been shown to be effective in type 1 GD and penetrates the CNS; however, its inhibitory effect on glucocerebrosidase, in particular GBA2, GI tolerability profile, ability to induce peripheral neuropathies, and high dose limits its overall utility and effectiveness (23, 25–27). Although anecdotal reports in individual patients suggest an impact on neurological manifestations in type 3 GD, a controlled clinical trial involving 25 subjects over a two year time course failed to demonstrate effectiveness on neurological endpoints such as saccadic eye movements (28). However, newer small molecule modulators with the potential to gain access to the CNS and impact glycolipid metabolism are now emerging (29–32).
Fig. 2.
Pathways of glycosphingolipid biosynthesis and catabolism and enzymes discussed in this review.
A significant challenge to the development of novel therapies for impacting glycosphingolipid-related synucleinopathies is the slow and variable onset of disease symptoms. In many of these disorders, onset of symptoms occurs over decades and shows substantial inter-patient variability in onset, severity, and manifestations, suggesting that disease-modifying therapies will require chronic administration to asymptomatic individuals. Although individual populations at high risk of early onset disease can now be identified through genetics and imaging techniques, the identification of biomarkers of disease progression and validation of these as endpoints or markers of disease remains in its infancy. Lastly, given the chronic nature of drug administration, therapies must have adequate safety and tolerability to be administered over the life of the patient
There is solid preclinical evidence supporting the rationale for augmenting glucocerebrosidase activity in treatment of neuronopathic GD and potentially other synucleinopathies. As discussed above, it is now apparent that glucocerebrosidase and synuclein form a feedback loop whereby GlcCer can stabilize synuclein and prevent its degradation, and synuclein, in turn, inhibits glucocerebrosidase (17). AAV delivery of recombinant glucocerebrosidase in D409V/D409V mice, a model of type 3 GD, increased GBA activity in discreet brain regions, lowered glycosphingolipid substrate levels and synuclein, and improved motor and cognitive symptoms of disease in these mice (33, 34). Although approaches utilizing gene therapy or direct administration of enzyme to the brain are fraught with challenges, these studies do provide strong evidence that augmenting glucocerebrosidase activity where it is deficient will provide a therapeutic benefit.
Glycosphingolipid biosynthesis inhibitors
As briefly discussed above, inhibition of glycosphingolipid biosynthesis at the level of GlcCer synthetase with small molecules represents a possible therapeutic approach, provided that the compound can gain access to the CNS. Miglustat is an iminosugar that inhibits GlcCer biosynthesis (35) and has been studied extensively. It displays modest selectivity toward GlcCer synthase and also inhibits several glycosidases, including the lysosomal glucocerebrosidase, GBA1, and the non-lysosomal glucocerebrosidase, GBA2 (35). Miglustat can also display chaperone-like activity, facilitating the translocation of glucocerebrosidase from the endoplasmic reticulum (ER) to the lysosome (36, 37). Miglustat shows good brain penetration and thereby provides the opportunity to impact CNS diseases (26). The effects of miglustat in type 3 GD have been evaluated. Early anecdotal reports suggested that miglustat could improve neurological symptoms (saccadic eye movement velocity, ataxia) in patients with type 3 GD. These reports were followed by an open label 2 year study in 20 patients with type 3 GD (28). Treatment with miglustat had no impact on neurological measures in these patients in spite of improvements in peripheral signs of the disease (hemoglobin, platelets on splenic volume).
The utility of miglustat in the treatment of Niemann-Pick C (NPC) disease has been evaluated in both preclinical and clinical studies. In NPC disease, a defect in the cholesterol transporters, NPC1 or NPC2, leads to lysosomal accumulation of cholesterol, GlcCer, and downstream glycosphingolipids (38, 39), along with accumulation of synuclein in brain tissue (7, 8). Recent studies have suggested that, as with GBA mutations, patients heterozygous for mutations in NPC1 may develop PD (40). Treatment of NPC mice with miglustat resulted in improvements of survival in these mice (41). In a cat model of NPC disease, miglustat delayed the onset of neurological symptoms and improved survival (42). These effects were associated with improved survival of cerebellar Purkinje cells and reduced micogliosis and inflammatory markers. Miglustat also reduces several glycosphingolipid species, including lactosylceramide and GM3. Paradoxically, miglustat increased levels of monohexosylceramides, an effect that was attributed to the inhibition of GBA2 by the compound (24, 43). The impact on neuronal synuclein levels remains to be established in this disease. In clinical studies, miglustat has been demonstrated to improve symptoms of disease in small open-label studies (44). In 20 patients treated for 12 months, miglustat improved saccadic eye movement velocity (the primary end point for the study), swallowing, hearing, and walking in these patients (45). Follow-up studies have confirmed the activity of miglustat in these patients (46). Based upon these observations, miglustat is approved in several countries for the treatment of NPC disease. Consistent with its actions in animal models, miglustat is associated with reductions of several glycolipid species, including lactosylceramide and GM3, while CSF monohexosylceramides increase (43).
