Background: Dephosphorylation of R-Smads in the nucleus shuts off TGF-β superfamily signaling.
Results: SCP4 specifically dephosphorylates BMP-activated Smad1/5/8, but not TGF-β-activated Smad2/3, and ectopic expression of SCP4 inhibits BMP signaling, whereas SCP4 depletion enhances BMP signaling.
Conclusion: SCP4 is a nuclear phosphatase terminating BMP signaling.
Significance: Identification of SCP4 may suggest its physiological functions in BMP-induced cellular processes and relevant diseases.
Keywords: Bone Morphogenetic Protein (BMP), Osteoblast, Phosphatase, Phosphorylation, SMAD Transcription Factor, Transforming Growth Factor Beta (TGF-β)
Abstract
The bone morphogenetic protein (BMP) signaling pathway regulates a wide range of cellular responses in metazoans. A key step in the canonical BMP signaling is the phosphorylation and activation of transcription factors Smad1, Smad5, and Smad8 (collectively Smad1/5/8) by the type I BMP receptors. We previously identified PPM1A as a phosphatase toward dephosphorylation of all receptor-regulated Smads (R-Smads), including Smad1/5/8. Here we report another nuclear phosphatase named SCP4/CTDSPL2, belonging to the FCP/SCP family, as a novel Smad phosphatase in the nucleus. SCP4 physically interacts with and specifically dephosphorylates Smad1/5/8, and as a result attenuates BMP-induced transcriptional responses. Knockdown of SCP4 in multipotent mesenchymal C2C12 cells leads to increased expression of BMP target genes and consequently promotes BMP-induced osteogenic differentiation. Collectively, our results demonstrate that SCP4, as a Smad phosphatase, plays a critical role in BMP-induced signaling and cellular functions.
Introduction
Bone morphogenetic proteins (BMPs),3 except protease BMP1, are growth factors belonging to the transforming growth factor-β (TGF-β) superfamily (1). The BMP signaling pathway, which is highly conserved during evolution, controls a wide range of cellular responses and diverse developmental processes including cell and tissue differentiation, morphogenetic processes, limb development, axis specification, generation of primordial germ cells, and patterning of the neural tube (2–5). Furthermore, BMPs play a vital regulatory role in the maintenance and differentiation of mesoderm-derived stem cells (6, 7). Deregulation of BMP signaling contributes to the pathogenesis of many human diseases in the mesoderm-derived tissues and organs such as bone diseases, kidney diseases, vascular disorders, and heritable cancer (8, 9). The activity of the BMP-initiated signaling pathways is under tight control by processes including regulation of the ligands, the receptors, secreted antagonists (e.g. noggin), and the downstream intracellular effector Smad proteins.
Smad proteins are central signal transducers in the canonical BMP/TGF-β signaling pathway (10). Eight Smads are divided into three subgroups in mammals: five R-Smads, one common Smad (Smad4), and two inhibitory Smads (Smad6 and Smad7). Among R-Smads, Smad1/5/8 transduce BMP signals, whereas Smad2 and Smad3 are specific for TGF-β and activin signaling (10). The most critical step in canonical BMP signaling is the ligand-induced phosphorylation of Smad1/5/8 in the C-terminal SXS motif, which is carried out by the BMP type I receptors. The SXS phosphorylation triggers a cascade of intracellular events from Smad complex assembly in the cytoplasm to transcriptional control in the nucleus (10, 11). Smads are also regulated by and cross-talked with multiple kinases (12–14). Despite substantial effort devoted to understanding the actions of BMP and Smads, the regulation of Smad signaling termination remains enigmatic.
