Abstract
Axitinib is a potent and selective inhibitor of vascular endothelial growth factor receptors 1, 2, and 3, approved for second-line therapy for advanced renal cell carcinoma (RCC). Axitinib population pharmacokinetic and pharmacokinetic/pharmacodynamic relationships were evaluated. Using nonlinear mixed effects modeling with pooled data from 383 healthy volunteers, 181 patients with metastatic RCC, and 26 patients with other solid tumors in 17 trials, the disposition of axitinib was best described by a 2-compartment model with first-order absorption and a lag time, with estimated mean systemic clearance (CL) of 14.6 L/h and central volume of distribution (Vc) of 47.3 L. Of 12 covariates tested, age over 60 years and Japanese ethnicity were associated with decreased CL, whereas Vc increased with body weight. However, the magnitude of predicted changes in exposure based on these covariates does not warrant dose adjustments. Multivariate Cox proportional hazard regression and logistic regression analyses showed that higher exposure and diastolic blood pressure were independently associated with longer progression-free and overall survivals and higher probability of partial response in metastatic RCC patients. These findings support axitinib dose titration to increase plasma exposure in patients who tolerate axitinib, and also demonstrate diastolic blood pressure as a potential marker of efficacy.
Keywords: axitinib, metastatic renal cell carcinoma, population pharmacokinetics and pharmacodynamics, VEGF receptor inhibitor, diastolic blood pressure
Axitinib is a potent and selective second-generation inhibitor of vascular endothelial growth factor receptors (VEGFR) 1, 2, and 3.1–3 It has demonstrated promising single-agent antitumor activity in phase II clinical trials for the treatment of various solid tumors, including metastatic renal cell carcinoma (mRCC),4–6 thyroid cancer,7 advanced non-small cell lung cancer,8 and metastatic melanoma.9 Recently, in a randomized phase III clinical study (AXIS trial), axitinib demonstrated superior efficacy over sorafenib as second-line therapy for mRCC.10 The safety profile of axitinib is consistent with that expected for this class of agents, with hypertension, fatigue, and diarrhea being common adverse events.4–10 Axitinib is now approved in the United States, European Union, Japan, and other countries for the treatment of advanced RCC after failure of a prior systemic therapy.
In a dose-finding phase I study in patients with various tumor types, axitinib pharmacokinetics (PK) displayed linear correlations between dose and maximum plasma concentration as well as area under plasma concentration– time curve (AUC).11 Axitinib was absorbed relatively rapidly, reaching a peak plasma concentration within 2–6 hours following oral administration in the fed state. The rate and extent of absorption increased after fasting. Axitinib plasma levels declined with a terminal plasma half-life between 2 and 5 hours.11 Consistent with the short plasma half-life, there is a minimal observed accumulation with continuous twice-daily (bid) dosing. Since axitinib exposure may be associated with efficacy and/or toxicity, as shown for other tyrosine kinase inhibitors such as imatinib and sunitinib,12,13 it is important to elucidate factors contributing to variability in axitinib PK.
A population PK approach has been widely used to model the PK of drugs and identify covariates responsible for variability in plasma concentrations in the target patient population. In addition, a marker for efficacy can facilitate early identification of patients who would most benefit from the treatment and guide individualized dosing during treatment. For example, an association between hypertension and clinical outcome has previously been reported in cancer patients treated with other antiangiogenic agents, such as sorafenib, sunitinib, and bevacizumab.14–16 A similar association between diastolic blood pressure (dBP) and efficacy endpoints has also been observed in patients treated with axitinib.17 However, the previous retrospective report did not include PK data to evaluate whether the observed dBP association with efficacy is independent of drug level. The objectives of the current retrospective analyses using a population-based approach were: to establish a model that describes axitinib PK following single-dose administration in healthy volunteers and multiple-dose administration in patients with solid tumors, including mRCC; to identify covariates that may contribute to variability in axitinib PK; and to evaluate relationships between axitinib plasma exposure, efficacy, and dBP in mRCC patients.
Methods
Study Design
Study designs and populations for the 17 trials included in the population PK analysis are summarized in Table 1. For some studies, only subsets of subjects that were clinically relevant were included in the analysis. All study protocols were approved by institutional review boards (IRBs) or independent ethics committees (IECs), and studies were conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines.
Table 1.
Axitinib Studies Included in the Population PK Analysis
| Study no. | Design | Population (enrolled/analyzed, n) |
Axitinib dose and regimen | PK sampling post-dosing (hour) |
|---|---|---|---|---|
| 111 | Phase I, OL, SA, dose-escalating |
Patients with solid tumors (36/20a) |
Tx 1: 5 mg bid, fed, 4-week cycles, Form IV Tx 2: 5 mg bid, fasted, 4-week cycles, Form IV Tx 3: 2 mg bid (day 1), 5 mg bid thereafter, fasted, 4-week cycles, Form IV |
0 (pre-dose), 0.5, 1, 2, 4, 8, 12 |
| 25 | Phase II, OL, SA | Cytokine-refractory mRCC patients (52/52) |
5 mg bid, fasted, 4-week cycles, dose titration, Form IV |
0 (pre-dose) and 1–2 |
| 318 | Phase I, OL, SA, Japanese | Japanese patients with solid tumors (12/12) |
5 mg single, then 5 mg bid, fed, 4-week cycles, dose titration, Form IV |
0 (pre-dose), 0.5, 1, 2, 4, 6, 8, 12, 24, 32 (following single-dose) 0 (pre-dose), 0.5, 1, 2, 4, 6, 8, 12 (following continuous dosing) |
| 46 | Phase II, OL, SA | Sorafenib-refractory mRCC patients (62/59b) |
5 mg bid, fed, 4-week cycles, dose titration, Form IV |
0 (pre-dose) and 1–2 |
| 54 | Phase II, OL, SA, Japanese | Japanese cytokine-refractory mRCC patients (64/64) |
5 mg bid, fed, 4-week cycles, dose titration, Form IV |
0 (pre-dose) and 1–2 |
| 639 | SB, randomized, 2-way CO, DDI with ketoconazole |
Healthy volunteers (35/32c) | 5 mg, fasted, Form IV | 0 (pre-dose), 1, 1.5, 2, 3, 4, 6, 8, 12, 16, 24, 36, 48 |
| 728 | OL, food effect | Healthy volunteers (42/41d) | Tx 1: 5 mg, fasted, Form IV Tx 2: 5 mg, fed high fat, Form IV |
0 (pre-dose), 0.5, 1, 1.5, 2, 4, 6, 8, 12, 16, 24, 36, 48 |
| 8 | OL, 2-way CO, F | Healthy volunteers (16/16) | Tx 1: 5 mg, fasted, Form IV Tx 2: 5 mg, fed moderate fat, Form IV Tx 3: 1 mg intravenous, fasted, Form IV |
0 (pre-dose), 0.5, 1, 1.5, 2, 4, 6, 8, 12, 16, 24, 36 |
| 9 | OL, randomized, CO, relative F | Healthy volunteers (40/20e) | 5 mg, fasted, Form IV | 0 (pre-dose), 0.5, 1, 1.5, 2, 4, 6, 8, 12, 16, 24, 36 |
| 10 | OL, randomized, 2-sequence, 3-period CO, BE |
Healthy volunteers (40/40) | 5 mg, fasted, Form IV | 0 (pre-dose), 0.5, 1, 1.5, 2, 4, 6, 8, 12, 16, 24, 32 |
| 1129 | OL, 2-period, 2-Tx CO, DDI with rifampin, Caucasian and Japanese |
Caucasian healthy volunteers (20/20) Japanese healthy volunteers (20/20) |
5 mg, fasted, Form IV | 0 (pre-dose), 0.5, 1, 1.5, 2, 4, 6, 8, 12, 16, 24, 32 |
| 12 | OL, randomized, 4-sequence, 4-period CO, relative F |
Healthy volunteers (56/54e) | Tx 1: 5 mg, fed moderate fat, Form IV Tx 2: 5 mg, fed moderate fat, Form XLI |
0 (pre-dose), 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, 16, 24, 32 |
| 1330 | OL, PG, normal, mild to moderate hepatic impairment |
Healthy volunteers (24/8f) | 5 mg, fed moderate fat, Form IV | 0 (pre-dose), 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 12, 16,24,36,48,96, 144 |
| 14 | OL, randomized, 4-sequence, 4-period CO, relative bioavailability |
Healthy volunteers (20/20) | 5 mg, fed moderate fat, Form IV | 0 (pre-dose), 0.5, 1, 1.5, 2, 3, 4, 5, 6, 7, 8, 10, 12, 16,24,36,48 |
| 15 | OL, randomized, 2-sequence, 4-period CO bioequivalence |
Healthy volunteers (68/68) | Tx 1: 5 mg, fasted, Form IV Tx 2: 5 mg, fasted, Form XLI |
0 (pre-dose), 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 16, 24, 32 |
| 1640 | OL, fixed-sequence, 3-period CO, Chinese | Healthy volunteers (14/14) | Single-dose 5, 7, and 10 mg with washout period, fed, Form XLI |
0 (pre-dose), 1, 1.5, 2, 2.5, 3, 4, 6, 12, 16, 24, 32 |
| 1728 | OL, randomized, 3-period CO food effect | Healthy volunteers (30/30) | Tx 1: 5 mg, fasted, Form XLI Tx 2: 5 mg, fed high fat, Form XLI Tx 3: 5 mg, fed moderate fat, Form XLI |
0 (pre-dose), 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 6, 8, 12, 16, 24 |
BE, bioequivalence; bid, twice daily; CO, crossover; DDI, drug–drug interaction; OL, open-label; PG, parallel-group; PK, pharmacokinetics; SA, single-agent; SB, single-blind; Tx, treatment. Reasons for excluding individual data from the analysis:
Axitinib starting dose higher than 5 mg bid.
