Abstract
Protein disulfide isomerase (PDI) has fundamental roles in the oxidative folding of proteins in the endoplasmic reticulum (ER) of eukaryotic cells. The study of this molecule has been attracting considerable attention due to its association with other cell functions and human diseases. In leukocytes, such as neutrophils, PDI is involved with cell adhesion, signaling and inflammation. However, the expression of PDI in other leukocytes, such as eosinophils, important cells in inflammatory, allergic and immunomodulatory responses, remains to be defined. Here we used different approaches to investigate PDI expression within human eosinophils. Western blotting and flow cytometry demonstrated high PDI expression in both unstimulated and CCL11/eotaxin-1-stimulated eosinophils, with similar levels in both conditions. By using an immunogold electron microscopy technique that combines better epitope preservation and secondary Fab-fragments of antibodies linked to 1.4-nm gold particles for optimal access to microdomains, we identified different intracellular sites for PDI. In addition to predictable strong PDI labeling at the nuclear envelope, other unanticipated sites, such as secretory granules, lipid bodies and vesicles, including large transport vesicles (eosinophil sombrero vesicles), were also labeled. Thus, we provide the first identification of PDI in human eosinophils, suggesting that this molecule may have additional/specific functions in these leukocytes.
Keywords: transmission electron microscopy, human eosinophils, immunonanogold electron microscopy, cell activation, endoplasmic reticulum, protein disulfide isomerase, secretory granules, lipid bodies, vesicular traffic
Introduction
The process of disulfide bond formation in the endoplasmic reticulum (ER) of eukaryotic cells was one of the first mechanisms of catalyzed protein folding to be discovered. This process involves protein disulfide isomerase (PDI) enzymes, a family composed of several well-characterized proteins that are encountered in the ER of both prokaryotic and eukaryotic cells. The name PDI indicates not only the family but also a particular protein, which was the first to be characterized and also the one studied in more detail. The specific protein PDI is localized to the ER lumen where it catalyzes the formation and breakage of disulfide bonds between cysteine residues within proteins [reviewed in (Appenzeller-Herzog and Ellgaard 2008; Laurindo et al. 2012; Turano et al. 2002)]. This allows proteins to quickly find the correct arrangement of disulfide bonds in their fully folded state. Therefore, it has been suggested that PDI plays a role similar to the chaperones in the folding process, promoting folding of the polypeptide chains as well as formation of disulfide bonds inside the protein structure [reviewed in (Laurindo et al. 2012; Turano et al. 2002)].
Despite the well-characterized function of PDI in the ER, this enzyme has been reported at distinct cytoplasmic sites and implicated in other cell functions. In leukocytes, such as neutrophils, PDI is closely associated with nicotinamide adenine dinucleotide phosphate (NADPH) oxidase subunits and exerts a functional regulatory role in reactive oxygen species (ROS) generation (Laurindo et al. 2008). Recent work has also identified another important function for PDI in leukocytes during vascular inflammation in vivo, demonstrating that this enzyme is required for neutrophil adhesion and crawling by interacting with integrins (Hahm et al. 2013). Interestingly, PDI may act as an intracellular anti-inflammatory molecule in leukocytes. Decreased levels of PDI were reported in monocytes primed by lipopolysaccharide (LPS) (Gadgil et al. 2003) and in neutrophils infected with Staphylococcus aureus (Zhang et al. 2010).
The expression of PDI in other leukocytes such as eosinophils remains to be defined. Eosinophils are innate immune leukocytes recruited in large numbers to sites of allergic inflammation and parasitic infections. More recently appreciated are the additional pleiotropic effects of recruited eosinophils that have an impact on immunomodulation and tissue homeostasis and repair [reviewed in (Melo et al. 2013; Rosenberg et al. 2013)].
Our group has been using a pre-embedding immunogold electron microscopy technique, which combines better preservation of subcellular compartments and protein epitopes with the use of very small gold particles conjugated with secondary antibodies, to localize specific proteins in human eosinophil subcellular sites (Melo et al. 2005a; Melo et al. 2005b). For example, by applying this technique, which enables optimal access to membrane microdomains, we identified major basic protein (MBP) and cytokines at large vesicles, termed Eosinophil Sombrero Vesicles (EoSVs), involved in the transport of these proteins from secretory granules to the cell surface (Melo et al. 2005a; Melo et al. 2008; Melo et al. 2005b; Melo et al. 2009).
Here we have applied the pre-embedding immunonanogold electron microscopy (immunoEM) technique to human eosinophils to investigate the expression and subcellular localization of PDI within these cells. Our findings reveal that PDI is highly expressed in human eosinophils and that this enzyme is also present in non-ER locations, such as secretory granules, vesicular compartments and lipid bodies. Thus, we provide the first identification of PDI in human eosinophils, suggesting that this molecule may have additional/specific functions in these leukocytes.
