Abstract
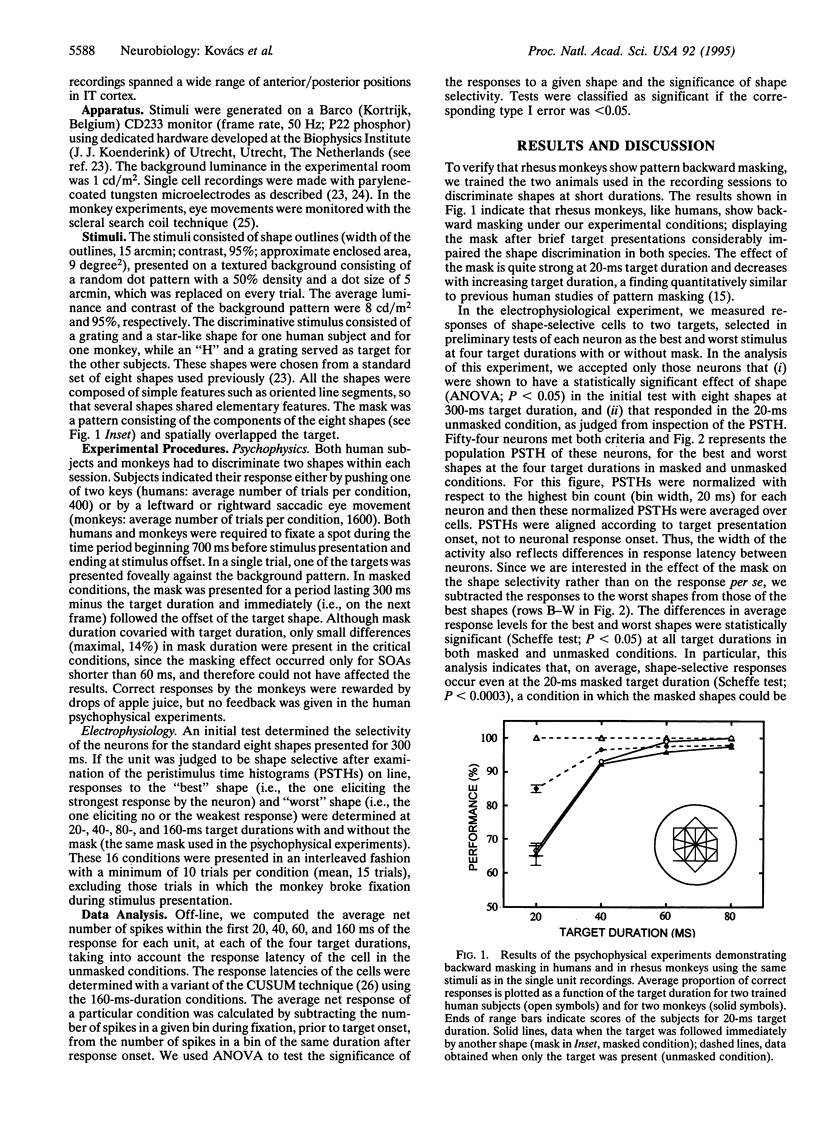
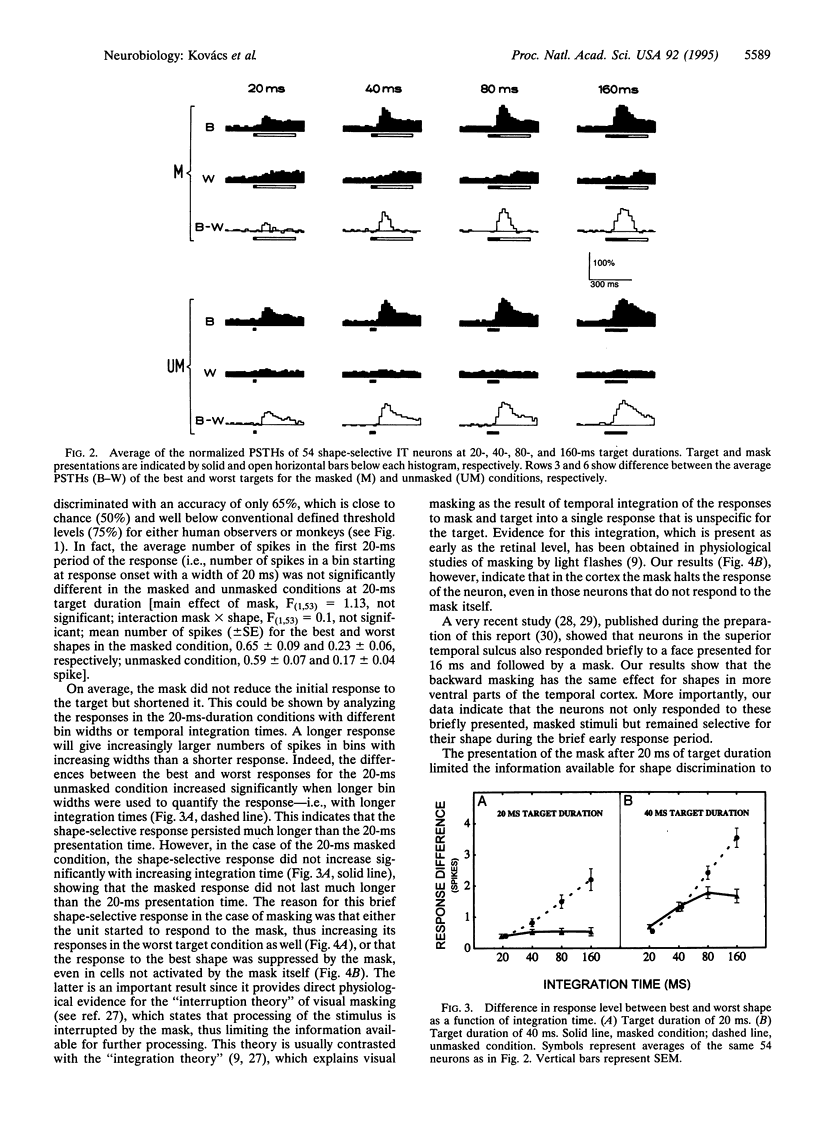
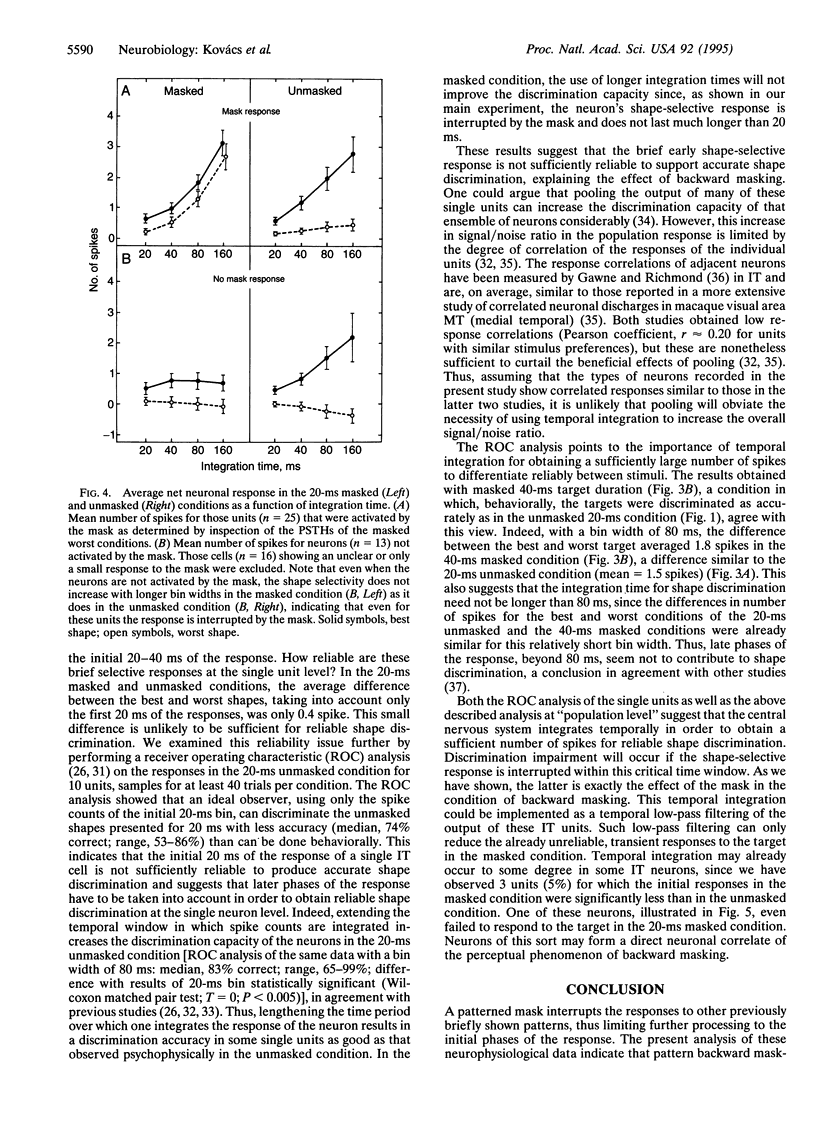
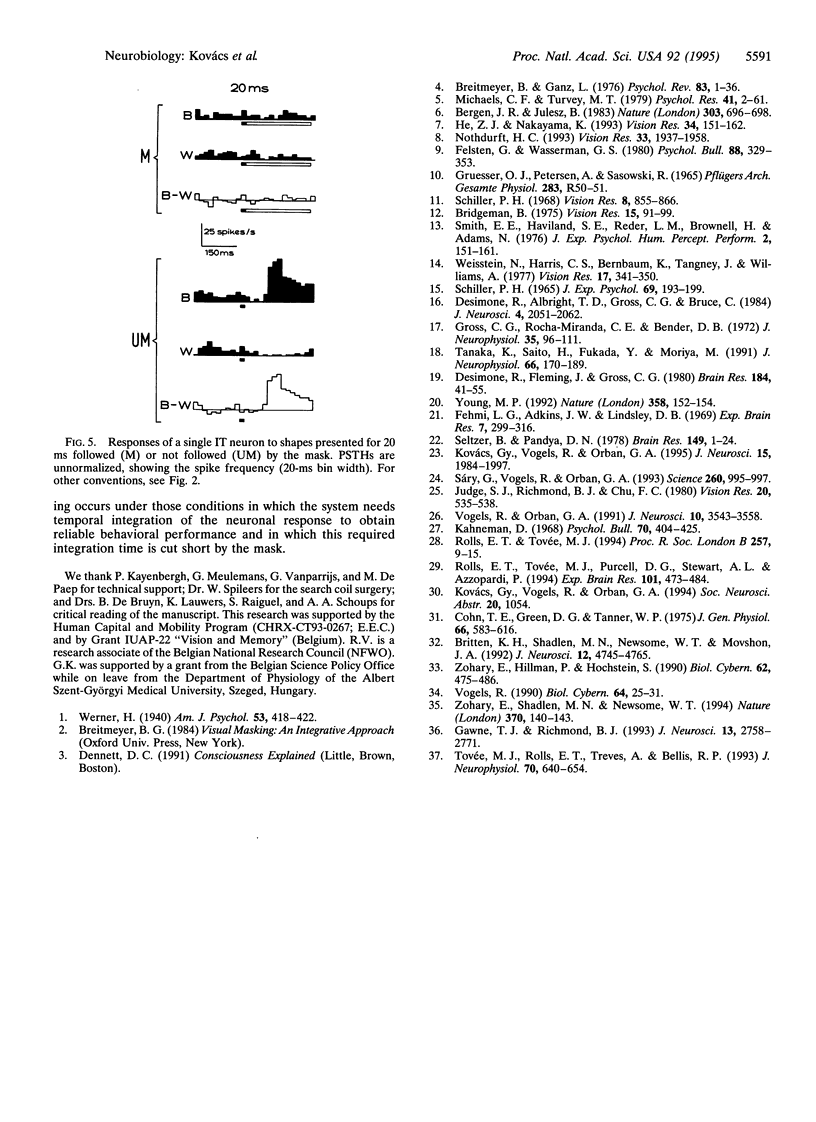
The perception of a briefly presented shape is strongly impaired when it is followed by another pattern, a phenomenon called backward masking. We found that the vast majority of a sample of shape-selective neurons in the macaque inferior temporal cortex respond selectively to backward-masked shapes, although these shapes could not be discriminated by human and monkey subjects. However, this selective response was brief, since it was either interrupted by the mask or overridden by a response to the mask itself. We show that reliable discrimination of briefly presented shapes by single neurons depends on the temporal integration of the response. Presentation of the mask, however, reduces the number of spikes available for integration, explaining backward masking. These results also provide direct neurophysiological evidence for the "interruption theory" of backward masking.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergen J. R., Julesz B. Parallel versus serial processing in rapid pattern discrimination. Nature. 1983 Jun 23;303(5919):696–698. doi: 10.1038/303696a0. [DOI] [PubMed] [Google Scholar]
- Breitmeyer B. G., Ganz L. Implications of sustained and transient channels for theories of visual pattern masking, saccadic suppression, and information processing. Psychol Rev. 1976 Jan;83(1):1–36. [PubMed] [Google Scholar]
- Bridgeman B. Correlates of metacontrast in single cells of the cat visual system. Vision Res. 1975 Jan;15(1):91–99. doi: 10.1016/0042-6989(75)90065-6. [DOI] [PubMed] [Google Scholar]