Because signal transduction pathways are regulated by dynamic interplay between protein kinases and phosphatases, the SXS motif must be dephosphorylated by phosphatases to ensure proper balance of Smad signaling. Recent work demonstrates that R-Smads exported from the nucleus are dephosphorylated (15–18). Several phosphatases were identified as the phosphatases toward the dephosphorylation of R-Smads, including PPM1A as a pan-Smad phosphatase (19, 20). Smad1 is also reported to be dephosphorylated by pyruvate dehydrogenase phosphatase (PDP) (21), small C-terminal phosphatases (SCP1/2/3) (22), and endosomal phosphatase MTMR4 (23). Meanwhile, SCP1/2/3 can target the linker region of R-Smads (22, 24, 25)
In this study, we showed that knockdown of PPM1A failed to completely restore the level of phospho-Smad1 (P-Smad1), suggesting the existence of other phosphatases toward P-Smad1. We thus undertook a screen for additional Smad phosphatases and identified CTDSPL2 (named SCP4 for small C-terminal domain Phosphatase-4) as a novel nuclear phosphatase for Smad1/5/8. We have demonstrated that SCP4 physically interacts with and specifically dephosphorylates Smad1/5/8, but not Smad2/3, at the C-terminal SXS motif. Furthermore, overexpression of SCP4 attenuates BMP-induced transcription. Conversely, depletion of SCP4 increases the C-terminal SXS motif phosphorylation and enables an increased sensitivity of cells to BMP2. Hence, SCP4-mediated dephosphorylation of Smad1/5/8 provides an additional mechanism in turning off the BMP signaling.
EXPERIMENTAL PROCEDURES
Mammalian Expression Plasmids
Expression plasmids for epitope-tagged Smads, HA-tagged constitutively active ALK3(Q233D), and FLAG-tagged constitutively active TβRI(T202D) have been previously described (19, 20). Full-length SCP4, its phosphatase-dead mutant (D293E, D295N), and deletion mutants were obtained by PCR and cloned into EcoRI (5′) and SalI (3′) of pXF6F (derivative of pRK5, Genentech). Their sequence integrity was confirmed by sequencing. Reporter gene plasmids SBE-luc, PAI-1-luc, and Id1-luc were obtained from Dr. Bert Vogelstein, David Luskutoff, and Peter ten Dijke, respectively.
Antibodies and Reagents
Antibodies against Smad1, P-Smad1, Smad2, P-Smad2 (Cell Signaling), Id1 (Epitomics), RNA polymerase II (PolII), and P-PolII-Ser-5 (phospho-Ser-5 in the CTD of RNA polymerase II (RNAPII)) (Covance) were obtained commercially. Rabbit anti-SCP4 polyclonal antibody was generated against the N-terminal 1–262 amino acids of SCP4 (Bethyl Laboratories), and P-Ser-206 (Smad1) antibody was raised against phospho-Ser-206 in the linker of Smad1 (Rockland Immunochemicals). Doxycycline was purchased from Clontech.
Cell Culture, Cell Transfection, Immunoprecipitation, and Western Blotting
HEK293T, HeLa, C2C12, ATDC5, HaCaT, and C3H10T1/2 cells were cultured and transfected using Lipofectamine (Invitrogen) as described previously (19). Methods for establishing C2C12 stable cells with shRNA-based gene knockdown were performed as described previously (19).
Immunoprecipitation (IP) was carried out as described previously (19). HEK293T cells were transiently transfected with expression plasmids for Myc-Smad1 and FLAG-SCP4. Twenty-four h after transfection, cell lysates were harvested by Myc lysis buffer (138 mm NaCl, 20 mm Tris-HCl (pH 8.0), 1% Nonidet P-40), and anti-FLAG antibodies were used to immunoprecipitate FLAG-SCP4 from transfected cell lysates by incubating with protein A-Sepharose CL-4B (GE Healthcare) at 4 °C for 4 h. After extensive washes, immunoprecipitated proteins were separated by SDS-PAGE, transferred onto PVDF membrane, immunostained with anti-Myc or anti-FLAG antibodies, and finally detected by horseradish peroxidase-conjugated secondary antibodies and visualized by chemiluminescence (Pierce).
In Vitro GST Pulldown Assay
In vitro protein translation was performed from the pRK5-derived vector using SP6 RNA polymerase and the TnT® quick coupled transcription/translation system (Promega). Proteins fused with GST in pGEX vector were expressed in Escherichia coli BL21 (DE3) strain and purified according to the manufacturer's instructions. GST pulldown experiments were carried out as described previously (26).
RNA Interference
Small interference siRNA targeting mouse SCP4 were made by RiboBio Co. (#1 target sequence, nucleotides 796–814 of coding region, GCAGTTCAAGTGAGGCCAT; #2 target sequence, nucleotides 1506–1524 of coding region, GAAGCTTGTAGAACTGAAT). Cells were transfected with siControl or siSCP4 using Lipofectamine® RNAiMAX (Invitrogen).