Other iminosugar-based inhibitors of GlcCer synthesis have been evaluated in animal models of Niemann-Pick disease (24). Genz 529468, N-(5′-adamantane-1′-yl-methoxy)-phenyl-1-deoxynorjirimycin (AMP-DNM), is significantly more potent than miglustat on the enzyme (47). Although its selectivity profile on several glycosidases is improved over that of miglustat, the compound still potently inhibits GBA2 and increases GlcCer in brain tissue (24). AMP-DNM improves motor function, inflammation, and survival in murine models of NPC disease and Sandhoff’s disease. As with miglustat, significant reductions in liver GlcCer and significant increases in brain GlcCer were observed in both models (24, 48). The basis for the increase in brain GlcCer is likely due to the inhibition of GBA2 by both inhibitors and the key role of this enzyme in brain GlcCer turnover.
The basis for the efficacy of these agents in the absence of significant reductions in GlcCer remains to be established. It is possible that the impact of these agents on other glycolipids is sufficient to offset the potential negative impact of elevating GlcCer. In this regard, biochemical studies have shown that other glycosphingolipids will interact with and stabilize synuclein (49, 50). As the impact on synuclein, or other aggregation-prone proteins, has not been evaluated in many of these studies, the connection between glycosyphingolipid reductions and aggregation-prone proteins cannot be established. Alternatively, off-target effects of these compounds on other pathways, e.g., autophagy, ER stress, and inflammation, may be responsible for their protective actions.
Inhibition of glycosphingolipid biosynthesis will reduce the formation and tissue levels of downstream glycolipids including glucosylsphingosine, GM2, and GM3. Two such clinical diseases associated with glycolipid elevations are Tay-Sachs disease and Sandhoff disease. In these diseases, a deficiency of hexosaminidase A and/or B leads to a buildup of GM2 that leads to neurodegeneration. Both diseases are associated with development of Lewy bodies and synuclein aggregates (7, 8). As in the case of GlcCer, GM2 also has been shown to interact with synuclein and may contribute to neurodegeneration via a similar feedback loop, as proposed for glucocerebrosidase and synuclein (49, 50). Miglustat has been evaluated in models of Tay-Sachs disease and Sandhoff disease (51–53). In a mouse model, dietary administration of miglustat for 12 weeks was shown to lower GM2 levels and prevent the histological evidence of lipid accumulation in neurons. In the Sandhoff mouse model, miglustat also initially reduced brain GM2 levels, delayed onset of motor deterioration, and prolonged survival (51). Similar results on motor function and survival were obtained in another study with both miglustat and Genz 529468 (48); however, GM2 levels were increased slightly and GL1 levels increased dramatically in this study. Liver glycolipids were reduced in both studies. The impact of miglustat and Genz 529468 on synuclein levels was also evaluated in this study (48). Drug treatment was associated with a 50% reduction in synuclein-positive cell staining. The basis for the differences in these studies on brain glycolipid levels is not clear, but may be related to the different doses of drug used, potentially different tolerability profiles at these doses, differences in exposures, or intrinsic differences in glycolipid turnover. The differences in liver response versus neuronal response on glycolipids likely reflect the dominant role of GBA2 in brain glycolipid metabolism.
Anecdotal observations suggest that miglustat may be of some benefit in treatment of patients with Sandhoff disease; however, no controlled clinical trials have been performed to date (54, 55). In a controlled clinical trial of 12 months duration in 20 patients with late onset Tay-Sachs disease, miglustat was shown to provide no benefit in terms of measures of muscle strength or neurological symptoms (56). Although a benefit of miglustat can be observed in some animal models of neuronal glycophingolipidoses, its clinical effects in these disorders has been very limited at best. The off-target actions of this drug on other enzymes, e.g., intestinal glycosidases leading to dose limitations and intolerability and its inhibitory effect on glucocerebrosidase leading to accumulation rather than reduction of GlcCer, may be important contributors to its overall lack of effect.
A structurally distinct class of GlcCer synthase inhibitors has also been elaborated. Molecules based upon ceramide mimicry have been described. As these agents are not based on a glycomimetic functionality, they lack the off-target effects of the iminosugars and may offer a more effective means of reducing glycolipid levels. D-threo-1-phenyl-decanoylamino-3-morpholinepropanol (PDMP) represents the first such agent (23). It is a 20 uM inhibitor of the enzyme. Its weak potency, cytotoxicity, and nonspecificity have limited its overall use as a proof-of-concept molecule. However, structural manipulation of the agent in which the morpholino has been replaced by an ethylene dioxy phenyl group and the decanoylamino group has been replaced with an octanoylamino functionality has resulted in the potent GlcCer inhibitor eliglustat (D-threo-1-(3,4 ethylenedioxyphenyl)-3-octanoylamino-3-pyrrolidinyl-propanol) (23). Eliglustat selectively inhibits GlcCer synthetase with a potency of approximately 100 nM and does not inhibit a variety of glycosidases, including GBA1 and GBA2. The compound lowers glycosphingolipid levels in animals and humans. As discussed above, the compound has shown high efficacy in type 1 GD patients. Although an effective inhibitor of the enzyme, the compound is a substrate for P-glycoprotein and does not achieve adequate brain concentrations to impact the enzyme in the brain. In fact, the compound has failed to demonstrate efficacy in animal models of NPC disease or Sandhoff disease (24, 48)
Recent studies have reported the discovery of ethylenedioxy PIP-2, CCG 203586, a further structural elaboration of the ceramide-based series (29, 30). The compound is a potent and selective inhibitor of GlcCer synthetase with a Ki of 27 nM. In contrast to eliglustat, it is not a substrate for P-glycoprotein, gains access to the CNS, and lowers GlcCer in mouse brain. Studies in Sandhoff mice demonstrated that the compound reduces gangliosides in the brain regions of these mice. The impact of the compound in GD mouse models or on brain pathology and synuclein levels or levels of other aggregation-prone proteins has not been reported to date.
