Abstract
Endothelial lipase (EL) is a major determinant of plasma HDL concentration, its activity being inversely proportional to HDL levels. Although it is known that it preferentially acts on HDL, compared to LDL and VLDL, the basis for this specificity is not known. Here we tested the hypothesis that sphingomyelin, a major phospholipid in lipoproteins is a physiological inhibitor of EL, and that the preference of the enzyme for HDL may be due to low sphingomyelin/ phosphatidylcholine (PtdCho) ratio in HDL, compared to other lipoproteins. Using recombinant human EL, we showed that sphingomyelin inhibits the hydrolysis of PtdCho in the liposomes in a concentration-dependent manner. While the enzyme showed lower hydrolysis of LDL PtdCho, compared to HDL PtdCho, this difference disappeared after the degradation of lipoprotein sphingomyelin by bacterial sphingomyelinase. Analysis of molecular species of PtdCho hydrolyzed by EL in the lipoproteins showed that the enzyme preferentially hydrolyzed PtdCho containing polyunsaturated fatty acids (PUFA) such as 22:6, 20:5, 20:4 at sn-2 position, generating the corresponding PUFA-lyso PtdCho. This specificity for PUFA-PtdCho species was not observed after depletion of sphingomyelin by sphingomyelinase. These results show that sphingomyelin not only plays a role in regulating EL activity, but also influences its specificity towards PtdCho species.
Keywords: sphingomyelin/phosphatidyl choline ratio, lysophsphatidylcholine, substrate specificity, molecular species of phosphatidylcholine, sphingomyelinase, LC/MS, enzyme regulation
Introduction
Endothelial lipase (EL, EC 3.1.1.3) is a unique member of the lipase family that functions more like a phospholipase than as a lipase (1). It is expressed predominantly in the vascular endothelial cells, and to a lesser extent in macrophages and smooth muscle cells, and preferentially hydrolyzes the phospholipids of HDL, compared to those of LDL and VLDL (1). It is known to play an important role in the metabolism of HDL because the HDL levels are inversely correlated with the EL activity in plasma (2, 3). HDL from the EL-knockout mice stimulates greater efflux of cholesterol compared to normal HDL, although the overall reverse cholesterol transport is not increased (4). The metabolism of apo B lipoproteins in vivo is also significantly affected by EL (5). The basis for the preference of the enzyme for HDL is not clear, since unlike LCAT (EC 2.3.1.43) or lipoprotein lipase (EC 3.1.1.34), it does not require a specific apoprotein activator. Furthermore, because of its specificity for the sn-1 position of phosphatidylcholine (PtdCho), it produces predominantly unsaturated species of lyso PtdCho, in contrast to LCAT or secretory phospholipases, which produce mostly saturated species of lyso PtdCho. This property of EL may be physiologically important since polyunsaturated fatty acids (PUFA) are transported more efficiently across the blood brain barrier in the form of lyso PtdCho, than as corresponding free fatty acids (6). The recent demonstration of a membrane transporter in endothelial cells of blood brain barrier that specifically transports lyso PtdCho containing docosahexaenoic acid (DHA), but not free DHA (7), further supports this mechanism Thus, EL may have an important role in supplying PUFA such as arachidonic acid and DHA to the brain, especially since it has been shown to be expressed and secreted by the endothelial cells of the brain capillaries (8). Recent studies from our laboratory showed that EL preferably hydrolyzes the sn-2 DHA - PtdCho, producing DHA-lyso PtdCho (9), lending additional support for this role.
While both the catalytic and non-catalytic (bridging) functions of EL appear to be involved in HDL clearance, the catalytic function is by far more important (10). Enzymatic action of EL has also been shown to be critical for the HDL-induced angiogenesis (11), and for the apo A1-mediated cholesterol efflux from the macrophages (12). Therefore, identification of the factors that regulate the activity of EL would be important in understanding the regulation of HDL metabolism and function. Though the regulation of the expression of this enzyme has been well studied (5, 13–15), very little is known about the physiological factors that regulate the activity of the enzyme in the plasma. It has been reported that the in vitro activity of the enzyme is inhibited by the addition of whole serum (1), although the specific factors responsible for the inhibition have not been identified. The inhibition of EL by angiopoietin like protein 3 (ANGPTL3) has been reported by Shinamura et al (16), apparently through binding to the heparin-binding region of the enzyme. Since EL does not require an apoprotein activator, its low activity towards VLDL and LDL compared to HDL, suggests that a normal constituent of LDL and VLDL may play an inhibitory role. We have previously shown that sphingomyelin, the second most abundant phospholipid in the plasma lipoproteins inhibits many of the phospholipases that utilize PtdCho as a substrate. These enzymes include LCAT (17), and secretory phospholipases II, V, and X (18, 19). The inhibition of lipoprotein lipase by sphingomyelin has also been reported (20, 21). Since the sphingomyelin/PtdCho ratio in LDL and VLDL is much higher than that of HDL (17), it is possible that one reason for the lower activity of EL on LDL and VLDL is the inhibition by sphingomyelin. The present studies were aimed at testing this hypothesis, and the results show that EL activity is indeed significantly inhibited by sphingomyelin in both artificial and lipoprotein substrates. The results also show that sphingomyelin affects the substrate specificity of EL towards the molecular species of PtdCho, leading to the generation of more polyunsaturated lyso PtdCho species as products.
Materials and Methods
Di- [14C] palmitoyl PtdCho (55mCi/mmol) was purchased from American Radiochemicals Inc (St. Louis, MO). Egg PtdCho, egg sphingomyelin, unlabeled dipalmitoyl PtdCho were purchased from Avanti Polar Lipids (alabaster, AL). Sphingomyelinase (EC 3.1.4.12) from B. cereus was obtained from Sigma- Aldrich (St. Louis, MO).
Lipoproteins and EL
Pooled normal human plasma (never frozen) was obtained from a local blood bank, and VLDL, LDL and HDL were prepared by sequential ultracentrifugation as described previously (17). The lipoproteins were dialyzed extensively against 10 mM Tris-0.15 M NaCl- 1mM EDTA pH 7.4, and stored at 4 °C. The phospholipid composition was analyzed by separation of the lipids by TLC, followed by determination of lipid phosphorus (22). The lipoproteins were used for the enzyme reactions within 3 weeks of preparation. The expression of EL in COS cells transiently transfected with the adenovirus carrying human EL has been previously described (1). In some studies, the enzyme expressed in FLP-IN 293 cells (12) was used. The conditioned medium was stored in aliquots at −80 °C until use, and was thawed only once before use. Medium from control cells transfected with GFP-adenovirus was used as negative control in all experiments.