Lentivirus Production and Stable Cell Line Generation
SCP4 cDNA was subcloned into pWPI-puro vector at EcoRI and PmeI sites to generate pWPI-SCP4. HEK293T cells were transfected with pWPI-SCP4 together with lentiviral packaging plasmid psPAX2 and envelope plasmid pMD2.G. After 48 h of culture, lentiviruses were collected from medium, purified by centrifuge, and then used to infect host cells. Stable cells were selected in the presence of 2 ng/ml puromycin (Sigma).
Transcription Reporter Assay
Eighteen h after transfection, cells were treated with BMP2 (5 ng/ml, 8 h) or TGF-β (2 ng/ml, 8 h) as described (26). Cells were then harvested and analyzed with the Dual-Luciferase reporter assay system (Promega). All assays were done in triplicates, and all values were normalized for transfection efficiency against Renilla luciferase activities.
Quantitative Real-time RT-PCR (qRT-PCR)
Total RNAs were extracted using TRIzol (Invitrogen). One μg of total RNAs was reverse-transcribed to complementary DNA using PrimeScript® RT reagent kit (TaKaRa). qRT-PCR was performed using SYBR Green (Applied Biosystems) with β-actin as an internal loading control on an ABI PRISM 7500 sequence detector system (Applied Biosystems). Samples were done in triplicate, and data were analyzed using the 2−ΔCT method. Primers used for specific mouse genes are listed as below: Id1, 5′-AGAACCGCAAAGTGAGCAAGGT-3′ (forward) and 5′-GGTGGTCCCGACTTCAGACT-3′ (reverse); β-actin, 5′-TGAGCGCAAGTACTCTGTGTGGAT-3′ (forward) and 5′-ACTCATCGTACTCCTGCTTGCTGA-3′ (reverse); osteocalcin 5′-TAGCAGACACCATGAGGACCATCT-3′ (forward) and 5′-CCTGCTTGGACATGAAGGCTTTGT-3′ (reverse).
Immunofluorescence
Cells grown on coverslips were fixed with 4% formaldehyde for 15 min, treated by 0.5% Triton X-100 treatment for 20 min, and blocked with 5% BSA. For staining, cells were probed with the indicated primary antibody, Alexa Fluor 546 anti-mouse or anti-rabbit antibody, and then visualized under a Zeiss Axio confocal scanning laser microscope.
Osteoblast Differentiation and Alkaline Phosphatase Assay
Multipotent C2C12 cells were cultured in DMEM supplemented with 10% FBS. After cell density reaching 50% confluence, BMP2 (50 ng/ml) was added to induce osteoblast-like lineage differentiation. Alkaline phosphatase (ALP) is a marker for osteoblast differentiation. After BMP2 stimulation, C2C12 cells were washed with TB buffer twice (20 mm Tris, pH 7.5, 150 mm NaCl) and then lysed with the lysis buffer (TB buffer, plus 0.1% Triton). After centrifugation at 12,000 rpm for 10 min, 50 μl of whole cell lysates were incubated with 100 μl of ALP substrate p-nitrophenyl phosphate (pNPP) at 37 °C for 1 h, and absorbance at 405 nm was measured according to the manufacturer's instructions (Sigma).
For histochemical analysis of ALP activity, cells were fixed for 10 min with 3.7% formaldehyde at room temperature. After washing with PBS, cells were incubated for 20 min with a mixture of 0.1 mg/ml naphthol AS-MX phosphate (Sigma), 0.5% N, N-dimethylformamide, 2 mm MgCl2, and 0.6 mg/ml Fast Blue BB salt (Sigma) in 0.1 m Tris-HCl, pH 8.5, at room temperature. Cells were examined by phase contrast microscopy Nikon SMZ 1500.
Statistical Analysis
Results are shown as means ± S.E. All experiments were repeated at least three times. Mean values were compared with controls by Student's t test.