Recently a novel synthetic GCS inhibitor, GZ 161, has been described, although its structure was not disclosed (31). The compound penetrates the CNS in mice and lowers GlcCer and glucosylsphingosine levels in mouse brain. In the K14 lnl/lnl mouse model of type 2 GD, the compound prolonged survival by up to 20% and reduced signs of inflammation in the brains of these mice. A greater benefit on survival was observed when the compound was administered in combination with administration of recombinant human glucocerebrosidase.
Not all studies support the utility of inhibitors of GlcCer synthesis for treatment of glycosphingolipid-related synucleinopathies. Depletion of GM1 leads to the development of synuclein deposits and Parkinson-like disease in mice (57). Moreover, GM1 ganglioside treatment of PD patients has shown some benefit in some studies (58). High concentrations of D-PDMP promoted synuclein accumulation, suppressed lysosomal function as measured by cathepsin B activity, inhibited autophagy, and exacerbated cytotoxicity in neuroblastoma cells expressing mutated β- and α-synucleins. These effects were reversed by addition of a mixture of gangliosides (59, 60). The basis for the difference between these results and those reported with other inhibitors remains to be established, but may relate to off-target actions of PDMP, the high concentrations required to elicit effects, and subtle differences in impact on different species of glycosphingolipids and/or their precursors, e.g., ceramide levels. Studies with other more potent inhibitors are required to further define these interactions. In either case, these data suggest that the interplay of glycolipids, synuclein, and neuropathology is complex. Moreover, it is possible that the potential beneficial effects of GlcCer biosynthesis inhibitors may be partially offset by depletion of GM1.
Pharmacological chaperones
An alternative approach to GlcCer synthesis inhibition is to enhance GCase activity by facilitating its translocation from the ER to the lysosome. Pharmacological chaperones based on the n-butyl deoxynojirimicin chemotype have been identified and evaluated in preclinical and clinical studies. Isofagomine is the most-studied of such agents. The molecule is an active site inhibitor of the enzyme GBA1 and functions to stabilize the enzyme thermodynamically, allowing for its translocation and delivery to the lysosome (61–65). The subtle differences in pH dependency of inhibition allow for the enzyme to function when it reaches the lysosome. Because this pH dependency is not absolute, and the molecules inhibit GBA2 as well, the overall efficacy in activating the enzyme and lowering GlcCer levels is limited. Studies in cells and in vivo have confirmed its ability to induce translocation of the wild-type and mutant GCase and activate the enzyme. Like other iminosugars, isofagomine gains some access to the CNS and may have utility in neurological settings (66). Isofagomine has been evaluated in the setting of neuronopathic GD (67). Transgenic mice made homozygous for the V394L mutation in GCase and in which saposin C, a key protein cofactor for lysosomal GCase, has been deleted, exhibit significant neurological deficits that include motor defects and premature death. Treatment of these mice with isofagomine prolonged survival by 10–20 days. There was no effect on glycosphingolipids, if anything levels increased slightly, and there was no major effect on pathology. The lack of effect on glycolipid levels may likely be due to the inhibitory effect of the drug on the enzyme. The basis for its impact on survival is not clear, but may be related to an anti-inflammatory action of the compound. Because of its toxicity and inhibitory effects on GBA1, limited conclusions can be drawn from this study on the utility of enzyme chaperones in this setting.
The Amicus group has reported, in abstract form, on the impact of isofagomine (AT 2101) and AT 3375 in the V394L/V394L prosaposin−/− mouse model that accumulates α-synuclein and GlcCer in brain tissue (32). AT 2101 increased glucocerebrosidase activity, lowered synuclein and GlcCer, and improved behavioral performance in these mice. AT 3375 provided better brain penetration and also increased GCase activity in the mice. These data provide important proof of concept support that activation of GCase in the brain will reduce synuclein levels and improve behavior performance deficiencies. The differences between these results and those described in the previous study may relate to the dosing regimens, as efficacy was observed with an on-off dosing regimen.
Non-iminosugar-based chaperones, which also act as inhibitors of GCase, have been described. The expectorant agent ambroxol was recently reported to inhibit GCase in a pH-dependent manner (68–70). At concentrations of 10–100 uM, the compound promoted the translocation of wild-type and various mutant GCases to the lysosome. Ambroxol was shown to stabilize both wild-type and several mutant forms of GCase. Moreover, the compound was shown to alleviate ER stress and morphological defects in the eyes of Drosophila expressing mutant human GCase (RecNeil allele containing L444P mutation), suggesting a possible neuroprotective action (71). Its effects in vivo and on neuronal accumulation of glycosphingolipids have not been evaluated to date. Pilot studies in GD patients suggest the drug may have some activity (72).
The group at National Institutes of Health has reported on a family of noninhibitory pyrazolopyrimidines that are activator-chaperones of GCase that were discovered through a high throughput screen utilizing human splenic homogenates (73, 74). The most potent compound, compound 40, was shown to activate the enzyme with an EC50 of approximately 300 nM (74). The compound also facilitated translocation of the enzyme to the lysosome in dermal fibroblasts from a GD patient. Pharmacokinetic studies suggested that the compound penetrates the CNS and is unlikely to be a substrate for PGP. The effect of the compound on glycolipid levels in normal and diseased animal models has not yet been assessed.
