Summary
Complex and diverse communities of bacteria establish mutualistic and symbiotic relationships with the gut after birth. The intestinal immune system responds to bacterial colonization by acquiring a state of hypo-responsiveness against commensals and active readiness against pathogens. The resulting homeostatic balance involves a continuous dialog between the microbiota and lymphocytes with the intermediation of epithelial and dendritic cells. This dialog causes massive production of immunoglobulin A (IgA), a non-inflammatory antibody specialized in mucosal protection. Here, we discuss recent advances on the regulation of intestinal IgA responses and their role in host-microbe interaction.
Keywords: mucosal immunity, immunoglobulin, class switching, B cells, T cells, dendritic cells, epithelial cells
Introduction
The gut mucosa is a dynamic interface encompassing an epithelial monolayer that separates the local immune system from diverse communities of commensal bacteria. This microbiota confers defensive and metabolic capabilities to the intestinal mucosa by competing with pathogens, breaking down otherwise indigestible food components, and generating essential vitamins (1). Commensals also stimulate the growth of intestinal epithelial cells (IECs) and enhance the development of the local immune system (1). To maintain a peaceful bacteria-host interaction, the gut mucosa releases anti-microbial proteins and immunoglobulin A (IgA), an antibody isotype specialized in mucosal protection (2, 3).
Anti-microbial proteins and IgA constrain the topography, composition, and pro-inflammatory activity of commensal bacteria (4). This protective activity involves the binding of both anti-microbial proteins and IgA to a mucus layer that separates commensal bacteria from the apical surface of IECs (5). The building block of intestinal mucus is MUC2, a gutspecific gel-forming mucin secreted by goblet cells (5). Besides providing glycan-dependent anchoring sites and nutrients to the microbiota (5), MUC2 helps the gut immune system to generate homeostasis (6).
Intestinal homeostasis is characterized by a state of hypo-responsiveness against commensals and active readiness against pathogens and involves an intimate interplay of the microbiota with IECs as well as dendritic cells (DCs) of the innate immune system (7). By using microbial sensors such as Toll-like receptors (TLRs), IECs and DCs orchestrate tonic non-inflammatory immune responses that involve massive generation of IgA by B cells of the adaptive immune system. This review discusses the regulation of IgA production and how IgA controls host-microbe interactions.
Function of intestinal IgA
IgA is the most abundant antibody in mucosal secretions (3, 8). In the intestine, monomeric IgA interacts with a small plasma cell-derived polypeptide termed joining (J) chain to form IgA dimers that recognize polymeric immunoglobulin receptor (pIgR) on the basolateral surface of mucosal IECs (9-11). By shuttling IgA dimers across IECs through a complex process called transcytosis, pIgR facilitates the release of secretory IgA (SIgA) onto the surface of the gut (12). The resulting, SIgA includes a pIgR-derived polypeptide termed secretory component (SC) that increases the stability of SIgA in the intestinal lumen and anchors SIgA to mucus (13-15).
SIgA favors both maintenance of non-invasive commensal bacteria and neutralization of invasive pathogens through multiple mechanisms (12, 16). By using the antigen-binding variable (V) region of IgA, SIgA specifically blocks certain bacterial epitopes to prevent the adhesion of commensal bacteria with the apical surface of IECs (12). In addition, SIgA limits the microbial motility by non-specifically binding bacteria through glycans associated with the SC and constant region α (Cα) of IgA (12). Besides neutralizing pathogens in the intestinal lumen, SIgA can intercept microbes and toxins inside IECs (12). Of note, SIgA delivers these protective functions without activating the complement cascade (12, 17), thus impeding inflammatory damage to the epithelial barrier.
Origin and reactivity of intestinal IgA
Intestinal SIgA originates from B cells undergoing somatic hypermutation (SHM) and class switch recombination (CSR) in the germinal center (GC) of gut-associated lymphoid follicles (18). SHM and CSR require activation-induced cytidine deaminase (AID), a B-cell-specific enzyme highly expressed in the GC (19). SHM introduces point mutations in the recombined V(D)J exons that encode the antigen-binding V regions of Igs (20). This process generates structural changes that promote the selection of B cells expressing high-affinity Ig variants by antigen (21). In contrast, CSR alters the effector function of Igs without changing their antigen specificity by replacing Cμ and Cδ exons encoding IgM and IgD (two antibody isotypes expressed by naïve B cells) with Cγ, Cα, or Cε exons encoding IgG, IgA, and IgE, respectively (22).
Intestinal B cells undergo class switching to IgA and affinity maturation within organized follicular structures associated with the gut-associated lymphoid tissue (GALT) (18). Affinity matured and IgA class-switched B cells emerging from intestinal follicles enter the general circulation and then home to the intestinal lamina propria (LP), an effector site that fosters the differentiation of IgA-secreting plasma cells (18). These plasma cells cooperate with IECs to release SIgA onto the mucosal surface (23, 24).
Recent evidence indicates that IgA-secreting plasma cells arise from either newly activated naïve B cells or previously selected memory B cells that become re-activated by antigen (24). In general, the intestinal IgA repertoire is comprised of high-frequency clones, which probably recognize highly prevalent and stable components of our microbiota, and low-frequency clones, which may reflect adaptive adjustments to minor changes in the microbiota or exposure to pathogens (24). After postnatal gut colonization by bacteria, the gut IgA repertoire becomes progressively more diverse through the introduction of additional mutations in highly expanded B-cell clones and the generation of new mutated B-cell clones (25). Commensals likely provide some of the signals required for the induction of mutated plasma cells (26). However, the nature of these bacteria remains unclear (26). Remarkably, commensals not only shape the reactivity of gut B cells, but may also provide checkpoint signals to remove autoreactivity form systemic B cells (27).
Intestinal IgA inductive sites
Two functional compartments known as inductive and effector sites characterize the geography of IgA responses in the intestine (17). Highly organized follicular structures termed Peyer’s patches (PPs) develop in the small intestine during fetal life independently of gut colonization by bacteria and constitute the most prominent IgA inductive site in the GALT (18). Additional organized structures called isolated lymphoid follicles (ILFs) develop after birth in both small and large intestinal segments in response to bacteria (18). Unlike systemic lymph nodes, PPs and ILFs lack afferent lymphatic vessels and therefore receive antigen directly from the epithelial surface via antigen-transporting DCs (18, 28). Further IgA responses occur in the follicles of mesenteric lymph nodes (MLNs), which develop during fetal life and receive antigens from PPs via afferent lymphatics (18). While PPs, ILFs, and MLNs function as IgA inductive sites, the intestinal LP mainly serves as an IgA effector site that supports the differentiation and survival of IgA-secreting plasmablasts and plasma cells (18).
