Abstract
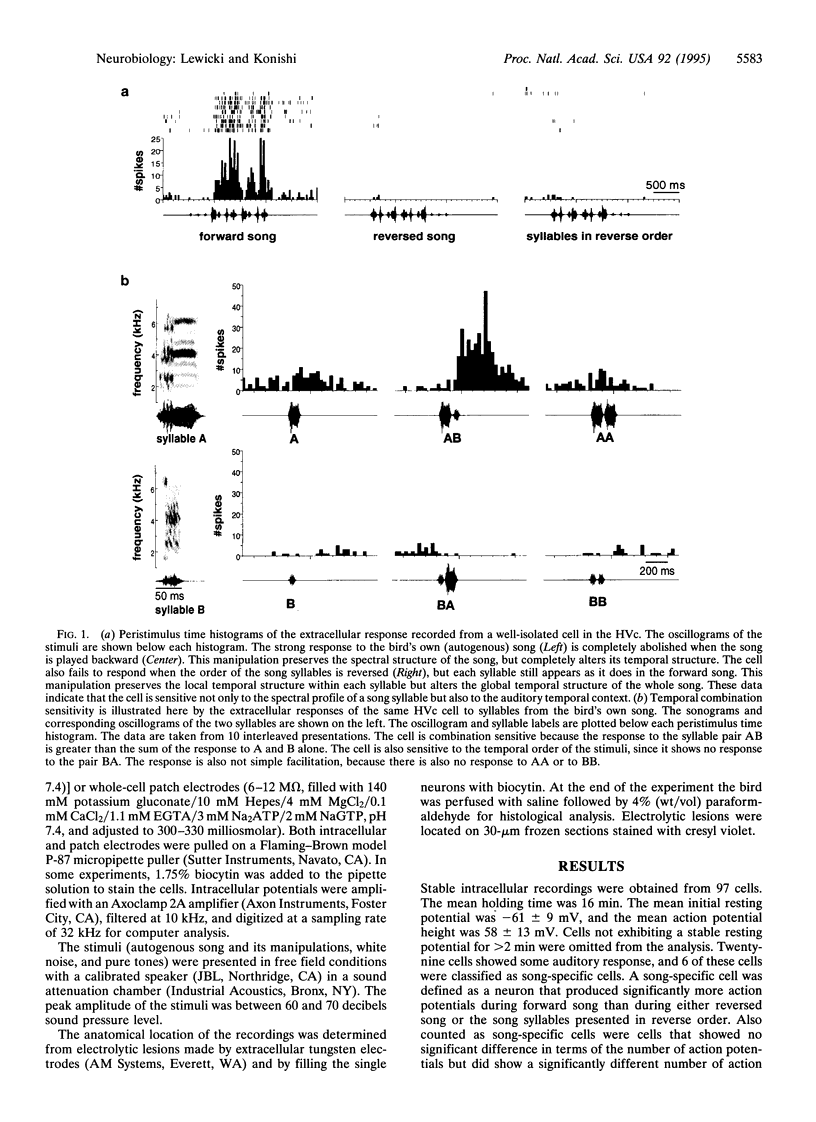
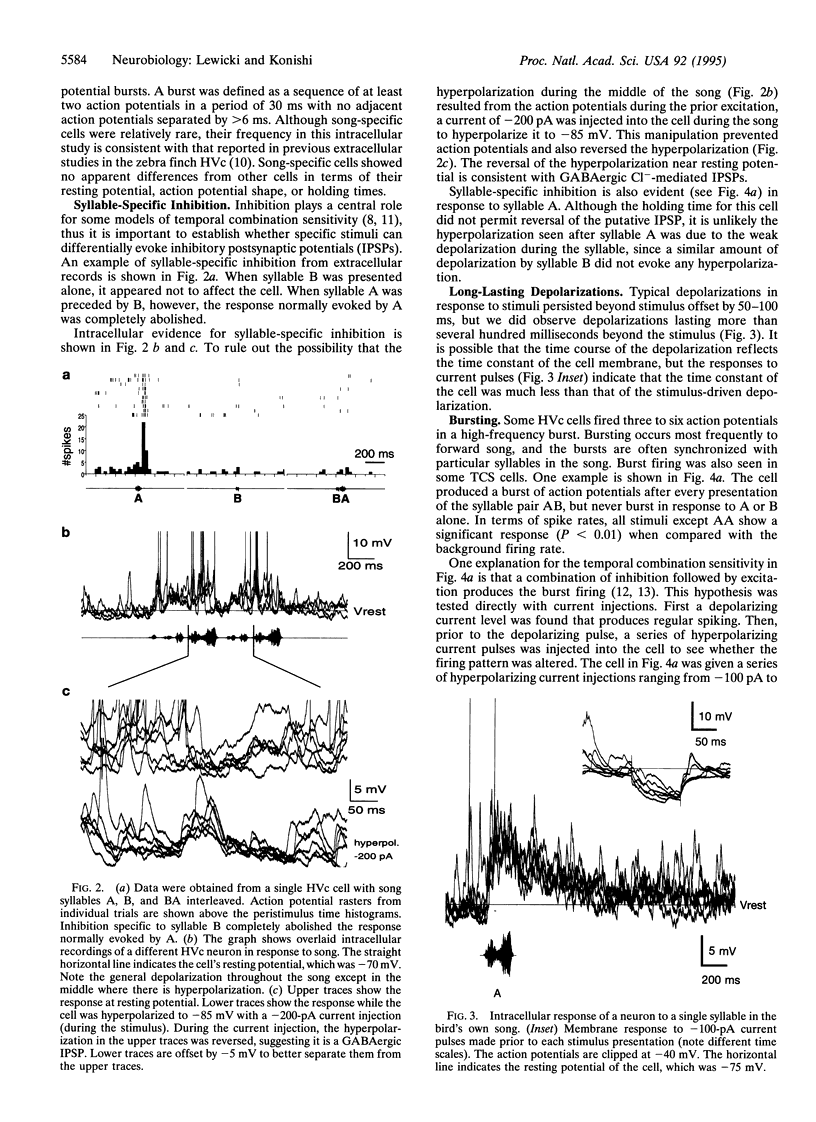
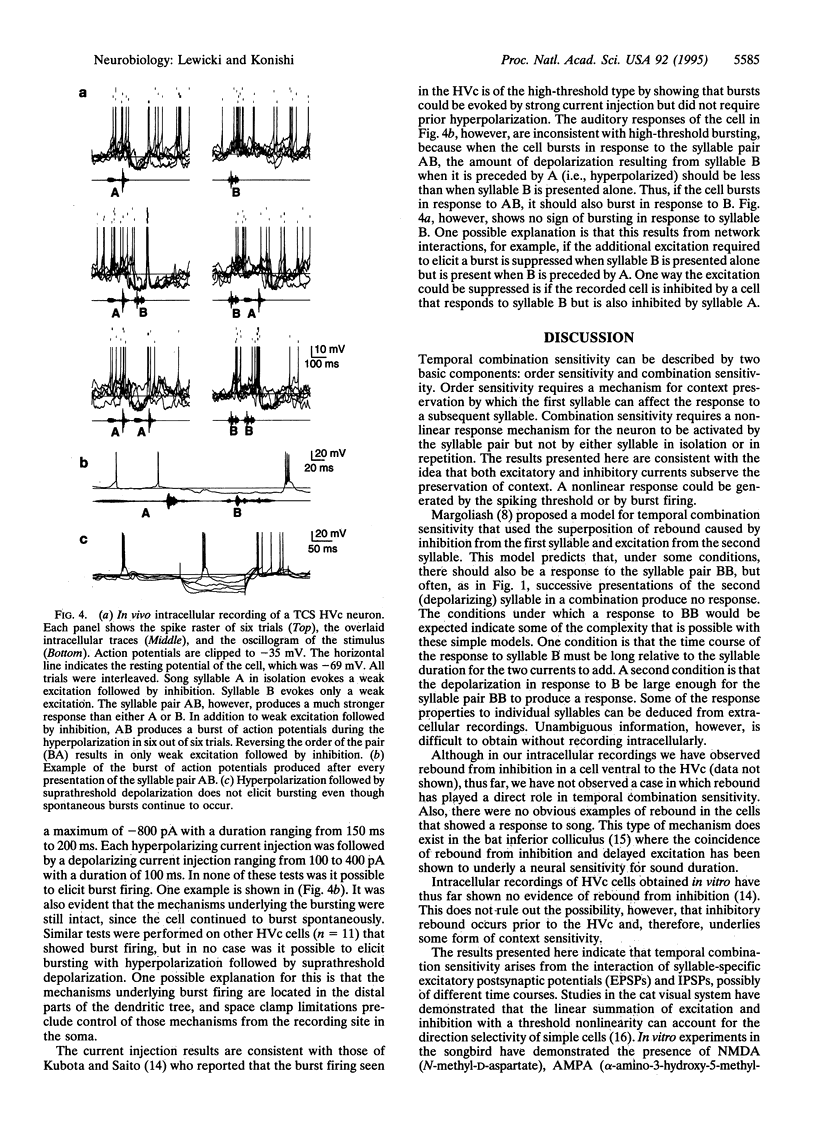
Neurons in the songbird forebrain area HVc (hyperstriatum ventrale pars caudale or high vocal center) are sensitive to the temporal structure of the bird's own song and are capable of integrating auditory information over a period of several hundred milliseconds. Extracellular studies have shown that the responses of some HVc neurons depend on the combination and temporal order of syllables from the bird's own song, but little is known about the mechanisms underlying these response properties. To investigate these mechanisms, we recorded intracellular responses to a set of auditory stimuli designed to assess the degree of dependence of the responses on temporal context. This report provides evidence that HVc neurons encode information about temporal structure by using a variety of mechanisms including syllable-specific inhibition, excitatory postsynaptic potentials with a range of different time courses, and burst-firing nonlinearity. The data suggest that the sensitivity of HVc neurons to temporal combinations of syllables results from the interactions of several cells and does not arise in a single step from afferent inputs alone.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Casseday J. H., Ehrlich D., Covey E. Neural tuning for sound duration: role of inhibitory mechanisms in the inferior colliculus. Science. 1994 May 6;264(5160):847–850. doi: 10.1126/science.8171341. [DOI] [PubMed] [Google Scholar]