Materials & Methods
Eosinophil Isolation, Stimulation and Viability
Granulocytes were isolated from the blood of different healthy donors. Eosinophils were enriched and purified by negative selection using human eosinophil enrichment cocktail (StemSep™, StemCell Technologies; Seattle, WA) and the MACS bead procedure (Miltenyi Biotec; Auburn, CA), as described [Melo et al., 2005a], with the exception that hypotonic red blood cell (RBC) lysis was omitted to avoid any potential effect of RBC lysis on eosinophil function. Experiments were approved by the Beth Israel Deaconess Medical Center Committee on Clinical Investigation, and informed consent was obtained from all subjects. Purified eosinophils (106 cells/mL) were stimulated with recombinant human eotaxin-1 (CCL11) (100 ng/mL; R&D Systems; Minneapolis, MN) in RPMI-1640 medium plus 0.1% ovalbumin (OVA) (Sigma-Aldrich; St. Louis, MO), or medium alone at 37C, for 1 hr. Eosinophil viability and purity were greater than 99%, as determined by ethidium bromide (Molecular Probes, Life Technologies; Carlsbad, CA) incorporation and cytocentrifuged smears stained with HEMA 3 stain kit (Fisher Scientific; Pittsburgh, PA), respectively.
Antibody Reagents
Anti-human mouse IgG2a PDI (clone RL90), whose PDI specificity has been well validated in previous studies (Alhamidi et al. 2011; Gill et al. 2013; Peterfi et al. 2009; Turner et al. 2009), and irrelevant isotype control monoclonal antibodies (Abcam; Cambridge, MA) were used for EM (5 μg/mL), flow cytometry (10 μg/mL) and western blotting (1:1000). Secondary antibody for immunoEM studies was an affinity-purified goat anti-mouse Fab fragment conjugated to 1.4-nm gold particles (1:100, Nanogold, Nanoprobes; Stony Brook, NY). Secondary antibodies for flow cytometry were goat anti-mouse conjugated to Alexa Fluor 488 (Molecular Probes, Life Technologies) and for western blotting were goat anti-mouse conjugated to HRP (1:5,000, Jackson ImmunoResearch Laboratories, Inc.; West Grove, PA).
Flow Cytometry
Human eosinophils were stimulated or not with CCL11 as detailed above. Immediately after stimulation, cells were fixed with 3.7% paraformaldehyde, permeabilized and blocked with 2.5% human serum. Cells were incubated with anti-PDI or isotype control antibodies, followed by anti-mouse secondary antibodies as described above. Data were acquired using the LSRII flow cytometer (BD Biosciences; Franklin Lakes, NJ) and the analysis software, Flow Jo (Tree Star Inc., Ashland, OR).
Western Blotting
Human eosinophils were stimulated as above, and lysed in lysis buffer: Tris buffer (10 mM, pH 7.4) containing 100 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 0.5% hexadecyl trimethylammonium bromide (CTAB), 10% glycerol, 1 mM PMSF, protease inhibitors mixture (Sigma-Aldrich), and phosphatase inhibitor cocktails 2 and 3 (Sigma-Aldrich). Samples were loaded onto 4-12% Bis-Tris gels (Invitrogen, Life Technologies; Carlsbad, CA) under denaturing conditions. Gels were transferred to PVDF membranes (Pierce Biotechnology; Rockford, IL), blocked for 1 hr at room temperature with 5% non-fat dry milk (Bio-Rad; Hercules, CA), and probed with mouse anti-PDI antibodies followed by goat anti-mouse secondary antibodies as described above. Membranes were developed with SuperSignal West Femto chemiluminescence kits (Pierce Biotechnology).
Cell Preparation for EM
For immunoEM, purified eosinophils were immediately fixed in fresh 4% paraformaldehyde in 0.02 M phosphate-buffered saline (0.15 M NaCl) (PBS), pH 7.4. Alternatively, fixation was performed in a mixture of freshly prepared aldehydes (1% paraformaldehyde and 1.25% glutaraldehyde) in 0.05 M sodium cacodylate buffer (pH 7.4). Cells were fixed for 30 min at room temperature, washed in PBS and centrifuged at 1500 ×g for 1 min. Samples were then resuspended in molten 2% agar in PBS and quickly re-centrifuged. Pellets were immersed in 30% sucrose in PBS overnight at 4C, embedded in OCT compound (Miles Laboratories; Elkhart, IN), and stored in -180C liquid nitrogen for subsequent use.
ImmunoEM
Pre-embedding immunolabeling was carried out before standard EM processing (dehydration, infiltration, resin embedding and resin sectioning). Pre-embedding immunoEM optimizes antigen preservation and is more sensitive to detect small molecules than post-embedding labeling that is carried out after conventional EM processing. Moreover, to reach antigens at membrane microdomains, such as small vesicles, we used labeling with very small (1.4-nm) gold particles (Nanogold). After testing different section thicknesses, we found that 10 μm enabled optimal penetration of the antibodies. Immunonanogold staining was then performed on 10-μm cryostat sections mounted on glass slides. All labeling steps were carried out at room temperature as before (Melo et al. 2009) as follows: (a) one wash in 0.02 M PBS, pH 7.6 (5 min); (b) immersion in 50 mM glycine in 0.02 M PBS, pH 7.4 (10 min); (c) incubation in a mixture of PBS and bovine serum albumin (PBS-BSA buffer; 0.02 M PBS plus 1% BSA) containing 0.1% gelatin (20 min) followed by PBS-BSA plus 10% normal goat serum (NGS) (30 min): This step is crucial to block non-specific binding sites. (d) Incubation with primary antibody (1 hr); (e) blocking with PBS-BSA plus NGS (30 min); (f) incubation with secondary antibody (1 hr); (g) washes in PBS-BSA (three times, 5 min each); (h) post-fixation in 1% glutaraldehyde (10 min); (i) five washes in distilled water; (j) incubation with HQ silver enhancement solution in a dark room according to the manufacturer’s instructions (Nanoprobes) (10 min): This step enables a nucleation of silver ions around gold particles. These ions precipitate as silver metal and the particles grow in size facilitating observation under TEM. (k) Three washes in distilled water; (l) immersion in freshly prepared 5% sodium thiosulfate (5 min); (m) post-fixation with 1% osmium tetroxide in distilled water (10 min); (n) staining with 2% uranyl acetate in distilled water (5 min); (o) embedding in Eponate (Eponate 12 Resin; Ted Pella, Redding, CA); (p) polymerization at 60C for 16 h; (q) embedding by inverting eponate-filled plastic capsules over the slide-attached tissue sections; and (r) separation of eponate blocks from glass slides by brief immersion in liquid nitrogen. Thin sections were cut using a diamond knife on an ultramicrotome (Leica, Bannockburn, IL). Sections were mounted on uncoated 200-mesh copper grids (Ted Pella) before staining with lead citrate and viewed with a transmission electron microscope (CM 10; Philips, Eindhoven, the Netherlands) at 60 kV. Two controls were performed: (1) primary antibody was replaced by an irrelevant antibody, and (2) primary antibody was omitted. Specimens were examined as described for conventional TEM. Electron micrographs from different experiments (n=4) were randomly taken at different magnifications to study the entire cell profile and subcellular features.