Structure of PPs and ILFs
PPs consist of large structures built on a stromal scaffold composed of several follicles that include B cells separated by interfollicular areas containing CD4+ T cells and DCs (18). The formation of PPs involves coordinated interactions between non-hematopoietic stromal cells and bone marrow-derived lymphoid tissue-inducing (LTi) cells expressing the transcription factors Id2 and retinoic acidrelated orphan receptor γt (RORγt) (29). These LTi cells belong to a heterogeneous family of group-3 RORγt+ innate lymphoid cells (ILCs) that regulate lymphoid organogenesis, homeostasis, and immunity (30). Interactions between stromal cells and LTi cells involve bidirectional signals that include lymphotoxin (LT) and tumor necrosis factor (TNF) from ILCs and interleukin-7 (IL-7) from stromal cells (31).
After colonization with bacteria, PPs become larger and form GCs, which foster the cognate interaction of B cells with CD4+ T cells (18). In the adult life, follicles from PPs always show prominent GCs due to the continuous stimulation of B cells by luminal antigens (18). These antigens are transported from the subepithelial dome to the follicles of PPs by DCs that receive antigen from specialized microfold (M) cells positioned in the follicle-associated epithelium (32). By constantly supplying antigen, this process permits PPs to generate a massive and diverse repertoire of high-affinity IgA (17).
ILFs consist of solitary B-cell clusters built on a scaffold of stromal cells with a few interspersed CD4+ T cells and more abundant perifollicular DCs (18). Scattered throughout the intestine, ILFs develop from the anlagen of cryptopatches in response to postnatal exposure to bacteria (18). This anlagen includes RORγt+ ILCs that guide the formation of ILFs by establishing an extensive crosstalk with stromal cells as well as DCs and B cells through LT and TNF (33).
Intestinal IgA responses through the TD pathway
Intestinal IgA production mostly occurs in the follicles of PPs and MLNs through a T-cell-dependent (TD) pathway (3, 17, 18). This pathway involves the activation of follicular B cells by T-follicular helper (Tfh) cells and the generation of IgA-secreting plasma cells that seed the LP along the whole length of the intestine (Fig. 1). While IgA responses to the microbiota may be multi-centered and highly diverse, IgA responses to simple protein antigens appear to be highly synchronized and involve the reutilization of pre-existing GCs in multiple PPs (34). This process leads to the generation of extensive clonal relationships in the pools of B cells and plasma cells that occupy PPs and the gut LP, respectively (24, 25).
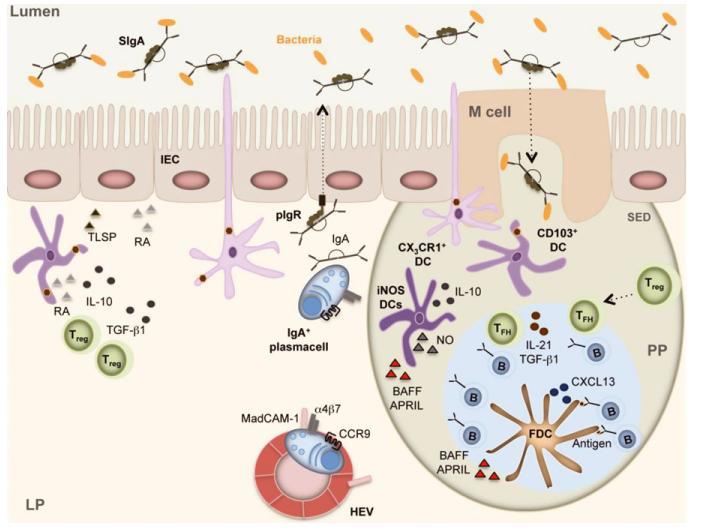
Fig. 1. Intestinal IgA production in Peyer’s patches (PPs).
M cells intercalated in the follicle-associated epithelium of PPs capture luminal SIgA-coated antigens through SIgA receptors. Luminal antigens are also captured by transepithelial projections emanating from non-migratory macrophage-like CX3CR1+ dendritic cells (DCs) in the subepithelial dome (SED) of PPs. Then, M cells and CX3CR1+ DCs transfer antigen to migratory CD103+ DCs, which move to the interfollicular area of PPs to establish cognate interactions with CD4+ T cells, including Treg cells. These tolerogenic CD4+ T cells have IgA-inducing function and require RA, TGF-β1, IL-10, and TSLP from intestinal epithelial cells (IECs) and DCs. Treg cells further differentiate to Tfh cells, which establish cognate interactions with antigen-specific B cells at the T-B cell border. Tfh cellactivated B cells migrate to the follicle together with Tfh cells by following a CXCL13 gradient formed by follicular dendritic cells (FDCs). In the presence of antigen-retaining FDCs, B cells undergo expansion, SHM, IgA CSR, and affinity maturation in response to CD40L, IL-21, and TGF-β1 from Tfh cells. TGF-β1, IL-10, RA, and nitric oxide (NO) from FDCs and TipDCs further enhance IgA CSR and production in the GC. B cells emerging from the GC upregulate α4β7 and CCR9 gut-homing receptors in response to RA, reach the general circulation, and finally enter the lamina propria (LP) through MAdCAM-1-expressing high endothelial venules. In the LP, B cells differentiate to plasmablasts and plasma cells that secrete polymeric IgA. Following pIgR-mediated transcytosis across IECs, polymeric IgA forms SIgA, which interacts with commensal bacteria and neutralizes pathogens.
Antigen sampling by gut DCs
Follicular IgA responses require the activation of CD4+ T cells by antigen-presenting DCs. But how do DCs sample antigens from the intestinal lumen? In agreement with their major role in IgA induction, PPs are equipped with a follicle-associated epithelium that contains M cells specialized in antigen capture (35). These M cells sample SIgA-free bacteria through an antigen recognition system that involves the glycoprotein 2 receptor (36). In addition, M cells sample SIgA-coated bacteria by using a poorly characterized IgA receptor and Dectin-1, a C-type lectin receptor that interacts with glycans associated with SIgA (37-39).
