Abstract
The genetic basis of type 2 diabetes remains incompletely defined despite the use of multiple genetic strategies. Multiparental populations such as heterogeneous stocks (HS) facilitate gene discovery by allowing fine mapping to only a few megabases, significantly decreasing the number of potential candidate genes compared to traditional mapping strategies. In the present work, we employed expression and sequence analysis in HS rats (Rattus norvegicus) to identify Tpcn2 as a likely causal gene underlying a 3.1-Mb locus for glucose and insulin levels. Global gene expression analysis on liver identified Tpcn2 as the only gene in the region that is differentially expressed between HS rats with glucose intolerance and those with normal glucose regulation. Tpcn2 also maps as a cis-regulating expression QTL and is negatively correlated with fasting glucose levels. We used founder sequence to identify variants within this region and assessed association between 18 variants and diabetic traits by conducting a mixed-model analysis, accounting for the complex family structure of the HS. We found that two variants were significantly associated with fasting glucose levels, including a nonsynonymous coding variant within Tpcn2. Studies in Tpcn2 knockout mice demonstrated a significant decrease in fasting glucose levels and insulin response to a glucose challenge relative to those in wild-type mice. Finally, we identified variants within Tpcn2 that are associated with fasting insulin in humans. These studies indicate that Tpcn2 is a likely causal gene that may play a role in human diabetes and demonstrate the utility of multiparental populations for positionally cloning genes within complex loci.
Keywords: Tpcn2, heterogeneous stock rats, expression QTL mapping, type 2 diabetes, glucose, insulin, Multiparent Advanced Generation Inter-Cross (MAGIC), multiparental populations, MPP, gene mapping
TO date, human genome-wide association studies (GWAS) have identified >60 genes involved in type 2 diabetes (T2D) (Zeggini et al. 2008; Voight et al. 2010; Morris et al. 2012; DIAbetes Genetics Replication and Meta-analysis (DIAGRAM) Consortium et al. 2014) and an additional 53 involved in related metabolic traits such as fasting glucose and insulin levels (Dupuis et al. 2010; Scott et al. 2012). Despite the relative success of human GWAS, when combined, these genes explain only a small percentage of the heritable variance (So et al. 2011; Morris et al. 2012), indicating many more genes have yet to be identified. Improving power with increased sample size, deep sequencing to identify rare variants, and identification of gene–gene and gene–environment interactions will help to identify at least some of this missing heritability in humans (Hu and Daly 2012). Animal models, however, offer an additional, complementary, method for identifying candidate genes and their related pathways. Advantages of animal models include the ability to control environment and genetics and assess gene expression levels in tissues that are not readily available from humans. Importantly, genes found for complex traits in animal models are frequently translated to humans (Swanberg et al. 2005; Aitman et al. 2006; Samuelson et al. 2007; Behmoaras et al. 2008; Petretto et al. 2008), demonstrating the utility of animal models for uncovering at least some of the missing heritability in humans.
Particularly useful are multiparental or outbred populations such as heterogeneous stocks (HS). HS animals are derived from crossing eight inbred strains and outbreeding them for many generations (see Flint and Eskin 2012 or Solberg Woods 2014 for a review on HS populations). The genetic makeup of the resulting progeny represents a random mosaic of the founder animals, with each HS rat being phenotypically and genetically distinct. The distance between recombination events decreases with each generation of breeding, such that HS mice and rats have been used to map multiple complex traits to generally <5 Mb (Talbot et al. 1999, 2003; Demarest et al. 2001; Valdar et al. 2006; Solberg Woods et al. 2010, 2012; Johnsen et al. 2011; Baud et al. 2013). This fine-resolution mapping significantly decreases the number of potential candidate genes within each locus relative to those in conventional mapping methods (such as those using an F2 intercross or backcross). Once narrowed, HS founder sequence can be used to rapidly identify potentially causative genes within at least some of these loci (Keane et al. 2011; Baud et al. 2013), thus making these populations ideal for positional cloning of genes involved in complex traits.
We have used HS rats to fine-map traits involved in diabetes and related traits. Our work has shown that HS rats harbor alleles for glucose tolerance (Solberg Woods et al. 2010), as well as insulin resistance and β-cell dysfunction, the underlying causes of type 2 diabetes (Solberg Woods et al. 2012). We have identified a 3.1-Mb region on rat chromosome 1 that maps both fasting and postprandial glucose (Solberg Woods et al. 2010, 2012), and this region overlaps a larger 7-Mb region that maps fasting insulin and insulin sensitivity (Solberg Woods et al. 2012). Although a great improvement over previous mapping studies in F2 intercrosses (Galli et al. 1996; Gauguier et al. 1996; Chung et al. 1997; Kanemoto et al. 1998; Wei et al. 1999; Nobrega et al. 2009; Solberg Woods et al. 2009; Wallis et al. 2009), this region still contains a relatively large number of genes: 86 within the 3.1-Mb glucose region and >200 genes in the insulin region. Although a few plausible genes (including Kcnq1 and Igf2) stand out as likely candidates within the 7-Mb insulin region, none of the 86 genes within the 3.1-Mb region present themselves as obvious potential biological candidate genes.
The goal of the present study was to use expression analysis and next-generation sequencing to evaluate positional candidate genes within this 3.1-Mb glucose locus, located on rat chromosome 1: 204.38–207.48 Mb, a complex region that may harbor multiple causal genes (Granhall et al. 2006; Solberg Woods et al. 2010, 2012). Expression and sequence analysis in the HS rats identified two-pore segment channel 2 (Tpcn2) as a plausible candidate gene within this region. We confirm the role of Tpcn2 by demonstrating that Tpcn2 knockout mice exhibit altered fasting glucose and insulin in response to a glucose challenge. We also demonstrate significant association between SNPs in Tpcn2 and fasting insulin in humans. Together this work indicates that Tpcn2 is a strong candidate for being a causal gene that underlies the glucose and insulin phenotypes at this QTL.
Materials and Methods
Animals
Heterogeneous stock colony:
The NMcwi:HS colony, hereafter referred to as HS, was initiated by the National Institutes of Health (NIH) in 1984, using the following eight inbred founder strains: ACI/N, BN/SsN, BUF/N, F344/N, M520/N, MR/N, WKY/N, and WN/N (Hansen and Spuhler 1984). This colony has been maintained at the Medical College of Wisconsin (MCW) since 2006 and has been through >60 generations of breeding (see Solberg Woods et al. 2010 for history of the breeding colony).
Founding inbred substrains:
Expression analysis was conducted in the following substrains (abbreviated names used throughout the article are in parentheses): ACI/Eur (ACI), BN/SsnHsd (BN), BUF/NHsd (BUF), F344/NHsd (F344), M520/N, and WKY/NHsd (WKY). Other than M520/N, which is the original founder of the HS colony, substrains were chosen based on origin similarities to the original founders, as determined by data obtained from the Rat Genome Database: http://rgd.mcw.edu/strains/. The M520 and ACI rats are maintained at MCW. All other substrains were ordered from Harlan Sprague Dawley (Indianapolis). We were unable to identify substrains for two of the founders (MR/N and WN/N), so these strains were not tested.
Cluster analysis of glucose and insulin levels in HS rats
Rats for expression analysis were chosen from a group of 522 HS rats previously used to map glucose tolerance (Solberg Woods et al. 2010) (see Supporting Information, File S2). As previously described, HS rats underwent an intraperitoneal glucose tolerance test (IPGTT) test at 16 weeks of age in which blood glucose and plasma insulin were measured at fasting and at 15, 30, 60, and 90 min after a 1-g/kg glucose injection (Solberg Woods et al. 2010, 2012). Glucose area under the curve (glucose_AUC) and insulin_AUC were determined using a trapezoidal analysis. The quantitative insulin sensitivity check (QUICKI), a measure of insulin sensitivity that has been validated in both rats and humans (Katz et al. 2000; Cacho et al. 2008), was calculated as where is fasting insulin and is fasting glucose (Solberg Woods et al. 2012). HS rats were killed at 17 weeks of age, 1 week after the glucose tolerance test. Fasting cholesterol, triglycerides, and c-peptide were determined from plasma at the time the animals were killed. Livers were immediately removed and frozen in liquid nitrogen for subsequent determination of transcript abundance. Rats were killed by decapitation to avoid gene expression changes that can occur when using inhalant drugs such as CO2 or isoflurane (Kadar et al. 2011; Taylor and Cummins 2011). All protocols were approved by the Institutional Animal Care and Use Committee at MCW.
To determine how many phenotypic groups existed within the HS colony, a K-means cluster analysis was conducted. Clusters were determined using the following glucose and insulin measurements: glucose_AUC, insulin_AUC, peak time, the difference between baseline and the peak value, the slope of the line from right before the peak to peak time, and slope from peak time to right after the peak. The K-means clustering has an animal’s phenotype values added to a cluster based on minimizing differences from the means of the K clusters. Canonical discrimination using SAS 9.1 was used to determine how the clustering related to the phenotype measures.