Liposome and emulsion substrates
Defined substrates containing either labeled PtdCho alone or with egg sphingomyelin were prepared by sonication. Chloroform solutions of labeled PtdCho (2 × 106 dpm of di-[14C] palmitoyl PtdCho), 2.5 μmol of egg PtdCho or unlabeled dipalmitoyl PtdCho, and varying amounts of egg sphingomyelin were first dried under nitrogen, re-dissolved in 0.5 ml ethanol, and dried under nitrogen again. The lipids were then dispersed in 2 ml of 10 mM Tris-0.15 NaCl pH 7.4 by vortexing, and incubated at 37 °C for 30 min. The sample was then sonicated with a microtip for 1 min at 4 °C in a Sonics Vibro cell sonicator (Newton, CT, USA) at 40% full power. In some experiments the liposomes were prepared by extrusion through 0.1 μ filters at 40 °C (Mini- Extruder, Avanti Polar Lipids). The enzyme activity with the liposomes prepared by the two methods was comparable (results not shown).
To prepare glycerol-stabilized emulsions, 2.0 μmol of labeled dipalmitoyl PtdCho (with or without 1.0 μmol egg sphingomyelin) was mixed with 18 μmol of cholesteryl oleate, and after evaporation of the solvent thoroughly, dispersed in 1 ml glycerol. The dispersion was sonicated for 2 min at room temperature, followed by 2 min in an ice bath, and finally at room temperature for 1 min. The emulsion was stored at 4 °C, and was used within one week.
Labeling of lipoproteins
VLDL, LDL, and HDL freshly isolated from pooled human plasma were labeled with di[14C] palmitoyl PtdCho in order to trace the hydrolysis of lipoprotein PtdCho by EL. To 1 ml of the lipoprotein containing 10 mg protein, 50 μl of ethanolic solution of di- [14C] palmitoyl PtdCho (0.2 μCi) was added, and the solvent was evaporated under nitrogen at room temperature. The labeled lipoprotein was further incubated under nitrogen for 30 min at 37 °C, and stored at 4 °C until use. Re-isolation of the labeled lipoproteins by ultracentrifugation (d= 1.21g/ml, at 100,000 x g, 16 h) showed that 94–97% of the radioactivity was associated with the lipoproteins, showing that the labeled PtdCho was incorporated into the lipoprotein particles. The labeled lipoprotein was used for the EL assay within one week.
EL assays
The incubation mixture for the enzyme assay with the liposome substrate contained 350 nmol of PtdCho, varying amounts of egg sphingomyelin to give the indicated sphingomyelin:PtdCho ratios, 200 μl of EL-conditioned medium (about 350 μg protein), and 10 mM Tris-HCl buffer pH 7.4 containing 0.15 M NaCl in a final volume of 0.4 ml. Control samples contained the conditioned medium from GFP control cells. The incubation was carried out for 2–4 h at 37 °C and was stopped by the addition of 1 ml methanol. The lipids were extracted by Bligh and Dyer procedure (23), and separated on a silica gel TLC plate with the solvent system of chloroform/ methanol/ water (65:25:4 by vol). The lipids were visualized by exposure to iodine vapors, and the spots corresponding to the standards of egg PtdCho, egg lyso PtdCho, and free fatty (oleic) acid were scraped, and their radioactivity determined in a liquid scintillation counter. In some experiments, the samples were acidified with HCl (to 0.1 N final) before lipid extraction, in order ensure complete extraction of free fatty acids. The enzyme activity was expressed as % of PtdCho hydrolyzed or the products formed, after correcting for the GFP control values.
In the case of emulsion substrates the reaction mixture contained 150 μl of glycerol-stabilized emulsion (containing 300 nmol of [14C]-dipalmitoyl PtdCho and 150 nmol of sphingomyelin where indicated), 3 mg of BSA, 10 mM Tris buffer containing 0.15 M NaCl, and 200 μl of conditioned medium (EL) containing 350 μg total protein in a final volume of 0.4 ml. The incubation was performed for 2 h at 37 °C, the reaction was stopped by the addition of 1 ml methanol, and the percent of PtdCho hydrolyzed was determined as described above.
Sphingomyelinase treatment of lipoproteins
VLDL, LDL or HDL (100 μg protein) was incubated with 0.05 units of sphingomyelinase from B. cereus (Sigma) for 1h in 100 mM Tris buffer, pH 7.4. Then the EL-conditioned medium was added and the incubation continued for the indicated periods of time. LC/MS analysis of samples after sphingomyelinase treatment showed complete degradation of sphingomyelin in all the lipoproteins (Supplemental Fig. S1)
LC/MS analysis
The analysis of lipids was carried out using an AB Sciex QTRAP 5500 mass spectrometer coupled to an Agilent 1260 HPLC system. The total lipid extract was mixed with internal standards (50 ng each) of 17:0-17:0 PtdCho, 17:0 lysoPtdCho, and 12:0 sphingomyelin, and was separated on a normal phase column (Supelco Ascentis Si 3μ, 10cm × 2.1 mm). The solvent system used was: 100% solvent A (chloroform/ methanol/2 mM aqueous ammonium chloride, 80:19.5:0.5 by vol.) from 0 to 5 min; then a linear gradient of 100% solvent A to 100% solvent B (chloroform/methanol/water/2 mM aqueous ammonium chloride, 60:34.5:5:0.5; by vol.) from 5 to 30 min; and 100% solvent B from 30 to 35 min. The flow rate was 0.35 mL min−1. The ESI source temperature was set at 450°C. Multiple reaction monitoring (MRM) was used to quantify the various PtdCho and lyso PtdCho species. Data acquisition and processing were controlled using the Analyst 1.5 software (Applied Biosystems, Foster City, CA, USA).