Statistical Analysis
A total of 175 electron micrographs were evaluated and the number of secretory granules (labeled and not labeled) as well as the number of gold particles/lipid body or vesicular compartment was counted using the software ImageJ (National Institutes of Health, Bethesda, MD). The number of gold particles per micrometer of nuclear envelope was quantitated using the same software. Data were compared using the Mann–Whitney U-test (p<0.05).
Results
PDI Is Highly Expressed within Human Eosinophils
First, the presence of PDI within eosinophils was investigated by immunodetection studies using western blotting and flow cytometry. Both approaches demonstrated high levels of PDI within human eosinophils (Fig. 1). Second, we evaluated whether PDI expression changed after stimulation with the eosinophil agonist, CCL11. There were no differences in PDI levels when unstimulated were compared with stimulated cells by both western blotting (Fig. 1A- lane 2 vs. 3) and flow cytometry (Fig. 1Bii).
Figure 1.
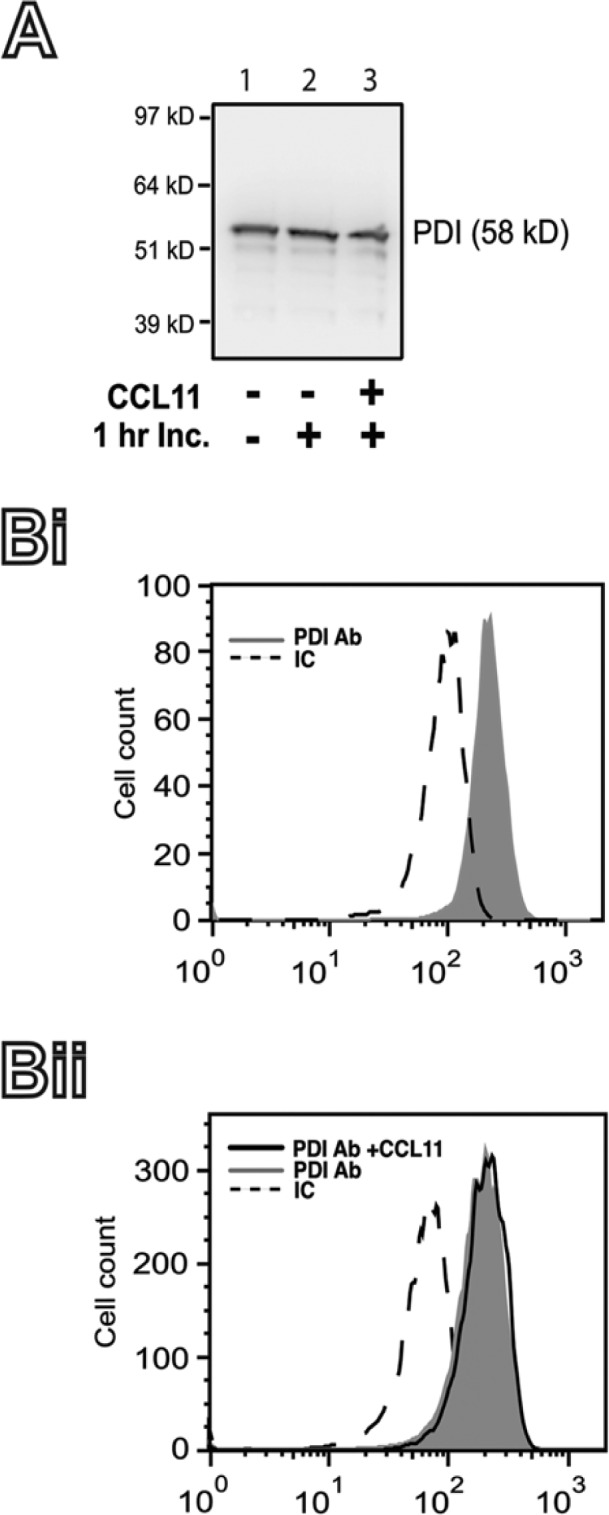
Protein disulfide isomerase (PDI) expression in human eosinophils. (A) The expression of PDI in fresh isolated human eosinophils (lane 1) or after 1 hr incubation (Inc.) at 37C in the absence (lane 2) or presence (3) of CCL11 was detected by western blotting, as detailed in the Materials & Methods. (B) The intracellular content of PDI in freshly isolated human eosinophils (Bi), or after 1 hr incubation at 37C in the absence, or presence of CCL11 (Bii) was measured by flow cytometry, as detailed in the Materials & Methods. IC, irrelevant antibody control. Representative blot and histograms are shown.