Expression analysis
Expression analysis was conducted in 46 male HS rats and 2 males from each of six inbred substrains, for a total of 58 animals. Total RNA was extracted from frozen liver tissue, using TRIzol reagent [Invitrogen (now Life Technologies), Carlsbad, CA]. Purified RNA (50 ng) was amplified using an Affymetrix two-cycle cDNA synthesis kit, and complementary RNA was synthesized, labeled, fragmented, and hybridized to the Gene-Chip rat genome 230_2.0 array in accordance with standard protocols (Affymetrix, Santa Clara, CA). The rat genome 230_2.0 array contains >31,000 probe sets representing >17,734 unique UniGenes, containing probes for 57 of the 86 genes (66.3%) within our region of interest [rat chromosome 1: 204.38–207.48 Mb (Solberg Woods et al. 2010, 2012)]. After hybridization, arrays were washed and stained with an Affymetrix Wash and Stain kit plus an Affymetrix GeneChip Fluidics Station 450 and scanned with an Affymetrix 7G laser scanner. Data were analyzed with Expression Console 1.1.2 software (Affymetrix) and normalized with robust multichip analysis (RMA) (http://www.bioconductor.org/) to determine signal log ratios (log2R). The statistical significance of differential gene expression was determined through ANOVA, using Partek (St. Louis) Genomics Suite 6.5. Differential expression (DE) was defined as those genes with P ≤ 0.05 and log2R ≥ 0.5. Because we planned to verify differential expression of genes of interest with quantitative RT-PCR (qRT-PCR), we did not correct for multiple testing (Hessner et al. 2004).
Real-time qRT-PCR
We initially confirmed differential expression of Tpcn2 using qRT-PCR in 10 HS rats with glucose intolerance and 10 with normal glucose regulation. qRT-PCR was also conducted in the founder strains, using 2 animals from each inbred strain. Upon confirming Tpcn2 as a plausible candidate gene, we used qRT-PCR to assess gene expression levels in a total of 120 male HS rats. qRT-PCR was conducted on an ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA). Specific oligonucleotide primers were designed with Primer3 (http://binoinfo.ut.ee/primer3-0.4.0/primer3/) (Koressaar and Remm 2007; Untergasser et al. 2012). To avoid differences in expression levels based on sequence variation (Huang et al. 2009), we ensured no sequence variants were present in any of the HS founders at the location of the Tpcn2 primers. The primers for Tpcn2 were forward 5′-TATGTGTTCGCCATGATTGG-3′ and reverse 5′-AGCAGCAAAGTCGTCGAAGT (Integrated DNA Technologies). We used primers for GAPDH, forward 5′-CATGGAGAAGGCTGGGGCTC-3′ and reverse 5′-AACGGATACATTGGGGGTAG-3′, and β-2 microglobulin (B2M), forward 5′-CCGTGATCTTTCTGGTGCTT-3′ and reverse 5′-TTTTGGGCTCCTTCAGAGTG-3′ (Integrated DNA Technologies), as internal controls. We used iQ SYBR Green Supermix (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions. Synthesis of first-strand cDNA from 3 μg of RNA per animal was accomplished with random hexamers (Invitrogen, now Life Technologies) and Superscript III (Invitrogen, now Life Technologies), according to the manufacturer’s instructions. Triplicate Tpcn2 and GAPDH or B2M were performed for each sample in 20-μl reactions, which included 1 μl of cDNA and 10 μl of iQ SYBR Green Supermix (Bio-Rad) possessing 1.2 μl of Tpcn2 (10 μM), GAPDH, or B2M specific primers and 7.8 μl of deionized water. Reactions were cycled as follows: stage 1, 95°, 3 min; stage 2, 55 cycles of 95°, 30 sec, 55°, 30 sec, 72°, 30 sec; and stage 3, melt curve of 95°, 10 sec, 60°, 1 min, 95°, 1 sec, 40°, 10 sec. Standard curves were created using 1:10, 1:100, and 1:1000 concentrations of Tpcn2. Specificity for a qRT-PCR was verified by a melting curve analysis. The data were analyzed with Sequence Detection System 2.3 software (Applied Biosystems), using the cycle threshold (ct) for quantification. Relative gene expression data (fold change) between samples was accomplished using the mathematical model described by Pfaffl (2001), using the mean ct score of 120 animals as the reference.
Genome-wide genotyping
Tail samples from the original eight inbred founders were obtained from the NIH. We extracted DNA from tail tissue from HS and founder strains, using the QIAGEN (Valencia, CA) DNeasy kit. All eight original founder inbred strains and the 46 HS rats used for expression analysis were genotyped using the Affymetrix GeneChip Targeted Genotyping technology on a custom 10K SNP array panel as previously described (Saar et al. 2008). The samples were genotyped by the Vanderbilt Microarray Shared Resource center at Vanderbilt University in Nashville, Tennessee (currently VANTAGE: http://vantage.vanderbilt.edu). From the 10,846 SNPs that are on the array, 7634 were informative and produced reliable results in the HS rats. From these final informative markers, the average spacing was 353 kb apart, with an average heterozygosity of 28.8%. These data have been deposited in the Gene Expression Omnibus (GEO) database under accession no. GSE57118 (see: http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=sjmjgkwulbebtmd&acc=GSE57118).
Genetic mapping of expression QTL
Expression mapping was conducted for all genes on the expression array that were located within the QTL (57 of 86). Genome-wide fine mapping was conducted by linkage disequilibrium mapping, testing for the significance of association between expression levels and inferred haplotype descent at each locus, as previously described (Solberg Woods et al. 2010, 2012). Briefly, haplotype descent along each HS rat genome was inferred using the haplotype reconstruction method HAPPY (Mott et al. 2000), which applies a hidden Markov model simultaneously to the genotypes of the eight founder strains and the 46 HS rats. At each interval between adjacent pairs of markers, HAPPY produces for each rat i a vector containing the probabilities of descent from each of the unique haplotype pairs (diplotypes) (Valdar et al. 2009). Using Bagpipe software (Valdar et al. 2009), this is then used in the following mixed-model regression to predict rat i’s expression levels
| (1) |
where is a transformed version of the expression levels, models the putative effect of the QTL, is the (random) effect of the sibship k to which i belongs, and residuali accounts for individual variation (further parameters defined as in Solberg Woods et al. 2010). Models were fitted by restricted-estimate maximum likelihood, and the significance of association at each interval m was assessed by comparing the fit of Equation 1 with that of the null model, which is Equation 1 without the QTL term. Genome-wide significance thresholds were estimated by parametric bootstrap from the fitted null model (Valdar et al. 2009; Solberg Woods et al. 2010). Prior to genetic analysis, expression levels for each gene were normalized using a van der Waerden normal score.
Sequencing and analysis
We sequenced 5 Mb within rat chromosome 1: 200–208 Mb in the eight inbred founder strains of the HS colony. We designed a custom 385K NimbleGen array that included all coding regions and all regions that are highly conserved. The array was composed of 385,000 long oligonucleotide probes and used a tiling design to target our unique genomic region. Five to eight μg of genomic DNA from each strain was nebulized and polished, and linkers were added according to the manufacturer’s standards (Roche NimbleGen). Samples were then hybridized to the custom NimbleGen 385K array. DNA not specific to these regions were washed off and targeted DNA sequences were eluted and amplified by emulsion PCR. To calculate whether targeted sequences were enriched, both pre- and postcapture DNAs were analyzed by qRT-PCR, using four real-time PCR assays developed and optimized by our laboratory. The region was then sequenced on the Roche GS-FLX 454 system (Roche 454, Branford, CT) according to the manufacturer’s protocol. Briefly, Roche “A” and “B” adapters were annealed onto captured fragments of template DNA. The library was single stranded and quantified on an Agilent 2100 Bioanalyzer. Library fragments were annealed to beads and emulsion PCR was performed. After clonal amplification by PCR, the emulsion was broken chemically and beads were collected and enriched. Enriched beads were then inserted into the Roche Picotiter Plate, using the appropriate gasket, and loaded onto the machine. Nucleotides flowed over the plate in a specific order and enzymatic cascade reaction caused incorporation of the specific nucleotides to illuminate. An image of every flow was taken with a CCD camera and images were strung together into a flowgram, which was transformed into usable sequence data.
Reads were separated by their multiplex identifiers, using the program sfftools (Roche). For each sample, demultiplexed reads were then trimmed and assembled against the rat chromosome 1 reference sequences (Rn4), using the GS Reference Mapper (http://454.com/products/analysis-software/index.asp). Variant detection was performed using the high-confidence differences strategies implemented in GS Reference Mapper software. The high-confidence differences (HCDiff) strategy requires the following criteria for a variant to be reported: (1) There must be at least three reads with the difference; (2) there must be both forward and reverse reads showing the difference, unless there are at least five reads with quality scores >20 (or 30 if the difference involves a 5-mer or higher); and (3) if the difference is a single-base overcall or undercall, then the reads with the difference must form the consensus of the sequenced reads.
Since the time of this work, all founder strains have been fully sequenced (Baud et al. 2013), and this information is currently available in the Rat Genome Database (http://rgd.mcw.edu). We have compared our sequence to what is available online. Some discrepancies were noted between the data sets. To determine the accurate sequence, these regions were sequenced using Sanger sequence technology.