Chaperones that may enhance the activity other glycolytic enzymes that may be involved in synucleinopathies have been described, but to date, limited proof-of-concept data in animals are available. As discussed above, hexosaminidase deficiency leads to accumulation of GM2 and synuclein in Tay-Sachs disease and Sandhoff disease. The antimalarial DHFR inhibitor, pyrimethamine, was identified as a chaperone of hexosaminidase in a screen of a 1,000 compound library (75, 76). The compound binds to the active site of the enzyme and inhibits enzymatic activity in a pH-dependent manner. Because the compound is FDA-approved and also gains access to the CNS, it has been evaluated in 20 patients with Tay-Sachs or Sandhoff disease (77). In this study, the compound increased leukocyte hexosaminidase activity up to four times, however significant neurological side effects were evident at the higher dose used in this study. There is no indication of further development of the compound for this indication. A second screening campaign by this same group identified a naphthalimide and two other compounds with micromolar potency that acted as inhibitors and enhanced enzymatic activity in Tay-Sachs disease fibroblasts (78).
A series of iminosugars has been reported that are inhibitors and chaperones of β-hexosaminidase. In cell-based studies, NBn-LABNAc was shown to act as a chaperone and restore deficient enzyme activity (79). To date, little information is available on whether these can function as chaperones in vivo.
To summarize, the modulation of glycolipid metabolism offers a viable approach to treating neuronopathic disorders associated with synuclein accumulation. To date, the compounds that have been described show some utility in animal models; however, they either lack the ability to penetrate the CNS or have off-target effects that may counteract or limit their capabilities to mediate the desired pharmacological action. Selective inhibitors of glycosphingolipid biosynthesis and noninhibitory pharmacological chaperones of glycosphingolipid processing enzymes that gain access to the CNS provide novel approaches that may overcome some of the limitations to the compounds reported to date.
Acknowledgments
The authors thank Alison S. Hofer for help with the figures.
Footnotes
Abbreviations:
- a-syn
- α-synuclein
- ER
- endoplasmic reticulum
- GCase
- glucocerebrosidase
- GD
- Gaucher’s disease
- GlcCer
- glucosylceramide
- LSD
- lysosomal storage disorder
- NPC
- Niemann-Pick C
- PD
- Parkinson’s disease
- PDMP
- D-threo-1-phenyl-decanoylamino-3-morpholinepropanol
This work was supported by National Institutes of Health Grant R01NS076054 (to D.K.).
REFERENCES
- 1.Levine B., Kroemer G. 2008. Autophagy in the pathogenesis of disease. Cell. 132: 27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krainc D. 2010. Clearance of mutant proteins as a therapeutic target in neurodegenerative diseases. Arch. Neurol. 67: 388–392. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto A., Lucas J. J., Hen R. 2000. Reversal of neuropathology and motor dysfunction in a conditional model of Huntington’s disease. Cell. 101: 57–66. [DOI] [PubMed] [Google Scholar]
- 4.Zu T., Duvick L. A., Kaytor M. D., Berlinger M. S., Zoghbi H. Y., Clark H. B., Orr H. T. 2004. Recovery from polyglutamine-induced neurodegeneration in conditional SCA1 transgenic mice. J. Neurosci. 24: 8853–8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim Y., Kehm V. M., Lee E. B., Soper J. H., Li C., Trojanowski J. Q., Lee V. M. 2011. α-Syn suppression reverses synaptic and memory defects in a mouse model of dementia with Lewy bodies. J. Neurosci. 31: 10076–10087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devine M. J., Gwinn K., Singleton A., Hardy J. 2011. Parkinson’s disease and α-synuclein expression. Mov. Disord. 26: 2160–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellettato C. M., Scarpa M. 2010. Pathophysiology of neuropathic lysosomal storage disorders. J. Inherit. Metab. Dis. 33: 347–362. [DOI] [PubMed] [Google Scholar]
- 8.Shachar T., Lo Bianco C., Recchia A., Wiessner C., Raas-Rothschild A., Futerman A. H. 2011. Lysosomal storage disorders and Parkinson’s disease: Gaucher disease and beyond. Mov. Disord. 26: 1593–1604. [DOI] [PubMed] [Google Scholar]
- 9.Sack G. H., Jr 1980. Clinical diversity in Gaucher’s disease. Johns Hopkins Med. J. 146: 166–170. [PubMed] [Google Scholar]
- 10.Sidransky E. 2005. Gaucher disease and parkinsonism. Mol. Genet. Metab. 84: 302–304. [DOI] [PubMed] [Google Scholar]
- 11.Neudorfer O., Giladi N., Elstein D., Abrahamov A., Turezkite T., Aghai E., Reches A., Bembi B., Zimran A. 1996. Occurrence of Parkinson’s syndrome in type I Gaucher disease. QJM. 89: 691–694. [DOI] [PubMed] [Google Scholar]