After capturing antigen, M cells deliver antigen to DCs positioned in the subepithelial dome of PPs (35). Various DC subsets orchestrate the IgA response in PPs (28, 40, 41). Some of these DCs directly capture luminal antigens by extending dendrites through M cell-specific transcellular pores (42). Similarly, macrophage-like DCs expressing the chemokine receptor CX3CR1 project antigen-sampling dendrites across IECs in response to a chemotactic gradient established by the epithelial chemokine CX3CL1 (43, 44). As they sample antigen, CX3CR1+ DCs preserve the integrity of the epithelial barrier by expressing tight junction proteins identical to those that seal the intercellular surfaces of IECs, including occludin, claudin 1, and zonula occludin 1 (45).
Owing to their sessile nature, CX3CR1+ DCs transfer sampled antigens to migratory CD103+ DCs through the formation of intercellular gap junctions that contain the gap junction protein connexin-43 (46). Of note, CD103+ DCs have an ontogeny distinct from that of CX3CR1+ DCs. Indeed, CD103+ DCs emerge from circulating pre-DCs, whereas CX3CR1+ DCs originate from circulating monocytes (47). After capturing antigen, CD103+ DCs move to the interfollicular areas of PPs and MLNs to establish cognate interactions with CD4+ T cells, including tolerogenic Treg cells that eventually differentiate to Tfh cells (48, 49). Yet, in the presence of dysbiosis, also CX3CR1+ DCs can acquire migratory and T-cell-stimulating capabilities (50). Conversely, CD103+ DCs form antigen-sampling transepithelial projections in response to pathogens (51). These observations point to the presence of a certain degree of functional flexibility in both macrophage-like CX3CR1+ DCs and CD103+ DCs.
Non-inflammatory priming of gut DCs
Intestinal IgA responses provide protection without causing inflammation (52). Given the key role of DCs in IgA induction, it is not surprising that the gut mucosa has developed several strategies to imprint antigen-sampling DCs with non-inflammatory properties. By releasing retinoic acid (RA), which is a metabolic product of dietary vitamin A, IECs stimulate the development of tolerogenic CD103+ DCs, which suppress Th1 cells secreting pro-inflammatory inter-feron-γ as well as Th17 cells secreting pro-inflammatory IL-17 (53, 54). This suppressive activity involves the release of multiple anti-inflammatory factors by CD103+ DCs, including TGF-β1, IL-10, and RA (55). Concomitantly, TGF-β1, IL-10, and RA promote the formation of Foxp3+ Treg cells that secrete tolerogenic TGF-β1 and IL-10 (56, 57).
By inducing tolerogenic Foxp3+ Treg cells, RA skews intestinal B-cell responses toward IgA production (48, 49). Indeed, Foxp3+ Treg cells release the IgA-inducing factor TGF-β1 and further differentiate to Foxp3+ Tfh cells that express CD40L and IL-21, which cooperate with TGF-β1 to induce IgA but not IgG production (48, 49, 58). Remark-ably, RA couples its IgA-inducing activity with a strong propensity to trigger expression of gut-homing CCR9 and a4b7 receptors on IgA-expressing B cells (59, 60). Besides RA, IECs release TGF-β1 and the IL-7-like cytokine thymic stromal lymphopoietin (TLSP) (53, 54, 61). Similar to RA and TGF-β1, TSLP mitigates DC production of IL-12, a pro-inflammatory cytokine required for the generation of Th1 cells (53, 54, 61).
In addition to capturing antigen from CX3CR1+ DCs, CD103+ DCs acquire antigen from goblet cells (62), a glandular epithelial cell specialized in the secretion of the mucin MUC2 (63). This hyperglycosylated protein constitutes the building block of gut mucus and provides anchoring sites to both commensal bacteria and SIgA (5). Furthermore, MUC2 imprints antigen-sampling CD103+ and CX3CR1+ DCs with tolerogenic properties following its interaction with a carbohydrate-binding complex comprised of galactin-3, Dectin-1, and FcγRIIB (6). Signals emanating from this MUC2 receptor inhibit inflammatory but not tolerogenic responses in antigen-sampling DCs through a mechanism involving β-catenin, a transcription factor required for the formation of tolerogenic gut DCs (6, 64).
Commensal bacteria also interact with SIgA and thus may use SIgA to deliver additional tolerogenic signals to antigen-sampling DCs (65). Consistent with this possibility, SIgA interacts with DC-specific ICAM-3-grabbing non-integrin (DC-SIGN), specific ICAM-3-grabbing non-integrin receptor-1 (SIGNR1), and Dectin-1, three carbohydrate receptors of the C-type lectin family that confer tolerogenic properties to DCs (39, 66, 67). Collectively, this evidence indicates that DCs collaborate with SIgA to orchestrate non-inflammatory immune responses in the gut.
Role of gut T and B cells in the GC reaction
Antigen-loaded DCs migrate to the interfollicular areas of PPs to establish cognate interactions with precursors of Tfh cells (18, 68, 69). In general, Tfh cells are essential for the maintenance and function of GCs and for the generation of both memory B cells and plasma cells (68, 69). The generation of Tfh cells requires their stimulation through the T-cell receptor via stable cognate interactions with DCs in the interfollicular area and with B cells at the T-B cell border of the follicle (68, 69).
Tfh cells to home to the follicular dendritic cell (FDC) network of the lymphoid follicle in response to CXCL13, a CXCR5-binding chemokine produced by FCDs (21, 68). Accordingly, both Tfh cells and Tfh cell-activated B cells upregulate CXCR5 to enter the follicle in response to CXCL13 (68, 69). In addition to CXCR5, Tfh cells express the co-stimulatory molecules CD40L, inducible costimulator (ICOS), and programed cell death-1 (PD-1) and release the B-cell-helper cytokines IL-21 and IL-4 (18, 68, 69). In PPs, Tfh cells also release TGF-β1, which cooperates with IL-21 to induce IgA (49, 58).
Tfh cells acquire GC B-cell-helper activity by following a genetic program that includes the induction of the transcriptional activator B-cell lymphoma-6 (Bcl-6) and the downregulation of the transcription repressor B-lymphocyte inducer of maturation-1 (BLIMP-1) (21, 70). Tfh cells are further regulated by T-follicular regulatory (Tfr) cells (71), a subset of GC CD4+ T cells that share several features with Tfh cells, including high expression of CXCR5 and PD-1, strong dependence on Bcl-6 and preferential GC location (72). Unlike Tfh cells, Tfr cells express the Treg cell-associated transcription factor Foxp3 and likely derive from natural (central) Treg cells (73). In addition, Tfr cells lack CD40L and secrete less IL-21 than Tfh cells (72).
