Abstract
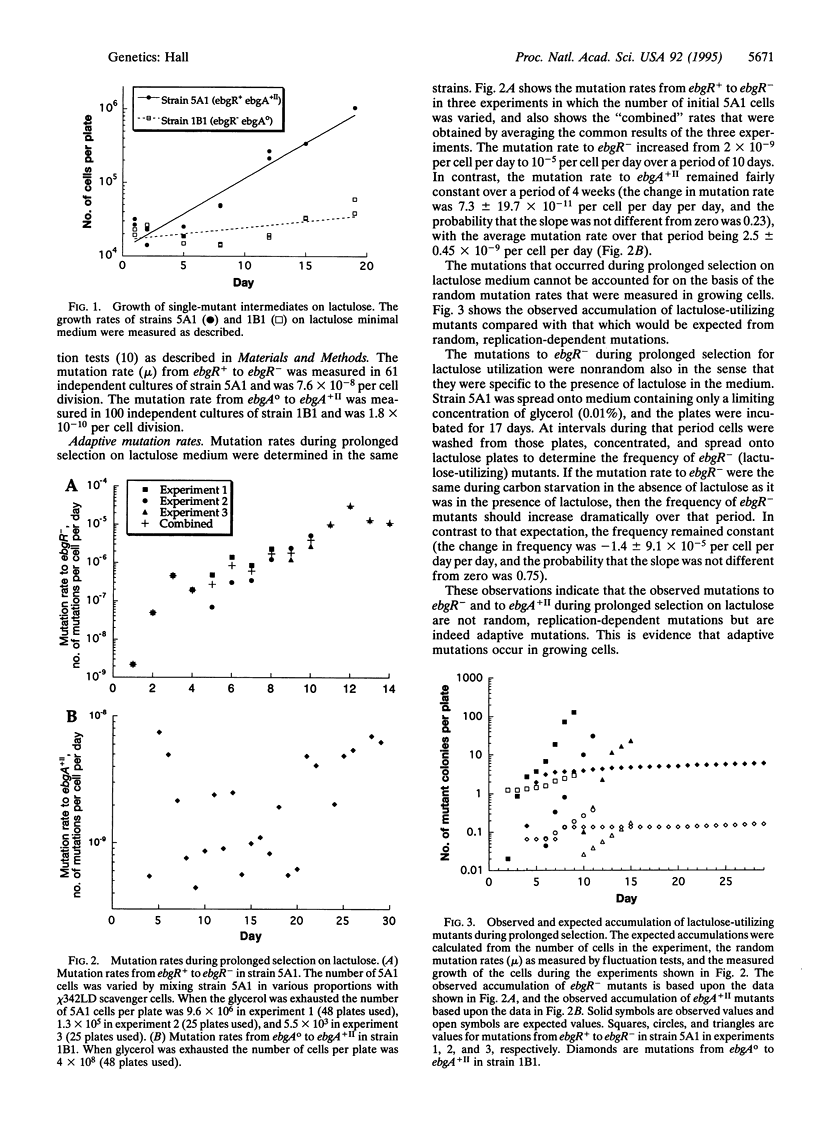
The cells in most tumors are found to carry multiple mutations; however, based upon mutation rates determined by fluctuation tests, the frequency of such multiple mutations should be so low that tumors are never detected within human populations. Fluctuation tests, which determine the cell-division-dependent mutation rate per cell generation in growing cells, may not be appropriate for estimating mutation rates in nondividing or very slowly dividing cells. Recent studies of time-dependent, "adaptive" mutations in nondividing populations of microorganisms suggest that similar measurements may be more appropriate to understanding the mutation origins of tumors. Here I use the ebgR and ebgA genes of Escherichia coli to measure adaptive mutation rates where multiple mutations are required for rapid growth. Mutations in either ebgA or ebgR allow very slow growth on lactulose (4-O-beta-D-galactosyl-D-fructose), with doubling times of 3.2 and 17.3 days, respectively. However, when both mutations are present, cells can grow rapidly with doubling times of 2.7 hr. I show that during prolonged (28-day) selection for growth on lactulose, the number of lactulose-utilizing mutants that accumulate is 40,000 times greater than can be accounted for on the basis of mutation rates measured by fluctuation tests, but is entirely consistent with the time-dependent adaptive mutation rates measured under the same conditions of prolonged selection.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhattacharyya N. P., Skandalis A., Ganesh A., Groden J., Meuth M. Mutator phenotypes in human colorectal carcinoma cell lines. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6319–6323. doi: 10.1073/pnas.91.14.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boe L. Mechanism for induction of adaptive mutations in Escherichia coli. Mol Microbiol. 1990 Apr;4(4):597–601. doi: 10.1111/j.1365-2958.1990.tb00628.x. [DOI] [PubMed] [Google Scholar]
- Bronner C. E., Baker S. M., Morrison P. T., Warren G., Smith L. G., Lescoe M. K., Kane M., Earabino C., Lipford J., Lindblom A. Mutation in the DNA mismatch repair gene homologue hMLH1 is associated with hereditary non-polyposis colon cancer. Nature. 1994 Mar 17;368(6468):258–261. doi: 10.1038/368258a0. [DOI] [PubMed] [Google Scholar]
- Cairns J., Foster P. L. Adaptive reversion of a frameshift mutation in Escherichia coli. Genetics. 1991 Aug;128(4):695–701. doi: 10.1093/genetics/128.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns J., Overbaugh J., Miller S. The origin of mutants. Nature. 1988 Sep 8;335(6186):142–145. doi: 10.1038/335142a0. [DOI] [PubMed] [Google Scholar]
- Cairns J. The evolution of cancer research. Cancer Cells. 1989 Sep;1(1):1–8. [PubMed] [Google Scholar]
- Cairns J. The origin of human cancers. Nature. 1981 Jan 29;289(5796):353–357. doi: 10.1038/289353a0. [DOI] [PubMed] [Google Scholar]
- Farber E., Rubin H. Cellular adaptation in the origin and development of cancer. Cancer Res. 1991 Jun 1;51(11):2751–2761. [PubMed] [Google Scholar]