Taqman genotyping
We genotyped 18 SNPs within the 3.1-Mb QTL in 508 male HS rats and all eight inbred founders. SNPs were chosen based on the founder strain distribution pattern (SDP) as a means of defining general haplotype blocks (Yalcin et al. 2004a). To ensure most haplotyes were represented, we used a measure of entropy as previously described (Yalcin et al. 2005). Briefly, a sliding window was used in which each SNP within the window is prioritized based on its ability to explain the diversity between the founder strains. SNPs were then given a score and those with the highest scores were chosen for genotyping. In addition to SNPs with high entropy scores, we also genotyped nonsynonymous coding variants in the F344 strain, the founder that contributes the susceptibility allele at the QTL (Solberg Woods et al. 2010, 2012). SNPs were spaced an average of 164.5 kb apart.
Samples were genotyped using 5′-exonuclease TaqMan technology (Applied Biosystems) with differently fluorescence-labeled probes. Custom TaqMan SNP Genotyping Assays (Applied Biosystems) were used. Ten nanograms of DNA was used in a total volume of 5 μl containing 2 μl of 5ng/μl DNA, 2 μl 1× TaqMan Universal PCR Master Mix (Applied Biosystems), 0.125 μl probe, and 0.875 μl water for each sample. PCR was carried out on a GeneAmp PCR System 9700 (Applied Biosystems) and the post-PCR plate was read on the ViiA 7 real-time PCR system (Applied Biosystems), following the manufacturer’s instructions. ViiA 7 RUO version 1.2.1 software was used to assign genotypes, applying the allelic discrimination test. The overall call rate was 98.8%.
Glucose tolerance test in Tpcn2 knockout mice
Wild-type and knockout mice were created using embryonic stem cells from the 129P2 strain carrying a gene trap vector and injected into C57BL/6 blastocysts, as previously described (Calcraft et al. 2009). We confirmed that Tpcn2 is not transcribed in the knockout mice by running qPCR on multiple tissues in knockout and wild-type mice (see Figure S1). PCR was conducted as described above, using primers for GAPDH (forward 3′-CTGAAGGGCATCTTGGGCTA-5′ and reverse 3′-GCCGTATTCATTGTCATACCA-5′) and Tpcn2 (forward 3′-CCAGGCTACCTGTCCTACCA-5′ and reverse 3′-CAGGAAGCGAAACACAATCA-5′). Heterozygous mice were bred and set up as breeder pairs. Male Tpcn2 knockout, heterozygote, and wild-type mice from the heterozygote breeders were phenotyped at 9–13 weeks of age (n = 5–7 in each group), using the IPGTT, described above and in Solberg Woods et al. (2010, 2012). Blood was collected after an overnight fast and at 15, 30, 60, 90, and 120 min after a 1-g/kg glucose injection. We used the Ascensia Elite system for reading blood glucose values (Bayer, Elkhart, IN). We also collected blood at each time point for subsequent analysis of plasma insulin levels, which was assayed using an ultrasensitive ELISA kit from Alpco Diagnostics (Salem, NH).
Statistical analysis
A mixed model was used to determine association between 18 SNPs within the chromosome 1 QTL and diabetic phenotypes that map to this region (glucose_AUC, fasting glucose, fasting insulin, insulin_AUC, and QUICKI) and Tpcn2 expression levels (as determined by qPCR). The model included all appropriate covariates (location, injector, collector, and number of glucose injections) and accounted for the complex family relationships of the HS, as previously described (Solberg Woods et al. 2010, 2012). The significance threshold was determined using the Bonferroni method to account for multiple comparisons (18 SNPs by six traits). Correlations between Tpcn2 expression levels (as determined by qRT-PCR) and these traits were assessed using Pearson’s correlation coefficient. Pearson’s correlation coefficient was also used to determine correlations between all microarray probes within the chromosome 1 region and metabolic traits, using a Bonferroni correction to determine significance. A one-way ANOVA was used to determine whether there were statistical differences between wild-type, heterozygote, and Tpcn2 knockout mice. If a trend toward significance was noted, a t-test was used to assess significance between wild-type and knockout mice only.
Tpcn2 lookup in human GWAS
We used data from two large consortiums to determine whether Tpcn2 may play a role in human diabetes. Diabetes Genetics Replication and Meta-analysis (DIAGRAM) has collected 2.4 million SNPs in >47,000 cases and controls (Zeggini et al. 2008; Voight et al. 2010), with 34,840 cases and 114,981 controls recently genotyped on the custom Metabochip (Morris et al. 2012). The Meta-Analyses of Glucose and Insulin-related traits Consortium (MAGIC) has collected phenotypic information for quantitative glycemic traits such as glucose and insulin in >40,000 individuals (Dupuis et al. 2010), with 133,010 individuals genotyped on the custom Metabochip (Scott et al. 2012). Both DIAGRAM and MAGIC cohorts consist of males and females mainly of European descent. Through collaboration with these investigators, we inquired whether a subset of 39 SNPs within Tpcn2 was associated with diabetic traits in humans. The 39 SNPs were chosen based on information from the HapMap project (www.hapmap.org), choosing SNPs with an average r2 > 0.8. All but 2 SNPs had an average r2 > 0.9. Fourteen of the 39 SNPs were upstream and 4 were downstream of the Tpcn2 gene (see Table S1). For those SNPs that reached an unadjusted level of significance at P ≤ 0.05, we used the Benjamini and Hochberg (1995) false discovery rate to account for multiple comparisons.
Results
Glucose and insulin phenotypes in HS rats separate into five main clusters
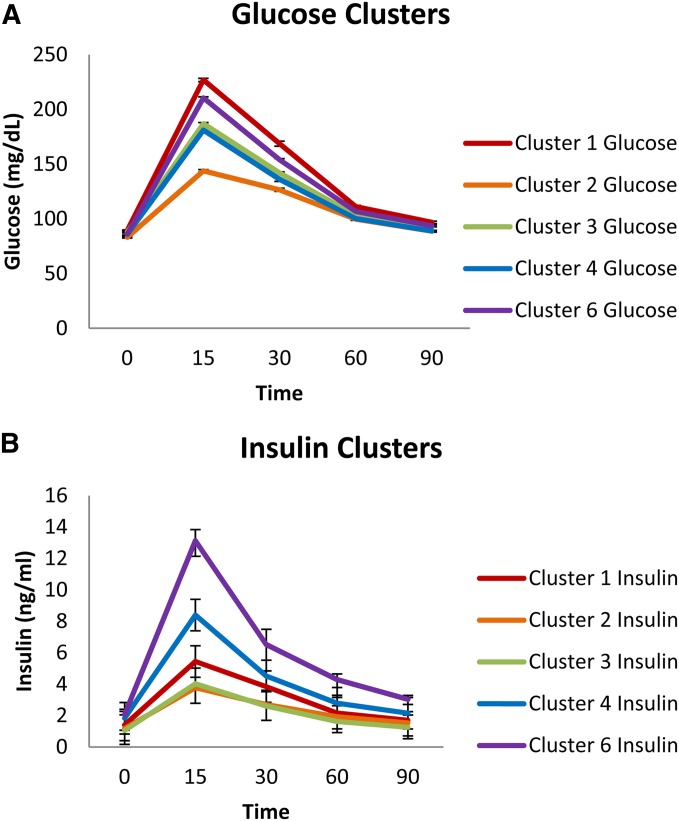
Although we initially identified six clusters based on glucose and insulin values, one of the clusters (cluster 5) contained a single animal and was excluded from subsequent analysis. There are two dominant phenotypic factors that characterized the clusters. The first (which explained 65% of the variability between clusters) consists of the magnitude of the peak of the insulin curve and the glucose curve, the rate of increase of the glucose from baseline to peak, the rate of increase of insulin from baseline to peak, and glucose_AUC. The second factor (which explained 31% of the variability between the clusters) consists of the rate of decrease of the insulin curve after the peak and the rate of decrease of the glucose curve after the peak.
The five clusters are shown in Figure 1 and demonstrate the importance of taking into account both glucose and insulin in the clustering. Note that animals in clusters 2 and 3 exhibit very similar plasma insulin levels, but differ significantly in blood glucose levels. Similarly, animals from clusters 3 and 4 exhibit similar blood glucose levels, but differ significantly in plasma insulin levels. For the expression analysis, glucose intolerant HS rats were chosen from cluster 1 (high glucose, moderate insulin) and HS rats with normal glucose regulation were chosen from clusters 2 and 4 (low glucose and insulin and moderate glucose and insulin, respectively). The glucose intolerant group is highly significantly different from the normal glucose group (glucose_AUC = 13,458.9 vs. 9061, F1,44 = 398.7, P < 2e-16). Animals in the glucose intolerant group also exhibit differences in several other phenotypes, including significantly higher levels of body weight (349.1 vs. 314.4 g, F1,44 = 15.47, P = 0.000294), fasting glucose (90.3 vs. 77.5 mg/dl, F1,44 = 42.65, P = 5.59e-08), fasting insulin (1.8 vs. 1.1 ng/ml, F1,44 = 7.52, P = 0.009), insulin_AUC (311.3 vs. 204.3, F1,44 = 17.10, P = 0.000157), QUICKI (0.48 vs. 0.54, F1,44 = 7.96, P = 0.007) fasting c-peptide (1808.0 vs. 1505.8 pg/ml, F1,44 = 5.75, P = 0.021), and fasting triglycerides (86.2 vs. 72.3 mg/dl, F1,44 = 8.0, P = 0.007). No differences are seen between the groups for fasting cholesterol or fat pad weights (see Table S2).