Results
Effect of sphingomyelin in synthetic substrates
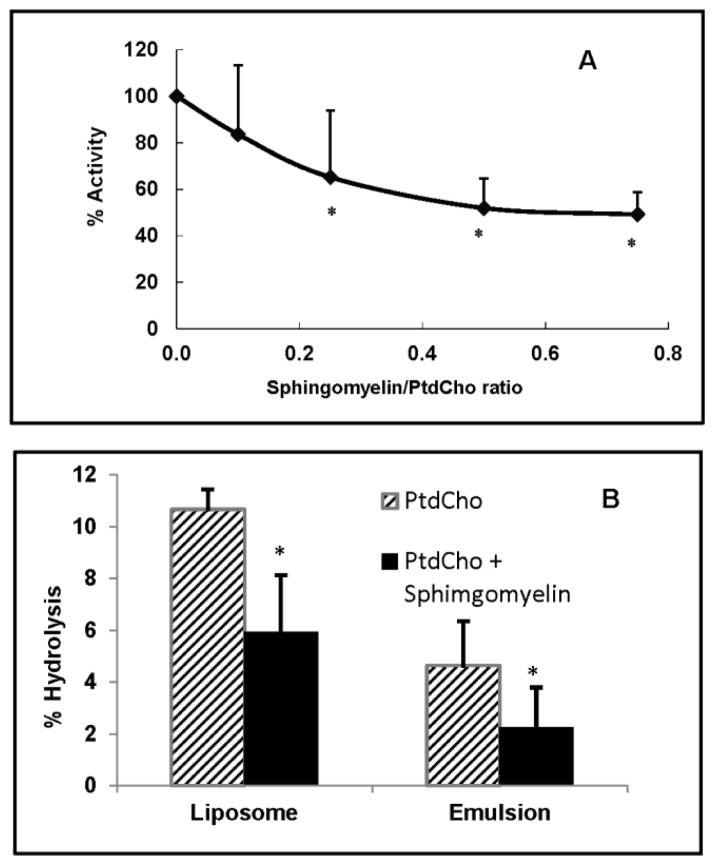
We first determined the effect of sphingomyelin on the hydrolysis of PtdCho in liposome substrates containing egg PtdCho and increasing amounts of egg sphingomyelin. Trace amount of dipalmitoyl [14C] PtdCho was included in the substrate to measure the enzyme activity. The activity was determined from the loss of radioactivity in PtdCho, as well as from the appearance of radioactivity in lyso PtdCho and FFA. As shown in Fig 1A, there was a concentration-dependent inhibition of EL activity by sphingomyelin, with about 35% inhibition when the ratio of sphingomyelin/PtdCho was 0.25, and 50% inhibition when the ratio was 0.5. The sphingomyelin/PtdCho ratios in normal human lipoproteins range from 0.15 to 0.45 (17), and therefore sphingomyelin inhibits the EL activity at physiological concentrations. In Fig 1B, the effect of sphingomyelin was compared in the liposome and emulsion substrates containing only 16:0–16:0 PtdCho (no egg PtdCho) at sphingomyelin/PtdCho ratio of 0.5, using a different preparation of EL. The inhibition was about 42% in the liposome substrate, and about 50% in the emulsion substrate, showing that sphingomyelin inhibition is not dependent upon the physical properties of the substrate. Although the labeled substrate used here (diplamitoyl-14C PtdCho) had equal amount of label in sn-1 and sn-2 positions, the label appearing in FFA exceeded that in lyso PtdCho in most cases (results not shown), apparently due to the lysophospholipase activity of EL reported previously (24).
Fig. 1. Inhibition of EL activity by sphingomyelin in synthetic substrates.
1A: Effect of sphingomyelin concentration in liposomes. Liposomes containing egg PtdCho and increasing amounts of egg sphingomyelin were prepared by sonication as described in the text. Trace amounts of [14C]-dipalmitoyl PtdCho were incorporated in all liposomes. After incubation of the liposomes with EL-conditioned medium (200 μl, 350 μg protein) for 4h, the lipids were extracted and the radioactivity in PtdCho, lyso PtdCho, and FFA was measured as described in Methods. The decrease in PtdCho radioactivity due to EL activity was calculated and the results (mean ± SD of 3 experiments) are shown as the percent decrease in enzyme activity compared to control (no sphingomyelin).
1B: Comparison of the effect of sphingomyelin in liposomes and emulsions. Liposomes and emulsions were prepared with [14C]-dipalmitoyl PtdCho (no egg PtdCho): egg sphingomyelin ratio of 2:1, as described in Methods. Incubations were carried out for 2h with 200μl of EL-conditioned medium and the decrease in PtdCho radioactivity was determined. The values shown are percent decrease in PtdCho radioactivity compared to the no-enzyme control (GFP control medium), and are mean ± SD of 3 experiments.
* p< 0.05 compared PtdCho alone.
EL activity on lipoproteins
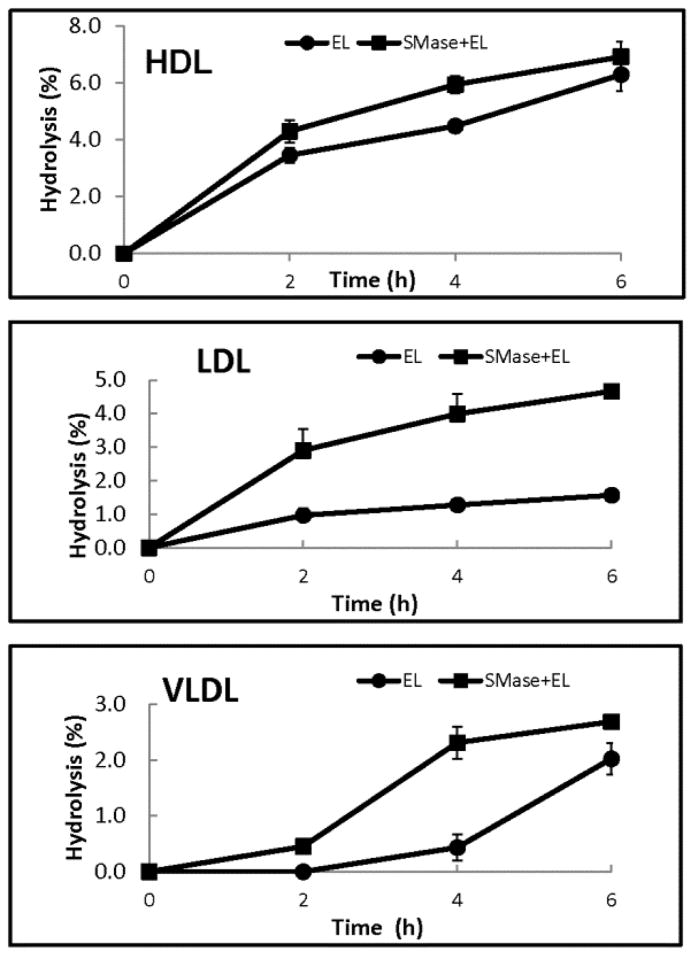
To test the effect of sphingomyelin on EL activity in the native lipoproteins, we first labeled VLDL, LDL, and HDL (isolated from normal human plasma) with trace amounts of 14C-dipalmitoyl PtdCho, and then treated them either directly with EL, or first with sphingomyelinase C for 1h, followed by EL. The hydrolysis of the labeled PtdCho was determined from the loss of radioactivity in PtdCho as well as by the increase in the radioactivity in lyso PtdCho and free fatty acid spots after TLC separation. In addition, since sphingomyelinase is known to exhibit lysophospholipase activity (24), we have also measured the radioactivity in the monoglyceride spot. As shown in Fig. 2, EL hydrolyzed the labeled PtdCho in HDL more efficiently than in LDL or VLDL. Pre-treatment of the lipoproteins with sphingomyelinase, however, activated the PtdCho hydrolysis significantly in VLDL and LDL, but less so in HDL. This shows that the higher concentrations of sphingomyelin in VLDL and LDL may be responsible for the lower EL activity against these lipoproteins, compared to HDL. LC/MS analysis of lipids showed that almost all sphingomyelin was degraded by sphingomyelinase in the lipoproteins under the conditions employed (Supplement, Fig. S1).
Fig. 2. Hydrolysis of labeled PtdCho in lipoproteins.
The lipoproteins were labeled with [14C]-dipalmitoyl PtdCho as described in Methods. An aliquot of the labeled lipoprotein was then treated with bacterial (B. cereus) sphingomyelinase (SMase) (0.05 units/mg lipoprotein) for 1h to deplete the sphingomyelin. Intact and sphingomyelinase-treated lipoproteins (100 μg protein each) were then incubated with EL-conditioned medium for the indicated period of time and the decrease in PtdCho radioactivity compared to the control (incubated with no EL) was determined. The values shown are mean ± SD of 3 experiments.