- Foster P. L. Directed mutation: between unicorns and goats. J Bacteriol. 1992 Mar;174(6):1711–1716. doi: 10.1128/jb.174.6.1711-1716.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall B. G., Hartl D. L. Regulation of newly evolved enzymes. II. The ebg repressor. Genetics. 1975 Nov;81(3):427–435. doi: 10.1093/genetics/81.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B. G. Adaptive evolution that requires multiple spontaneous mutations. I. Mutations involving an insertion sequence. Genetics. 1988 Dec;120(4):887–897. doi: 10.1093/genetics/120.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B. G. Adaptive evolution that requires multiple spontaneous mutations: mutations involving base substitutions. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5882–5886. doi: 10.1073/pnas.88.13.5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B. G., Betts P. W., Wootton J. C. DNA sequence analysis of artificially evolved ebg enzyme and ebg repressor genes. Genetics. 1989 Dec;123(4):635–648. doi: 10.1093/genetics/123.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B. G. Changes in the substrate specificities of an enzyme during directed evolution of new functions. Biochemistry. 1981 Jul 7;20(14):4042–4049. doi: 10.1021/bi00517a015. [DOI] [PubMed] [Google Scholar]
- Hall B. G., Clarke N. D. Regulation of newly evolved enzymes. III Evolution of the ebg repressor during selection for enhanced lactase activity. Genetics. 1977 Feb;85(2):193–201. doi: 10.1093/genetics/85.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B. G. Experimental evolution of a new enzymatic function. Kinetic analysis of the ancestral (ebg) and evolved (ebg) enzymes. J Mol Biol. 1976 Oct 15;107(1):71–84. doi: 10.1016/s0022-2836(76)80018-6. [DOI] [PubMed] [Google Scholar]
- Hall B. G. Genetics of selection-induced mutations: I. uvrA, uvrB, uvrC, and uvrD are selection-induced specific mutator loci. J Mol Evol. 1995 Jan;40(1):86–93. doi: 10.1007/BF00166599. [DOI] [PubMed] [Google Scholar]
- Hall B. G., Hartl D. L. Regulation of newly evolved enzymes. I. Selection of a novel lactase regulated by lactose in Escherichia coli. Genetics. 1974 Mar;76(3):391–400. doi: 10.1093/genetics/76.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B. G. On alternatives to selection-induced mutation in the Bgl operon of Escherichia coli. Mol Biol Evol. 1994 Mar;11(2):159–168. doi: 10.1093/oxfordjournals.molbev.a040100. [DOI] [PubMed] [Google Scholar]
- Hall B. G. Regulation of newly evolved enzymes. IV. Directed evolution of the Ebg repressor. Genetics. 1978 Dec;90(4):673–681. doi: 10.1093/genetics/90.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B. G. Selection, adaptation, and bacterial operons. Genome. 1989;31(1):265–271. doi: 10.1139/g89-044. [DOI] [PubMed] [Google Scholar]
- Hall B. G. Selection-induced mutations occur in yeast. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4300–4303. doi: 10.1073/pnas.89.10.4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B. G. Spectrum of mutations that occur under selective and non-selective conditions in E. coli. Genetica. 1991;84(2):73–76. doi: 10.1007/BF00116545. [DOI] [PubMed] [Google Scholar]