Figure 1.
(A and B) Cluster analysis of (A) glucose and (B) insulin levels during an intraperitoneal glucose tolerance test in >500 HS rats. Values are expressed in means ± SE. We identified six clusters based on glucose and insulin values. Because cluster 5 included only one animal, however, it is not included in the graphs or in subsequent analyses. Note that clusters with similar glucose values (clusters 1 and 6 or 3 and 4) have drastically different insulin values. Based on this cluster analysis, we selected animals for the expression analysis, choosing glucose intolerant HS from cluster 1 and HS with normal glucose from clusters 2 and 4.
Tpcn2 is the only gene measured within the 3.1-Mb QTL that is differentially expressed between HS rats with different glucose profiles
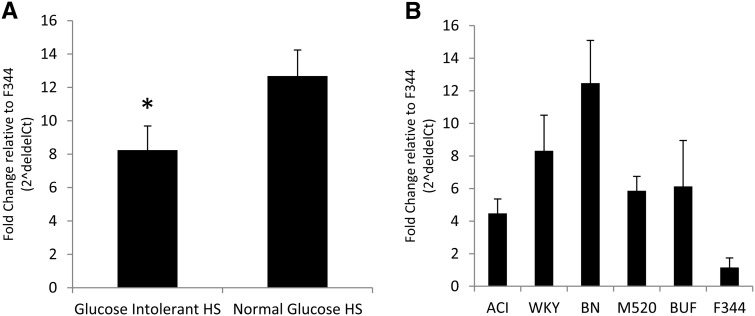
We identify 48 probes genome-wide that are differentially expressed between HS rats with glucose intolerance and those with normal glucose regulation, 3 of which had an unknown location (see Table S3). These data have been deposited in the GEO database under accession no. GSE57118 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=sjmjgkwulbebtmd&acc=GSE57118). Of these 48 genes, only one falls within the 3.1-Mb glucose region on rat chromosome 1: Tpcn2. Statistics for the other probes within the 3.1-Mb region are listed in Table S4. Using qRT-PCR, we confirm differential expression of Tpcn2 against both GAPDH (F1,19 = 4.36, P = 0.05; see Figure 2A) and B2M (F1,18 = 7.03, P = 0.017; data not shown). HS founder strains also show different levels of Tpcn2 expression, with the F344 strain showing the least amount of Tpcn2 expression and the BN strain showing the greatest amount of Tpcn2 expression (see Figure 2B). All fold-change differences are expressed relative to the F344 founder substrain.
Figure 2.
(A and B) Differential expression of Tpcn2 by qRT-PCR in (A) HS rats and (B) HS founder substrains. Bars show the mean ± SE. Glucose intolerant HS rats exhibit an approximately twofold decrease in expression levels of Tpcn2 relative to HS rats with normal glucose levels (*P = 0.05). Large variation is seen in HS founder substrains with BN exhibiting the greatest levels of Tpcn2 and the F344 strain exhibiting the least. All fold-change differences are relative to the F344 founder strain. Note that the scales in A and B are the same.
Tpcn2 maps as a cis-eQTL to the same region as the physiological QTL
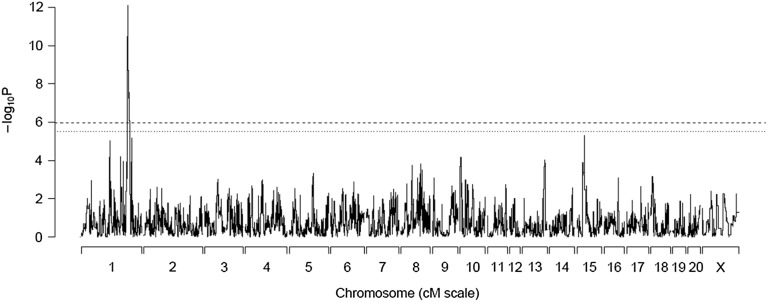
Using haplotype reconstruction and mixed-model linear regression, which takes into account sibship relationships, we identify a single sharp peak (marker interval gko.18d13.rp2.b1.157 and Cpn.1207450582, 205.22–207.45 Mb; log P = 12.09) for expression levels of Tpcn2 (see Figure 3). Only one other gene within this region, Acy3, maps as a cis-eQTL, but with a much lower log P (marker interval WKYOa01b03.r1.818 and rat106.025.f16.p1ca.195, 204.32–207.50 Mb; log P = 6.28). The genome-wide significance threshold is log P = 5.95 for both Tpcn2 and Acy3 expression levels, where −log P is defined as –log10(P-value).
Figure 3.
Genome-wide eQTL scan of Tpcn2 expression levels using 10K SNP markers. The x-axis shows chromosome number and position in centimorgans. The y-axis gives the –log10 P-value of association. The dashed lines represent the genome-wide significance thresholds (bottom line represents the suggestive threshold at P = 0.1 and the top line represents significance at P = 0.05). Tpcn2 expression levels map to within 1 Mb of the gene itself on rat chromosome 1, indicating this gene is cis-regulated.
Nonsynonymous coding variants are identified within three genes, including Tpcn2
As expected, we identify ∼2000–3000 SNPs between each strain and the BN reference genome (Saar et al. 2008). Within the 3.1-Mb glucose locus, we identify three genes with a nonsynonymous amino acid change in the F344 founder strain: Tpcn2, RGD1311946, and Gpr152 (see Table 1). We focused on variants in the F344 strain because our previous work demonstrates that the F344 strain contributes to the susceptibility allele at the QTL for glucose tolerance (Solberg Woods et al. 2010) as well as for fasting glucose and insulin (Solberg Woods et al. 2012). It is important to note that loci for fasting glucose and insulin exhibit a complex genetic architecture with several other founder haplotype combinations leading to an increase in fasting glucose or insulin. Because of this, we were unable to run a simple haplotype analysis, as demonstrated by other groups (Svenson et al. 2012; Logan et al. 2013). The variants within Tpcn2 and RGD1311946 are not predicted to be damaging by PolyPhen or SIFT. The variant within Gpr152, however, results in a premature stop codon and could potentially lead to changes in protein function.
Table 1. HS rat SNPs that pass the region-wide significance threshold for fasting glucose and/or Tpcn2 expression levels.
| SNP location (bp) | Gene/functional location | Trait (−log P) | Variant | Founder strain(s) with variant | Amino acid change (Polyphen and SIFT predictions) |
|---|---|---|---|---|---|
| 205,653,821 | Rmt1/intron | Fasting glucose (3.62) | T to C | ACI, BUF, M520, MR, WKY, WN | NA |
| Tpcn2 expression (6.58) | |||||
| 205,715,459 | Tpcn2/exon | Fasting glucose (3.35) | G to A | F344 | P to L (benign/tolerated) |
| Tpcn2 expression (6.17) | |||||
| 205,898,738 | Cpt1a/exon | Tpcn2 expression (4.90) | G to A | ACI, BUF, M520, MR, WN | V to I (benign/tolerated) |
| 206,242,238 | RGD1311946/exon | Tpcn2 expression (3.69) | G to A | F344 | A to T (unknown/tolerated) |
| 206,726,856 | Gpr152/exon | Tpcn2 expression (4.43) | C to T | F344 | Q to * (unknown/unknown) |
indicates a stop codon.
Variants within and near Tpcn2 are significantly associated with fasting glucose and Tpcn2 expression levels
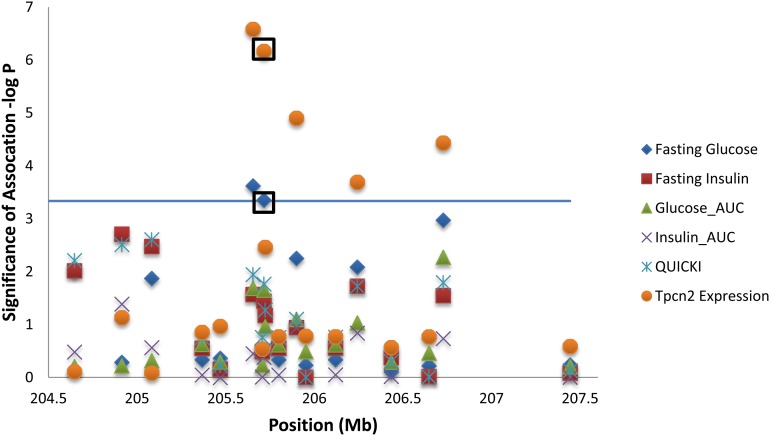
Using a Bonferroni-adjusted region-wide threshold of 3.33, we found that 2 SNPs, located at 205,653,821 bp (−log P = 3.62) and 205,715,459 bp (−log P = 3.35), are significantly associated with fasting glucose (see Figure 4, Table 1, and Table S5). The SNP at 205,715,459 bp harbors the nonsynonymous coding variant within Tpcn2, while the SNP at 205,653,821 bp is located in the intron of the neighboring mammary cancer-associated protein Rmt1 gene. These SNPs and 3 additional SNPs are also associated with Tpcn2 expression levels: 205,653,821 bp (−log P = 6.58), 205,715,459 bp (−log P = 6.17), 205,898,738 bp (−log P = 4.90), 206,242,238 bp (−log P = 3.69), and 206,726,856 bp (−log P = 4.43). The additional SNPs include the nonsynonymous coding variants within RGD1311946 and Gpr152, as well as a coding variant within Cpt1a. No associations are found between the 18 SNPs and glucose_AUC, fasting insulin, insulin_AUC, or QUICKI. Genotypes for the 18 SNPs can be found in File S3.