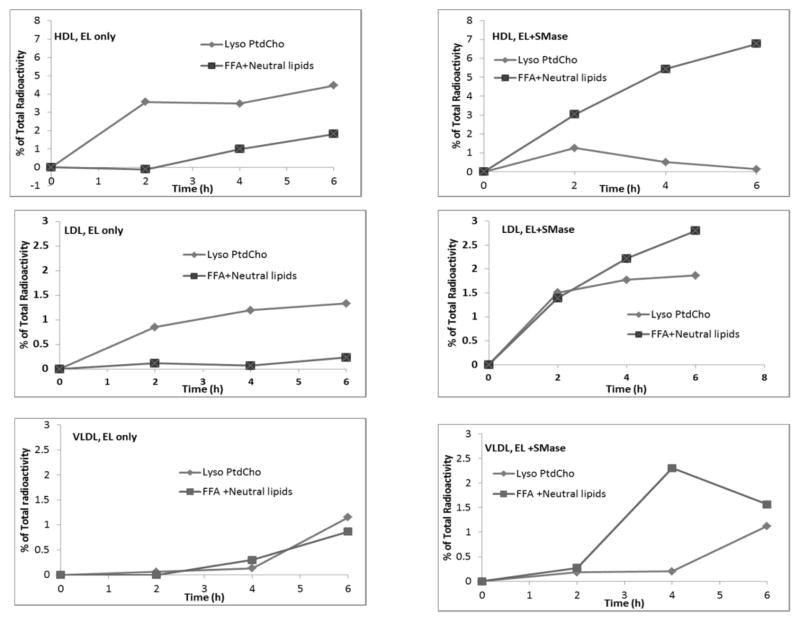
The results in Fig. 2 were calculated from the loss of radioactivity in PtdCho. We have also determined the distribution of radioactivity in the two products, namely lyso PtdCho and FFA. Since the PtdCho was labeled equally between sn-1 and sn-2 positions, the radioactivity in lyso PtdCho and FFA should be equal if there is no further hydrolysis of lyso PtdCho by the enzyme. On the other hand, the counts on FFA will exceed that of lyso PtdCho if the enzyme shows lysophospholipase activity, as suggested by earlier studies (24). Furthermore, since sphingomyelinase has been shown to hydrolyze the phosphodiester bond of lyso PtdCho (25), the radioactivity in the neutral lipids (mainly monoglyceride) was also measured. As shown in Fig. 3, in HDL and LDL not pre-treated with sphingomyelinase, the radioactivity in lyso PtdCho actually exceeded that of FFA and neutral lipids, indicating that no hydrolysis of lyso PtdCho has occurred. The lower amount of radioactivity in FFA is apparently due to an incomplete extraction of FFA, although the samples were acidified before extraction. In samples treated with sphingomyelinase, however, the radioactivity in FFA and neutral lipids exceeded that of lyso PtdCho, suggesting hydrolysis of lyso PtdCho by EL, or sphingomyelinase, or both. The effect of sphingomyelinase on lyso PtdCho hydrolysis was most apparent in HDL, and least evident in LDL. Sphingomyelinase by itself (in the absence of EL) showed very little lysophospholipase activity, and this baseline activity was subtracted from the total activity in the above calculations. These results suggest that the lyso PtdCho generated by the EL is further hydrolyzed by both EL and sphingomyelinase. Another possibility is that the lysophospholipase activity of EL also is inhibited by sphingomyelin, and that the degradation of sphingomyelin by sphingomyelinase released this inhibition.
Fig. 3. Analysis of products of PtdCho hydrolysis.
Lipoproteins labeled with di-[14C] palmitoyl PtdCho were treated with EL alone, or with sphingomyelinase (SMase) followed by EL, as described under Fig. 2, for the indicated periods of time. The radioactivity in lyso PtdCho, FFA, and the neutral lipids (area above the FFA spot) was determined after TLC separation of the lipids. The counts were corrected for any radioactivity in the control (no enzyme) samples. The data shown are averages of 2 separate experiments.
Hydrolysis of lipoprotein PtdCho: LC/MS analysis
The data in Fig. 2 and Fig. 3 represent the hydrolysis of labeled 16:0–16:0 PtdCho incorporated into the lipoproteins. While this measures the overall activity of the enzyme on each lipoprotein, it may not accurately reflect the hydrolysis of endogenous PtdCho or the effect of sphingomyelin on this hydrolysis. Since the lipoproteins contain several different molecular species of PtdCho in varying percentages, it is also necessary to determine the specificity of EL toward the molecular species of PtdCho, and whether the specificity is influenced by sphingomyelin. Therefore we analyzed the PtdCho composition of the lipoproteins by LC/MS before and after treatment with EL alone or with sphingomyelinase followed by EL.
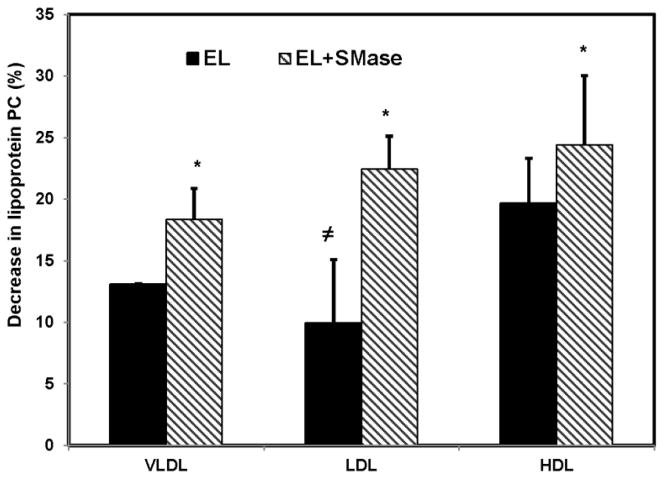
Fig. 4 shows the decrease in PtdCho mass following incubation of each lipoprotein with EL before and after sphingomyelin depletion by sphingomyelinase. The percent hydrolysis of total PtdCho was greater in HDL than in LDL or VLDL before the depletion of sphingomyelin, although these differences were less striking than those observed with the PtdCho-labeled lipoproteins (Fig. 2). Following sphingomyelin depletion, the PtdCho hydrolysis was stimulated significantly in all cases, and the differences between the lipoproteins disappeared, indicating that the concentration of sphingomyelin in the lipoprotein is an important physiological modulator of EL activity.
Fig. 4. Decrease in lipoprotein PtdCho after EL treatment.
Unlabeled VLDL, LDL, and HDL (100 μg protein) were treated with EL-conditioned medium (200 μl) for 4h, either directly or following treatment with bacterial sphingomyelinase (0.05 units, 1h). The total lipids were extracted and the decrease in PtdCho quantified by LC/MS analysis as described in the text. The values shown are mean ± SD of 3 analyses for VLDL, and mean ± SD of 5 analyses fro LDL and HDL.