PDI Is Located in Different Subcellular Compartments
Immunonanogold EM revealed an intense PDI labeling at the nuclear envelope in both unstimulated (not shown) and CCL11-stimulated cells (Figs. 2, 3A, 3Ai). Moreover, PDI was clearly marked within vesicular compartments distributed in the cytoplasm (Figs. 2B, 3A, 3Aii) including EoSVs (Figs. 2B, 4A, 4Ai). These distinct vesicles are easily identifiable within human eosinophils because of their large size and typical ‘mexican hat’ (sombrero) appearance in cross sections with a central area of cytoplasm and a brim of circular membrane-delimited vesicles (Fig. 4A) (Melo et al. 2005b). Of note, vesicular compartments were found adjacent to lipid bodies as well as in close contact with these organelles (Fig. 3A, 3Aii).
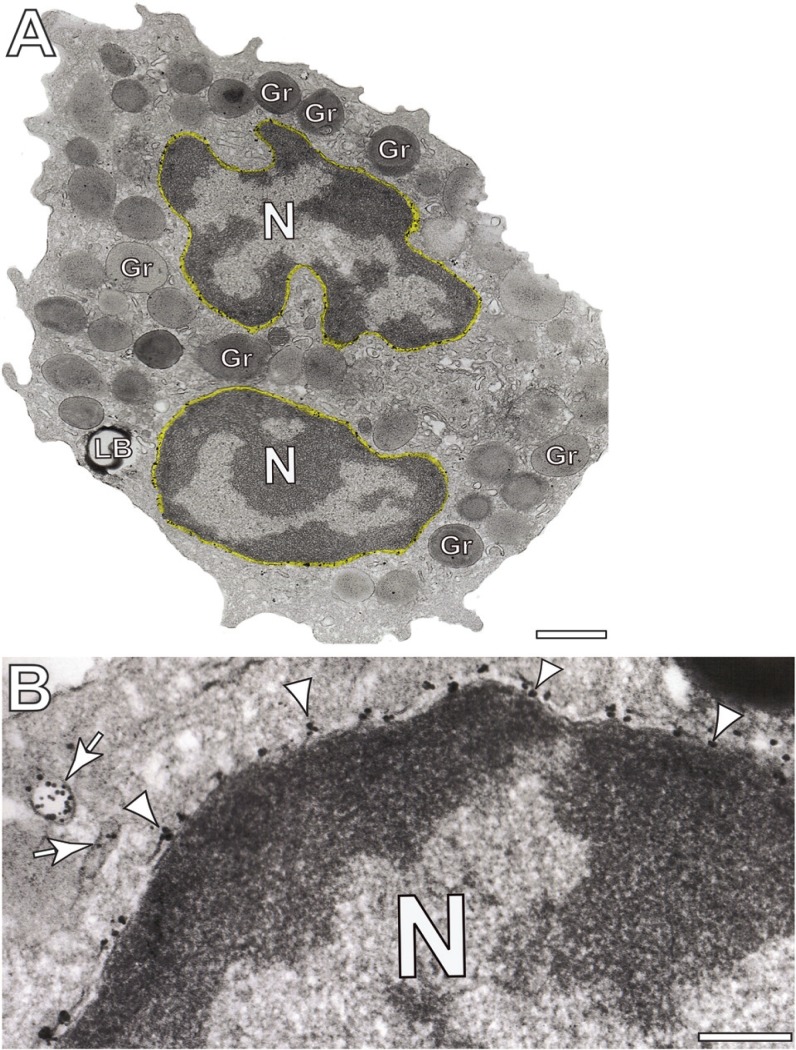
Figure 2.
Immunonanogold electron microscopy of protein disulfide isomerase (PDI) in human eosinophils. (A, B) PDI is densely labeled at the nuclear envelope (highlighted in yellow in A). Gold particles are indicated by arrowheads in (B). Arrows point to PDI-positive vesicles. Eosinophils were isolated from the blood of healthy donors, stimulated with CCL11 and processed for immunonanogold electron microscopy as before (Melo et al. 2005a). N, nucleus, Gr, secretory granule. Scale bars: (A) 2.0 μm; (B) 0.8 μm.