- 12.Tayebi N., Walker J., Stubblefield B., Orvisky E., LaMarca M. E., Wong K., Rosenbaum H., Schiffmann R., Bembi B., Sidransky E. 2003. Gaucher disease with parkinsonian manifestations: does glucocerebrosidase deficiency contribute to a vulnerability to parkinsonism? Mol. Genet. Metab. 79: 104–109. [DOI] [PubMed] [Google Scholar]
- 13.Goker-Alpan O., Schiffmann R., LaMarca M. E., Nussbaum R. L., McInerney-Leo A., Sidransky E. 2004. Parkinsonism among Gaucher disease carriers. J. Med. Genet. 41: 937–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goker-Alpan O., Stubblefield B. K., Giasson B. I., Sidransky E. 2010. Glucocerebrosidase is present in alpha-synuclein inclusions in Lewy body disorders. Acta Neuropathol. 120: 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong K., Sidransky E., Verma A., Mixon T., Sandberg G. D., Wakefield L. K., Morrison A., Lwin A., Colegial C., Allman J. M., et al. 2004. Neuropathology provides clues to the pathophysiology of Gaucher disease. Mol. Genet. Metab. 82: 192–207. [DOI] [PubMed] [Google Scholar]
- 16.Sidransky E., Nalls M. A., Aasly J. O., Aharon-Peretz J., Annesi G., Barbosa E. R., Bar-Shira A., Berg D., Bras J., Brice A., et al. 2009. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N. Engl. J. Med. 361: 1651–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazzulli J. R., Xu Y. H., Sun Y., Knight A. L., McLean P. J., Caldwell G. A., Sidransky E., Grabowski G. A., Krainc D. 2011. Gaucher disease glucocerebrosidase and α-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell. 146: 37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gegg M. E., Burke D., Heales S. J., Cooper J. M., Hardy J., Wood N. W., Schapira A. H. 2012. Glucocerebrosidase deficiency in substantia nigra of Parkinson disease brains. Ann. Neurol. 72: 455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yap T. L., Velayati A., Sidransky E., Lee J. C. 2013. Membrane-bound α-synuclein interacts with glucocerebrosidase and inhibits enzyme activity. Mol. Genet. Metab. 108: 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cullen V., Sardi S. P., Ng J., Xu Y. H., Sun Y., Tomlinson J. J., Kolodziej P., Kahn I., Saftig P., Woulfe J., et al. 2011. Acid β-glucosidase mutants linked to Gaucher disease, Parkinson disease, and Lewy body dementia alter α-synuclein processing. Ann. Neurol. 69: 940–953. [DOI] [PubMed] [Google Scholar]
- 21.Lukina E., Watman N., Arreguin E. A., Banikazemi M., Dragosky M., Iastrebner M., Rosenbaum H., Phillips M., Pastores G. M., Rosenthal D. I., et al. 2010. A phase 2 study of eliglustat tartrate (Genz-112638), an oral substrate reduction therapy for Gaucher disease type. Blood. 116: 893–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lukina E., Watman N., Arreguin E. A., Dragosky M., Iastrebner M., Rosenbaum H., Phillips M., Pastores G. M., Kamath R. S., Rosenthal D. I., et al. 2010. Improvement in hematological, visceral, and skeletal manifestations of Gaucher disease type 1 with oral eliglustat tartrate (Genz-112638) treatment: 2-year results of a phase 2 study. Blood. 116: 4095–4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shayman J. A. 2010. Eliglustat tartrate: glucosylceramide synthase inhibitor for the treatment of type 1 Gaucher disease. Drugs Future. 35: 613–620. [PMC free article] [PubMed] [Google Scholar]
- 24.Nietupski J. B., Pacheco J. J., Chuang W. L., Maratea K., Li L., Foley J., Ashe K. M., Cooper C. G., Aerts J. M., Copeland D. P., et al. 2012. Iminosugar-based inhibitors of glucosylceramide synthase prolong survival but paradoxically increase brain glucosylceramide levels in Niemann-Pick C mice. Mol. Genet. Metab. 105: 621–628. [DOI] [PubMed] [Google Scholar]
- 25.Elstein D., Hollak C., Aerts J. M., van Weely S., Maas M., Cox T. M., Lachmann R. H., Hrebicek M., Platt F. M., Butters T. D., et al. 2004. Sustained therapeutic effects of oral miglustat (Zavesca, N-butyldeoxynojirimycin, OGT 918) in type 1 Gaucher disease. J. Inherit. Metab. Dis. 27: 757–766. [DOI] [PubMed] [Google Scholar]
- 26.Treiber A., Morand O., Clozel M. 2007. The pharmacokinetics and tissue distribution of the glucosylceramide synthase inhibitor miglustat in the rat. Xenobiotica. 37: 298–314. [DOI] [PubMed] [Google Scholar]
- 27.Cox T., Lachmann R., Hollak C., Aerts J., van Weely S., Hrebícek M., Platt F., Butters T., Dwek R., Moyses C., et al. 2000. Novel oral treatment of Gaucher’s disease with N-butyldeoxynojirimycin (OGT 918) to decrease substrate biosynthesis. Lancet. 355: 1481–1485. [DOI] [PubMed] [Google Scholar]