Figure 4.
Association analysis between 18 SNPs within the 3.1-Mb QTL and all traits that map to this region, including five diabetic traits and Tpcn2 expression levels. A mixed model, which takes into account the complex family structure of the HS, was used. The nonsynonymous coding variant within Tpcn2, which is significant for both fasting glucose and Tpcn2 expression levels, has been marked with a black square. The x-axis shows the position in megabases, and the y-axis give the –log10 P of association. The solid line represents the Bonferroni-adjusted region-wide significance threshold (3.33). Expression levels are based on fold change from the qPCR data, n = 120 HS rats; for all other phenotypes, n = 508 HS rats.
Tpcn2 expression levels negatively correlate with fasting glucose
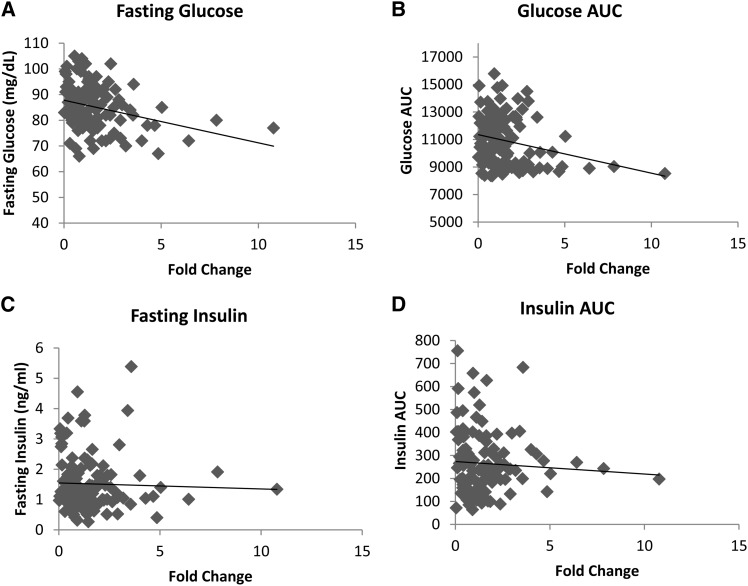
Using the qRT-PCR data in 120 HS rats, we demonstrate a significant negative correlation between Tpcn2 expression levels and fasting glucose and glucose_AUC (r = −0.302, P = 0.001 and r = −0.246, P = 0.007, respectively; see Figure 5). After removing 6 animals that have extremely high expression values (>2 SD from the mean), only fasting glucose remains significantly correlated with expression levels (r = −0.232, P = 0.013). Tpcn2 expression levels are not significantly correlated with fasting insulin, insulin_AUC, and QUICKI (r = −0.034, −0.063, and 0.078, respectively). qRT-PCR data can be found in File S4. To determine whether any other genes in the region are correlated with the diabetic traits, we determined correlation coefficients between expression levels of the microarray probes within the chromosome 1 region and six metabolic traits. We confirm the significant negative correlation between fasting glucose and Tpcn2 expression levels (r = −0.559, P = 0.0000549). Based on a Bonferroni-adjusted significance level of 0.00009, we also find a marginally significant correlation between RGD1307603, a gene with no known function, and QUICKI (r = 0.544, P = 0.00000925). Correlation coefficients and unadjusted P-values are reported in Table S6.
Figure 5.
(A–D) Expression of Tpcn2 in liver of 120 HS rats as determined by qRT-PCR and correlations with (A) fasting glucose, (B) glucose_AUC, (C) fasting insulin, and (D) QUICKI. Fold change is relative to the mean ct of all HS animals. The black line represents the best fit. A significant negative correlation is found between Tpcn2 expression levels and fasting glucose (r = −0.302, P = 0.001) and glucose_AUC (r = −0.246, P = 0.007). When the outliers are removed, fasting glucose remains significant (r = −0.232, P = 0.013).
Tpcn2 knockout mice exhibit decreased fasting glucose and insulin_AUC
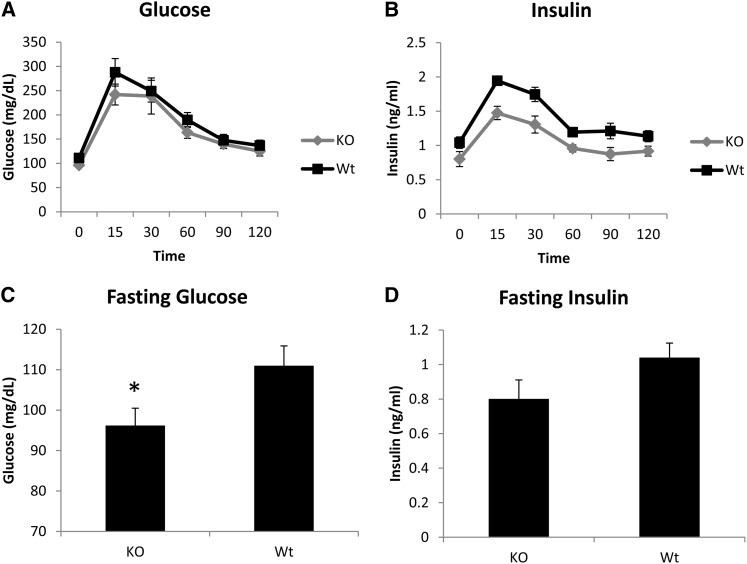
We find a significant main effect of genotype on insulin_AUC (F2,15 = 4.41, P = 0.031), with knockout mice exhibiting significantly decreased insulin response relative to wild-type mice (KO = 126.2, Het = 147.2, WT = 165.4, P = 0.029, Tukey’s post-hoc test; see Figure 6 and Table S7). There is a trend toward significance for fasting glucose (KO = 96.2, Het = 116.3, WT = 111.0, F2,15 = 3.56, P = 0.056) and QUICKI (KO = 0.54, Het = 0.49, WT = 0.49, F2,15 = 3.32, P = 0.066). Upon running a t-test between knockout and wild-type mice only, we find that Tpcn2 knockout mice exhibit significantly decreased fasting glucose levels relative to wild-type mice (t(8.9) = 2.28, P = 0.048). Tpcn2 knockout mice do not differ significantly from wild-type mice in fasting insulin levels or glucose_AUC. Means for all traits are reported in Table S7. Raw data for the knockout, wild-type, and heterozygote mice can be accessed in File S5.
Figure 6.
(A–D) Glucose and insulin levels after a glucose tolerance test (A and B) and at fasting (C and D) in wild-type (WT) and Tpcn2 knockout (KO) mice. Knockout mice exhibit significantly lower fasting glucose levels (*P = 0.048). Although fasting insulin levels do not differ significantly between wild-type and knockout mice, insulin_AUC is highly significantly different (P = 0.031).
Association between Tpcn2 and fasting insulin and HOMA_IR in humans
Although we looked up associations in 39 SNPs, MAGIC was able to provide statistics on only 14 SNPs and DIAGRAM provided statistics on 11 SNPs (see Table S1). Information was not available for the other SNPs likely because they were either rare variants or failed imputation quality control for other reasons. Of the 14 SNPs from MAGIC, 4 SNPs reach unadjusted levels of significance for fasting insulin, 2 of which remain marginally significant after accounting for multiple comparisons (rs10736671, P = 0.051, and rs11604251, P = 0.051; see Table 2). Three of the 4 SNPs reach unadjusted levels of significance for HOMA_IR, a measure of insulin sensitivity (Matthews et al. 1985), but do not remain significant after adjusting for multiple comparisons. Significance is not found between SNPs within Tpcn2 and type 2 diabetes or fasting glucose (data not shown).