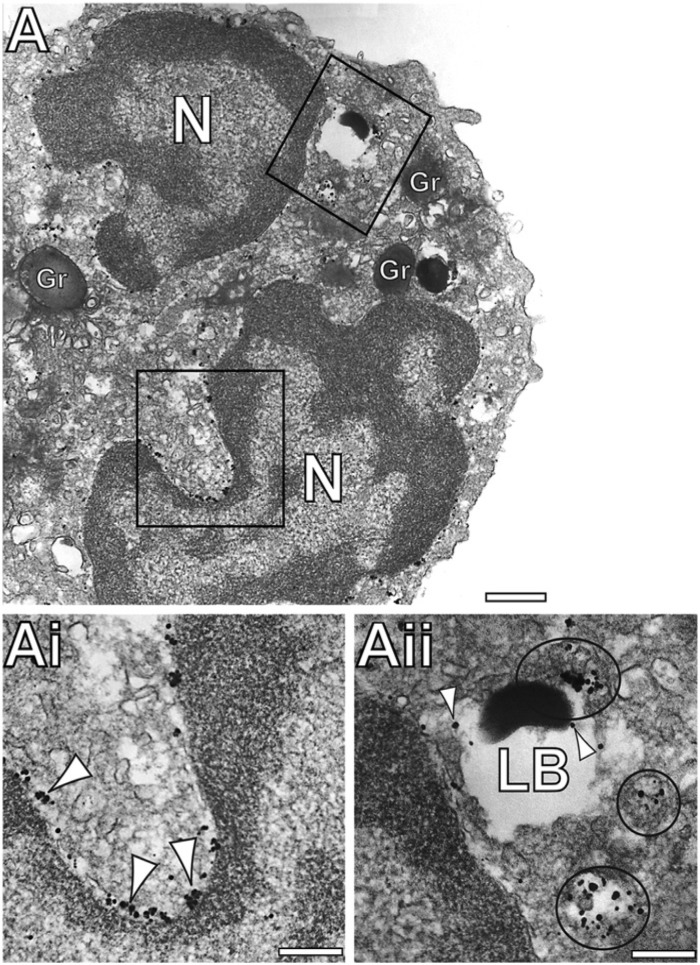
Figure 3.
Immunonanogold electron microscopy demonstrates protein disulfide isomerase (PDI) labeling within vesicles and lipid bodies (LBs). (Ai-Aii) Large vesicles (circles in Aii) around and in association with LBs are densely positive for PDI. PDI labeling is also seen within LBs (arrowheads in Aii). Note in (Ai) the consistent PDI labeling (arrowheads) around the nuclear envelope. (Ai) and (Aii) are boxed areas of (A) seen in high magnification. N, nucleus, Gr, secretory granule; LB, lipid body. Scale bars: (A) 1.1 μm; (Ai) 0.9 μm; (Aii) 0.5 μm.
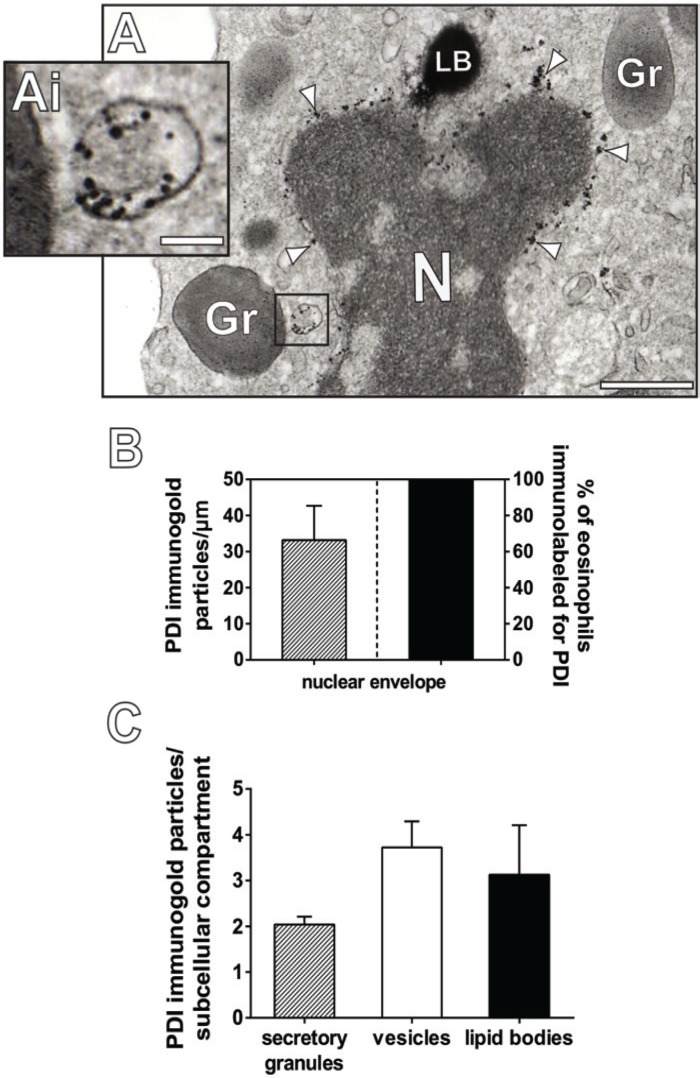
Figure 4.
Different subcellular compartments are labeled for protein disulfide isomerase (PDI) within human eosinophils. (A, Ai) A typical Eosinophil Sombrero Vesicle (EoSV, Ai) is observed in the cytoplasm of a human eosinophil by transmission electron microscopy after immunonanogold labeling for PDI. Note that PDI is localized within the vesicle lumen and that the vesicle is close to a secretory granule (Gr). (Ai) is the boxed area of (A) seen in high magnification. Arrowheads indicate PDI labeling at the nuclear envelope. (B and C) Quantitation of PDI immunogold labeling in eosinophil subcellular compartments. Data shown represent the mean and SEM of gold particle counts for each compartment. The total number of organelles/structures evaluated was as follows: 386 secretory granules, 24 lipid bodies and 314 vesicular compartments. The entire extension of the nuclear envelope of 30 cell sections was measured and the number of gold particles counted. Eosinophils were isolated from the blood of healthy donors, stimulated with CCL11 and processed for immunonanogold electron microscopy as before (Melo et al. 2005a). Scale bars: (A) 1.9 μm; (Ai) 70 nm. N, nucleus; LB, lipid body.