≠ p< 0.01 vs HDL; * p< 0.05 EL vs EL + SMase.
Substrate specificity towards molecular species of PtdCho
Although EL is known to be specific for the sn-1 position of lipoprotein PtdCho, the substrate preference for various molecular species present in native lipoproteins is not established. The studies of Duong et al (26), who compared recombinant HDLs containing single PtdCho species, showed that the catalytic efficiency of the enzyme for different HDL species was in the order 16:0–22:6 > 16:0–20:4 >16:0–18:1 >16:0–18:2. Since the native lipoproteins contain unequal amounts of different PtdCho species, and since other components of the lipoproteins (including sphingomyelin and apoproteins) could affect the specificity, we determined the PtdCho species hydrolyzed in native VLDL, LDL and HDL before and after sphingomyelin depletion.
All the major PtdCho species in the lipoproteins were hydrolyzed by EL, quantitatively the most predominant being 16:0–18:2, 16:0–20:4, 18:0–18:2, and 18:0–20:4 species (Supplement Fig. S2). Since the PtdCho species are present in widely varying concentrations in the lipoproteins, the true specificity of the enzyme cannot be assessed by the decrease in absolute amount of each PtdCho species. To get a more accurate measure of the specificity, we calculated the contribution of individual PtdCho species to the total decrease (in percentage) and divided it by its initial concentration (percent of total PtdCho) in the lipoprotein. By this measure, a value above 1.0 indicates a relative preference of the enzyme for the given PtdCho species compared to the ‘average’ PtdCho, while a value lower than 1.0 indicates that it is less preferred than ‘average’ PtdCho. By this criterion, the enzyme preferentially hydrolyzed PtdChos containing polyunsaturated fatty acids (20:4, 20:5, 22:5, 22:6) at sn-2 in the native VLDL and LDL (Fig. 5). Treatment with sphingomyelinase, however, virtually eliminated this preference in VLDL, indicating a possible effect of sphingomyelin in determining the specificity of the enzyme. Similarly treatment of LDL with sphingomyelinase decreased the EL specificity for most of the unsaturated PtdChos. In native HDL, only PtdCho species containing 22:6 and 20:5 in the sn-2 position showed a slight preferential hydrolysis by EL. Pre-treatment with sphingomyelinase eliminated this preference, as found for VLDL and LDL. These results suggest that in the native lipoproteins the polyunsaturated PtdCho species are the preferred substrates for EL, and that this specificity is at least in part due to the presence of sphingomyelin, since the depletion of sphingomyelin virtually eliminated the differences between PtdCho species.
Fig. 5. Specificity of EL for the molecular species of PtdCho in lipoproteins.
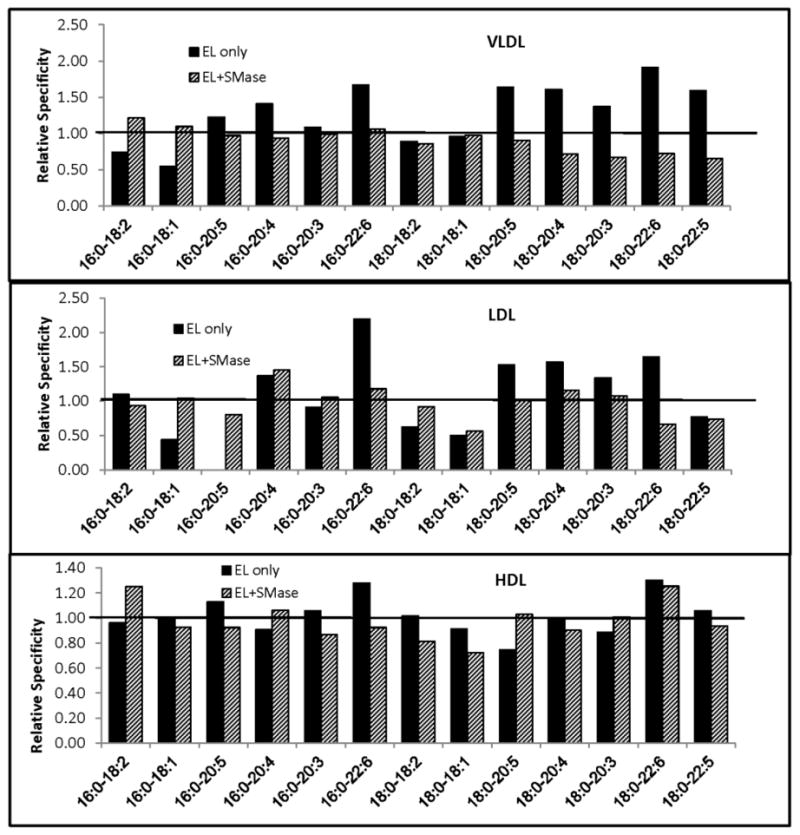
Sphingomyelinase(SMase)-treated or untreated lipoproteins were incubated with EL for 4 h and the PtdCho molecular species composition was analyzed as described in Materials and Methods. The decrease in individual PtdCho species after EL treatment was determined (compared to control sample incubated in the absence of EL) from this data. The relative specificity was calculated by dividing the contribution of individual PtdCho species to the decrease in total PtdCho (in %) by the concentration of the same species (% of total) in the untreated sample. A value above 1.0 indicates that the given PtdCho species is preferentially hydrolyzed by EL. The values shown are averages of 2 experiments.
Changes in lyso PtdCho species
Fig. 6 shows the lyso PtdCho species formed by the action of EL in the presence and absence of added sphingomyelinase. The major lyso PtdCho formed was 18:2 lyso PtdCho in all lipoproteins, followed by 20:4, 20:3 and 18:1. These results agree with the decrease in the PtdCho species containing the corresponding fatty acids in the sn-2 position (Fig. S2 Supplement), and also confirm the specificity of the enzyme for the sn-1 position of PtdCho. The omega-3 polyunsaturated lyso PtdChos (22:6 and 20:5) were minor products of the enzyme reaction because of the low concentrations of the corresponding PtdCho species in all lipoproteins, although EL shows preference for these species of PtdCho. An interesting observation is that the saturated lyso PtdCho (16:0 and 18:0), which are the predominant lyso PtdCho in the native lipoproteins, actually decreased in concentration after treatment with a combination of EL and sphingomyelinase, but not in the presence of EL alone. These results suggest that lyso PtdCho is either hydrolyzed by sphingomyelinase C alone, or by EL alone after the depletion of sphingomyelin, or by a combination of the two activities. It should be pointed out that the lyso PtdCho values shown are corrected for the controls containing only sphingomyelinase C (no EL), and therefore it appears that EL hydrolyzed the lyso PtdCho, but only after the depletion of sphingomyelin by sphingomyelinase C.
Fig. 6. Molecular species of lyso PtdCho produced by EL action on lipoproteins.