- Hall B. G. Spontaneous point mutations that occur more often when advantageous than when neutral. Genetics. 1990 Sep;126(1):5–16. doi: 10.1093/genetics/126.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B. G. The role of single-mutant intermediates in the generation of trpAB double revertants during prolonged selection. J Bacteriol. 1993 Oct;175(20):6411–6414. doi: 10.1128/jb.175.20.6411-6414.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Kaden D. A., Bardwell L., Newmark P., Anisowicz A., Skopek T. R., Sager R. High frequency of large spontaneous deletions of DNA in tumor-derived CHEF cells. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2306–2310. doi: 10.1073/pnas.86.7.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy A. R., Fox M., Murphy G., Little J. B. Relationship between x-ray exposure and malignant transformation in C3H 10T1/2 cells. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7262–7266. doi: 10.1073/pnas.77.12.7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C. C., Gadi I. K., Kalvonjian S., Anisowicz A., Sager R. Plasmid-induced "hit-and-run" tumorigenesis in Chinese hamster embryo fibroblast (CHEF) cells. Proc Natl Acad Sci U S A. 1985 May;82(9):2839–2843. doi: 10.1073/pnas.82.9.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach F. S., Nicolaides N. C., Papadopoulos N., Liu B., Jen J., Parsons R., Peltomäki P., Sistonen P., Aaltonen L. A., Nyström-Lahti M. Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell. 1993 Dec 17;75(6):1215–1225. doi: 10.1016/0092-8674(93)90330-s. [DOI] [PubMed] [Google Scholar]
- Lei X., Hall B. G. SASA: a simplified, reliable method for allele-specific amplification of polymorphic sites. Biotechniques. 1994 Jan;16(1):44–45. [PubMed] [Google Scholar]
- Loeb L. A. Microsatellite instability: marker of a mutator phenotype in cancer. Cancer Res. 1994 Oct 1;54(19):5059–5063. [PubMed] [Google Scholar]
- Loeb L. A. Mutator phenotype may be required for multistage carcinogenesis. Cancer Res. 1991 Jun 15;51(12):3075–3079. [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler J. E., Lenski R. E. Experimental evidence for an alternative to directed mutation in the bgl operon. Nature. 1992 Apr 2;356(6368):446–448. doi: 10.1038/356446a0. [DOI] [PubMed] [Google Scholar]
- Nowell P. C. The clonal evolution of tumor cell populations. Science. 1976 Oct 1;194(4260):23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Rubin A. L., Yao A., Rubin H. Relation of spontaneous transformation in cell culture to adaptive growth and clonal heterogeneity. Proc Natl Acad Sci U S A. 1990 Jan;87(1):482–486. doi: 10.1073/pnas.87.1.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager R. Mutation rates and mutational spectra in tumorigenic cell lines. Cancer Surv. 1988;7(2):325–333. [PubMed] [Google Scholar]
- Steele D. F., Jinks-Robertson S. An examination of adaptive reversion in Saccharomyces cerevisiae. Genetics. 1992 Sep;132(1):9–21. doi: 10.1093/genetics/132.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein W. D. Analysis of cancer incidence data on the basis of multistage and clonal growth models. Adv Cancer Res. 1991;56:161–213. doi: 10.1016/s0065-230x(08)60481-9. [DOI] [PubMed] [Google Scholar]
- Stewart F. M., Gordon D. M., Levin B. R. Fluctuation analysis: the probability distribution of the number of mutants under different conditions. Genetics. 1990 Jan;124(1):175–185. doi: 10.1093/genetics/124.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss B. S. The origin of point mutations in human tumor cells. Cancer Res. 1992 Jan 15;52(2):249–253. [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Kern S. E., Hamilton S. R., Preisinger A. C., Nakamura Y., White R. Allelotype of colorectal carcinomas. Science. 1989 Apr 14;244(4901):207–211. doi: 10.1126/science.2565047. [DOI] [PubMed] [Google Scholar]