- 28.Schiffmann R., Fitzgibbon E. J., Harris C., DeVile C., Davies E. H., Abel L., van Schaik I. N., Benko W., Timmons M., Ries M., et al. 2008. Randomized, controlled trial of miglustat in Gaucher’s disease type 3. Ann. Neurol. 64: 514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larsen S. D., Wilson M. W., Abe A., Shu L., George C. H., Kirchhoff P., Showalter H. D., Xiang J., Keep R. F., Shayman J. A. 2012. Property-based design of a glucosylceramide synthase inhibitor that reduces glucosylceramide in the brain. J. Lipid Res. 53: 282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arthur J. R., Wilson M. W., Larsen S. D., Rockwell H. E., Shayman J. A., Seyfried T. N. 2013. Ethylenedioxy-PIP2 oxalate reduces ganglioside storage in juvenile Sandhoff disease mice. Neurochem. Res. 38: 866–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cabrera-Salazar M. A., Deriso M., Bercury S. D., Li L., Lydon J. T., Weber W., Pande N., Cromwell M. A., Copeland D., Leonard J., et al. 2012. Systemic delivery of a glucosylceramide synthase inhibitor reduces CNS substrates and increases lifespan in a mouse model of type 2 Gaucher disease. PLoS ONE. 7: e43310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richter F., Fleming S. M., Watson M., Lemesre V., Pelligrino L., Ranes B., Zhu C., Montazavi F., Mulligan C. K., Sioshansi P. C., et al. A GCase chaperone improves motor function in a mouse model of synucleinopathy. Neurotherapeutics. Epub ahead of print. July 20, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sardi S. P., Clarke J., Viel C., Chan M., Tamsett T. J., Treleaven C. M., Bu J., Sweet L., Passini M. A., Dodge J. C., et al. 2013. Augmenting CNS glucocerebrosidase as a therapeutic strategy for Parkinsonism and other Gaucher-related synucleinopathies. Proc. Natl. Acad. Sci. USA. 110: 3537–3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sardi S. P., Clarke J., Kinnecom C., Tamsett T. J., Li L., Stanek L. M., Passini M. A., Grabowski G. A., Schlossmacher M. G., Sidman R. L., et al. 2011. CNS expression of glucocerebrosidase corrects alpha-synuclein pathology and memory in a mouse model of Gaucher-related synucleinopathy. Proc. Natl. Acad. Sci. USA. 108: 12101–12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ridley C. M., Thur K. E., Shanahan J., Thillaiappan N. B., Shen A., Uhl K., Walden C. M., Rahim A. A., Waddington S. N., Platt F. M., et al. 2013. β-Glucosidase 2 (GBA2) activity and imino sugar pharmacology. J. Biol. Chem. 288: 26052–26066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abian O., Alfonso P., Velazquez-Campoy A., Giraldo P., Pocovi M., Sancho J. 2011. Therapeutic strategies for Gaucher disease: miglustat (NB-DNJ) as a pharmacological chaperone for glucocerebrosidase and the different thermostability of velaglucerase alfa and imiglucerase. Mol. Pharm. 8: 2390–2397. [DOI] [PubMed] [Google Scholar]
- 37.Alfonso P., Pampín S., Estrada J., Rodríguez-Rey J. C., Giraldo P., Sancho J., Pocoví M. 2005. Miglustat (NB-DNJ) works as a chaperone for mutated acid beta-glucosidase in cells transfected with several Gaucher disease mutations. Blood Cells Mol. Dis. 35: 268–276. [DOI] [PubMed] [Google Scholar]
- 38.Boomkamp S. D., Butters T. D. 2008. Glycosphingolipid disorders of the brain. Subcell. Biochem. 49: 441–467. [DOI] [PubMed] [Google Scholar]
- 39.Mukherjee S., Maxfield F. R. 2004. Lipid and cholesterol trafficking in NPC. Biochim. Biophys. Acta. 1685: 28–37. [DOI] [PubMed] [Google Scholar]
- 40.Kluenemann H. H., Nutt J. G., Davis M. Y., Bird T. D. 2013. Parkinsonism syndrome in heterozygotes for Niemann-Pick C1. J. Neurol. Sci. 335: 219–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davidson C. D., Ali N. F., Micsenyi M. C., Stephney G., Renault S., Dobrenis K., Ory D. S., Vanier M. T., Walkley S. U. 2009. Chronic cyclodextrin treatment of murine Niemann-Pick C disease ameliorates neuronal cholesterol and glycosphingolipid storage and disease progression. PLoS ONE. 4: e6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stein V. M., Crooks A., Ding W., Prociuk M., O’Donnell P., Bryan C., Sikora T., Dingemanse J., Vanier M. T., Walkley S. U., et al. 2012. Miglustat improves purkinje cell survival and alters microglial phenotype in feline Niemann-Pick disease type C. J. Neuropathol. Exp. Neurol. 71: 434–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fan M., Sidhu R., Fujiwara H., Tortelli B., Zhang J., Davidson C., Walkley S. U., Bagel J. H., Vite C., Yanjanin N. M., et al. 2013. Identification of Niemann-Pick C1 disease biomarkers through sphingolipid profiling. J. Lipid Res. 54: 2800–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chien Y. H., Lee N. C., Tsai L. K., Huang A. C., Peng S. F., Chen S. J., Hwu W. L. 2007. Treatment of Niemann-Pick disease type C in two children with miglustat: initial responses and maintenance of effects over 1 year. J. Inherit. Metab. Dis. 30: 826. [DOI] [PubMed] [Google Scholar]