In PPs, Tfh cells originate from Treg cells and possibly also Th17 cells and exert their IgA-inducing function under the control of constraining signals from Tfr cells (49, 74). Indeed, excessive expansion of Tfh cells resulting from the lack of Tfr cells perturbs the affinity selection of GC B cells in PPs and thereby alters the quality of IgA released by plasma cells in the LP (75). The mechanism by which Tfr cells control IgA production remains unclear, but one possibility is that Tfr cells inhibit the proliferation and cytokine secretion of antigenprimed Tfh cells. This process may involve constraining signals from programed cell death-1 (PD-1), an inhibitory receptor that binds at least two distinct PD-1 ligands on various cell types, including GC B cells (75).
Role of gut FDCs in the GC reaction
In PPs, FDCs expose gut antigens on their surface for extended periods, thereby facilitating the selection of GC B cells that express high-affinity BCRs (18, 76). In addition, FDCs release CXCL13 to induce CXCR5-dependent follicular homing of Tfh cells, Tfr cells, and B cells (18, 77). Moreover, FDCs support the preferential generation of IgA by producing large amounts of TGF-β1, B cell-activating factor of the TNF family (BAFF), and IL-6 in response to tissuespecific conditioning signals (77). In particular, TLR signals from the microbiota cooperate with RA signals from dietary vitamin A to induce FDC expression of latent TGF-β-binding proteins and matrix metalloproteinases (MMPs), which are required for the activation and release of the IgA-inducing cytokine TGF-β1 by FDCs (77).
However, it must be noted that FDCs are not the only intestinal cell type capable to skew antibody production toward IgA. For instance, PPs may contain TNF and inducible nitric-oxygen synthase (iNOS)-producing DCs (TipDCs) that release nitric oxygen (NO) in response to TLR signals from the microbiota (78). Nitric oxide increases the expression of TGF-β receptors on follicular B cells, thereby enhancing IgA CSR and production (78).
Homing of PP-derived B cells to the gut LP
In addition to activating CD4+ T cells and initiating IgA production, DCs from PPs release RA to imprint IgA classswitched B cells with gut-homing properties through the induction of a4b7 (79). This integrin binds mucosal addressin cell-adhesion molecule-1 (MadCAM-1) on high endothelial venules located in the gut LP (79). RA also induces B-cell expression of CCR9, a chemokine receptor that binds CCL25 from IECs (79). After entering the general circulation via efferent lymphatics, MLNs and the thoracic duct, PP-derived IgA+ α4b7+ CCR9+ B cells migrate to the LP of the small intestine in response to CCL25. The mechanism underlying the colonization of the large intestine by PP-derived B cells remains unclear, although CCR10 ligands such as CCL28 may be involved.
Homing of IgA-expressing B cells to the LP is coupled with their differentiation into plasmablasts and plasma cells (17). Newly generated plasma cells displace pre-existing plasma cells in the LP through a process of antigenic attrition (26). In this competitive scenario and given the high rate of intestinal plasma cell generation, one might expect that intrinsic factors limit the survival of plasma cells in the LP. However, gut plasma cells are not intrinsically short-lived, but can survive for more than 3 months in the LP (26), possibly due to the release of powerful plasma cell survival factors such as IL-6 and a proliferation-inducing ligand (APRIL) (24).
Extensive remodeling of the plasma cell pool by antigenic attrition might counteract the establishment of stable hostmicrobiota symbiosis and limit the efficacy of mucosal vaccination. However, the human microbiota is remarkably stable and mucosal vaccination confers IgA-mediated protection (80, 81). In agreement with these observations, recent data indicate that the intestinal IgA system recalls previously selected specificities expressed by dominant B-cell clones (25). Thus, the generation of IgA likely occurs partly through the activation of naive B cells and partly through the re-activation of previously selected memory B cells.
Accordingly, the pool of gut plasma cells consists of an oligoclonal component, in which a few clones are highly expanded, and a polyclonal component, in which many different clones are present at low frequency (25). Dominant clones could respond to new antigens if these antigens have sufficient structural similarity with the ones previously encountered (24). This strategy could provide the IgA system with the necessary flexibility to respond to pathogens, while offering sufficient stability to accommodate minor changes in the resident microbiota.
A DC-mediated α4b7-CCR9-dependent mechanism also orchestrates the homing of antigen-specific CD4+ T cells such as Treg and Th17 cells to the LP. This process is controlled by soluble LT from LP-based RORγt+ ILCs, which appear to selectively guide the influx of CD4+ T cells expressing CD40L (82). Given the strong IgA-inducing function of CD40L, the LP may function not only as an IgA effector site but to some extent also as an IgA inductive site (82).
Intestinal IgA responses through the TI pathway
In the absence of CD4+ T cells, mice retain significant intestinal IgA production (33, 83, 84), which points to the existence of mucosal T cell-independent (TI) pathways that may generate some degree of IgA-mediated protection (Fig. 2). These TI IgA responses appear to occur at both follicular and extrafollicular sites, including the LP.
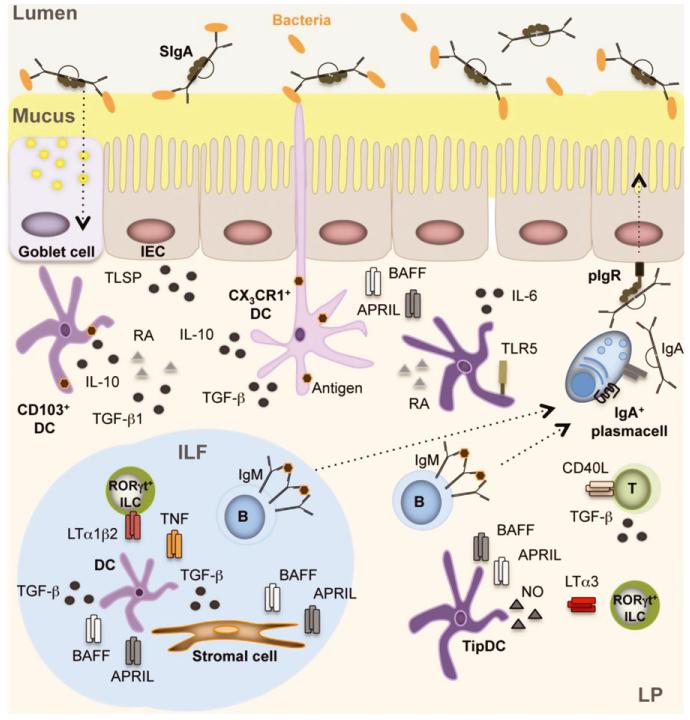
Fig. 2. Intestinal IgA production in isolated lymphoid follicles (ILFs) and lamina propria (LP).