Table 2. Association between Tpcn2 SNPs and diabetic traits in humans.
| Fasting insulin | HOMA_IR | ||||
|---|---|---|---|---|---|
| SNP | Location | Unadjusted P-value (effect) | BH adjusted P-value | Unadjusted P-value (effect) | BH adjusted P-value |
| rs11604251 | chr11: 68,820,429 | 0.0073 (+) | 0.0511 | 0.6643 (+) | 0.6643 |
| rs7127082 | chr11: 68,822,023 | 0.02475 (−) | 0.0937 | 0.0392 (−) | 0.2415 |
| rs753559 | chr11: 68,851,605 | 0.02676 (+) | 0.0937 | 0.03936 (+) | 0.2415 |
| rs10736671 | chr11: 68,858,907 | 0.0038 (+) | 0.0511 | 0.05174 (−) | 0.2415 |
Only the 4 most significant SNPs (of 14) are reported. Statistics on the additional SNPs are reported in Table S1. SNPs that remain marginally significant after adjusting for multiple comparisons using the Benjamini and Hochberg FDR method (BH) are in boldface type.
Discussion
In our previous work, we used HS rats to identify a 3.1-Mb region that contains a QTL for glucose tolerance and fasting glucose (Solberg Woods et al. 2010, 2012) and that overlaps larger QTL for fasting insulin and insulin sensitivity (Solberg Woods et al. 2012). In the present study, we use both analysis of gene expression and association analysis to identify Tpcn2 as a likely causal gene within this locus. These findings are supported by altered glucose and insulin levels in a Tpcn2 knockout mouse, as well as by an association between variants within Tpcn2 and fasting insulin levels in humans.
The role of Tpcn2 in regulating glucose and insulin levels is currently unknown. Tpcn2 channels localize to the lysosome and are likely receptors for the calcium-mobilizing agent NAADP (Brailoiu et al. 2009; Calcraft et al. 2009; Schieder et al. 2010). Several studies indicate that NAADP may play a role in insulin signaling of the β-cells (Kim et al. 2008; Naylor et al. 2009; Shawl et al. 2009; Alejandro et al. 2010; Arredouani et al. 2010), and a recent study suggests NAADP is involved in glucose homeostasis (Park et al. 2013). It is also interesting to note that Tpcn2 and other calcium-handling genes (Tpcn1 and IP3R1) have recently been found to be differentially expressed in patients with heart failure (Garcia-Rua et al. 2012). Although previous studies have not looked at the role of Tpcn2 in liver, alterations of calcium channels such as CaMKII have been shown to lead to impaired liver gluconeogenesis (Ozcan et al. 2012), while Cav2.3 calcium channel knockout mice exhibit altered glucose levels, likely as a result of reduced insulin sensitivity (Matsuda et al. 2001). Together these studies suggest a possible role for Tpcn2 in both the β-cell and the liver. Future studies will test the hypothesis that Tpcn2 is involved in glucose-stimulated insulin secretion of the β-cells as well as probe a potential role of this gene in regulating insulin sensitivity and gluconeogenesis.
Global gene expression analysis in HS rats was used to initially identify Tpcn2 as a candidate gene within the glucose QTL. We show that Tpcn2 is differentially expressed in HS rats with glucose intolerance relative to those with normal glucose regulation and demonstrate that this gene maps as a cis-eQTL to the same region as the physiological QTL. Several previous studies have found that genes that map as cis-eQTL within previously identified physiological QTL can be considered prime candidate genes (Hubner et al. 2005; Chen et al. 2008; Morrissey et al. 2011). Demonstrating a correlation between expression levels and fasting glucose levels further supports that this gene may be playing a causal role at the QTL (Farber et al. 2009; Leduc et al. 2011; Morrissey et al. 2011).
Upon assessing association between 18 SNPs within the QTL and traits that map to this region (including five metabolic traits and Tpcn2 expression levels), we find that the nonsynonymous coding variant within Tpcn2, as well as an intronic variant in the neighboring Rmt1 gene, exhibits the highest level of association with fasting glucose levels and Tpcn2 expression levels, further supporting a potential causal role for this gene. Despite having a slightly higher level of association, we do not believe that Rmt1 plays a causal role in diabetes because it is neither correlated with the diabetic traits nor exhibits expression differences. It is interesting to note that three additional SNPs in the region exhibit significant association with Tpcn2 expression levels, indicating that Tpcn2 expression may be regulated by other genes in the region. Three of these SNPs, including the Tpcn2 SNP, are from the F344 founder, the strain that exhibits very low Tpcn2 expression levels (Figure 2) and that contributes the susceptibility allele at this locus (Solberg Woods et al. 2010, 2012). It is unlikely that any of the identified variants are causal, however, because the significance levels of these SNPs do not surpass that of the haplotype mapping for either fasting glucose (Solberg Woods et al. 2012) or Tpcn2 expression levels (Figure 3). A merge analysis, in which genotypes for all SNPs in the region are imputed, may be useful to identify the causative variant (Yalcin et al. 2005; Keane et al. 2011; Baud et al. 2013). However, because this method is most successful when a single causative variant underlies the locus (Baud et al. 2013), it may prove less useful for the current region in which multiple genes/variants may be playing a role.
Upon investigating glucose and insulin levels in Tpcn2 knockout mice, we find that knockout mice exhibit significantly decreased fasting glucose and decreased insulin response to a glucose challenge relative to wild-type mice. These findings contrast with the results in the HS rats, in which decreased Tpcn2 expression levels are associated with increased, as opposed to decreased, fasting glucose and insulin levels. There are several possible reasons for this discrepancy. Because the mice were created on a mixed C57/129 background, we cannot rule out the possibility that alleles from the 129 strain, as opposed to the gene knockout, are causing the differences in phenotype. To minimize this effect, however, only siblings from heterozygous breeder pairs were tested. Another possibility is related to the fact that a full gene knockout can have a very different effect on a phenotype than natural allelic variation (Flint and Eskin 2012). Furthermore, several studies have shown that different variants within the same gene, or within the same GWAS location, can have opposite effects on a single phenotype (Cohen et al. 2005; Flister et al. 2013). To test the role specifically of the F344 variant on glucose and insulin regulation, quantitative complementation can be used (Yalcin et al. 2004b, 2010). Alternatively, if we are able to identify the causal variant(s) within this region, we can use genetic techniques to investigate the specific role of this (or these) variant(s) (Katter et al. 2013).
To determine whether Tpcn2 may be playing a role in human diabetes, we collaborated with investigators from the DIAGRAM and MAGIC consortiums. Tpcn2 has not previously reached significance levels in any of the published genome-wide scans (Morris et al. 2012; Scott et al. 2012). However, genome-wide thresholds are conservative because of the need to account for the multiple testing across the genome. In the present study, we were able to assess 14 of 39 SNPs within the Tpcn2 gene and find that two are marginally significantly associated with fasting insulin levels in humans. Although no significant associations were found between these SNPs and diabetes or fasting glucose levels, the majority of SNPs that we investigated were not represented within the MAGIC or DIAGRAM meta-analyses. A potential reason for this is that some of these SNPs may be rare variants and thus could not be properly imputed. It is therefore possible that rare variants within Tpcn2 may be associated with diabetes and related traits (see Steinthorsdottir et al. 2014; Zuk et al. 2014). The fact that variants within all three species alter diabetic phenotypes, however, is strong evidence that this gene plays a role in metabolic regulation.
This work demonstrates the power of highly recombinant animal models such as the HS for relative rapid identification of a candidate gene within a QTL. Despite relatively complex genetic architecture at the QTL, we were able to identify a highly plausible causal gene within only a few years. It is important to recognize, however, that we are not able to rule out any other genes within this QTL at this time, particularly the 29 genes that were not assessed on the expression array. In fact, our mapping studies indicate that more than one gene likely underlies the insulin locus (Solberg Woods et al. 2012), and it is reasonable to expect that Tpcn2 may interact with one or more genes within this region. Of particular interest is Gpr152, a G-protein coupled receptor in which the F344 variant encodes a premature stop codon, thus likely affecting protein function. The variant within Gpr152 was also significantly associated with Tpcn2 expression levels, suggesting a potential interaction between these genes. Future studies will investigate a potential role for these other genes, as well as potential interactions between genes within this region.
In sum, we have identified Tpcn2 as a highly plausible causal gene within a QTL for both glucose and insulin traits. We have used expression and sequence analysis in a highly recombinant population to identify this gene in only a few years. A causal role for this gene is supported by alterations in both glucose and insulin in a Tpcn2 knockout mouse as well as data from clinical cohorts. Future work will identify the causative variant within this gene and begin to unravel the underlying mechanisms involved, as well as investigate a potential role for other genes in the region. The gene expression data, association analysis, and confirmation in both mice and humans demonstrate Tpcn2 as a new gene contributing to glucose and insulin regulation.
Supplementary Material
Acknowledgments
We thank Shawn Levy and Melanie Robinson from Vanderbilt University (currently at Hudson Alpha, Inc.) for running the 10K genotyping arrays. We thank Caitlin O’Meara and Josef Lazar for helpful discussions regarding follow-up studies of Tpcn2. This work was funded by grant R01 DK-088975 (to L.C.S.W.), a Basic Research Award from Advancing a Healthier Wisconsin Research (to L.C.S.W.), a Children’s Research Institute Pilot and Innovation award (to L.C.S.W.), and funding from the Individualized Medicine Institute at the Medical College of Wisconsin (to L.C.S.W.). MAGIC lookup was funded by grant K24 DK080140 (to J.M.). Knockout mice were created using funding from the Medical Research Council (United Kingdom) programme grant G0901521 (to J.P.).