RESULTS
Depletion of PPM1A Does Not Fully Sustain Smad1 Phosphorylation
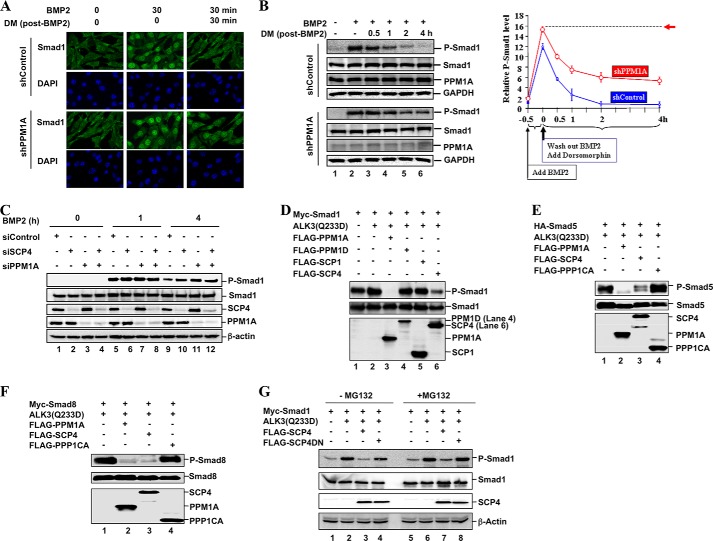
Ligand-induced phosphorylation of Smad1/5/8 is the key step in activation of canonical BMP signaling. Conversely, dephosphorylation of Smad1/5/8 represents a critical event in terminating BMP signaling. Phosphatases that have been reported to dephosphorylate Smad1/5/8 include the pan-Smad phosphatase PPM1A (19, 20), SCP1/2/3 (22, 24), PDPs (21), and MTMR4 (23). In various cell types, we found that PPM1A, but not the others, plays a major role of pan-Smad dephosphorylation (19). Here we used C2C12 cells, a mouse multipotent mesenchymal cell line, to study BMP physiological responses. As shown in Fig. 1A, nuclear accumulation of Smad1 was significantly increased upon 30 min of BMP2 stimulation, which was completely abolished by Dorsomorphin, a small molecule inhibitor for type I BMP receptor (27). When compared with control cells, stable PPM1A depletion promoted profound accumulation of Smad1 in the nucleus of shPPM1A cells (Fig. 1A). Because dephosphorylation is required for nuclear export of Smads, Smad1 should be retained in the nucleus in shPPM1A cells if PPM1A were the sole phosphatase. We found that in shPPM1A cells, simultaneous BMP withdrawal and Dorsomorphin treatment (30 min after BMP stimulation) reduced the accumulation of Smad1 in the nucleus, suggesting that depletion of PPM1A is not sufficient to maintain full retention of Smad1 in the nucleus (Fig. 1A).
FIGURE 1.
SCP4 dephosphorylates BMP-specific R-Smads. A, PPM1A depletion increases Smad1 accumulation in the nucleus. C2C12 cells were stably expressed control short hairpin RNA or shPPM1A. C2C12-shPPM1A or control cells were treated with BMP2 (100 ng/ml) for 30 min followed by BMP2 washout and simultaneous addition of 10 μm Dorsomorphin (DM) for 30 min. Smad1 was visualized using anti-Smad1 antibody. DNA was stained using DAPI dye. B, PPM1A depletion does not fully restore P-Smad1 level. C2C12-shPPM1A or control cells were treated with BMP2 (100 ng/ml) for 0.5 h and then with Dorsomorphin for 0.5, 1, 2 or 4 h. Left, levels of P-Smad1 and total Smad1 were analyzed by Western blotting. Knockdown of PPM1A in shPPM1A cells is also indicated by anti-PPM1A Western blotting. Right, graphical analysis of intensity of P-Smad1 relative to total Smad1 was quantified by Kodak Image software, and the values are the mean of three independent experiments; error bars are ± S.D. of the mean. The dotted line (pointed with an arrow) indicates the highest P-Smad1 level. C, depletions of SCP4 and PPM1A display additive effects on the P-Smad1 level. C2C12 cells were transfected with siRNA against SCP4 or PPM1A or control and then treated with BMP2 (100 ng/ml) for 0, 1, or 4 h before harvest. Levels of P-Smad1, Smad1, SCP4, and PPM1A were analyzed by Western blotting. siControl, control siRNA. D, SCP4 dephosphorylates Smad1. HEK293T cells were transfected with Myc-Smad1 and a FLAG-tagged phosphatase. To stimulate Smad1 phosphorylation, constitutively active BMP type 1 receptor ALK3(Q233D) was co-transfected (lanes 2–6). Total Smad1, P-Smad1, and phosphatases were detected by Western blotting with the appropriate antibodies. E, SCP4 dephosphorylates Smad5. Experiment was carried out as described for panel D. F, SCP4 dephosphorylates Smad8. The experiment was carried out as described for panel D. G, 26 S proteasome inhibitor MG-132 has no effect on Smad1 dephosphorylation. HEK293T cells were treated with 20 μm MG-132 for 4 h. Total Smad1, P-Smad1, SCP4, and β-actin levels were determined by Western blotting. SCP4DN, catalytically inactive mutant of SCP4 with two amino acids mutated to D293E and D295N.