CX3CR1+ dendritic cells (DCs) capture luminal antigens through transepithelial projections. Luminal antigens are also captured by CD103+ DCs through transient passages formed by MUC2-secreting goblet cells. These DCs receive conditioning TSLP, RA, TGF-β1, and MUC2 signals from intestinal epithelial cells (IECs) or goblet cells and then present antigen to B cells located in ILFs or in the diffuse lymphoid tissue of the LP. In ILFs, B cells receive IgA-inducing signals from BAFF, APRIL, and TGF-β1 produced by DCs and stromal cells in response to TNF and LT from LTi cells and TLR ligands from the microbiota. In the LP, B cells receive IgA-inducing signals from BAFF, APRIL, and nitric oxide (NO) produced by TipDCs and TLR ligands from the microbiota. Of note, NO enhances BAFF and APRIL produced by TLR-activated DCs. DC release of NO is further enhanced by LTα1β2 on RORγt+ ILCs. Additional IgA-inducing signals originate from RA and IL-6 produced by TLR5+ DCs and from BAFF, APRIL, and IL-10 produced by DCs and/or IECs in response to TLR ligands. IECs further enhance DC production of BAFF and APRIL by releasing TSLP. Together with IL-6 from stromal cells and DCs, BAFF and APRIL enhance the survival of IgA-secreting plasma cell in addition to inducing IgA CSR in B cells. Finally, CD4+ T cells (possibly Treg cells) further enhance IgA production in the LP by expressing CD40L. The homing of these CD4+ T cells to the LP is regulated by soluble LTα3 from RORγt+ ILCs.
Some intestinal IgA persists in mice lacking the GALT (85), which raises the possibility that limited IgA production takes place in the diffuse lymphoid tissue of the LP (Fig. 2). Indeed, variable amounts of IgA can be detected in the gut of RORγt-deficient or Id2-deficient mice, which have no PPs, ILFs, and lymph nodes due to the lack of LTi cells (33). Accordingly, the LP from both gut and upper respiratory tracts of immunodeficient patients with no CD40L-CD40 interaction or CD4+ T cells contains subepithelial B cells that express both AID and IgA, a hallmark of ongoing IgA CSR (86-88). Similarly, AID can be detected in IgA class-switched B cells from the intestinal LP of mice that lack GCs (89).
Despite these findings, the notion that the intestinal LP functions as an IgA inductive site remains debated (90). Indeed, some studies show that, when compared to PPs, the LP contains little or no AID and little or no molecular byproducts of IgA CSR (91-94). One caveat of these studies is that the LP contains more scattered B cells than PPs and that B cells in the LP express less AID transcripts than B cells in PPs (90). Thus, IgA CSR can be easily underestimated or pass completely unrecognized in the LP, unless appropriate controls and sensitive methodologies are used (90). Accordingly, recent works confirm that gut B cells can indeed undergo IgA production through a TI pathway that takes place in ILFs or LP (33, 82).
Some B cells may initiate TI IgA production in the gut LP after acquiring antigen from DCs. Indeed, some AID-expressing B cells can be detected beneath the intestinal epithelium (18, 86, 88). This sub-epithelial area also contains CX3CR1+ DCs and CD103+ DCs that sample luminal antigens through transepithelial projections or transient passages formed by goblet cells. Besides presenting luminal antigens to B cells, DCs inhabiting the LP release various cytokines that support local IgA production in a TI manner. In particular, TLR5-expressing DCs highly responsive to bacterial flagellin (a TLR5 ligand) induce IgA production by stimulating B cells through IL-6 and RA (95). In addition to promoting plasma cell differentiation, RA enhances both IgA CSR and production by cooperating with TGF-β (59, 96).
Another subset of LP-based DCs called TipDCs triggers TI IgA production by releasing CD40L-related members of the TNF family known as BAFF and APRIL in response to TLR signals from the microbiota (78). TipDCs also release nitric oxide, which up-regulates BAFF and APRIL production in LP-based DCs (78). Recent findings suggest that this TI pathway further involves iNOS-inducing signals from LT expressed on RORγt+ ILCs (82). Of note, also IECs release BAFF and APRIL and cooperate with DCs to promote APRIL-dependent CSR from IgM to IgA1 or IgA2, particularly in the distal gut (86, 88). IECs may further amplify their IgA-inducing function by releasing TSLP, which augments BAFF and APRIL production by DCs (86, 88). In the presence of TGF-β or IL-10, BAFF and APRIL deliver IgA CSR signals to B cells by engaging transmembrane activator and calciummodulating cyclophilin-ligand interactor, a receptor that signals in cooperation with BCR and TLR ligands (97, 98). In addition to inducing local CSR from IgM to IgA, BAFF and APRIL likely cooperate with IL-6 from DCs and stromal cells to enhance the survival of IgA-secreting plasmablasts and plasma cells (24).
IgA induction in ILFs
As discussed earlier, ILFs contain RORγt+ ILCs that cooperate with stromal cells, DCs, and macrophages to sustain IgA production through a TI pathway (33). In the presence of TNF and LT signals from LTi cells and TLR signals from the microbiota, SCs produce BAFF, APRIL, and TGF-β1 in addition to CXCL13, CCL19, and CCL20, which recruit both B cells and DCs (33). Together with LTi cells, macrophages and DCs produce abundant TNF, which triggers DC and stromal cell expression of MMP2, MMP9, and MMP13 required for the release of active TGF-β1 (33). Similar to stromal cells, DCs and macrophages also produce BAFF and APRIL, which cooperate with TGF-β1 and TLR ligands to induce IgA CSR and production in B cells (33). Of note, this IgA induction requires neither T cells nor a GC reaction (33).
IgA induction in MLNs and PPs
TI IgA production can also occur in MLNs through a pathway involving plasmacytoid DCs (pDCs) (99). By producing type-I interferon in response to TLR signals from the microbiota, stromal cells stimulate pDC release of BAFF and APRIL, which thereafter trigger IgA CSR and production in B cells (99). In MLNs as well as PPs, also FDCs may induce TI IgA production by stimulating B cells through BAFF, APRIL, TGF-β1 and antigen-containing immunocomplexes (77). Indeed, imprinting signals from dietary RA and microbial TLR ligands stimulate FDC release of BAFF and CXCL13 as well as FDC processing of TGF-β1 via MMP9 and MMP13 (77). This FDC-dependent pathway may permit PPs to support IgA production in the absence of CD40L-CD40 interaction (92, 100).