To evaluate the level of PDI labeling in intracellular sites, we next performed quantitative EM studies. Our analyses revealed that 100% of the eosinophils had abundant labeling for PDI on the nuclear envelope with a mean level of labeling of 33.2 ± 9.5 gold particles/μm (mean ± SEM) (Fig. 4B). The level of immunonanogold labeling was also evaluated in secretory granules, vesicular compartments and lipid bodies. Quantitative analysis showed a mean level of labeling in secretory granules and lipid bodies of 2.0 ± 0.1 and 3.13 ± 1.0 gold particles/organelle (mean ± SEM), respectively, whereas vesicular compartments exhibited a mean level of labeling of 3.7 ± 0.5 gold particles/structure (mean ± SEM) (Fig. 4C). In accord with our results obtained with western blotting and flow cytometry (Fig. 1), non-stimulated and stimulated cells displayed a similar distribution of labeling for PDI at the organelles/structures analyzed (not shown). Control cells, for which the primary antibody was replaced by an irrelevant antibody, were negative (compare Fig. 5A with 5B).
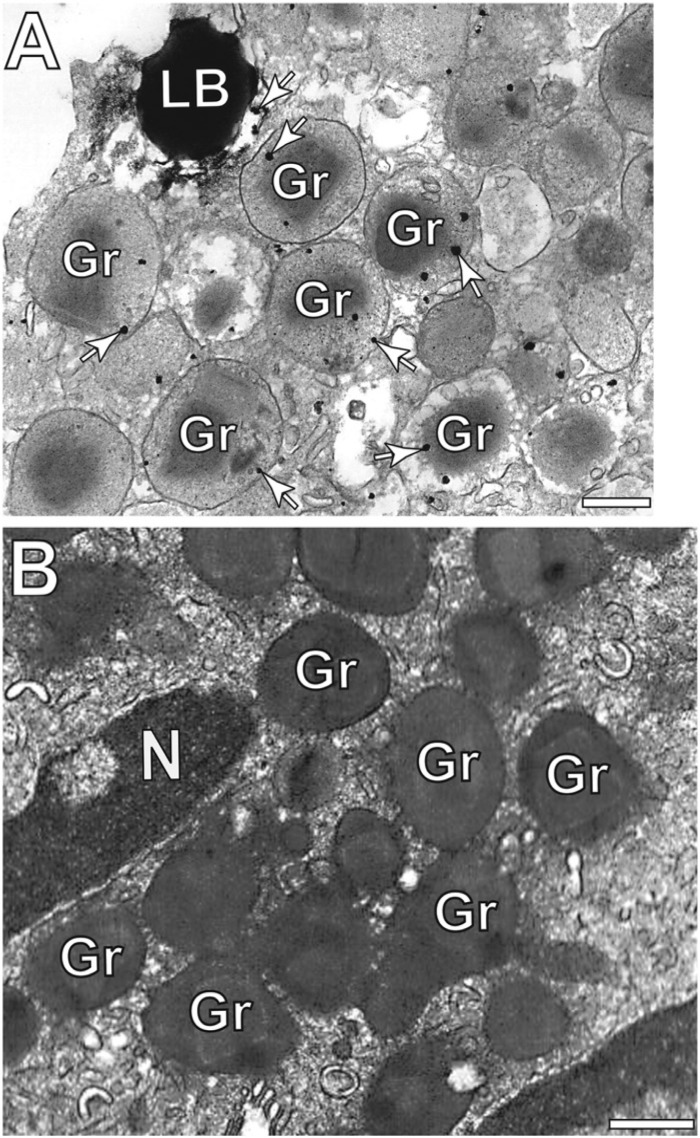
Figure 5.
Eosinophil secretory granules (Gr) are positive for protein disulfide isomerase (PDI). (A) Gold particles (arrows) are seen inside granules and close to a lipid body (LB). In (B), a representative control cell, for which the primary antibody was replaced by an irrelevant antibody, was negative for PDI. N, nucleus. Scale bars: (A, B) 400 nm.
Discussion
We demonstrated here, for the first time, that PDI is expressed in human eosinophils. Eosinophils are not rich in the peripheral ER. Using pre-embedding immunoEM, our studies revealed that PDI is densely associated with the nuclear envelope (Figs. 2, 3Ai). Early electron microscopy (EM) images showed that the inner and outer nuclear membranes are continuous with the endoplasmic reticulum (Watson 1955). It is now well established that the ER is a continuous membrane system that is comprised of the nuclear envelope as well as a peripheral network of tubules and sheets (Baumann and Walz 2001; Friedman and Voeltz 2011; Hu et al. 2011; Shibata et al. 2010). PDI is highly labeled on the nuclear envelope in different cells, as demonstrated by earlier immunogold EM studies in cells such as hepatocytes (Akagi et al. 1988), chondrocytes (Akagi et al. 1989) and exocrine pancreatic cells (Akagi et al. 1988), and our quantitative immunoEM results confirm the high level of PDI labeling on this ER domain (Fig. 2A and 2B).