- 45.Patterson M. C., Vecchio D., Prady H., Abel L., Wraith J. E. 2007. Miglustat for treatment of Niemann-Pick C disease: a randomised controlled study. Lancet Neurol. 6: 765–772. [DOI] [PubMed] [Google Scholar]
- 46.Chien Y. H., Peng S. F., Yang C. C., Lee N. C., Tsai L. K., Huang A. C., Su S. C., Tseng C. C., Hwu W. L. 2013. Long-term efficacy of miglustat in paediatric patients with Niemann-Pick disease type C. J. Inherit. Metab. Dis. 36: 129–137. [DOI] [PubMed] [Google Scholar]
- 47.Wennekes T., van den Berg R. J., Donker W., van der Marel G. A., Strijland A., Aerts J. M., Overkleeft H. S. 2007. Development of adamantan-1-yl-methoxy-functionalized 1-deoxynojirimycin derivatives as selective inhibitors of glucosylceramide metabolism in man. J. Org. Chem. 72: 1088–1097. [DOI] [PubMed] [Google Scholar]
- 48.Ashe K. M., Bangari D., Li L., Cabrera-Salazar M. A., Bercury S. D., Nietupski J. B., Cooper C. G., Aerts J. M., Lee E. R., Copeland D. P., et al. 2011. Iminosugar-based inhibitors of glucosylceramide synthase increase brain glycosphingolipids and survival in a mouse model of Sandhoff disease. PLoS ONE. 6: e21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fantini J., Yahi N. 2011. Molecular basis for the glycosphingolipid-binding specificity of α-synuclein: key role of tyrosine 39 in membrane insertion. J. Mol. Biol. 408: 654–669. [DOI] [PubMed] [Google Scholar]
- 50.Martinez Z., Zhu M., Han S., Fink A. L. 2007. GM1 specifically interacts with alpha-synuclein and inhibits fibrillation. Biochemistry. 46: 1868–1877. [DOI] [PubMed] [Google Scholar]
- 51.Jeyakumar M., Butters T. D., Cortina-Borja M., Hunnam V., Proia R. L., Perry V. H., Dwek R. A., Platt F. M. 1999. Delayed symptom onset and increased life expectancy in Sandhoff disease mice treated with N-butyldeoxynojirimycin. Proc. Natl. Acad. Sci. USA. 96: 6388–6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Platt F. M., Neises G. R., Reinkensmeier G., Townsend M. J., Perry V. H., Proia R. L., Winchester B., Dwek R. A., Butters T. D. 1997. Prevention of lysosomal storage in Tay-Sachs mice treated with N-butyldeoxynojirimycin. Science. 276: 428–431. [DOI] [PubMed] [Google Scholar]
- 53.Platt F. M., Jeyakumar M., Andersson U., Heare T., Dwek R. A., Butters T. D. 2003. Substrate reduction therapy in mouse models of glycosphingolipidosis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 358: 947–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wortmann S. B., Lefeber D. J., Dekomien G., Willemsen M. A., Wevers R. A., Morava E. 2009. Substrate deprivation therapy in juvenile Sandhoff disease. J. Inherit. Metab. Dis. 32(Suppl 1): S307–S311. [DOI] [PubMed] [Google Scholar]
- 55.Masciullo M., Santoro M., Modoni A., Ricci E., Guitton J., Tonali P., Silvestri G. 2010. Substrate reduction therapy with miglustat in chronic GM2 gangliosidosis type Sandhoff: results of a 3-year follow-up. J. Inherit. Metab. Dis. 33(Suppl 3): S355–S361. [DOI] [PubMed] [Google Scholar]
- 56.Shapiro B. E., Pastores G. M., Gianutsos J., Luzy C., Kolodny E. H. 2009. Miglustat in late-onset Tay-Sachs disease: a 12-month, randomized, controlled clinical study with 24 months of extended treatment. Genet. Med. 11: 425–433. [DOI] [PubMed] [Google Scholar]
- 57.Wu G., Lu Z. H., Kulkarni N., Ledeen R. W. 2012. Deficiency of ganglioside GM1 correlates with Parkinson’s disease in mice and humans. J. Neurosci. Res. 90: 1997–2008. [DOI] [PubMed] [Google Scholar]
- 58.Schneider J. S., Gollomp S. M., Sendek S., Colcher A., Cambi F., Du W. 2013. A randomized, controlled, delayed start trial of GM1 ganglioside in treated Parkinson’s disease patients. J. Neurol. Sci. 324: 140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wei J., Fujita M., Nakai M., Waragai M., Sekigawa A., Sugama S., Takenouchi T., Masliah E., Hashimoto M. 2009. Protective role of endogenous gangliosides for lysosomal pathology in a cellular model of synucleinopathies. Am. J. Pathol. 174: 1891–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wei J., Fujita M., Sekigawa A., Sekiyama K., Waragai M., Hashimoto M. 2009. Gangliosides’ protection against lysosomal pathology of synucleinopathies. Autophagy. 5: 860–861. [DOI] [PubMed] [Google Scholar]