Conclusions
The intestine is a major site of pathogen entry, contains trillions of commensal bacteria, and is exposed to large amounts of food antigens. To protect the integrity of the epithelial barrier and avoid potentially catastrophic inflammatory reactions, SIgA not only restricts the access of microbes and other antigens to the mucosal surface, but also modulates the sampling of antigens and the quality of the local immune response. Although better known for its ability to neutralize toxins and some pathogens, IgA also maintains a diverse and spatially diversified community of commensal bacteria. To achieve these functions, the intestine has developed multiple follicular and extrafollicular pathways for the induction of IgA with or without help from T cells. The precise contribution of these pathways to mucosal immunity and homeostasis remains to be fully elucidated. Further studies are also needed to characterize how intestinal IgA discriminates commensals from pathogens and whether specific commensals are needed to optimize homeostatic IgA responses. This information may not only facilitate the development of novel oral vaccines, but also contribute to a better understanding of intestinal inflammatory disorders and food allergies.
Acknowledgements
The authors are supported by US National Institutes of Health grants R01 AI57653, U01 AI95613, P01 AI61093, and U19 096187 to A. C. The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review. The authors have no conflict of interest to declare.
References
- 1.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 2.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10:159–169. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 3.Cerutti A, Chen K, Chorny A. Immunoglobulin responses at the mucosal interface. Annu Rev Immunol. 2011;29:273–293. doi: 10.1146/annurev-immunol-031210-101317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamada N, Seo SU, Chen GY, Nunez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13:321–335. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- 5.Johansson ME, Sjovall H, Hansson GC. The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol. 2013;10:352–361. doi: 10.1038/nrgastro.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shan M, et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science. 2013;342:447–453. doi: 10.1126/science.1237910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sansonetti PJ. To be or not to be a pathogen: that is the mucosally relevant question. Mucosal Immunol. 2011;4:8–14. doi: 10.1038/mi.2010.77. [DOI] [PubMed] [Google Scholar]
- 8.Corthesy B. Multi-faceted functions of secretory IgA at mucosal surfaces. Front Immunol. 2013;4:185. doi: 10.3389/fimmu.2013.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandtzaeg P. Presence of J chain in human immunocytes containing various immunoglobulin classes. Nature. 1974;252:418–420. doi: 10.1038/252418a0. [DOI] [PubMed] [Google Scholar]
- 10.Mostov KE, Deitcher DL. Polymeric immunoglobulin receptor expressed in MDCK cells transcytoses IgA. Cell. 1986;46:613–621. doi: 10.1016/0092-8674(86)90887-1. [DOI] [PubMed] [Google Scholar]
- 11.Mestecky J, McGhee JR. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- 12.Strugnell RA, Wijburg OL. The role of secretory antibodies in infection immunity. Nat Rev Microbiol. 2010;8:656–667. doi: 10.1038/nrmicro2384. [DOI] [PubMed] [Google Scholar]
- 13.Brandtzaeg P, Prydz H. Direct evidence for an integrated function of J chain and secretory component in epithelial transport of immunoglobulins. Nature. 1984;311:71–73. doi: 10.1038/311071a0. [DOI] [PubMed] [Google Scholar]
- 14.Crottet P, Corthesy B. Secretory component delays the conversion of secretory IgA into antigen-binding competent F(ab’)2: a possible implication for mucosal defense. J Immunol. 1998;161:5445–5453. [PubMed] [Google Scholar]
- 15.Phalipon A, Cardona A, Kraehenbuhl JP, Edelman L, Sansonetti PJ, Corthesy B. Secretory component: a new role in secretory IgA-mediated immune exclusion in vivo. Immunity. 2002;17:107–115. doi: 10.1016/s1074-7613(02)00341-2. [DOI] [PubMed] [Google Scholar]
- 16.Johansen FE, et al. Absence of epithelial immunoglobulin A transport, with increased mucosal leakiness, in polymeric immunoglobulin receptor/secretory component-deficient mice. J Exp Med. 1999;190:915–922. doi: 10.1084/jem.190.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macpherson AJ, McCoy KD, Johansen FE, Brandtzaeg P. The immune geography of IgA induction and function. Mucosal Immunol. 2008;1:11–22. doi: 10.1038/mi.2007.6. [DOI] [PubMed] [Google Scholar]
- 18.Fagarasan S, Kawamoto S, Kanagawa O, Suzuki K. Adaptive immune regulation in the gut: T cell-dependent and T cell-independent IgA synthesis. Annu Rev Immunol. 2010;28:243–273. doi: 10.1146/annurev-immunol-030409-101314. [DOI] [PubMed] [Google Scholar]
- 19.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 20.Odegard VH, Schatz DG. Targeting of somatic hypermutation. Nat Rev Immunol. 2006;6:573–583. doi: 10.1038/nri1896. [DOI] [PubMed] [Google Scholar]
- 21.Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 22.Chaudhuri J, Alt FW. Class-switch recombination: interplay of transcription, DNA deamination and DNA repair. Nat Rev Immunol. 2004;4:541–552. doi: 10.1038/nri1395. [DOI] [PubMed] [Google Scholar]
- 23.Benckert J, et al. The majority of intestinal IgA+ and IgG+ plasmablasts in the human gut are antigen-specific. J Clin Invest. 2011;121:1946–1955. doi: 10.1172/JCI44447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pabst O. New concepts in the generation and functions of IgA. Nat Rev Immunol. 2012;12:821–832. doi: 10.1038/nri3322. [DOI] [PubMed] [Google Scholar]
- 25.Lindner C, et al. Age, microbiota, and T cells shape diverse individual IgA repertoires in the intestine. J Exp Med. 2012;209:365–377. doi: 10.1084/jem.20111980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hapfelmeier S, et al. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science. 2010;328:1705–1709. doi: 10.1126/science.1188454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vossenkamper A, et al. A role for gut-associated lymphoid tissue in shaping the human B cell repertoire. J Exp Med. 2013;210:1665–1674. doi: 10.1084/jem.20122465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2008;8:435–446. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 30.Spits H, et al. Innate lymphoid cells - a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 31.van de Pavert SA, Mebius RE. New insights into the development of lymphoid tissues. Nat Rev Immunol. 2010;10:664–674. doi: 10.1038/nri2832. [DOI] [PubMed] [Google Scholar]