Lack of dose time data or only one measurable axitinib concentration.
Did not receive axitinib.
Did not complete the study.
Received I mg axitinib dose in a spray-dried dispersion or self-emulsifying drug dispersion system.
Having hepatic impairment based on Child-Pugh classification.
The IRBs were as follows. Study 1: Committee on Human Research, University of California San Francisco, San Francisco, CA, USA; University of Wisconsin– Madison Health Sciences Human Subjects Committee, Madison, WI, USA; and the University of Texas, M. D. Anderson Cancer Center Surveillance Committee, Houston, TX, USA. Study 2: Comité de protection des personnes, Hôpital Pitié Salpêtrière, Paris, France; Ethik-Kommission der Medizinischen Hochschule Hannover, Hannover, Germany; Cleveland Clinic Foundation IRB, Cleveland, OH, USA; Committee on Human Research, University of California San Francisco, San Francisco, CA, USA; IRB at Memorial Sloan Kettering Cancer Center, New York, NY, USA; Fox Chase Cancer Center IRB, Philadelphia, PA, USA; Dana Farber IRB/Office for the Protection of Research Subjects, Boston, MA, USA; and the University of Wisconsin–Madison Health Sciences Human Subject Committee, Madison, WI, USA. Study 3: the IRB of the National Cancer Center, Tokyo, Japan. Study 4: University of Wisconsin Health Sciences IRB, Madison, WI, USA; Fox Chase Cancer Center IRB, Philadelphia, PA, USA; Cleveland Clinic Foundation IRB, Cleveland, OH, USA; University of Chicago IRB, Chicago, IL, USA; and Our Lady of Mercy Medical Center IRB, Bronx, NY, USA. Study 5: Hokkaido University Hospital IRB, Sapporo, Hokkaido, Japan; Yamagata University Hospital IRB, Yamagata, Yamagata, Japan; Tsukuba University Hospital IRB, Tsukuba, Ibaraki, Japan; Tokyo Women’s Medical University Hospital IRB, Shinjyuku-ku, Tokyo, Japan; National Cancer Center Hospital IRB, Chuo-ku, Tokyo, Japan; Shizuoka Cancer Center IRB, Sunto-gun, Shizuoka, Japan; Kinki University Hospital IRB, Osaka-sayama, Osaka, Japan; Osaka University Hospital IRB, Suita, Osaka, Japan; Tokushima University Hospital IRB, Tokushima, Tokushima, Japan; Kochi Medical School Hospital IRB, Nankoku-shi, Kochi, Japan; Kyushu Cancer Center IRB, Fukuoka, Fukuoka, Japan; Kyushu University Hospital IRB, Higashi-ku, Fukuoka, Fukuoka, Japan; Hamamatsu Medical University, Faculty of Medicine IRB, Hamamatsu, Shizuoka, Japan; Iwate Medical University Hospital IRB, Morioka, Iwate, Japan; University Hospital Kyoto IRB, Kyoto, Kyoto, Japan; Akita University Hospital IRB, Akita, Akita, Japan; Nihon University Itabashi Hospital IRB, Itabashi, Tokyo, Japan; National Cancer Center Hospital East IRB, Chuo-ku, Tokyo, Japan; and Kagoshima University Hospital IRB, Kagoshima, Kagoshima, Japan. Study 6: the Research Consultants’ Review Committee, Austin, TX, USA. Studies 7 and 8: PRACS Institute IRB, Fargo, ND, USA. Study 9: RCRC IRB, Austin, TX, USA. Study 10: IntegReview, Austin, TX, USA. Study 11: Aspire IRB, La Mesa, CA, USA. Study 13: IRB Board Inc, Plantation, FL, USA.
The IECs were as follows: studies 14 and 17, Comite d’Ethique de l’Hôpital Erasme, Bruxelles, Belgium; studies 12 and 15, Comite d’Ethique de l’Hôpital Erasme, Bruxelles, Belgium, and Parkway IEC, Parkway Hospitals, Singapore; and study 16, the Ethics Committee of Peking Union Medical College Hospital for Clinical Trials, Beijing, China. Written informed consent was obtained from each participant prior to study entry.
Study Treatment
Healthy volunteers received a single 5-mg oral dose of axitinib, except study 8 in which subjects also received 1 mg axitinib intravenously to determine absolute bioavailability (F) (Table 1). Patients received axitinib at a starting dose of 5 mg orally bid, with dose adjustments to a maximum of 10 mg bid permitted, based on tolerability, except dose-finding study 1 in which some patients received up to 30 mg bid (Table 1).4–6,11,18 Axitinib was administered as crystal polymorph Form IV or Form XLI (commercial) formulation (Table 1). In study 1, the maximum tolerated dose (MTD) for axitinib was found to be 5 mg bid, which was recommended as a starting dose in all subsequent studies. Therefore, patients treated with starting doses higher than MTD in study 1 were not included in the population PK analysis. Axitinib was administered in patients continuously on a bid schedule until disease progression, unacceptable toxicity, or withdrawal of consent.
Patients who developed treatment-related hypertension (defined by at least 2 in-clinic readings of systolic blood pressure [sBP] >150 mm Hg or dBP >100 mm Hg separated by at least 1 hour) were to be given either new or additional antihypertensive medications at the discretion of the treating physician. For patients with in-clinic readings of sBP ≥160 mm Hg or dBP ≥105 mm Hg despite maximal antihypertensive therapy, axitinib administration was to be withheld until BP decreased to < 150/100 mm Hg, at which time axitinib treatment could be resumed at a 20% or one level lower dose (or at the same dose in study 4).
PK Assessment
All PK blood samples were collected prior to and at various time intervals following single-dose and continuous-dose administration (Table 1). Axitinib plasma concentrations were measured using a validated high-performance liquid chromatography–tandem mass spectrometric method (Charles River Laboratories, Shrewsbury, MA). Linear range, precision (% coefficient of variation [CV]), and bias (% relative error) were 0.1–25 ng mL−1, <16%, and <13%, respectively, in studies 1, 2, 3, 4, 6, 7, 8, 9, and 11; 0.1–100 ng mL−1, <16%, and <13%, respectively, in study 10; and 0.5–100 ng mL−1, <8%, and <8%, respectively, in studies 5, 12, 13, 14, 15, 16, and 17.
Efficacy Assessment
In mRCC studies, tumor assessments were performed by measuring changes in the sum of the longest diameters for all target and non-target lesions using radiologic methods at baseline and every 8 weeks. Objective response rate (defined as a complete response or partial response) was determined according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0. Other efficacy endpoints included progression-free survival (PFS) and overall survival (OS). Efficacy was assessed by investigators.