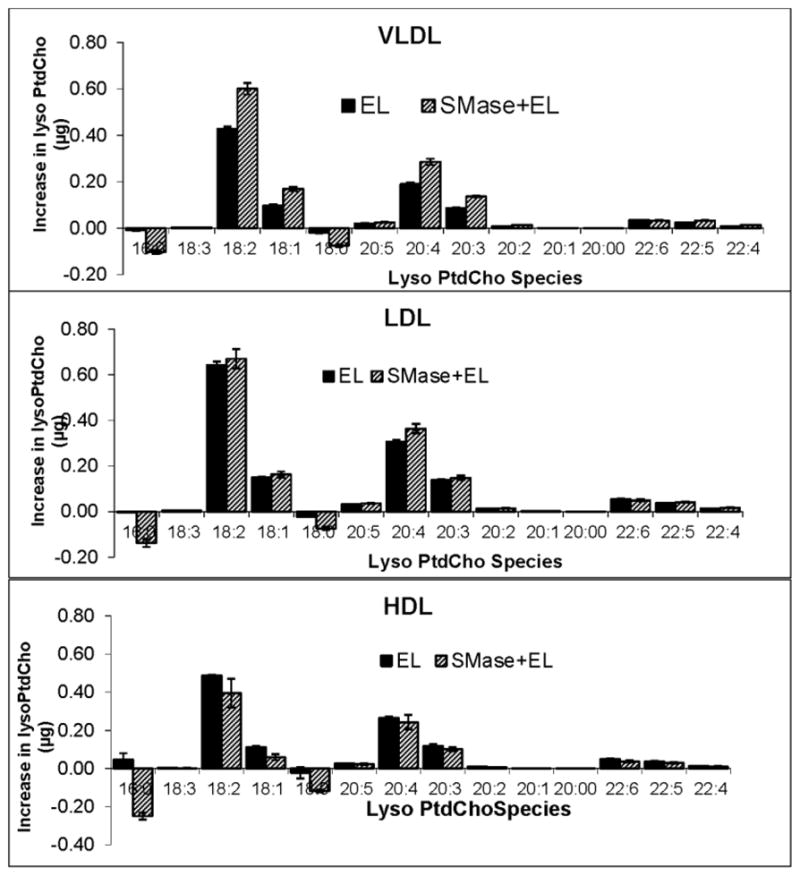
Lipoproteins (100 μg protein) were incubated with EL before or after treatment with SMase as described in text. The lyso PtdCho composition was determined by LC/MS. The increase in individual molecular species of lyso PtdCho over the control (sample incubated in the absence of EL) was calculated. The values shown are mean ± SD of 3 experiments.
Discussion
Although sphingomyelin is one of the major phospholipids in human plasma, comprising up to 30% of the total phospholipids in some lipoproteins (27, 28), its physiological role in the lipoproteins is not clearly known. Several reports suggested that sphingomyelin is an independent risk factor for atherosclerosis (29–31), although a more recent multi-center, multi- ethnic study showed that high plasma sphingomyelin is actually associated with a modest reduction in coronary heart disease (32). Previous studies from our laboratory and from others have shown that one important function of sphingomyelin is the inhibition of hydrolysis of lipoprotein PtdCho by LCAT (17, 33–35) and various secretory phospholipases (18, 19, 36), thereby preventing uncontrolled degradation of this important structural component. The results presented here show that sphingomyelin may also be involved in regulating the activity of EL, which is an important determinant of HDL concentration in human plasma. In addition, these results provide a possible explanation for the low activity of EL on LDL and VLDL, compared to HDL, because LDL and VLDL have higher sphingomyelin/PtdCho ratios than HDL (17). It is of interest to note that the EL activity is increased during inflammatory conditions (37). This increase in EL activity could be partly due to the increased presence of secretory sphingomyelinase during inflammation (38), and the consequent degradation of sphingomyelin in the lipoproteins, which in turn activates the EL activity.
The results presented here also show that EL preferentially hydrolyzes PtdCho species containing the polyunsaturated acyl groups (22:6, 20:5, 20:4) at the sn-2 position, generating the corresponding polyunsaturated lyso PtdCho species because of its specificity for the sn-1 position. This specificity appears to be abolished after depletion of sphingomyelin, suggesting a possible role for sphingomyelin in not only regulating the activity of the enzyme, but also its substrate specificity. It is possible that sphingomyelin associates with the less unsaturated PtdCho species such as 16:0–18:1 and 16:0–18:2, and thus specifically inhibits their hydrolysis, but does not affect the hydrolysis of the polyunsaturated species such as 16:0–22:6 PtdCho. The weak interaction of sphingomyelin with the polyunsaturated PtdCho species as reported in the model membranes (39) supports this possibility. The generation of polyunsaturated lyso PtdCho in the plasma may be physiologically significant, since the brain has been shown to take up the long chain polyunsaturated fatty acids such as 20:4 and 22:6 in the form of lyso PtdCho up to 12 times more efficiently than the corresponding free fatty acids (40). The presence of a specific transporter in the blood brain barrier that preferentially transports the lyso PtdCho form of DHA (7) further supports this possibility. It would be of interest to determine whether the levels of 20:4 and 22:6 in the brain are decreased in the EL knockout mice, since these mice would be expected to have a defect in generating the polyunsaturated lyso PtdChos.
Supplementary Material
Supplement Fig. S1. LC/MS analysis of lipoprotein phospholipids before and after treatment with sphingomyelinase.
HDL or LDL (100 μg protein) was treated with sphingomyelinase from B. cereus (0.05 units) for 1 h at 37°C, and the total lipids were extracted and analyzed by LC/MS as described in the text. The total ion current is shown. There was a complete loss of sphingomyelin after the sphingomyelinase treatment. Similar results were obtained with VLDL (results not shown)
Supplement Fig. S2: Molecular species composition of PtdCho.
Aliquots of VLDL, LDL, and HDL (100 μg protein) were first treated with sphingomyelinase (0.05 units) for 1h. sphingomyelinase-treated and untreated samples were then incubated with EL (200 μl EL-conditioned medium) for 4h. Control samples were incubated with GFP-control medium. The lipids were extracted and analyzed for molecular species of PtdCho by LC/MS as described in Methods. The values shown are mean ± SD of 3 analyses.