- 32.Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006;6:148–158. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- 33.Tsuji M, et al. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity. 2008;29:261–271. doi: 10.1016/j.immuni.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 34.Bergqvist P, et al. Re-utilization of germinal centers in multiple Peyer’s patches results in highly synchronized, oligoclonal, and affinity-matured gut IgA responses. Mucosal Immunol. 2013;6:122–135. doi: 10.1038/mi.2012.56. [DOI] [PubMed] [Google Scholar]
- 35.Neutra MR, Mantis NJ, Kraehenbuhl JP. Collaboration of epithelial cells with organized mucosal lymphoid tissues. Nat Immunol. 2001;2:1004–1009. doi: 10.1038/ni1101-1004. [DOI] [PubMed] [Google Scholar]
- 36.Hase K, et al. Uptake through glycoprotein 2 of FimH(+) bacteria by M cells initiates mucosal immune response. Nature. 2009;462:226–230. doi: 10.1038/nature08529. [DOI] [PubMed] [Google Scholar]
- 37.Mantis NJ, Cheung MC, Chintalacharuvu KR, Rey J, Corthesy B, Neutra MR. Selective adherence of IgA to murine Peyer’s patch M cells: evidence for a novel IgA receptor. J Immunol. 2002;169:1844–1851. doi: 10.4049/jimmunol.169.4.1844. [DOI] [PubMed] [Google Scholar]
- 38.Kadaoui KA, Corthesy B. Secretory IgA mediates bacterial translocation to dendritic cells in mouse Peyer’s patches with restriction to mucosal compartment. J Immunol. 2007;179:7751–7757. doi: 10.4049/jimmunol.179.11.7751. [DOI] [PubMed] [Google Scholar]
- 39.Rochereau N, et al. Dectin-1 is essential for reverse transcytosis of glycosylated SIgA-antigen complexes by intestinal M cells. PLoS Biol. 2013;11:e1001658. doi: 10.1371/journal.pbio.1001658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iwasaki A. Mucosal dendritic cells. Annu Rev Immunol. 2007;25:381–418. doi: 10.1146/annurev.immunol.25.022106.141634. [DOI] [PubMed] [Google Scholar]
- 41.Schulz O, Pabst O. Antigen sampling in the small intestine. Trends Immunol. 2013;34:155–161. doi: 10.1016/j.it.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 42.Lelouard H, Fallet M, de Bovis B, Meresse S, Gorvel JP. Peyer’s patch dendritic cells sample antigens by extending dendrites through M cell-specific transcellular pores. Gastroenterology. 2012;142:592–601. e593. doi: 10.1053/j.gastro.2011.11.039. [DOI] [PubMed] [Google Scholar]
- 43.Niess JH, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 44.Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med. 2006;203:2841–2852. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rescigno M, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 46.Mazzini E, Massimiliano L, Penna G, Rescigno M. Oral tolerance can be established via gap junction transfer of fed antigens from CX3CR1(+) macrophages to CD103(+) dendritic cells. Immunity. 2014;40:248–261. doi: 10.1016/j.immuni.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 47.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cong Y, Feng T, Fujihashi K, Schoeb TR, Elson CO. A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc Natl Acad Sci USA. 2009;106:19256–19261. doi: 10.1073/pnas.0812681106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsuji M, et al. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer’s patches. Science. 2009;323:1488–1492. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- 50.Diehl GE, et al. Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX(3)CR1 (hi) cells. Nature. 2013;494:116–120. doi: 10.1038/nature11809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Farache J, et al. Luminal bacteria recruit CD103+ dendritic cells into the intestinal epithelium to sample bacterial antigens for presentation. Immunity. 2013;38:581–595. doi: 10.1016/j.immuni.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peterson DA, McNulty NP, Guruge JL, Gordon JI. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe. 2007;2:328–339. doi: 10.1016/j.chom.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 53.Iliev ID, Mileti E, Matteoli G, Chieppa M, Rescigno M. Intestinal epithelial cells promote colitis-protective regulatory T-cell differentiation through dendritic cell conditioning. Mucosal Immunol. 2009;2:340–350. doi: 10.1038/mi.2009.13. [DOI] [PubMed] [Google Scholar]
- 54.Iliev ID, et al. Human intestinal epithelial cells promote the differentiation of tolerogenic, et al. dendritic cells. Gut. 2009;58:1481–1489. doi: 10.1136/gut.2008.175166. [DOI] [PubMed] [Google Scholar]
- 55.Scott CL, Aumeunier AM, Mowat AM. Intestinal CD103+ dendritic cells: master regulators of tolerance? Trends Immunol. 2011;32:412–419. doi: 10.1016/j.it.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 56.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-βeta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dullaers M, et al. A T cell-dependent mechanism for the induction of human mucosal homing immunoglobulin A-secreting plasmablasts. Immunity. 2009;30:120–129. doi: 10.1016/j.immuni.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tokuyama H, Tokuyama Y. The regulatory effects of all-trans-retinoic acid on isotype switching: retinoic acid induces IgA switch rearrangement in cooperation with IL-5 and inhibits IgG1 switching. Cell Immunol. 1999;192:41–47. doi: 10.1006/cimm.1998.1438. [DOI] [PubMed] [Google Scholar]
- 60.Mora JR, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 61.Rimoldi M, et al. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol. 2005;6:507–514. doi: 10.1038/ni1192. [DOI] [PubMed] [Google Scholar]
- 62.McDole JR, et al. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. 2012;483:345–349. doi: 10.1038/nature10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johansson ME, Larsson JM, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4659–4665. doi: 10.1073/pnas.1006451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Manicassamy S, et al. Activation of beta-catenin in dendritic cells regulates immunity versustolerance in the intestine. Science. 2010;329:849–853. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Corthesy B. Role of secretory IgA in infection and maintenance of homeostasis. Autoimmun Rev. 2013;12:661–665. doi: 10.1016/j.autrev.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 66.Baumann J, Park CG, Mantis NJ. Recognition of secretory IgA by DC-SIGN: implications for immune surveillance in the intestine. Immunol Lett. 2010;131:59–66. doi: 10.1016/j.imlet.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Diana J, et al. Secretory IgA induces tolerogenic dendritic cells through SIGNR1 dampening autoimmunity in mice. J Immunol. 2013;191:2335–2343. doi: 10.4049/jimmunol.1300864. [DOI] [PubMed] [Google Scholar]