- Britten K. H., Shadlen M. N., Newsome W. T., Movshon J. A. The analysis of visual motion: a comparison of neuronal and psychophysical performance. J Neurosci. 1992 Dec;12(12):4745–4765. doi: 10.1523/JNEUROSCI.12-12-04745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn T. E., Green D. G., Tanner W. P., Jr Receiver operating characteristic analysis. Application to the study of quantum fluctuation effects in optic nerve of Rana pipiens. J Gen Physiol. 1975 Nov;66(5):583–616. doi: 10.1085/jgp.66.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R., Albright T. D., Gross C. G., Bruce C. Stimulus-selective properties of inferior temporal neurons in the macaque. J Neurosci. 1984 Aug;4(8):2051–2062. doi: 10.1523/JNEUROSCI.04-08-02051.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R., Fleming J., Gross C. G. Prestriate afferents to inferior temporal cortex: an HRP study. Brain Res. 1980 Feb 17;184(1):41–55. doi: 10.1016/0006-8993(80)90586-7. [DOI] [PubMed] [Google Scholar]
- Fehmi L. G., Adkins J. W., Lindsley D. B. Electrophysiological correlates of visual perceptual masking in monkeys. Exp Brain Res. 1969;7(4):299–316. doi: 10.1007/BF00237318. [DOI] [PubMed] [Google Scholar]
- Felsten G., Wasserman G. S. Visual masking: mechanisms and theories. Psychol Bull. 1980 Sep;88(2):329–353. [PubMed] [Google Scholar]