Blood Pressure Assessment
BP measurements were taken at the clinic with patients in the seated position after 5-minute rest, at baseline, and every 4 weeks (study 2); every 2 weeks for 6 visits and every 4 weeks thereafter (study 4); or every week for 4 visits, every 2 weeks for the next 6 visits, and every 4 weeks thereafter (study 5). Additionally, patients were provided with a BP monitoring device for daily self-assessment and instructed to notify their physician of sBP ≥150 mm Hg or dBP ≥90 mm Hg. Only in-clinic dBP data were used in the PK/pharmacodynamic (PD) analyses. Diastolic BP was used rather than sBP, since the latter tends to be more labile.
Population PK Modeling
Statistical analysis
Plasma concentration—time data were analyzed using nonlinear mixed effects modeling (NONMEM 7, level 1.0, ICON Development Solutions, Ellicott City, MD). First-order conditional estimation with interaction was used for the PK model. Model selection was based on assessment of diagnostic plots and comparison of the NONMEM change of minimum objective function values (ΔMOF >3.84 points, P < .05) using the log-likelihood ratio test. The selected base model was analyzed for covariate influence on the interindividual variability (IIV) error terms. The significance of potential covariates was systematically evaluated in a stepwise forward selection (ΔMOF <−3.84 points, P < .05) followed by backward elimination (ΔMOF <10.83 points, P < .001). Simulations were performed to determine the predicted effect of these covariates. Evaluation of model robustness was based on relative standard errors (RSE) of the model parameter estimates determined by non-parametric bootstrapping (n = 200).
Model development
Since only sparse PK samples were collected for the majority of patients in clinical trials, rich PK data from healthy volunteers were pooled with patient data to better characterize the basic PK structural model. Log-transformed axitinib plasma concentration–time data were modeled using a linear 2-compartment model defined in terms of systemic clearance (CL), volume of distribution in central compartment (Vc), apparent intercompartmental clearance (Q), volume of distribution in peripheral compartment (Vp), first-order absorption rate constant (ka), and absorption lag time. Absolute bioavailability after oral administration was estimated using the intravenous data from healthy volunteers (study 8). Residual variability (intravenous and oral) was modeled as a proportional error and IIV was modeled using an exponential error term on each PK parameter.
During base model development, alternative models were investigated to improve estimates (e.g., transit compartment model instead of a lag-time model,19 the M3 approach for censoring data below the lower limit of quantitation,20 and modeling of inter-occasion variability). The residual variability improved slightly using the alternate models; however, CL was not different across models.21
Following development of the base model, covariates of clinical interest were systematically evaluated in stepwise forward selection followed by backward elimination. Continuous covariates tested were age, body weight, body surface area, alanine aminotransferase (ALT), aspartate aminotransferase (AST), bilirubin, and creatinine clearance (CrCL, estimated using Cockcroft-Gault equation), whereas categorical covariates were gender, race, smoking status, baseline Eastern Cooperative Oncology Group performance score (ECOG PS), and study population. All variables were investigated for potential effect on axitinib CL; only gender and body weight were investigated for potential effect on Vc. Continuous covariates were modeled with a power function centered on a median value as:
where X is the covariate of interest and Xreference is the median value of that covariate. Categorical covariates were incorporated in a proportional model:
where indicator is a coding variable equal to 1 when the covariate is present and 0 when it is absent. θ2 is the proportional change, coded as relative to the most common covariate value.
PK/PD Modeling
Statistical analysis
Patients from the 3 mRCC studies were included in the analyses to elucidate potential relationships between axitinib exposure, dBP, and efficacy. Relationships between AUC, dBP, and categorical efficacy endpoints (RECIST-defined response) were evaluated using logistic regression (S-PLUS® 7.0, TIBCO Software, Palo Alto, CA) to predict the probability of a partial response as a function of either AUC or dBP. Probability was expressed as an odds ratio (OR) and calculated as:
where β denotes regression coefficient and x denotes unit change in the independent variable. Mean daily AUC was calculated from the average total daily dose (accounting for any dose reduction or missed doses), the population mean estimate of F, and individual post hoc estimates of CL as:
AUC was estimated for each patient at the end of 4 weeks of study treatment (prior to dose changes in most patients) and the entire study treatment. For dBP, the maximum observed dBP during the first 4 weeks, the first 8 weeks, and at any time during the study were assessed, as well as maximum observed dBP changes from baseline (ΔdBP).
Relationships between AUC, dBP, and time-to-event efficacy (PFS and OS) as well as prognostic factors were analyzed using multivariate Cox regression (R version 2.12.). Previous analyses have identified prognostic factors predictive for survival in second-line mRCC patients: serum hemoglobin (≤13 g/dL vs. >13 g/dL for male, ≤11.5 g/dL vs. >11.5 g/dL for female), corrected calcium level (<10 g/dL vs. ≥10 mg/dL), and Karnofsky Performance Status (KPS) <80%.22 These variables were evaluated in this analysis, except KPS which was not recorded in the mRCC studies. Additionally, baseline ECOG PS (0 vs. 1), prior therapy (cytokine- vs. sorafenib-refractory), age, and gender were evaluated.
Univariate Cox regression was initially performed on each prognostic factor to identify significant (P < .1, log-rank score test) variables for inclusion into a multivariate full model. Then, backward elimination with removal of each variable was performed until only significant (P < .05, log-likelihood ratio test) variables were retained in the multivariate model. Once the multivariate model with significant prognostic factors was developed, AUC and dBP were tested as predictors of response (using the same statistical criteria). AUC and dBP were assessed in both continuous and categorical forms. For categorical variable analyses, 300 hxng/mL was used as the cutoff value for AUC, which corresponded to the estimated total daily exposure that correlated with the maximum reduction in blood flow and permeability (based on the previous report,23 showing a correlation [Figures 3 and 4] between blood flow parameter and AUC). A cutoff value of 90 mm Hg was used for dBP (the definition of hypertension according to the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure24).
Results
Population PK Analysis
Subject characteristics
The population PK analysis was conducted using data from 590 subjects, including 383 healthy volunteers and 207 patients (181 with mRCC and 26 with other solid tumors). At screening, the median (range) for body weight, CrCL, AST, ALT, and bilirubin was 74 kg (37–136), 103 mL/min (8–214),22 U/L (9–154), 21 U/L (5–188), and 0.7 mg/dL (0.1–3), respectively. The overall median (range) age was 42 years (18–85). However, median [range] age of patients (60 years [32–85]) was higher than that of healthy volunteers (32 years [18–69]). Most of the subjects were male (85%) and Caucasian (61%).
PK model
After testing alternative models that included a transit compartment model, the disposition of axitinib was best described by a linear 2-compartment model with a lag time for absorption and first-order ka. Final parameter estimates (%CV) were 14.6 L/h (59.9%) and 47.3 L (39.7%) for CL and Vc, respectively (Table 2). In this population PK analysis, data from 16 subjects with hepatic impairment (study 13) and 16 patients who received a starting dose greater than 5 mg bid (dose-finding study 1) were excluded from the dataset, since dosing in hepatically impaired subjects or administration of doses exceeding the 5 mg bid starting dose would not be permitted in subsequent clinical trials for axitinib. However, inclusion of data from these subjects in a repeat population PK analysis did not result in substantial changes in the base model PK parameter estimates (data not shown). The equations describing typical axitinib CL and Vc are given as:
where Age>60, RaceJapanese, and Smokeractive are 1 if applicable and 0 otherwise.
Table 2.