- 68.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 69.Vinuesa CG, Cyster JG. How T cells earn the follicular rite of passage. Immunity. 2011;35:671–680. doi: 10.1016/j.immuni.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 70.Vinuesa CG, Sanz I, Cook MC. Dysregulation of germinal centres in autoimmune disease. Nat Rev Immunol. 2009;9:845–857. doi: 10.1038/nri2637. [DOI] [PubMed] [Google Scholar]
- 71.Washington K, Stenzel TT, Buckley RH, Gottfried MR. Gastrointestinal pathology in patients with common variable immunodeficiency and X-linked agammaglobulinemia. Am J Surg Pathol. 1996;20:1240–1252. doi: 10.1097/00000478-199610000-00010. [DOI] [PubMed] [Google Scholar]
- 72.Linterman MA, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chung Y, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17:983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hirota K, et al. Plasticity of Th17 cells in Peyer’s patches is responsible for the induction of T cell-dependent IgA responses. Nat Immunol. 2013;14:372–379. doi: 10.1038/ni.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kawamoto S, et al. The inhibitory receptor PD-1 regulates IgA selection and bacterial composition in the gut. Science. 2012;336:485–489. doi: 10.1126/science.1217718. [DOI] [PubMed] [Google Scholar]
- 76.Batista FD, Harwood NE. The who, how and where of antigen presentation to B cells. Nat Rev Immunol. 2009;9:15–27. doi: 10.1038/nri2454. [DOI] [PubMed] [Google Scholar]
- 77.Suzuki K, et al. The sensing of environmental stimuli by follicular dendritic cells promotes immunoglobulin A generation in the gut. Immunity. 2010;33:71–83. doi: 10.1016/j.immuni.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 78.Tezuka H, et al. Regulation of IgA production by naturally occurring TNF/iNOS-producing dendritic cells. Nature. 2007;448:929–933. doi: 10.1038/nature06033. [DOI] [PubMed] [Google Scholar]
- 79.Mora JR, von Andrian UH. Role of retinoic acid in the imprinting of gut-homing IgA-secreting cells. Semin Immunol. 2009;21:28–35. doi: 10.1016/j.smim.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Faith JJ, et al. The long-term stability of the human gut microbiota. Science. 2013;341:1237439. doi: 10.1126/science.1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Franco MA, Angel J, Greenberg HB. Immunity and correlates of protection for rotavirus vaccines. Vaccine. 2006;24:2718–2731. doi: 10.1016/j.vaccine.2005.12.048. [DOI] [PubMed] [Google Scholar]
- 82.Kruglov AA, et al. Nonredundant function of soluble LTalpha3 produced by innate lymphoid cells in intestinal homeostasis. Science. 2013;342:1243–1246. doi: 10.1126/science.1243364. [DOI] [PubMed] [Google Scholar]
- 83.Macpherson AJ, Gatto D, Sainsbury E, Harriman GR, Hengartner H, Zinkernagel RM. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science. 2000;288:2222–2226. doi: 10.1126/science.288.5474.2222. [DOI] [PubMed] [Google Scholar]
- 84.Macpherson AJ, et al. IgA production without mu or delta chain expression in developing B cells. Nat Immunol. 2001;2:625–631. doi: 10.1038/89775. [DOI] [PubMed] [Google Scholar]
- 85.Lopatin U, Blutt SE, Conner ME, Kelsall BL. Lymphotoxin alpha-deficient mice clear persistent rotavirus infection after local generation of mucosal IgA. J Virol. 2013;87:524–530. doi: 10.1128/JVI.01801-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.He B, et al. Intestinal bacteria trigger T cell-independent immunoglobulin A2 class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26:812–826. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 87.Xu W, et al. Viral double-stranded RNA triggers Ig class switching by activating upper respiratory mucosa B cells through an innate TLR3 pathway involving BAFF. J Immunol. 2008;181:276–287. doi: 10.4049/jimmunol.181.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu W, et al. HIV-1 evades virus-specific IgG2 and IgA responses by targeting systemic and intestinal B cells via long-range intercellular conduits. Nat Immunol. 2009;10:1008–1017. doi: 10.1038/ni.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Crouch EE, et al. Regulation of AID expression in the immune response. J Exp Med. 2007;204:1145–1156. doi: 10.1084/jem.20061952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cerutti A. The regulation of IgA class switching. Nat Rev Immunol. 2008;8:421–434. doi: 10.1038/nri2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barone F, Patel P, Sanderson JD, Spencer J. Gut-associated lymphoid tissue contains the molecular machinery to support T-cell-dependent and T-cell-independent class switch recombination. Mucosal Immunol. 2009;2:495–503. doi: 10.1038/mi.2009.106. [DOI] [PubMed] [Google Scholar]
- 92.Bergqvist P, Gardby E, Stensson A, Bemark M, Lycke NY. Gut IgA class switch recombination in the absence of CD40 does not occur in the lamina propria and is independent of germinal centers. J Immunol. 2006;177:7772–7783. doi: 10.4049/jimmunol.177.11.7772. [DOI] [PubMed] [Google Scholar]
- 93.Shikina T, et al. IgA class switch occurs in the organized nasopharynx- and gut-associated lymphoid tissue, but not in the diffuse lamina propria of airways and gut. J Immunol. 2004;172:6259–6264. doi: 10.4049/jimmunol.172.10.6259. [DOI] [PubMed] [Google Scholar]
- 94.Lin M, Du L, Brandtzaeg P, Pan-Hammarstrom Q. IgA subclass switch recombination in human mucosal and systemic immune compartments. Mucosal Immunol. 2013 doi: 10.1038/mi.2013.68. doi: 10.1038/mi.2013.68. [DOI] [PubMed] [Google Scholar]
- 95.Uematsu S, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol. 2008;9:769–776. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- 96.Seo GY, et al. Retinoic acid, acting as a highly specific IgA isotype switch factor, cooperates with TGF-βeta1 to enhance the overall IgA response. J Leukoc Biol. 2013;94:325–335. doi: 10.1189/jlb.0313128. [DOI] [PubMed] [Google Scholar]
- 97.Castigli E, et al. TACI and BAFF-R mediate isotype switching in B cells. J Exp Med. 2005;201:35–39. doi: 10.1084/jem.20032000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.He B, et al. The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. Nat Immunol. 2010;11:836–845. doi: 10.1038/ni.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tezuka H, et al. Prominent role for plasmacytoid dendritic cells in mucosal T cell-independent IgA induction. Immunity. 2011;34:247–257. doi: 10.1016/j.immuni.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 100.Bergqvist P, Stensson A, Lycke NY, Bemark M. T cell-independent IgA class switch recombination is restricted to the GALT and occurs prior to manifest germinal center formation. J Immunol. 2010;184:3545–3553. doi: 10.4049/jimmunol.0901895. [DOI] [PubMed] [Google Scholar]