- Gawne T. J., Richmond B. J. How independent are the messages carried by adjacent inferior temporal cortical neurons? J Neurosci. 1993 Jul;13(7):2758–2771. doi: 10.1523/JNEUROSCI.13-07-02758.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C. G., Rocha-Miranda C. E., Bender D. B. Visual properties of neurons in inferotemporal cortex of the Macaque. J Neurophysiol. 1972 Jan;35(1):96–111. doi: 10.1152/jn.1972.35.1.96. [DOI] [PubMed] [Google Scholar]
- He Z. J., Nakayama K. Perceiving textures: beyond filtering. Vision Res. 1994 Jan;34(2):151–162. doi: 10.1016/0042-6989(94)90328-x. [DOI] [PubMed] [Google Scholar]
- Judge S. J., Richmond B. J., Chu F. C. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res. 1980;20(6):535–538. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- Kahneman D. Method, findings, and theory in studies of visual masking. Psychol Bull. 1968 Dec;70(6):404–425. doi: 10.1037/h0026731. [DOI] [PubMed] [Google Scholar]
- Kovács G., Vogels R., Orban G. A. Selectivity of macaque inferior temporal neurons for partially occluded shapes. J Neurosci. 1995 Mar;15(3 Pt 1):1984–1997. doi: 10.1523/JNEUROSCI.15-03-01984.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels C. F., Turvey M. T. Central sources of visual masking: indexing structures supporting seeing at a single, brief glance. Psychol Res. 1979;41(1):2–61. doi: 10.1007/BF00309423. [DOI] [PubMed] [Google Scholar]
- Nothdurft H. C. The role of features in preattentive vision: comparison of orientation, motion and color cues. Vision Res. 1993 Sep;33(14):1937–1958. doi: 10.1016/0042-6989(93)90020-w. [DOI] [PubMed] [Google Scholar]
- Rolls E. T., Tovee M. J. Processing speed in the cerebral cortex and the neurophysiology of visual masking. Proc Biol Sci. 1994 Jul 22;257(1348):9–15. doi: 10.1098/rspb.1994.0087. [DOI] [PubMed] [Google Scholar]