Parameter Estimates for Axitinib Population PK Final Model
| Parameter | Estimate, mean (%CV)a | % RSEb | 95% CIc |
|---|---|---|---|
| CL (L/h) | 14.6 (59.9) | 8.5 (8.5) | 12.4, 17.2 |
| Age >60-yr effect on CL | −0.213 | 26 (29) | −0.320, −0.106 |
| Smoking status on CL | 1.02 | 44 (42) | 0.144, 1.90 |
| Japanese ethnicity effect on CL | −0.249 | 25 (27) | −0.370, −0.128 |
| Vc (L) | 47.3 (39.7) | 6.2 (7.8) | 41.9, 53.4 |
| Weight effect on Vc | 0.778 | 14 (13) | 0.591, 1.02 |
| Q (L/h) | 4.00 (86.8)d | 4.7 (12) | 3.65, 4.39 |
| Vp (L) | 393 | 16 (28) | 285, 542 |
| ka (hour–1): fed | 0.482 (77) | 4.8 (5.6) | 0.439, 0.529 |
| Fasting effect on kae | 1.97 | 13 (15) | 1.46, 2.48 |
| tlag (hour) | 0.454 | 0.41 (0.8) | 0.450, 0.458 |
| F: fed/Form IV | 0.457 | 6.6 (8) | 0.402, 0.520 |
| Fasting on F, Form IVf | 0.330 | 13 (13) | 0.243, 0.417 |
| Form XLI on Fg | −0.121 | 24 (26) | −0.179, −0.0630 |
| Residual error (%), oral | 58.2 | 2.0 (2.1) | 55.9, 60.6 |
| Residual error (%), intravenous | 33.5 | 16 | 24.4, 46.1 |
| η-shrinkage (%)h | |||
| CL | 7.58 | — | — |
| Vc | 17.5 | — | — |
| Q (and Vp) | 32.8 | — | — |
| ka | 17.8 | — | — |
CI, confidence interval; CL, systemic clearance; CV, coefficient of variation; η [eta], Empirical Bayes prediction of the interindividual random effect in a PK parameter; F, bioavailability; ka, first-order absorption rate constant; PK, pharmacokinetics; Q, apparent inter-compartmental clearance; RSE, relative standard error; Vc, volume of distribution in central compartment; Vp, volume of distribution in peripheral compartment.
Interindividual variability provided as %CV.
%RSE of model parameter estimates obtained from the NONMEM covariance step. %RSE in parenthesis were obtained from non-parametric bootstrap of 200 samples (86% successful minimization).
CI calculated as estimate × exp (± 1.96 %RSE/100) or for quantities that can be positive or negative (covariate parameters and covariances), as estimate ± 1.96 %RSE/100 to allow the interval to span 0; %RSE used for CI were obtained from NONMEM covariance step.
Correlation between Q and Vp was modeled to be 100% correlated and with the same interindividual variability.
For fasting effect, ka is estimated to be 1.43 hour−1; calculated as 0.482 hour−1 × (1 + 1.97).
For fasting effect with Form IV, F is estimated to be 0.608; calculated as 0.457 × (1 + 0.33).
F with Form XLI is estimated to be 0.402; calculated as 0.457 × (1 – 0.121). Note that there was no observed food effect with Form XLI.
Eta [η]-shrinkage calculated as (1 – SD (ηEBE)/ω) where SD is standard error, EBE is Empirical Bayes estimate, and ω is variance-covariance matrix of the interindividual random effects in the measurements.
Goodness of fit
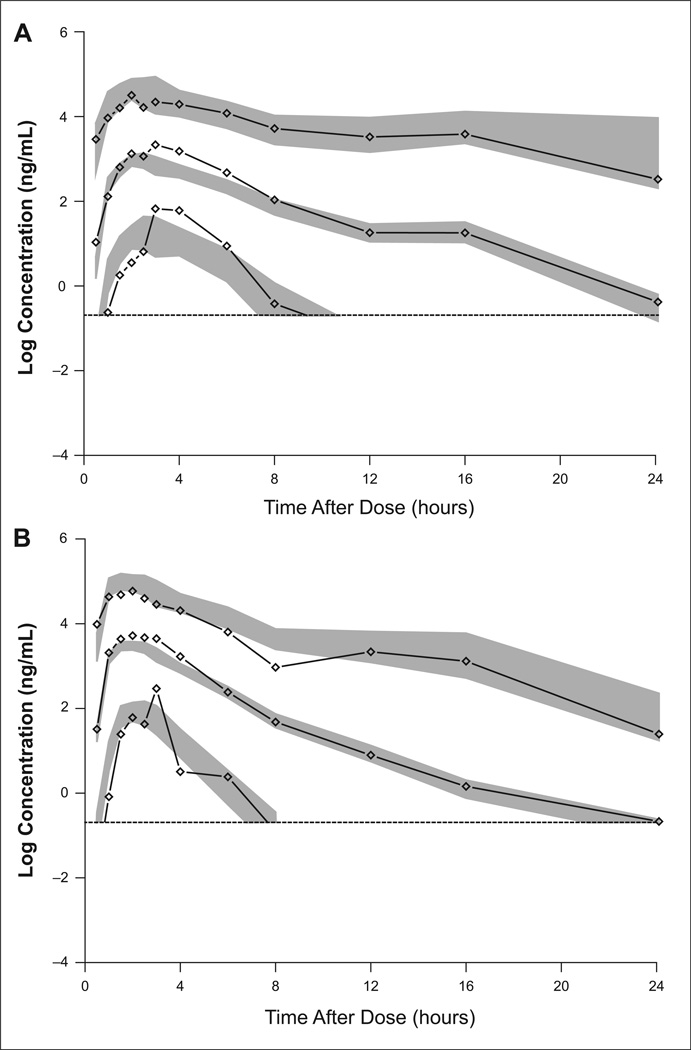
Diagnostic plots from the final model showed good agreement between population and individual predictions with observed data and a lack of systematic bias in the residuals (Supplementary Figure S1). The estimates of shrinkage (a metric to evaluate if variability in individual estimates differs from the population predicted values) for CL and Vc were 7.58% and 17.5%, respectively (Table 2). Since the random effects showed low shrinkage (<20%), the estimated random effects were considered to have good diagnostic value.25 Visual predictive check (VPC) with the final model also showed that the simulated concentrations appeared consistent with observed concentrations, with no systematic bias (Figure 1). The RSEs of the model parameter estimates obtained from non-parametric bootstrap of 200 samples (Table 2) were not statistically different from those obtained from the NONMEM covariance step.
Figure 1.
Visual predictive checks (VPCs) of Form IV in fed (A) and fasted (B) subjects with 2.5, 50, and 97.5 percentiles (lines with diamond shapes) and corresponding simulated 95% confidence intervals (shaded area) to lower limit of quantification (LLOQ; dashed lines). For each regimen, VPCs were calculated from 1,000 simulations of all subjects in the dataset with that regimen. CI, confidence interval.
Effects of covariates
In the final model, ka was 197% higher in the fasted state compared with the fed state, regardless of formulations (Table 2). The oral bioavail-ability for axitinib polymorph Form IV was increased 33% in the fasted state compared with the fed state, whereas food had only negligible effect on Form XLI. When comparing the 2 formulations in the fed state, F for Form XLI was 12.1% lower than Form IV.
Both age >60 years and Japanese ethnicity were associated with decreased CL (21.3% and 24.9%, respectively), resulting in correspondingly higher exposure. The effect of age on CL was initially evaluated as a continuous covariate, which showed that CL tended to decrease modestly in the higher range of age (Supplementary Figure S2-A). Hence, age was tested as a binary effect to avoid overestimation of CL at younger ages, due to a non-uniform distribution of range of ages across the studies. Different age thresholds were tested in the model and a cutoff of 60 years was the most significant (Supplementary Figure S2-B). Ethnic differences between Japanese and other East Asian populations have been reported;26 hence, in this analysis, both Asians and Japanese were tested separately and in combination for their potential influence on CL relative to other races (Caucasian, Black, Hispanic, and those coded as “Other/Not listed”). The results showed that among the different races and ethnic groups evaluated, only Japanese patients had a significant effect on CL.
While active smokers showed a greater CL (by 102%) compared with ex-smokers or non-smokers, the magnitude of the effect was not well-defined since only 19 of 590 subjects were active smokers and the precision of the estimate of the effect was poor (44% RSE). Therefore, the clinical significance of this relationship is unclear.
Body weight displayed no relationship with CL, but had a notable impact on axitinib Vc. Lighter subjects had smaller estimated Vc than heavier subjects, resulting in greater peak plasma concentrations. However, expected changes in peak plasma concentrations due to body weight were less than the estimated IIV for Vc. Furthermore, exposure is not affected by changes in Vc.