Acknowledgments
These studies were supported by a grant from NIH HL-68585, and in part by a Merit Review award I01 BX001090 from US Department of Veterans Affairs (to PVS). Research reported in this publication was supported by the Office of The Director, National Institutes of Health of the National Institutes of Health under Award Number S10OD010660. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We wish to acknowledge the technical assistance of Ms Amrith Rodriguez in the preparation of EL
Abbreviations
- DHA
Docosahexaenoic acid
- EL
Endothelial lipase
- FFA
Free fatty acid(s)
- GFP
Green fluorescent protein
- HDL
High density lipoproteins
- LC/MS
Liquid chromatography/mass spectroscopy
- LCAT
Lecithin-cholesterol acyltransferase
- LDL
Low density lipoproteins
- PtdCho
Phosphatidylcholine
- PUFA
Polyunsaturated fatty acids
- SMase
Sphingomyelinase
- TLC
Thin layer chromatography
- VLDL
Very low density lipoproteins
Reference List
- 1.McCoy MG, Sun GS, Marchadier D, Maugeais C, Glick JM, Rader DJ. Characterization of the lipolytic activity of endothelial lipase. J Lipid Res. 2002;43:921–929. [PubMed] [Google Scholar]
- 2.Ishida T, Choi S, Kundu RK, Hirata K, Rubin EM, Cooper AD, Quertermous T. Endothelial lipase is a major determinant of HDL level. J Clin Invest. 2003;111:347–355. doi: 10.1172/JCI16306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang J, Yu Y, Nakamura K, Koike T, Waqar AB, Zhang X, Liu E, Nishijima K, Kitajima S, Shiomi M, Qi Z, Yu J, Graham MJ, Crooke RM, Ishida T, Hirata Ki, Hurt-Camejo E, Chen YE, Fan J. Endothelial Lipase Mediates HDL Levels in Normal and Hyperlipidemic Rabbits. Journal of Atherosclerosis and Thrombosis. 2012;19:213–226. doi: 10.5551/jat.11148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown RJ, Lagor WR, Sankaranaravanan S, Yasuda T, Quertermous T, Rothblat GH, Rader DJ. Impact of combined deficiency of hepatic lipase and endothelial lipase on the metabolism of both high-density lipoproteins and apolipoprotein B-containing lipoproteins. Circ Res. 2010;107:357–364. doi: 10.1161/CIRCRESAHA.110.219188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yasuda T, Ishida T, Rader DJ. Update on the role of endothelial lipase in high-density lipoprotein metabolism, reverse cholesterol transport, and atherosclerosis. Circ J. 2010;74:2263–2270. doi: 10.1253/circj.cj-10-0934. [DOI] [PubMed] [Google Scholar]
- 6.Bernoud N, Fenart L, Moliere P, Dehouck MP, Lagarde M, Cecchelli R, Lecerf J. Preferential Transfer of 2-Docosahexaenoyl-1-Lysophosphatidylcholine Through an In Vitro Blood-Brain Barrier Over Unesterified Docosahexaenoic Acid. J Neurochem. 1999;72:338–345. doi: 10.1046/j.1471-4159.1999.0720338.x. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen LN, Ma D, Shui G, Wong P, Cazenave-Gassiot A, Zhang X, Wenk MR, Goh ELK, Silver DL. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature. 2014;509:503–506. doi: 10.1038/nature13241. [DOI] [PubMed] [Google Scholar]
- 8.Sovic A, Panzenboeck U, Wintersperger A, Kratzer I, Hammer A, Levak-Frank S, Frank S, Rader DJ, Malle E, Sattler W. Regulated expression of endothelial lipase by porcine brain capillary endothelial cells constituting the blood-brain barrier. J NEUROCHEM. 2005;94:109–119. doi: 10.1111/j.1471-4159.2005.03175.x. [DOI] [PubMed] [Google Scholar]
- 9.Chen S, Subbaiah PV. Phospholipid and fatty acid specificity of endothelial lipase: Potential role of the enzyme in the delivery of docosahexaenoic acid (DHA) to tissues. Biochim Biophys Acta. 2007;1771:1319–1328. doi: 10.1016/j.bbalip.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka H, Ishida T, Johnston TP, Yasuda T, Ueyama T, Kojima Y, Kundu RK, Quertermous T, Ishikawa Y, Hirata K. Role of endothelial lipase in plasma HDL levels in a murine model of hypertriglyceridemia. J Atheroscler Thromb. 2009;16:327–338. doi: 10.5551/jat.no844. [DOI] [PubMed] [Google Scholar]
- 11.Tatematsu S, Francis SA, Natarajan P, Rader DJ, Saghatelian A, Brown JD, Michel T, Plutzky J. Endothelial Lipase Is a critical determinant of high-density lipoprotein- stimulated sphingosine 1-phosphate- dependent signaling in vascular endothelium. Arterioscler Thromb Vasc Biol. 2013;33:1788–1794. doi: 10.1161/ATVBAHA.113.301300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiu G, Hill JS. Endothelial Lipase Promotes Apolipoprotein AI-Mediated Cholesterol Efflux in THP-1 Macrophages. Arterioscler Thromb Vasc Biol. 2009;29:84–91. doi: 10.1161/ATVBAHA.108.176487. [DOI] [PubMed] [Google Scholar]
- 13.Kivela AM, Dijkstra MH, Heinonen SE, Gurzeler E, Jauhiainen S, Levonen AL, Yla-Herttuala S. Regulation of endothelial lipase and systemic HDL cholesterol levels by SREBPs and VEGF-A. Atherosclerosis. 2012;225:335–340. doi: 10.1016/j.atherosclerosis.2012.09.039. [DOI] [PubMed] [Google Scholar]
- 14.Wu X, Huang H, Tang F, Le K, Xu S, Liu P. Regulated expression of endothelial lipase in atherosclerosis. Mol Cell Endocrinol. 2010;315:233–238. doi: 10.1016/j.mce.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Qiu G, Hill JS. Atorvastatin decreases lipoprotein lipase and endothelial lipase expression in human THP-1 macrophages. J Lipid Res. 2007;48:2112–2122. doi: 10.1194/jlr.M600510-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Shimamura M, Matsuda M, Yasumo H, Okazaki M, Fujimoto K, Kono K, Shimizugawa T, Ando Y, Koishi R, Kohama T, Sakai N, Kotani K, Komuro R, Ishida T, Hirata K, Yamashita S, Furukawa H, Shimomura I. Angiopoietin-Like Protein3 Regulates Plasma HDL Cholesterol Through Suppression of Endothelial Lipase. Arterioscler Thromb Vasc Biol. 2007;27:366–372. doi: 10.1161/01.ATV.0000252827.51626.89. [DOI] [PubMed] [Google Scholar]
- 17.Subbaiah PV, Liu M. Role of sphingomyelin in the regulation of cholesterol esterification in the plasma lipoproteins. Inhibition of lecithin- cholesterol acyltransferase. J Biol Chem. 1993;268:20156–20163. [PubMed] [Google Scholar]
- 18.Gesquiere L, Cho W, Subbaiah PV. Role of group IIa and group V secretory phospholipases A2 in the metabolism of lipoproteins. Substrate specificities of the enzymes and the regulation of their activities by sphingomyelin. Biochemistry. 2002;41:4911–4920. doi: 10.1021/bi015757x. [DOI] [PubMed] [Google Scholar]