- 61.Steet R. A., Chung S., Wustman B., Powe A., Do H., Kornfeld S. A. 2006. The iminosugar isofagomine increases the activity of N370S mutant acid beta-glucosidase in Gaucher fibroblasts by several mechanisms. Proc. Natl. Acad. Sci. USA. 103: 13813–13818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Steet R., Chung S., Lee W. S., Pine C. W., Do H., Kornfeld S. 2007. Selective action of the iminosugar isofagomine, a pharmacological chaperone for mutant forms of acid-beta-glucosidase. Biochem. Pharmacol. 73: 1376–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kornhaber G. J., Tropak M. B., Maegawa G. H., Tuske S. J., Coales S. J., Mahuran D. J., Hamuro Y. 2008. Isofagomine induced stabilization of glucocerebrosidase. ChemBioChem. 9: 2643–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lieberman R. L., D’aquino J. A., Ringe D., Petsko G. A. 2009. Effects of pH and iminosugar pharmacological chaperones on lysosomal glycosidase structure and stability. Biochemistry. 48: 4816–4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khanna R., Benjamin E. R., Pellegrino L., Schilling A., Rigat B. A., Soska R., Nafar H., Ranes B. E., Feng J., Lun Y., et al. 2010. The pharmacological chaperone isofagomine increases the activity of the Gaucher disease L444P mutant form of beta-glucosidase. FEBS J. 277: 1618–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun Y., Liou B., Xu Y. H., Quinn B., Zhang W., Hamler R., Setchell K. D., Grabowski G. A. 2012. Ex vivo and in vivo effects of isofagomine on acid β-glucosidase variants and substrate levels in Gaucher disease. J. Biol. Chem. 287: 4275–4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun Y., Ran H., Liou B., Quinn B., Zamzow M., Zhang W., Bielawski J., Kitatani K., Setchell K. D., Hannun Y. A., et al. 2011. Isofagomine in vivo effects in a neuronopathic Gaucher disease mouse. PLoS ONE. 6: e19037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maegawa G. H., Tropak M. B., Buttner J. D., Rigat B. A., Fuller M., Pandit D., Tang L., Kornhaber G. J., Hamuro Y., Clarke J. T., et al. 2009. Identification and characterization of ambroxol as an enzyme enhancement agent for Gaucher disease. J. Biol. Chem. 284: 23502–23516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bendikov-Bar I., Ron I., Filocamo M., Horowitz M. 2011. Characterization of the ERAD process of the L444P mutant glucocerebrosidase variant. Blood Cells Mol. Dis. 46: 4–10. [DOI] [PubMed] [Google Scholar]
- 70.Bendikov-Bar I., Maor G., Filocamo M., Horowitz M. 2013. Ambroxol as a pharmacological chaperone for mutant glucocerebrosidase. Blood Cells Mol. Dis. 50: 141–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suzuki T., Shimoda M., Ito K., Hanai S., Aizawa H., Kato T., Kawasaki K., Yamaguchi T., Ryoo H. D., Goto-Inoue N., et al. 2013. Expression of human Gaucher disease gene GBA generates neurodevelopmental defects and ER stress in Drosophila eye. PLoS ONE. 8: e69147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zimran A., Altarescu G., Elstein D. 2013. Pilot study using ambroxol as a pharmacological chaperone in type 1 Gaucher disease. Blood Cells Mol. Dis. 50: 134–137. [DOI] [PubMed] [Google Scholar]
- 73.Goldin E., Zheng W., Motabar O., Southall N., Choi J. H., Marugan J., Austin C. P., Sidransky E. 2012. High throughput screening for small molecule therapy for Gaucher disease using patient tissue as the source of mutant glucocerebrosidase. PLoS ONE. 7: e29861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Patnaik S., Zheng W., Choi J. H., Motabar O., Southall N., Westbroek W., Lea W. A., Velayati A., Goldin E., Sidransky E., et al. 2012. Discovery, structure-activity relationship, and biological evaluation of noninhibitory small molecule chaperones of glucocerebrosidase. J. Med. Chem. 55: 5734–5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maegawa G. H., Tropak M., Buttner J., Stockley T., Kok F., Clarke J. T., Mahuran D. J. 2007. Pyrimethamine as a potential pharmacological chaperone for late-onset forms of GM2 gangliosidosis. J. Biol. Chem. 282: 9150–9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Osher E., Fattal-Valevski A., Sagie L., Urshanski N., Amir-Levi Y., Katzburg S., Peleg L., Lerman-Sagie T., Zimran A., Elstein D., et al. 2011. Pyrimethamine increases β-hexosaminidase A activity in patients with late onset Tay Sachs. Mol. Genet. Metab. 102: 356–363. [DOI] [PubMed] [Google Scholar]
- 77.Clarke J. T., Mahuran D. J., Sathe S., Kolodny E. H., Rigat B. A., Raiman J. A., Tropak M. B. 2011. An open-label phase I/II clinical trial of pyrimethamine for the treatment of patients affected with chronic GM2 gangliosidosis (Tay-Sachs or Sandhoff variants). Mol. Genet. Metab. 102: 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tropak M. B., Blanchard J. E., Withers S. G., Brown E. D., Mahuran D. 2007. High-throughput screening for human lysosomal beta-N-acetyl hexosaminidase inhibitors acting as pharmacological chaperones. Chem. Biol. 14: 153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rountree J. S., Butters T. D., Wormald M. R., Boomkamp S. D., Dwek R. A., Asano N., Ikeda K., Evinson E. L., Nash R. J., Fleet G. W. 2009. Design, synthesis, and biological evaluation of enantiomeric beta-N-acetylhexosaminidase inhibitors LABNAc and DABNAc as potential agents against Tay-Sachs and Sandhoff disease. ChemMedChem. 4: 378–392. [DOI] [PubMed] [Google Scholar]