The PK analysis indicated no differences in axitinib CL between healthy volunteers and cancer patients, the majority of whom had mRCC. The random effects on CL had relatively low shrinkage (7.6% overall; 16.5% in healthy volunteers; and 6.5% in cancer patients) and appeared normally distributed and centered at a mean of zero in cancer patients, indicating no systemic bias (Supplementary Figure S3). In addition, log-transformed scatter plots of observed axitinib plasma concentrations versus population or individual predicted concentrations revealed no systematic bias as the concentrations for cancer patients were randomly distributed around the line of unity (Supplementary Figure S1–A, B). Since these diagnostic plots showed that the population PK model adequately predicted axitinib CL in cancer patients, the pooling of cancer patients and healthy volunteers was retained in the analysis. Overall, no dose adjustment is warranted based on age, Japanese ethnicity, or body weight. None of the other covariates studied, including body surface area and levels of CrCL ALT, AST, or bilirubin, were found to significantly affect the disposition of axitinib.
Simulations
To quantify the effect of covariates on axitinib exposures in the most extreme situations, subjects were categorized as having a low or high body weight (i.e., 10th [58 kg] or 90th [94 kg] percentile body weight, respectively), age ≤ or >60 years old, and Japanese or non-Japanese ethnicity (active smoker effect was not included since it was not a well-defined covariate). The results demonstrated that axitinib exposures with 95% predicted intervals estimated for each covariate combination substantially overlap with each other (Supplementary Figure S4). Additionally, Monte Carlo simulations (n = 1,000 simulations with IIV) were performed to predict axitinib steady-state concentrations with the 2 extreme covariate combinations: >60-year-old, 58-kg Japanese subjects and ≤60-year-old, 94-kg non-Japanese subjects. The results also show substantial overlap in the axitinib concentration profiles (Supplementary Figure S5).
PK/PD Analysis
mRCC patient characteristics
The RECIST-based overall response rates were 44% (study 2), 23% (study 4), and 50% (study 5). 4–6 Of the 178 patients from these mRCC studies, 168 were included in this PK/PD analysis (patients excluded from the analysis had missing values for hemoglobin, corrected serum calcium, post-baseline dBP, and/or axitinib exposure). The majority of the mRCC patients were male (71%) and had prior cytokine therapy (68%) and a baseline ECOG PS 0 (65%). The median (range) age, baseline hemoglobin, and baseline corrected serum calcium were 60 years (34–85), 12.4 g/dL (1.1–17.9), and 9.4 mg/dL (8.4–13.1), respectively (Table 3).
Table 3.
Characteristics of 168 mRCC Patients Included in the PK/PD Analyses
| Covariates | n |
|---|---|
| Age, years | |
| Median (range) | 60 (34–85) |
| Gender, (%) | |
| Male | 120 (71) |
| Female | 48 (29) |
| Prior therapy, (%) | |
| Cytokine-refractory | 114 (68) |
| Sorafenib-refractory | 54 (32) |
| ECOG PS, (%) | |
| 0 | 109 (65) |
| 1 | 59 (35) |
| Hemoglobin, g/dLa | |
| Median (range) | 12.4 (1.1–17.9) |
| ≤ 13 for male, ≤ 11.5 for female (%) | 89 (53) |
| >13 for male, >11.5 for female (%) | 79 (47) |
| Corrected serum calcium, mg/dLa | |
| Median (range) | 9.4 (8.4–13.1) |
| <10 (%) | 145 (86) |
| ≥ 10 (%) | 23 (14) |
| AUC, hxng/mLa,b | |
| Median (range) | 375 (32.8–1,728) |
| <300c (%) | 63 (38) |
| ≥300 (%) | 105 (62) |
| dBP, mm Hga,d | |
| Median (range) | 90 (58–116)e |
| <90 (%) | 80 (48)f |
| ≥90 (%) | 88 (52)g |
AUC, area under the plasma concentration–time curve; dBP, diastolic blood pressure; ECOG PS, Eastern Cooperative Oncology Group performance status; mRCC, metastatic renal cell carcinoma; PK/PD, pharmacokinetics/pharmacodynamics.
Of the 178 mRCC patients, 2 patients were missing hemoglobin, 3 were missing calcium, 2 were missing dBP, and 5 were missing AUC.
AUC at the end of 4 weeks of study treatment.
Total daily AUC correlated with the maximum reduction in blood flow and permeability was determined to be reached at ~300 hxng/mL (based on Figures 3 and 4 in the previous study evaluating axitinib exposure and blood flow23).
Maximum post-baseline dBP within the first 8 weeks.
Median baseline dBP for all patients was 76 mm Hg (range 55–96.5).
Median baseline dBP for patients with post-baseline dBP <90 mm Hg was 71.3 mm Hg (range 55–96).
Median baseline dBP for patients with post-baseline dBP ≥90 mm Hg was 80 mm Hg (range 56–96.5).
Correlation of prognostic factors with efficacy
With univariate Cox regression, prognostic factors found to be significant (P < .1) for PFS were gender, prior therapy, ECOG PS, and hemoglobin (Table 4). For OS, they were prior therapy, ECOG PS, hemoglobin, and corrected calcium. Age did not influence PFS or OS. These variables were included in the full model of multivariate analysis. Using a significance level of P < .05 for removing a variable in backward elimination, all prognostic factors except corrected calcium were retained in the multivariate model (Table 5).
Table 4.
Univariate Cox Proportional Hazards Analysis of Progression-Free and Overall Survival
| Covariates | mPFS, months | HR (95% CI) | Pa | mOS, months | HR (95% CI) | Pa |
|---|---|---|---|---|---|---|
| Age | ||||||
| Continuous | — | 1.006 (0.986, 1.026) | .593 | — | 0.992 (0.972, 1.01) | .480 |
| Gender | ||||||
| Male | 13.0 | 1 | — | 27.7 | 1 | — |
| Female | 7.63 | 1.63 (1.09, 2.46) | .018 | 19.6 | 1.26 (0.799, 1.97) | .324 |
| Prior therapy | ||||||
| Cytokine-refractory | 13.0 | 1 | — | 30.0 | 1 | — |
| Sorafenib-refractory | 7.63 | 1.55 (1.02, 2.34) | .038 | 15.8 | 2.15 (1.39, 3.34) | <.001 |
| ECOG PS | ||||||
| 0 | 13.7 | 1 | — | 41.6 | 1 | — |
| 1 | 7.13 | 2.17 (1.46, 3.23) | <.001 | 10.7 | 3.63 (2.40, 5.48) | <.001 |
| Hemoglobin (g/dL) | ||||||
| ≤ 13 for male, ≤ 11.5 for female | 7.69 | 1 | — | 15.9 | 1 | — |
| >13 for male, >11.5 for female | 14.6 | 0.537 (0.364, 0.792) | .001 | 43.3 | 0.282 (0.179, 0.443) | <.001 |
| Corrected serum calcium, mg/dL | ||||||
| <10 | 11.1 | 1 | — | 27.7 | 1 | — |
| ≥10 | 8.38 | 1.21 (0.688, 2.13) | .507 | 16.4 | 1.74 (1.03, 2.96) | .038 |
| AUC, hxng/mL | ||||||
| Continuousb | — | 0.871 (0.801, 0.947) | .001 | — | 0.810 (0.733, 0.897) | <.001 |
| <300 | 7.4 | 1 | — | 15.8 | 1 | — |
| ≥300 | 13.8 | 0.558 (0.379 0.823) | .003 | 37.4 | 0.489 (0.324, 0.738) | <.001 |
| dBP, mm Hg | ||||||
| Continuousc | — | 0.604 (0.487, 0.750) | <.001 | — | 0.652 (0.524, 0.811) | <.001 |
| <90 | 7.86 | 1 | — | 18.5 | 1 | — |
| ≥90 | 14.6 | 0.590 (0.402, 0.866) | .006 | 29.5 | 0.622 (0.411, 0.942) | .024 |
AUC, area under the plasma concentration–time curve; CI, confidence interval; dBP, diastolic blood pressure; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; mOS, median overall survival; mPFS, median progression-free survival.
Based on log-rank (score) test at significance level of P = .1 for inclusion in multivariate model.
Hazard ratio per 100 hxng/mL increase of AUC.
Hazard ratio per 10 mm Hg increase of dBP.
Table 5.