- Rolls E. T., Tovee M. J., Purcell D. G., Stewart A. L., Azzopardi P. The responses of neurons in the temporal cortex of primates, and face identification and detection. Exp Brain Res. 1994;101(3):473–484. doi: 10.1007/BF00227340. [DOI] [PubMed] [Google Scholar]
- SCHILLER P. H. MONOPTIC AND DICHOPTIC VISUAL MASKING BY PATTERNS AND FLASHES. J Exp Psychol. 1965 Feb;69:193–199. doi: 10.1037/h0021574. [DOI] [PubMed] [Google Scholar]
- Schiller P. H. Single unit analysis of backward visual masking and metacontrast in the cat lateral geniculate nucleus. Vision Res. 1968 Jul;8(7):855–866. doi: 10.1016/0042-6989(68)90135-1. [DOI] [PubMed] [Google Scholar]
- Seltzer B., Pandya D. N. Afferent cortical connections and architectonics of the superior temporal sulcus and surrounding cortex in the rhesus monkey. Brain Res. 1978 Jun 23;149(1):1–24. doi: 10.1016/0006-8993(78)90584-x. [DOI] [PubMed] [Google Scholar]
- Smith E. E., Haviland S. E., Reder L. M., Brownell H. When preparation fails: disruptive effects of prior information on perceptual recognition. J Exp Psychol Hum Percept Perform. 1976 May;2(2):151–161. doi: 10.1037//0096-1523.2.2.151. [DOI] [PubMed] [Google Scholar]
- Sáry G., Vogels R., Orban G. A. Cue-invariant shape selectivity of macaque inferior temporal neurons. Science. 1993 May 14;260(5110):995–997. doi: 10.1126/science.8493538. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Saito H., Fukada Y., Moriya M. Coding visual images of objects in the inferotemporal cortex of the macaque monkey. J Neurophysiol. 1991 Jul;66(1):170–189. doi: 10.1152/jn.1991.66.1.170. [DOI] [PubMed] [Google Scholar]