- Glass I., Wollberg Z. Auditory cortex responses to sequences of normal and reversed squirrel monkey vocalizations. Brain Behav Evol. 1983;22(1):13–21. doi: 10.1159/000121503. [DOI] [PubMed] [Google Scholar]
- Jagadeesh B., Wheat H. S., Ferster D. Linearity of summation of synaptic potentials underlying direction selectivity in simple cells of the cat visual cortex. Science. 1993 Dec 17;262(5141):1901–1904. doi: 10.1126/science.8266083. [DOI] [PubMed] [Google Scholar]
- Jahnsen H., Llinás R. Electrophysiological properties of guinea-pig thalamic neurones: an in vitro study. J Physiol. 1984 Apr;349:205–226. doi: 10.1113/jphysiol.1984.sp015153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota M., Saito N. Sodium- and calcium-dependent conductances of neurones in the zebra finch hyperstriatum ventrale pars caudale in vitro. J Physiol. 1991;440:131–142. doi: 10.1113/jphysiol.1991.sp018700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margoliash D. Acoustic parameters underlying the responses of song-specific neurons in the white-crowned sparrow. J Neurosci. 1983 May;3(5):1039–1057. doi: 10.1523/JNEUROSCI.03-05-01039.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margoliash D., Fortune E. S. Temporal and harmonic combination-sensitive neurons in the zebra finch's HVc. J Neurosci. 1992 Nov;12(11):4309–4326. doi: 10.1523/JNEUROSCI.12-11-04309.