Multivariate Cox Proportional Hazards Analysis of Progression-Free and Overall Survival
| PFS |
OS |
|||||||
|---|---|---|---|---|---|---|---|---|
| Covariates | Parameter estimatea |
SEb | HR (95% CI) | Pc | Parameter estimatea |
SEb | HR (95% CI) | Pc |
| Gender | 0.542 | 0.211 | 1.72 (1.14, 2.60) | .010 | — | — | — | — |
| Prior therapy | 0.472 | 0.221 | 1.60 (1.04, 2.47) | .033 | 0.751 | 0.232 | 2.12 (1.35, 3.34) | .001 |
| ECOG PS | 0.598 | 0.225 | 1.82 (1.17, 2.83) | .008 | 0.861 | 0.231 | 2.37 (1.50, 3.72) | <.001 |
| Hemoglobin, g/dL | −0.509 | 0.217 | 0.601 (0.393, 0.921) | .019 | −1.06 | 0.249 | 0.348 (0.213, 0.567) | <.001 |
| AUC, hxng/mLd,e | −0.0956 | 0.0453 | 0.909 (0.832, 0.993) | .035 | −0.145 | 0.0574 | 0.866 (0.773, 0.968) | .012 |
| dBP, mm Hge,f | −0.416 | 0.122 | 0.660 (0.519, 0.839) | <.001 | −0.303 | 0.118 | 0.739 (0.586, 0.931) | .010 |
AUC, area under the plasma concentration–time curve; CI, confidence interval; dBP, diastolic blood pressure; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; SE, standard error.
Regression coefficient.
Standard error of regression coefficient.
Based on log-rank (score) test at significance in multivariate model. Backward elimination of covariates was performed at significance level of P = .05 using the log-likelihood ratio test.
Hazard ratio per 100 hxng/mL increase of AUC.
Final estimates after adjusting baseline hazard ratios with prognostic factors (gender, prior therapy, ECOG PS, and hemoglobin).
Hazard ratio per 10 mm Hg increase of dBP.
Correlations between AUC and efficacy
For evaluation of axitinib AUC versus efficacy endpoints, AUC at the end of 4 weeks of treatment showed better correlation compared with AUC for the entire study treatment (data not shown). Hence, the results based on AUC at the end of 4 weeks of treatment are presented here. However, analyses based on AUC for the entire treatment showed similar results (data not shown). The median (range) for AUC at the end of 4 weeks was 375 hxng/mL (32.8–1,728) (Table 3).
To evaluate the relationship between RECIST-based objective responses and exposure, logistic regression was performed. The results revealed a significant (P < .0001) relationship between axitinib plasma exposure and the probability of a response (i.e., 1.5-fold increase in the probability of achieving a partial response for every 100 hxng/mL increase in AUC, data not shown). To explore the relationship between AUC and time-to-event endpoints (PFS and OS), univariate Cox proportional regression was performed using AUC as both a categorical and continuous variable. Patients were stratified by having an AUC greater or equal to axitinib total daily therapeutic exposure of 300 hxng/mL (high AUC) or <300 hxng/mL (low AUC). Median PFS in the high-AUC group was significantly longer than median PFS in the low-AUC group (13.8 months vs. 7.4 months, respectively; hazard ratio [HR] 0.558; P = .003) (Table 4). Similarly, median OS of 37.4 months in the high-AUC group was considerably longer than the 15.8 months in the low-AUC group (HR 0.489; P < .001) (Table 4). These results indicated significant associations between AUC and clinical responses for axitinib. When used as a continuous variable, the result was more significant than using a cutoff value of 300 hxng/mL. For PFS and OS, the HR was 0.871 (P = .001) and 0.810 (P < .001), respectively, for every 100 hxng/mL increase in AUC (Table 4).
Correlations between dBP and efficacy
For evaluation of dBP versus efficacy endpoints, the maximum observed post-baseline dBP within the first 8 weeks was used in all analyses to ensure that adequate numbers of measurements were available for most patients while avoiding any bias due to inherent confounding factors, that is, patients staying on the study longer have a greater chance of registering an increase in BP and also a better response to treatment.
To evaluate the relationship between RECIST-based objective responses and dBP, logistic regression was performed. The results demonstrated a strong association (P = .0042) between dBP and the probability of a response (i.e., 1.6-fold increase in the probability of achieving a partial response for every 10 mm Hg increase in dBP; data not shown). To explore the relationship between dBP and PFS or OS, univariate Cox proportional regression was performed using dBP as both a categorical and continuous variable. The first onset of dBP ≥90 mm Hg as a time-dependent covariate showed that median PFS was significantly longer in patients with at least one dBP measurement ≥90 mm Hg compared with those without elevated dBP. The median PFS was 14.6 months in patients with dBP ≥90 mm Hg compared with 7.8 6 months in those with dBP <90 mm Hg (HR 0.590; P = .006) (Table 4). Similarly, the median OS was longer in the group of patients with dBP ≥90 mm Hg compared with those with dBP <90 mm Hg (29.5 vs. 18.5 months, HR 0.622; P = .024) (Table 4). With the use of dBP as a continuous variable, the results were more significant compared with using a cutoff of 90 mm Hg. For PFS and OS, the HR was 0.604 (P < .001) and 0.652 (P < .001), respectively, for every 10 mm Hg increase of dBP (Table 4). The use of ΔdBP (i.e., dBP change from baseline) provided similar results and did not improve the correlation with efficacy compared with the use of absolute maximum observed dBP (data not shown).
Correlation between dBP and AUC
In order to assess if the axitinib-related BP increase was simply a surrogate for axitinib plasma exposure, the relationship between AUC and dBP was initially evaluated using linear regression. Results showed a weak correlation between exposure and dBP (r2 values < .10; data not shown). The use of ΔdBP instead of absolute observed dBP did not improve the correlation (data not shown).
AUC and dBP as predictors of efficacy
To assess whether the relationship between AUC and dBP were independent predictors of survival, multivariate Cox regression was performed, using the previously developed multivariate model with the significant prognostic factors. In the multivariate analysis, AUC and dBP were tested as continuous variables since they were both more significant as continuous variables. Both AUC and dBP were retained as significant predictors after backward elimination, indicating that they were independently associated with PFS and OS, with dBP being more significant (data not shown). The final model parameter estimates for PFS and OS versus AUC and dBP (after adjusting for the baseline HR for prognostic factors) are shown in Table 5.
Discussion
The current retrospective analyses expanded on a previous exploratory analysis (using data from 12 clinical trials)27 in order to develop a comprehensive population PK model for axitinib and identify factors contributing toward variability in axitinib disposition. The axitinib PK model developed here confirmed the results of individual clinical trials with respect to the effect of food.11,28 Among 12 covariates tested, age >60 years, Japanese ethnicity, and smoking status had a significant influence on axitinib systemic CL and body weight on Vc. However, the effect of weight on Vc is considered to have little impact on overall axitinib plasma exposure. For individuals older than 60 years or of Japanese ethnicity, higher exposures may occur. A formal comparison of axitinib PK previously conducted in healthy, young Caucasian and Japanese subjects demonstrated no difference in axitinib PK between these two groups.29 Since this pooled population PK analysis included Japanese patients who had an overall lower body weight and tended to be older than other patients, it is unclear if the observed effect on plasma exposure for Japanese ethnicity may have been confounded by demographic imbalances in the study dataset. The final model also predicted a greater CL in active smokers than non-smokers or ex-smokers. However, the precision of the estimate for this effect was highly variable, likely due to a small (3%) number of active smokers in the dataset, thus requiring additional testing in a larger population to draw a definitive conclusion.
The lack of impact of renal (based on CrCL) or hepatic (based on AST, ALT, and bilirubin) function on axitinib disposition was not surprising, since subjects enrolled in these clinical trials were required to have normal renal and hepatic function at study entry. A formal hepatic impairment study conducted in subjects with mild and moderate hepatic impairment using the Child-Pugh classification, a more comprehensive assessment of hepatic function, indicated that mild hepatic impairment did not alter axitinib exposures compared with normal hepatic function. However, subjects with moderate hepatic impairment demonstrated a 2-fold higher exposure,30 necessitating axitinib dose adjustment in these patients.
Although the 4 covariates—Japanese ethnicity, body weight, age >60 years, and active smokers—were identified as having a significant effect on axitinib PK, inclusion of these covariates resulted in modest reductions of the IIV for CL and Vc from 64% to 60% and 44% to 40% (from base to final model), respectively. Thus, inclusion of the covariates, while significant as determined by the modeling, did little (<5%) to explain the observed high variability noted for axitinib PK. As such, these covariate effects are not considered clinically meaningful, and dose adjustments for axitinib based on these covariates are not warranted. Additional studies are needed to elucidate the source(s) of the variability observed in axitinib PK.