- Tovée M. J., Rolls E. T., Treves A., Bellis R. P. Information encoding and the responses of single neurons in the primate temporal visual cortex. J Neurophysiol. 1993 Aug;70(2):640–654. doi: 10.1152/jn.1993.70.2.640. [DOI] [PubMed] [Google Scholar]
- Vogels R., Orban G. A. How well do response changes of striate neurons signal differences in orientation: a study in the discriminating monkey. J Neurosci. 1990 Nov;10(11):3543–3558. doi: 10.1523/JNEUROSCI.10-11-03543.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels R. Population coding of stimulus orientation by striate cortical cells. Biol Cybern. 1990;64(1):25–31. doi: 10.1007/BF00203627. [DOI] [PubMed] [Google Scholar]
- Weisstein N., Harris C. S., Berbaum K., Tangney J., Williams A. Contrast reduction by small localized stimuli: extensive spatial spread of above-threshold orientation-selective masking. Vision Res. 1977;17(3):341–350. doi: 10.1016/0042-6989(77)90022-0. [DOI] [PubMed] [Google Scholar]
- Young M. P. Objective analysis of the topological organization of the primate cortical visual system. Nature. 1992 Jul 9;358(6382):152–155. doi: 10.1038/358152a0. [DOI] [PubMed] [Google Scholar]
- Zohary E., Hillman P., Hochstein S. Time course of perceptual discrimination and single neuron reliability. Biol Cybern. 1990;62(6):475–486. doi: 10.1007/BF00205109. [DOI] [PubMed] [Google Scholar]
- Zohary E., Shadlen M. N., Newsome W. T. Correlated neuronal discharge rate and its implications for psychophysical performance. Nature. 1994 Jul 14;370(6485):140–143. doi: 10.1038/370140a0. [DOI] [PubMed] [Google Scholar]