Footnotes
Available freely online through the author-supported open access option.
Supporting information is available online at http://www.genetics.org/lookup/suppl/doi:10.1534/genetics.114.162982/-/DC1.
Communicating editor: S. F. Chenoweth
Literature Cited
- Aitman T. J., Dong R., Vyse T. J., Norsworthy P. J., Johnson M. D., et al. , 2006. Copy number polymorphism in Fcgr3 predisposes to glomerulonephritis in rats and humans. Nature 439(7078): 851–855 [DOI] [PubMed] [Google Scholar]
- Alejandro E. U., Kalynyak T. B., Taghizadeh F., Gwiazda K. S., Rawstron E. K., et al. , 2010. Acute insulin signaling in pancreatic beta-cells is mediated by multiple Raf-1 dependent pathways. Endocrinology 151(2): 502–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arredouani A., Evans A. M., Ma J., Parrington J., Zhu M. X., et al. , 2010. An emerging role for NAADP-mediated Ca2+ signaling in the pancreatic beta-cell. Islets 2(5): 323–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud A., Hermsen R., Guryev V., Stridh P., Graham D., et al. , 2013. Combined sequence-based and genetic mapping analysis of complex traits in outbred rats. Nat. Genet. 45(7): 767–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behmoaras J., Bhangal G., Smith J., McDonald K., Mutch B., et al. , 2008. Jund is a determinant of macrophage activation and is associated with glomerulonephritis susceptibility. Nat. Genet. 40(5): 553–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y., 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57: 289–300 [Google Scholar]
- Brailoiu E., Churamani D., Cai X., Schrlau M. G., Brailoiu G. C., et al. , 2009. Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J. Cell Biol. 186(2): 201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacho J., Sevillano J., de Castro J., Herrera E., Ramos M. P., 2008. Validation of simple indexes to assess insulin sensitivity during pregnancy in Wistar and Sprague-Dawley rats. Am. J. Physiol. Endocrinol. Metab. 295(5): E1269–E1276 [DOI] [PubMed] [Google Scholar]
- Calcraft P. J., Ruas M., Pan Z., Cheng X., Arredouani A., et al. , 2009. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 459(7246): 596–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Zhu J., Lum P. Y., Yang X., Pinto S., et al. , 2008. Variations in DNA elucidate molecular networks that cause disease. Nature 452(7186): 429–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung W. K., Zheng M., Chua M., Kershaw E., Power-Kehoe L., et al. , 1997. Genetic modifiers of Leprfa associated with variability in insulin production and susceptibility to NIDDM. Genomics 41(3): 332–344 [DOI] [PubMed] [Google Scholar]
- Cohen J., Pertsemlidis A., Kotowski I. K., Graham R., Garcia C. K., et al. , 2005. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat. Genet. 37(2): 161–165 [DOI] [PubMed] [Google Scholar]
- Demarest K., Koyner J., McCaughran J., Jr, Cipp L., Hitzemann R., 2001. Further characterization and high-resolution mapping of quantitative trait loci for ethanol-induced locomotor activity. Behav. Genet. 31(1): 79–91 [DOI] [PubMed] [Google Scholar]
- DIAbetes Genetics Replication and Meta-analysis (DIAGRAM) Consortium, Asian Genetic Epidemiology Network Type 2 Diabetes (AGEN-T2D) Consortium, South Asian Type 2 Diabetes (SAT2D) Consortium, Mexican American Type 2 Diabetes (MAT2D) Consortium, Type 2 Diabetes Genetic Exploration by Next-Generation Sequencing in Multi-Ethnic Samples (T2D-GENES) Consortium et al, 2014. Genome-wide trans-ancestry meta-analysis provides insight into the genetic architecture of type 2 diabetes susceptibility. Nat. Genet. 46(3): 234–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis J., Langenberg C., Prokopenko I., Saxena R., Soranzo N., et al. , 2010. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat. Genet. 42(2): 105–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber C. R., van Nas A., Ghazalpour A., Aten J. E., Doss S., et al. , 2009. An integrative genetics approach to identify candidate genes regulating BMD: combining linkage, gene expression, and association. J. Bone Miner. Res. 24(1): 105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint J., Eskin E., 2012. Genome-wide association studies in mice. Nat. Rev. Genet. 13(11): 807–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flister M. J., Tsaih S. W., O’Meara C. C., Endres B., Hoffman M. J., et al. , 2013. Identifying multiple causative genes at a single GWAS locus. Genome Res. 23(12): 1996–2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli J., Li L. S., Glaser A., Ostenson C. G., Jiao H., et al. , 1996. Genetic analysis of non-insulin dependent diabetes mellitus in the GK rat. Nat. Genet. 12(1): 31–37 [DOI] [PubMed] [Google Scholar]
- Garcia-Rua V., Otero M. F., Lear P. V., Rodriguez-Penas D., Feijoo-Bandin S., et al. , 2012. Increased expression of fatty-acid and calcium metabolism genes in failing human heart. PLoS ONE 7(6): e37505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauguier D., Froguel P., Parent V., Bernard C., Bihoreau M. T., et al. , 1996. Chromosomal mapping of genetic loci associated with non-insulin dependent diabetes in the GK rat. Nat. Genet. 12(1): 38–43 [DOI] [PubMed] [Google Scholar]
- Granhall C., Park H. B., Fakhrai-Rad H., Luthman H., 2006. High-resolution quantitative trait locus analysis reveals multiple diabetes susceptibility loci mapped to intervals <800 kb in the species-conserved Niddm1i of the GK rat. Genetics 174: 1565–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen C., Spuhler K., 1984. Development of the National Institutes of Health genetically heterogeneous rat stock. Alcohol. Clin. Exp. Res. 8(5): 477–479 [DOI] [PubMed] [Google Scholar]
- Hessner M. J., Wang X., Meyer L., Geoffrey R., Jia S., et al. , 2004. Involvement of eotaxin, eosinophils, and pancreatic predisposition in development of type 1 diabetes mellitus in the BioBreeding rat. J. Immunol. 173(11): 6993–7002 [DOI] [PubMed] [Google Scholar]
- Hu X., Daly M., 2012. What have we learned from six years of GWAS in autoimmune diseases, and what is next? Curr. Opin. Immunol. 24(5): 571–575 [DOI] [PubMed] [Google Scholar]
- Huang G. J., Shifman S., Valdar W., Johannesson M., Yalcin B., et al. , 2009. High resolution mapping of expression QTLs in heterogeneous stock mice in multiple tissues. Genome Res. 19(6): 1133–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubner N., Wallace C. A., Zimdahl H., Petretto E., Schulz H., et al. , 2005. Integrated transcriptional profiling and linkage analysis for identification of genes underlying disease. Nat. Genet. 37(3): 243–253 [DOI] [PubMed] [Google Scholar]
- Johnsen A. K., Valdar W., Golden L., Ortiz-Lopez A., Hitzemann R., et al. , 2011. Genome-wide and species-wide dissection of the genetics of arthritis severity in heterogeneous stock mice. Arthritis Rheum. 63(9): 2630–2640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadar B., Gombos K., Szele E., Ember I., Ivanyi J. L., et al. , 2011. Effects of isoflurane on Nfkappab p65, Gadd45a and Jnk1 expression in the vital organs of CBA/CA mice. In Vivo 25(2): 241–244 [PubMed] [Google Scholar]
- Kanemoto N., Hishigaki H., Miyakita A., Oga K., Okuno S., et al. , 1998. Genetic dissection of “OLETF”, a rat model for non-insulin-dependent diabetes mellitus. Mamm. Genome 9(6): 419–425 [DOI] [PubMed] [Google Scholar]
- Katter K., Geurts A. M., Hoffmann O., Mates L., Landa V., et al. , 2013. Transposon-mediated transgenesis, transgenic rescue, and tissue-specific gene expression in rodents and rabbits. FASEB J. 27(3): 930–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A., Nambi S. S., Mather K., Baron A. D., Follmann D. A., et al. , 2000. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J. Clin. Endocrinol. Metab. 85(7): 2402–2410 [DOI] [PubMed] [Google Scholar]
- Keane T. M., Goodstadt L., Danecek P., White M. A., Wong K., et al. , 2011. Mouse genomic variation and its effect on phenotypes and gene regulation. Nature 477(7364): 289–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B. J., Park K. H., Yim C. Y., Takasawa S., Okamoto H., et al. , 2008. Generation of nicotinic acid adenine dinucleotide phosphate and cyclic ADP-ribose by glucagon-like peptide-1 evokes Ca2+ signal that is essential for insulin secretion in mouse pancreatic islets. Diabetes 57(4): 868–878 [DOI] [PubMed] [Google Scholar]
- Koressaar T., Remm M., 2007. Enhancements and modifications of primer design program Primer3. Bioinformatics 23(10): 1289–1291 [DOI] [PubMed] [Google Scholar]
- Leduc M. S., Hageman R. S., Verdugo R. A., Tsaih S. W., Walsh K., et al. , 2011. Integration of QTL and bioinformatic tools to identify candidate genes for triglycerides in mice. J. Lipid Res. 52(9): 1672–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan R. W., Robledo R. F., Recla J. M., Philip V. M., Bubier J. A., et al. , 2013. High-precision genetic mapping of behavioral traits in the diversity outbred mouse population. Genes Brain Behav. 12(4): 424–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda Y., Saegusa H., Zong S., Noda T., Tanabe T., 2001. Mice lacking Ca(v)2.3 (alpha1E) calcium channel exhibit hyperglycemia. Biochem. Biophys. Res. Commun. 289(4): 791–795 [DOI] [PubMed] [Google Scholar]