Relationships between axitinib exposure, elevated dBP, and efficacy were evaluated using pooled data from the 3 mRCC phase II trials. Prognostic factors predictive of PFS that were significant in the multivariate model were gender, sorafenib-refractory patients, low ECOG PS, and low hemoglobin. They were also predictive of OS, with the exception of gender. In this analysis, corrected serum calcium level, previously established as one of the prognostic factors in second-line mRCC22 was not found to be predictive for survival. A possible explanation may be that a relatively high proportion of patients (n = 145/168) had normal values of serum calcium.
After accounting for significant prognostic factors potentially predictive of PFS and OS in the final multivariate model, axitinib exposure and dBP were tested as additional, independent predictors. The results revealed that high AUC and an increase in dBP were both associated with longer PFS and OS and were independent predictors of survival in mRCC patients. Furthermore, logistic regression indicated that patients with high AUC and an increase in dBP had a higher probability of achieving a partial response.
The current PK/PD analyses provide evidence that treatment-related increases in dBP may not be simply an indicator of higher axitinib plasma concentrations, since changes in dBP following axitinib administration were not strongly correlated with AUC in this pooled analysis. Absence of a strong correlation between dBP and AUC observed for axitinib may be explained partially by a narrow range of exposures obtained with axitinib ≤ 10 mg bid in these phase II mRCC studies. In the phase I dose-escalation study in patients with solid tumors,11 the frequency and severity of hypertension were found to be dose-dependent when higher doses of axitinib (up to 30 mg bid) were administered.
Increases in BP have been suggested as a marker for inhibition of the VEGF/VEGFR signaling pathway in cancer patients treated with other antiangiogenic agents.14–16,31–34 Although the mechanism(s) involved in the elevation of BP following inhibition of VEGF or VEGFR is not well understood, endothelial dysfunction and microvascular rarefaction via decreased availability of nitric oxide have been postulated.35–37 The time course of increases in BP is not clear, though recent studies using more frequent home BP monitoring or ambulatory BP monitoring suggest that BP elevations occurred early following initiation of an antiangiogenic agent.14,38 This retrospective analysis provides further support that dBP may serve as a useful and easy-to-measure predictor of axitinib efficacy and help to identify patients with mRCC and possibly other solid tumors who would benefit from axitinib treatment. BP was monitored regularly and patients with increased BP were promptly treated with axitinib dose reduction/interruption and/or antihypertensive medications to control BP. Therefore, it is unlikely that sustained increase in BP is required for efficacy; rather the transient increase in BP generally seen soon after initiation of treatment, which is subsequently managed with treatment, may be the predictor of efficacy. Early detection through close monitoring of dBP, and strict control of axitinib-related increase in dBP when it occurs, are critical for maintaining patients on treatment with axitinib to improve clinical outcomes.
In conclusion, the current population PK analysis identified age over 60 years, active smokers, and Japanese ethnicity as having a statistically significant influence on axitinib systemic CL, whereas body weight influenced Vc. However, none of the covariate effects substantially decreased the variability associated with axitinib PK. Consequently, no dose adjustments for axitinib are warranted based on these covariates. Evaluation of prognostic factors identified gender, prior therapy, ECOG PS, and hemoglobin as significant risk factors in mRCC patients. These risk factors may assist in clinical trial design in prospective studies in advanced mRCC patients. In the PK/PD analyses, increasing axitinib AUC correlated with longer PFS and OS and higher probability of a partial response in patients with mRCC. The observed variability in axitinib PK suggests some patients achieve only sub-therapeutic plasma concentrations at the starting dose of 5 mg bid. In these patients, dose increases above 5 mg bid may be justified to increase plasma exposures, which in turn would lead to improved clinical benefits, as long as patients tolerate the drug. In addition, dBP was shown to be an independent predictor of clinical efficacy, and may not be merely a reflection of higher axitinib exposures. Based on these findings, drug-induced BP elevation may be an early indicator of drug activity, serving as a potential marker. A prospective study to confirm the association between dBP and clinical response in axitinib-treated patients is warranted.
Supplementary Material
Acknowledgments
This study and all clinical trials included in the analyses were sponsored by Pfizer Inc. B. I. Rini: consultant/advisory board and commercial research grant from Pfizer Inc. B. Poland: employment Pharsight, which received funding from Pfizer Inc to provide modeling and simulation services. J. P. Dutcher: commercial research grant from Exelixis; honoraria from Speakers Bureaus for Pfizer Inc., Novartis, Genentech, and Prometheus; consultant/advisory boards for Pfizer Inc and Prometheus. O. Rixe: honoraria from Speakers Bureau for Pfizer Inc. W. M. Stadler: commercial research grants from Bayer, Pfizer Inc., Novartis, and Roche; honoraria from Speakers Bureau for Pfizer Inc; stock ownership (spouse), Abbott; consultant/advisory boards for Novartis, Pfizer Inc., Roche, CareMark, and AVEO. M. Garrett, Y. K. Pithavala, S. Kim, and J. Tarazi: employment and stock ownership, Pfizer Inc R. J. Motzer: commercial research grant and consultant/advisory board for Pfizer Inc. Medical writing support was funded by Pfizer Inc and was provided by Mariko Nagashima, PhD, of UBC Scientific Solutions (Southport, CT, USA).
Footnotes
Additional supporting information may be found in the online version of this article.
Declaration of Conflicting Interests
G. Wilding: nothing to disclose.
References
- 1.Hu-Lowe DD, Zou HY, Grazzini ML, et al. Nonclinical antiangio-genesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2 3. Clin Cancer Res. 2008;14:7272–7283. doi: 10.1158/1078-0432.CCR-08-0652. [DOI] [PubMed] [Google Scholar]
- 2.Kelly RJ, Rixe O. Axitinib—a selective inhibitor of the vascular endothelial growth factor (VEGF) receptor. Target Oncol. 2009;4:297–305. doi: 10.1007/s11523-009-0126-9. [DOI] [PubMed] [Google Scholar]
- 3.Choueiri TK. Axitinib, a novel anti-angiogenic drug with promising activity in various solid tumors. Curr Opin Invest Drugs. 2008;9:658–671. [PubMed] [Google Scholar]
- 4.Tomita Y, Uemura H, Fujimoto H, et al. Key predictive factors of axitinib (AG-013736)-induced proteinuria and efficacy: a phase II study in Japanese patients with cytokine-refractory metastatic renal cell cancer. Eur J Cancer. 2011;47:2592–2602. doi: 10.1016/j.ejca.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 5.Rixe O, Bukowski RM, Michaelson MD, et al. Axitinib treatment in patients with cytokine-refractory metastatic renal-cell cancer: a phase II study. Lancet Oncol. 2007;8:975–984. doi: 10.1016/S1470-2045(07)70285-1. [DOI] [PubMed] [Google Scholar]
- 6.Rini BI, Wilding G, Hudes G, et al. Phase II study of axitinib in sorafenib-refractory metastatic renal cell carcinoma. J Clin Oncol. 2009;27:4462–4468. doi: 10.1200/JCO.2008.21.7034. [DOI] [PubMed] [Google Scholar]
- 7.Cohen EE, Rosen LS, Vokes EE, et al. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol. 2008;26:4708–4713. doi: 10.1200/JCO.2007.15.9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schiller JH, Larson T, Ou SH, et al. Efficacy and safety of axitinib in patients with advanced non-small-cell lung cancer: results from a phase II study. J Clin Oncol. 2009;27:3836–3841. doi: 10.1200/JCO.2008.20.8355. [DOI] [PubMed] [Google Scholar]