Our pre-embedding immunonanogold EM studies applied to human eosinophils also revealed clear PDI labeling in secretory granules and lipid bodies (Figs. 3A, 3Aii and 5A). In fact, despite being considered primarily an ER protein, PDI has been reported at other cytoplasmic sites including mitochondria, nucleus and even at the cell surface [reviewed in (Laurindo et al. 2012)] and our study confirms that PDI is not restricted to the ER. Moreover, different studies using cell biological, biochemical and clinical approaches have demonstrated that the PDI family of proteins is involved in a wide range of physiological and disease processes (Benham 2012). As noted, PDI has non-canonical ER functions in leukocytes (Laurindo et al. 2008). The isomerase activity of extracellular PDI, for example, is critical for its regulatory effect on neutrophil recruitment (Hahm et al. 2013). Indeed, the participation of PDI and other chaperones in cell signaling is an emerging concept that has been discussed, especially in leukocytes in which PDI may function as an extracellular signal after being secreted (Henderson and Pockley 2010).
Based on proteomics studies of lipid bodies isolated by subcellular fractionation, PDI was previously associated with lipid bodies from Chinese hamster ovarian K2 (CHO K2), HeLa cells (Bartz et al. 2007) and U937 monocytic cells (Wan et al. 2007). This is the first time that PDI presence in situ has been associated with lipid bodies. In addition, PDI was localized within secretory granules from human eosinophils. Evidence indicative of protein synthesis within secretory granules (Behzad et al. 2010; Dvorak 2005; Wan et al. 2007) and even lipid bodies (Dvorak 2005) in cells from the immune system, including human eosinophils (Behzad et al. 2010; Wan et al. 2007) supports the view that PDI might be involved in the proper folding and in the formation and reshuffling of the disulfide bridges of the proteins synthesized within these organelles.
Immunonanogold EM also revealed that vesicular compartments distributed in the eosinophil cytoplasm were clearly labeled for PDI (Figs. 2B, 3Aii, 4A). Some of these vesicles likely represent part of the peripheral ER in which the process of protein folding is occurring. However, PDI was also labeled in the lumen of vesicles showing the typical morphology of EoSVs (Fig. 4A). Ultrastructural observations using refined techniques, such as electron tomography, showed that EoSVs are able not only to interact with eosinophil secretory granules but also to originate from these organelles (Melo et al. 2005b). Therefore, the presence of PDI in EoSVs may indicate a role for PDI in protein traffic. We also documented the interaction of PDI-positive vesicular compartments with lipid bodies (Fig. 3).
In contrast to activated monocytes (Gadgil et al. 2003) and neutrophils (Zhang et al. 2010), activated eosinophils did not clearly change the levels of intracellular PDI (Fig. 1).
Taken together, our present results demonstrate, for the first time, sites of localization of PDI within eosinophil leukocytes. The consistent expression of these molecules in non-ER sites, mainly in secretory granules and granule-associated vesicular compartments, indicates that this molecule may have additional/specific functions in these leukocytes.
Acknowledgments
We gratefully acknowledge the skillful assistance of Ellen Morgan and Rita Monahan-Earley (Electron Microscopy Unit, Department of Pathology, BIDMC, Harvard Medical School).
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Institutes of Health (NIH grants, USA- R37AI020241, R01AI022571) and by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil- 305983/2011-3, 477475/2013-2) and Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG, Brazil- APQ-04129-10, CBB PPM 00456/12).
References
- Akagi S, Yamamoto A, Yoshimori T, Masaki R, Ogawa R, Tashiro Y. (1989). Distribution of protein disulfide isomerase in rat epiphyseal chondrocytes. J Histochem Cytochem 37:1835-1844 [DOI] [PubMed] [Google Scholar]
- Akagi S, Yamamoto A, Yoshimori T, Masaki R, Ogawa R, Tashiro Y. (1988). Distribution of protein disulfide isomerase in rat hepatocytes. J Histochem Cytochem 36:1533-1542 [DOI] [PubMed] [Google Scholar]
- Alhamidi M, Kjeldsen Buvang E, Fagerheim T, Brox V, Lindal S, Van Ghelue M, Nilssen O. (2011). Fukutin-related protein resides in the Golgi cisternae of skeletal muscle fibres and forms disulfide-linked homodimers via an N-terminal interaction. PLoS One 6:e22968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appenzeller-Herzog C, Ellgaard L. (2008). The human PDI family: versatility packed into a single fold. Biochim Biophys Acta 1783:535-548 [DOI] [PubMed] [Google Scholar]
- Bartz R, Zehmer JK, Zhu M, Chen Y, Serrero G, Zhao Y, Liu P. (2007). Dynamic activity of lipid droplets: protein phosphorylation and GTP-mediated protein translocation. J Proteome Res 6:3256-3265 [DOI] [PubMed] [Google Scholar]
- Baumann O, Walz B. (2001). Endoplasmic reticulum of animal cells and its organization into structural and functional domains. Int Rev Cytol 205:149-214 [DOI] [PubMed] [Google Scholar]