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margoliash D. Preference for autogenous song by auditory neurons in a song system nucleus of the white-crowned sparrow. J Neurosci. 1986 Jun;6(6):1643–1661. doi: 10.1523/JNEUROSCI.06-06-01643.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCasland J. S., Konishi M. Interaction between auditory and motor activities in an avian song control nucleus. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7815–7819. doi: 10.1073/pnas.78.12.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna T. M., Weinberger N. M., Diamond D. M. Responses of single auditory cortical neurons to tone sequences. Brain Res. 1989 Feb 27;481(1):142–153. doi: 10.1016/0006-8993(89)90494-0. [DOI] [PubMed] [Google Scholar]
- Newman J. D., Wollberg Z. Multiple coding of species-specific vocalizations in the auditory cortex of squirrel monkeys. Brain Res. 1973 May 17;54:287–304. doi: 10.1016/0006-8993(73)90050-4. [DOI] [PubMed] [Google Scholar]
- Steriade M., Deschenes M. The thalamus as a neuronal oscillator. Brain Res. 1984 Nov;320(1):1–63. doi: 10.1016/0165-0173(84)90017-1. [DOI] [PubMed] [Google Scholar]
- Volman S. F. Development of neural selectivity for birdsong during vocal learning. J Neurosci. 1993 Nov;13(11):4737–4747. doi: 10.1523/JNEUROSCI.13-11-04737.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollberg Z., Newman J. D. Auditory cortex of squirrel monkey: response patterns of single cells to species-specific vocalizations. Science. 1972 Jan 14;175(4018):212–214. doi: 10.1126/science.175.4018.212. [DOI] [PubMed] [Google Scholar]