- 9.Fruehauf JP, Lutzky J, McDermott DF, et al. Multicenter, phase II study of axitinib, a selective second-generation inhibitor of vascular endothelial growth factor receptors 1, 2 3, in patients with metastatic melanoma. Clin Cancer Res. 2011;17:7462–7469. doi: 10.1158/1078-0432.CCR-11-0534. [DOI] [PubMed] [Google Scholar]
- 10.Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378:1931–1939. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 11.Rugo HS, Herbst RS, Liu G, et al. Phase I trial of the oral antiangiogenesis agent AG-013736 in patients with advanced solid tumors: pharmacokinetic and clinical results. rJ Clin Oncol. 2005;23:5474–5483. doi: 10.1200/JCO.2005.04.192. [DOI] [PubMed] [Google Scholar]
- 12.Larson RA, Druker BJ, Guilhot F, et al. Imatinib pharmacokinetics and its correlation with response and safety in chronic-phase chronic myeloid leukemia: a subanalysis of the IRIS study. Blood. 2008;111:4022–4028. doi: 10.1182/blood-2007-10-116475. [DOI] [PubMed] [Google Scholar]
- 13.Houk BE, Bello CL, Poland B, Rosen LS, Demetri GD, Motzer RJ. Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: results of a pharmacokinetic/pharmacodynamic meta-analysis. Cancer Chemother Pharmacol. 2010;66:357–371. doi: 10.1007/s00280-009-1170-y. [DOI] [PubMed] [Google Scholar]
- 14.Maitland ML, Kasza KE, Karrison T, et al. Ambulatory monitoring detects sorafenib-induced blood pressure elevations on the first day of treatment. Clin Cancer Res. 2009;15:6250–6257. doi: 10.1158/1078-0432.CCR-09-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rixe O, Billemont B, Izzedine H. Hypertension as a predictive factor of sunitinib activity. Ann Oncol. 2007;18:1117. doi: 10.1093/annonc/mdm184. [DOI] [PubMed] [Google Scholar]
- 16.Scartozzi M, Galizia E, Chiorrini S, et al. Arterial hypertension correlates with clinical outcome in colorectal cancer patients treated with first-line bevacizumab. Ann Oncol. 2009;20:227–230. doi: 10.1093/annonc/mdn637. [DOI] [PubMed] [Google Scholar]
- 17.Rini BI, Schiller JH, Fruehauf JP, et al. Diastolic blood pressure as a biomarker of axitinib efficacy in solid tumors. Clin Cancer Res. 2011;17:3841–3849. doi: 10.1158/1078-0432.CCR-10-2806. [DOI] [PubMed] [Google Scholar]
- 18.Mukohara T, Nakajima H, Mukai H, et al. Effect of axitinib (AG-013736) on fatigue, thyroid-stimulating hormone, and biomarkers: a phase I study in Japanese patients. Cancer Sci. 2010;101:963–968. doi: 10.1111/j.1349-7006.2009.01465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rousseau A, Leger F, Le Meur Y, et al. Population pharmacokinetic modeling of oral cyclosporin using NONMEM: comparison of absorption pharmacokinetic models and design of a Bayesian estimator. Ther Drug Monit. 2004;26:23–30. doi: 10.1097/00007691-200402000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Duval V, Karlsson MO. Impact of omission or replacement of data below the limit of quantification on parameter estimates in a two-compartment model. Pharm Res. 2002;19:1835–1840. doi: 10.1023/a:1021441407898. [DOI] [PubMed] [Google Scholar]
- 21.Garrett M, Amantea MA, Pithavala Y, Ruiz-Garcia A. Evaluation of the impact of omitting drug concentration data below the lower limit of quantification (LLOQ) on the pharmacokinetics of axitinib (AG-013736), an anti-angiogenic agent; Presented at the 2010 Annual Meeting of the American Society Clinical Pharmacology Therapeutics (ASCPT); Atlanta, GA. 2010. Mar 17–20, [Google Scholar]
- 22.Motzer RJ, Bacik J, Schwartz LH, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. 2004;22:454–463. doi: 10.1200/JCO.2004.06.132. [DOI] [PubMed] [Google Scholar]
- 23.Liu G, Rugo HS, Wilding G, et al. Dynamic contrast-enhanced magnetic resonance imaging as a pharmacodynamic measure of response after acute dosing of AG-013736, an oral angiogenesis inhibitor, in patients with advanced solid tumors: results from a phase I study. J Clin Oncol. 2005;23:5464–5473. doi: 10.1200/JCO.2005.04.143. [DOI] [PubMed] [Google Scholar]
- 24.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 25.Savic RM, Karlsson MO. Importance of shrinkage in Empirical Bayes estimates for diagnostics: problems solutions. AAPS J. 2009;11:558–569. doi: 10.1208/s12248-009-9133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ichimaru K, Toyoshima S, Uyama Y. Effective global drug development strategy for obtaining regulatory approval in Japan in the context of ethnicity-related drug response factors. Clin Pharmacol Ther. 2010;87:362–366. doi: 10.1038/clpt.2009.285. [DOI] [PubMed] [Google Scholar]
- 27.Rixe O, Dutcher J, Motzer R, et al. Diastolic blood pressure (dBP) and pharmacokinetics (PK) as predictors of axitinib efficacy in metastatic renal cell cancer (mRCC) J Clin Oncol. 2009;27(15s):5045. abstract. [Google Scholar]
- 28.Pithavala YK, Chen Y, Toh M, et al. Evaluation of the effect of food on the pharmacokinetics of axitinib in healthy volunteers. Cancer Chemother Pharmacol. 2012;70:103–112. doi: 10.1007/s00280-012-1888-9. [DOI] [PubMed] [Google Scholar]
- 29.Pithavala YK, Tortorici M, Toh M, et al. Effect of rifampin on the pharmacokinetics of axitinib (AG-013736) in Japanese and Caucasian healthy volunteers. Cancer Chemother Pharmacol. 2010;65:563–570. doi: 10.1007/s00280-009-1065-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tortorici MA, Toh M, Rahavendran SV, et al. Influence of mild and moderate hepatic impairment on axitinib pharmacokinetics. Invest New Drugs. 2011;29:1370–1380. doi: 10.1007/s10637-010-9477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu X, Wu S, Dahut WL, Parikh CR. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis. 2007;49:186–193. doi: 10.1053/j.ajkd.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 32.Wu S, Chen JJ, Kudelka A, Lu J, Zhu X. Incidence and risk of hypertension with sorafenib in patients with cancer: a systematic review and meta-analysis. Lancet Oncol. 2008;9:117–123. doi: 10.1016/S1470-2045(08)70003-2. [DOI] [PubMed] [Google Scholar]
- 33.Zhu X, Stergiopoulos K, Wu S. Risk of hypertension and renal dysfunction with an angiogenesis inhibitor sunitinib: systematic review and meta-analysis. Acta Oncol. 2009;48:9–17. doi: 10.1080/02841860802314720. [DOI] [PubMed] [Google Scholar]
- 34.Sleijfer S, Ray-Coquard I, Papai Z, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European Organisation for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC study 62043) J Clin Oncol. 2009;27:3126–3132. doi: 10.1200/JCO.2008.21.3223. [DOI] [PubMed] [Google Scholar]
- 35.Veronese ML, Mosenkis A, Flaherty KT, et al. Mechanisms of hypertension associated with BAY 43–9006. J Clin Oncol. 2006;24:1363–1369. doi: 10.1200/JCO.2005.02.0503. [DOI] [PubMed] [Google Scholar]
- 36.Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007;96:1788–1795. doi: 10.1038/sj.bjc.6603813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mourad JJ, des Guetz G, Debbabi H, Levy BI. Blood pressure rise following angiogenesis inhibition by bevacizumab. A crucial role for microcirculation. Ann Oncol. 2008;19:927–934. doi: 10.1093/annonc/mdm550. [DOI] [PubMed] [Google Scholar]
- 38.Azizi M, Chedid A, Oudard S. Home blood-pressure monitoring in patients receiving sunitinib. N Engl J Med. 2008;358:95–97. doi: 10.1056/NEJMc072330. [DOI] [PubMed] [Google Scholar]
- 39.Pithavala YK, Tong W, Mount J, et al. Effect of ketoconazole on the pharmacokinetics of axitinib in healthy volunteers. Invest New Drugs. 2012;30:273–281. doi: 10.1007/s10637-010-9511-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Y, Jiang J, Zhang J, et al. A Phase I study to evaluate the pharmacokinetics of axitinib (AG-13736) in healthy Chinese volunteers. Int J Clin Pharmacol Ther. 2011;49:679–687. doi: 10.5414/cp201570. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.