The immunofluorescence staining in Fig. 1A is further supported by Western blotting analysis. In control cells, the P-Smad1 level was induced upon BMP2 stimulation and quickly diminished by simultaneous BMP withdrawal and Dorsomorphin treatment (Fig. 1B). Knockdown of PPM1A caused a much slower, but still apparent, diminishment of the P-Smad1 level in shPPM1A cells (Fig. 1B). Thus, depletion of PPM1A did not fully restore the P-Smad1 level to the highest point (Fig. 1B), suggesting that phosphatases other than nuclear PPM1A can also specifically target Smad1 dephosphorylation.
SCP4 Is a Novel Phosphatase for Smad1/5/8
We searched for specific phosphatases for BMP-activated Smad1/5/8. A new member in the FCP/SCP family, CTDSPL2 or SCP4, was identified as a phosphatase for Smad1 in the screen (28). SCP4 contains a FCP1-like phosphatase domain in its C terminus (see Fig. 3D). To validate the initial screen, we compared the effects of SCP4 and PPM1A depletion on the P-Smad1 level. The P-Smad1 level significantly decreased in the presence of 4-h exposure to BMP2 in comparison with the 2-h exposure (Fig. 1C). Notably, whereas siRNA-mediated knockdown of either SCP4 or PPM1A could visibly restore the P-Smad1 level, double depletion of SCP4 and PPM1A caused an even higher P-Smad1 level (Fig. 1C).
FIGURE 3.
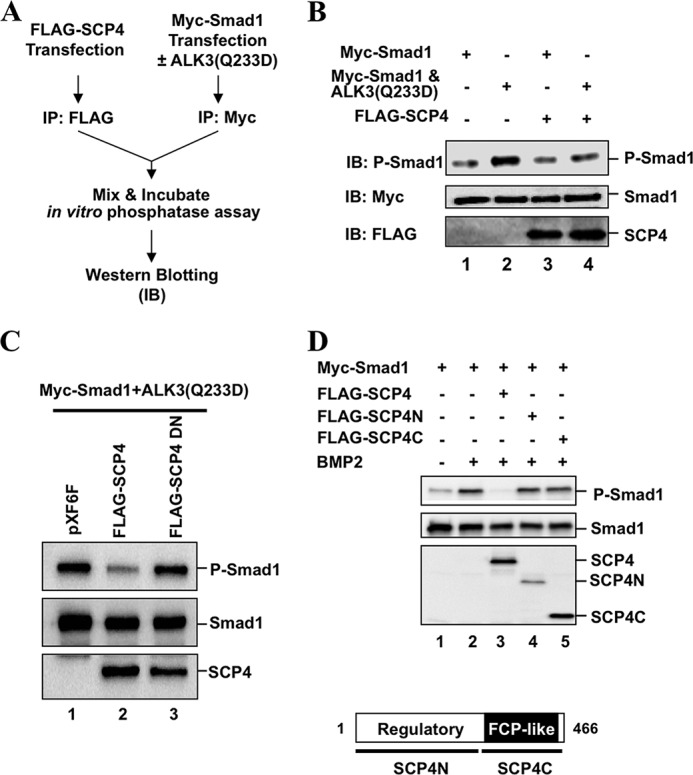
SCP4 dephosphorylates Smad1 in vitro. A, a schema of in vitro dephosphorylation assay. HEK293T cells were transfected with Myc-Smad1 (with or without HA-ALK3(Q233D)) or FLAG-SCP4 to express respective proteins. Myc-Smad1 and FLAG-SCP4 proteins were purified by IP with anti-Myc or anti-FLAG antibody, respectively. Purified FLAG-SCP4 and Myc-Smad1/P-Smad1 were incubated in in vitro phosphatase reaction buffer (50 mm Tris-HCl, pH 7.5, 1 mm dithiothreitol, 20 mm MgCl2) at 37 °C for 90 min. IB, Western blotting. B, SCP4 dephosphorylates Smad1 in vitro. Smad1 and P-Smad1 were detected and analyzed by Western blotting. C, SCP4DN has no phosphatase activity on Smad1 dephosphorylation in vitro. D, dephosphorylation of Smad1 by SCP4 requires its structural integrity. Experiment was carried out as described for Fig. 1D. Domains of SCP4 are shown at the bottom.