- Matthews D. R., Hosker J. P., Rudenski A. S., Naylor B. A., Treacher D. F., et al. , 1985. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7): 412–419 [DOI] [PubMed] [Google Scholar]
- Morris A. P., Voight B. F., Teslovich T. M., Ferreira T., Segre A. V., et al. , 2012. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat. Genet. 44(9): 981–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey C., Grieve I. C., Heinig M., Atanur S., Petretto E., et al. , 2011. Integrated genomic approaches to identification of candidate genes underlying metabolic and cardiovascular phenotypes in the spontaneously hypertensive rat. Physiol. Genomics 43(21): 1207–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott R., Talbot C. J., Turri M. G., Collins A. C., Flint J., 2000. A method for fine mapping quantitative trait loci in outbred animal stocks. Proc. Natl. Acad. Sci. USA 97(23): 12649–12654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor E., Arredouani A., Vasudevan S. R., Lewis A. M., Parkesh R., et al. , 2009. Identification of a chemical probe for NAADP by virtual screening. Nat. Chem. Biol. 5(4): 220–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobrega M. A., Woods L. C., Fleming S., Jacob H. J., 2009. Distinct genetic regulation of progression of diabetes and renal disease in the Goto-Kakizaki rat. Physiol. Genomics 39(1): 38–46 [DOI] [PubMed] [Google Scholar]
- Ozcan L., Wong C. C., Li G., Xu T., Pajvani U., et al. , 2012. Calcium signaling through CaMKII regulates hepatic glucose production in fasting and obesity. Cell Metab. 15(5): 739–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K. H., Kim B. J., Shawl A. I., Han M. K., Lee H. C., et al. , 2013. Autocrine/paracrine function of nicotinic acid adenine dinucleotide phosphate (NAADP) for glucose homeostasis in pancreatic beta-cells and adipocytes. J. Biol. Chem. 288(49): 35548–35558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petretto E., Sarwar R., Grieve I., Lu H., Kumaran M. K., et al. , 2008. Integrated genomic approaches implicate osteoglycin (Ogn) in the regulation of left ventricular mass. Nat. Genet. 40(5): 546–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl M. W., 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29(9): e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saar K., Beck A., Bihoreau M. T., Birney E., Brocklebank D., et al. , 2008. SNP and haplotype mapping for genetic analysis in the rat. Nat. Genet. 40(5): 560–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson D. J., Hesselson S. E., Aperavich B. A., Zan Y., Haag J. D., et al. , 2007. Rat Mcs5a is a compound quantitative trait locus with orthologous human loci that associate with breast cancer risk. Proc. Natl. Acad. Sci. USA 104(15): 6299–6304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieder M., Rotzer K., Bruggemann A., Biel M., Wahl-Schott C. A., 2010. Characterization of two-pore channel 2 (TPCN2)-mediated Ca2+ currents in isolated lysosomes. J. Biol. Chem. 285(28): 21219–21222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R. A., Lagou V., Welch R. P., Wheeler E., Montasser M. E., et al. , 2012. Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nat. Genet. 44(9): 991–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawl A. I., Park K. H., Kim U. H., 2009. Insulin receptor signaling for the proliferation of pancreatic beta-cells: involvement of Ca2+ second messengers, IP3, NAADP and cADPR. Islets 1(3): 216–223 [DOI] [PubMed] [Google Scholar]
- So H. C., Gui A. H., Cherny S. S., Sham P. C., 2011. Evaluating the heritability explained by known susceptibility variants: a survey of ten complex diseases. Genet. Epidemiol. 35(5): 310–317 [DOI] [PubMed] [Google Scholar]
- Solberg Woods L. C., 2014. QTL mapping in outbred populations: successes and challenges. Physiol. Genomics 46(3): 81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg Woods L. C., Ahmadiyeh N., Baum A., Shimomura K., Li Q., et al. , 2009. Identification of genetic loci involved in diabetes using a rat model of depression. Mamm. Genome 20(8): 486–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg Woods L. C., Holl K., Tschannen M., Valdar W., 2010. Fine-mapping a locus for glucose tolerance using heterogeneous stock rats. Physiol. Genomics 41(1): 102–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg Woods L. C., Holl K. L., Oreper D., Xie Y., Tsaih S. W., et al. , 2012. Fine-mapping diabetes-related traits, including insulin resistance, in heterogeneous stock rats. Physiol. Genomics 44(21): 1013–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinthorsdottir V., Thorleifsson G., Sulem P., Helgason H., Grarup N., et al. , 2014. Identification of low-frequency and rare sequence variants associated with elevated or reduced risk of type 2 diabetes. Nat. Genet. 46(3): 294–298 [DOI] [PubMed] [Google Scholar]
- Svenson K. L., Gatti D. M., Valdar W., Welsh C. E., Cheng R., et al. , 2012. High-resolution genetic mapping using the Mouse Diversity outbred population. Genetics 190: 437–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanberg M., Lidman O., Padyukov L., Eriksson P., Akesson E., et al. , 2005. MHC2TA is associated with differential MHC molecule expression and susceptibility to rheumatoid arthritis, multiple sclerosis and myocardial infarction. Nat. Genet. 37(5): 486–494 [DOI] [PubMed] [Google Scholar]
- Talbot C. J., Nicod A., Cherny S. S., Fulker D. W., Collins A. C., et al. , 1999. High-resolution mapping of quantitative trait loci in outbred mice. Nat. Genet. 21(3): 305–308 [DOI] [PubMed] [Google Scholar]
- Talbot C. J., Radcliffe R. A., Fullerton J., Hitzemann R., Wehner J. M., et al. , 2003. Fine scale mapping of a genetic locus for conditioned fear. Mamm. Genome 14(4): 223–230 [DOI] [PubMed] [Google Scholar]
- Taylor C. T., Cummins E. P., 2011. Regulation of gene expression by carbon dioxide. J. Physiol. 589(Pt. 4): 797–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Untergasser A., Cutcutache I., Koressaar T., Ye J., Faircloth B. C., et al. , 2012. Primer3–new capabilities and interfaces. Nucleic Acids Res. 40(15): e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdar W., Solberg L. C., Gauguier D., Burnett S., Klenerman P., et al. , 2006. Genome-wide genetic association of complex traits in heterogeneous stock mice. Nat. Genet. 38(8): 879–887 [DOI] [PubMed] [Google Scholar]
- Valdar W., Holmes C. C., Mott R., Flint J., 2009. Mapping in structured populations by resample model averaging. Genetics 182(4): 1263–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voight B. F., Scott L. J., Steinthorsdottir V., Morris A. P., Dina C., et al. , 2010. Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat. Genet. 42(7): 579–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis R. H., Wang K., Marandi L., Hsieh E., Ning T., et al. , 2009. Type 1 diabetes in the BB rat: a polygenic disease. Diabetes 58(4): 1007–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S., Wei K., Moralejo D. H., Ogino T., Koike G., et al. , 1999. Mapping and characterization of quantitative trait loci for non-insulin-dependent diabetes mellitus with an improved genetic map in the Otsuka Long-Evans Tokushima fatty rat. Mamm. Genome 10(3): 249–258 [DOI] [PubMed] [Google Scholar]
- Yalcin B., Fullerton J., Miller S., Keays D. A., Brady S., et al. , 2004a Unexpected complexity in the haplotypes of commonly used inbred strains of laboratory mice. Proc. Natl. Acad. Sci. USA 101(26): 9734–9739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcin B., Willis-Owen S. A., Fullerton J., Meesaq A., Deacon R. M., et al. , 2004b Genetic dissection of a behavioral quantitative trait locus shows that Rgs2 modulates anxiety in mice. Nat. Genet. 36(11): 1197–1202 [DOI] [PubMed] [Google Scholar]
- Yalcin B., Flint J., Mott R., 2005. Using progenitor strain information to identify quantitative trait nucleotides in outbred mice. Genetics 171: 673–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcin B., Nicod J., Bhomra A., Davidson S., Cleak J., et al. , 2010. Commercially available outbred mice for genome-wide association studies. PLoS Genet. 6(9): e1001085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeggini E., Scott L. J., Saxena R., Voight B. F., Marchini J. L., et al. , 2008. Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat. Genet. 40(5): 638–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk O., Schaffner S. F., Samocha K., Do R., Hechter E., et al. , 2014. Searching for missing heritability: designing rare variant association studies. Proc. Natl. Acad. Sci. USA 111(4): E455–E464 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.