- Behzad AR, Walker DC, Abraham T, McDonough J, Mahmudi-Azer S, Chu F, Shaheen F, Hogg JC, Pare PD. (2010). Localization of DNA and RNA in eosinophil secretory granules. Int Arch Allergy Immunol 152:12-27 [DOI] [PubMed] [Google Scholar]
- Benham AM. (2012). The protein disulfide isomerase family: key players in health and disease. Antioxid Redox Signal 16:781-789 [DOI] [PubMed] [Google Scholar]
- Dvorak AM. (2005). Mast cell secretory granules and lipid bodies contain the necessary machinery important for the in situ synthesis of proteins. Chem Immunol Allergy 85:252-315 [DOI] [PubMed] [Google Scholar]
- Friedman JR, Voeltz GK. (2011). The ER in 3D: a multifunctional dynamic membrane network. Trends Cell Biol 21:709-717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadgil HS, Pabst KM, Giorgianni F, Umstot ES, Desiderio DM, Beranova-Giorgianni S, Gerling IC, Pabst MJ. (2003). Proteome of monocytes primed with lipopolysaccharide: analysis of the abundant proteins. Proteomics 3:1767-1780 [DOI] [PubMed] [Google Scholar]
- Gill DJ, Tham KM, Chia J, Wang SC, Steentoft C, Clausen H, Bard-Chapeau EA, Bard FA. (2013). Initiation of GalNAc-type O-glycosylation in the endoplasmic reticulum promotes cancer cell invasiveness. Proc Natl Acad Sci U S A 110:E3152-3161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahm E, Li J, Kim K, Huh S, Rogelj S, Cho J. (2013). Extracellular protein disulfide isomerase regulates ligand-binding activity of alphaMbeta2 integrin and neutrophil recruitment during vascular inflammation. Blood 121:3789-3800, S3781-3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson B, Pockley AG. (2010). Molecular chaperones and protein-folding catalysts as intercellular signaling regulators in immunity and inflammation. J Leukoc Biol 88:445-462 [DOI] [PubMed] [Google Scholar]
- Hu J, Prinz WA, Rapoport TA. (2011). Weaving the web of ER tubules. Cell 147:1226-1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurindo FR, Fernandes DC, Amanso AM, Lopes LR, Santos CX. (2008). Novel role of protein disulfide isomerase in the regulation of NADPH oxidase activity: pathophysiological implications in vascular diseases. Antioxid Redox Signal 10:1101-1113 [DOI] [PubMed] [Google Scholar]
- Laurindo FR, Pescatore LA, Fernandes Dde C. (2012). Protein disulfide isomerase in redox cell signaling and homeostasis. Free Radic Biol Med 52:1954-1969 [DOI] [PubMed] [Google Scholar]
- Melo RCN, Liu L, Xenakis JJ, Spencer LA. (2013). Eosinophil-derived cytokines in health and disease: unraveling novel mechanisms of selective secretion. Allergy 68:274-284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo RCN, Perez SA, Spencer LA, Dvorak AM, Weller PF. (2005a). Intragranular vesiculotubular compartments are involved in piecemeal degranulation by activated human eosinophils. Traffic 6:866-879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo RCN, Spencer LA, Dvorak AM, Weller PF. (2008). Mechanisms of eosinophil secretion: large vesiculotubular carriers mediate transport and release of granule-derived cytokines and other proteins. J Leukoc Biol 83:229-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo RCN, Spencer LA, Perez SA, Ghiran I, Dvorak AM, Weller PF. (2005b). Human eosinophils secrete preformed, granule-stored interleukin-4 through distinct vesicular compartments. Traffic 6:1047-1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo RCN, Spencer LA, Perez SA, Neves JS, Bafford SP, Morgan ES, Dvorak AM, Weller PF. (2009). Vesicle-mediated secretion of human eosinophil granule-derived major basic protein. Lab Invest 89:769-781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterfi Z, Donko A, Orient A, Sum A, Prokai A, Molnar B, Vereb Z, Rajnavolgyi E, Kovacs KJ, Muller V, Szabo AJ, Geiszt M. (2009). Peroxidasin is secreted and incorporated into the extracellular matrix of myofibroblasts and fibrotic kidney. Am J Pathol 175:725-735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg HF, Dyer KD, Foster PS. (2013). Eosinophils: changing perspectives in health and disease. Nat Rev Immunol 13:9-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata Y, Shemesh T, Prinz WA, Palazzo AF, Kozlov MM, Rapoport TA. (2010). Mechanisms determining the morphology of the peripheral ER. Cell 143:774-788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turano C, Coppari S, Altieri F, Ferraro A. (2002). Proteins of the PDI family: unpredicted non-ER locations and functions. J Cell Physiol 193:154-163 [DOI] [PubMed] [Google Scholar]
- Turner NA, Nolasco L, Ruggeri ZM, Moake JL. (2009). Endothelial cell ADAMTS-13 and VWF: production, release, and VWF string cleavage. Blood 114:5102-5111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan HC, Melo RCN, Jin Z, Dvorak AM, Weller PF. (2007). Roles and origins of leukocyte lipid bodies: proteomic and ultrastructural studies. FASEB J 21:167-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson ML. (1955). The nuclear envelope; its structure and relation to cytoplasmic membranes. J Biophys Biochem Cytol 1:257-270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang PH, Li LL, Zeng JZ, Yang LR, Ren LC, Liang PF, Huang XY. (2010). Preliminary proteomic analysis of circulating polymorphonuclear neutrophils from rabbits experiencing scald injury and Staphylococcus aureus sepsis. Inflamm Res 59:307-314 [DOI] [PubMed] [Google Scholar]