- 19.Singh DK, Subbaiah PV. Modulation of the activity and arachidonic acid selectivity of group X secretory phospholipase A2 by sphingolipids. J Lipid Res. 2007;48:683–692. doi: 10.1194/jlr.M600421-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Olivera A, Romanowski A, Rani CSS, Spiegel S. Differential effects of sphingomyelinase and cell- permeable ceramide analogs on proliferation of swiss 3t3 fibroblasts. Biochim Biophys Acta. 1997;1348:311–323. doi: 10.1016/s0005-2760(97)00067-2. [DOI] [PubMed] [Google Scholar]
- 21.Arimoto I, Saito H, Kawashima Y, Miyajima K, Handa T. Effects of sphingomyelin and cholesterol on lipoprotein lipase- mediated lipolysis in lipid emulsions. J Lipid Res. 1998;39:143–151. [PubMed] [Google Scholar]
- 22.Marinetti GV. Chromatographic separation, identification, and analysis of phosphatides. J Lipid Res. 1962;3:1–20. [Google Scholar]
- 23.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 24.Gauster M, Rechberger G, Sovic A, Horl G, Steyrer E, Sattler W, Frank S. Endothelial lipase releases saturated and unsaturated fatty acids of high density lipoprotein phosphatidylcholine. J Lipid Res. 2005;46:1517–1525. doi: 10.1194/jlr.M500054-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Fujii S, Yoshida A, Sakurai S, Morita M, Tsukamoto K, Ikezawa H, Ikeda K. Chromogenic Assay for the Activity of Sphingomyelinase from Bacillus cereus and Its Application to the Enzymatic Hydrolysis of Lysophospholipids. Biol Pharm Bull. 2004;27:1725–1729. doi: 10.1248/bpb.27.1725. [DOI] [PubMed] [Google Scholar]
- 26.Duong M, Psaltis M, Rader DJ, Marchadier D, Barter PJ, Rye KA. Evidence that hepatic lipase and endothelial lipase have different substrate specificities for high-density lipoprotein phospholipids. Biochemistry. 2003;42:13778–13785. doi: 10.1021/bi034990n. [DOI] [PubMed] [Google Scholar]
- 27.Myher JJ, Kuksis A, Pind S. Molecular species of glycerophospholipids and sphingomyelins of human plasma: Comparison to red blood cells. Lipids. 1989;24:408–418. doi: 10.1007/BF02535148. [DOI] [PubMed] [Google Scholar]
- 28.Subbaiah PV, Rodby RA. Abnormal acyltransferase activities and accelerated cholesteryl ester transfer in patients with nephrotic syndrome. Metabolism. 1994;43:1126–1133. doi: 10.1016/0026-0495(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 29.Jiang XC, Paultre F, Pearson TA, Reed RG, Francis CK, Lin M, Berglund L, Tall AR. Plasma sphingomyelin level as a risk factor for coronary artery disease. Arterioscler Thromb Vasc Biol. 2000;20:2614–2618. doi: 10.1161/01.atv.20.12.2614. [DOI] [PubMed] [Google Scholar]
- 30.Park TS, Panek RL, Mueller SB, Hanselman JC, Rosebury WS, Robertson AW, Kindt EK, Homan R, Karathanasis SK, Rekhter MD. Inhibition of Sphingomyelin Synthesis Reduces Atherogenesis in Apolipoprotein E-Knockout Mice. Circulation. 2004;110:3465–3471. doi: 10.1161/01.CIR.0000148370.60535.22. [DOI] [PubMed] [Google Scholar]
- 31.Nelson JC, Jiang XC, Tabas I, Tall A, Shea S. Plasma sphingomyelin and subclinical atherosclerosis: findings from the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2006;163:903–912. doi: 10.1093/aje/kwj140. [DOI] [PubMed] [Google Scholar]
- 32.Yeboah J, McNamara C, Jiang XC, Tabas I, Herrington DM, Burke GL, Shea S. Association of Plasma Sphingomyelin Levels and Incident Coronary Heart Disease Events in an Adult Population: Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:628–633. doi: 10.1161/ATVBAHA.109.199281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bolin DJ, Jonas A. Sphingomyelin inhibits the lecithin-cholesterol acyltransferase reaction with reconstituted high density lipoproteins by decreasing enzyme binding. J Biol Chem. 1996;271:19152–19158. doi: 10.1074/jbc.271.32.19152. [DOI] [PubMed] [Google Scholar]
- 34.Rye KA, Hime NJ, Barter PJ. The influence of sphingomyelin on the structure and function of reconstituted high density lipoproteins. J Biol Chem. 1996;271:4243–4250. doi: 10.1074/jbc.271.8.4243. [DOI] [PubMed] [Google Scholar]
- 35.Sparks DL, Frank PG, Neville TAM. Effect of the surface lipid composition of reconstituted LpA-I on apolipoprotein A-I structure and lecithin: cholesterol acyltransferase activity. Biochim Biophys Acta. 1998;1390:160–172. doi: 10.1016/s0005-2760(97)00172-0. [DOI] [PubMed] [Google Scholar]
- 36.Koumanov K, Wolf C, Bereziat G. Modulation of human type II secretory phospholipase A2 by sphingomyelin and annexin VI, Biochem. J. 1997;326:227–233. doi: 10.1042/bj3260227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Broedl UC, Jin W, Rader DJ. Endothelial Lipase: A Modulator of Lipoprotein Metabolism Upregulated by Inflammation. Trend Cardiovasc Med. 2004;14:202–206. doi: 10.1016/j.tcm.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Wong ML, Xie B, Beatini N, Phu P, Marathe S, Johns A, Gold PW, Hirsch E, Williams KJ, Licinio J, Tabas I. Acute systemic inflammation up-regulates secretory sphingomyelinase in vivo: a possible link between inflammatory cytokines and atherogenesis. Proc Natl Acad Sci U S A. 2000;97:8681–8686. doi: 10.1073/pnas.150098097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams J, Batten S, Harris M, Rockett B, Shaikh S, Stillwell W, Wassall S. Docosahexaenoic and Eicosapentaenoic Acids Segregate Differently between Raft and Nonraft Domains. Biophys J. 2012;103:228–237. doi: 10.1016/j.bpj.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lagarde M, Bernoud N, Brossard N, Lemaitre-Delaunay D, Thies F, Croset M, Lecerf J. Lysophosphatidylcholine as a preferred carrier form of docosahexaenoic acid to the brain. J Mol Neurosci. 2001;16:201–204. doi: 10.1385/JMN:16:2-3:201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Fig. S1. LC/MS analysis of lipoprotein phospholipids before and after treatment with sphingomyelinase.
HDL or LDL (100 μg protein) was treated with sphingomyelinase from B. cereus (0.05 units) for 1 h at 37°C, and the total lipids were extracted and analyzed by LC/MS as described in the text. The total ion current is shown. There was a complete loss of sphingomyelin after the sphingomyelinase treatment. Similar results were obtained with VLDL (results not shown)
Supplement Fig. S2: Molecular species composition of PtdCho.
Aliquots of VLDL, LDL, and HDL (100 μg protein) were first treated with sphingomyelinase (0.05 units) for 1h. sphingomyelinase-treated and untreated samples were then incubated with EL (200 μl EL-conditioned medium) for 4h. Control samples were incubated with GFP-control medium. The lipids were extracted and analyzed for molecular species of PtdCho by LC/MS as described in Methods. The values shown are mean ± SD of 3 analyses.