We further determined the effect of SCP4 on Smad1 phosphorylation in HEK293T cells. To rule out the possibility that SCP4 dephosphorylates BMP receptors to prevent Smad1 phosphorylation and activation, we used a constitutively active mutant of BMP receptor ALK3(Q233D) to stimulate Smad1 phosphorylation. The P-Smad1 level was determined by a phospho-SXS motif-specific antibody. As expected, the level of P-Smad1 increased in the presence of HA-ALK3(Q233D) (Fig. 1D, lane 2), and it was reduced by pan-Smad phosphatase PPM1A (Fig. 1C, lane 3). Notably, FLAG-SCP4 also apparently decreased the ALK3(Q233D)-induced P-Smad1 level (Fig. 1C, lane 6). As controls, PPM1D or SCP1 exhibited no detectable effects on the phosphorylation level of Smad1 (Fig. 1D, lanes 4 and 5).
Smad5 and Smad8 are two other BMP-activated R-Smads in vertebrates. We found that SCP4 could effectively dephosphorylate both Smad5 (Fig. 1E) and Smad8 (Fig. 1E). Consistent with our previous finding (20), PPM1A also reduced the levels of both P-Smad5 and P-Smad8 induced by ALK3(Q233D) (Fig. 1, E and F, lane 2). In contrast, PPP1CA (PP1α) had no effect on Smad5/8 dephosphorylation (Fig. 1, E and F, lane 4). Taken together, SCP4 is a novel phosphatase targeting dephosphorylation of BMP-specific R-Smads.
We next determined whether the phosphatase activity of SCP4 is essential in reducing the P-Smad1 level. Amino acids Asp-293 and Asp-295 in the catalytic domain of SCP4 are predicted to be the key amino acids for the phosphatase activity. We generated a phosphatase-dead mutant of SCP4 with D293E and D295N substitutions, named SCP4DN. As shown in Fig. 1G, SCP4DN had no effect on ALK3(Q233D)-induced P-Smad1, suggesting that the phosphatase activity of SCP4 is necessary to dephosphorylate Smad1 (lane 4). To exclude the possibility that SCP4 causes reduction in Smad1 phosphorylation dependent on the 26 S proteasome, the proteasome inhibitor MG-132 was included. MG-132 had no apparent effect on the levels of total Smad1 and P-Smad1 and did not reverse the effect of SCP4 on the P-Smad1 level (Fig. 1G, compare lane 7 with lane 3). This suggests that SCP4-induced diminishment of the P-Smad1 level does not require the proteasome pathway.
SCP4 Specifically Targets the SXS Motif of BMP-activated Smads
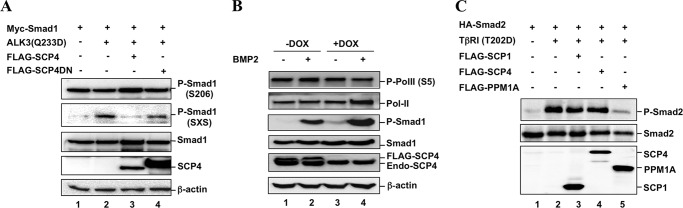
In addition to the C-terminal SXS motif, there are additional phosphorylation sites on Smad1 (29). To test whether SCP4 dephosphorylates these other sites, we chose to examine the effect of SCP4 on the linker region, especially Ser-206. Results in Fig. 2A demonstrate that SCP4 only decreased ALK3(Q233D)-induced P-Smad1(SXS), but not Ser-206 (P-Ser-206) (lane 3). This suggests that SCP4 is a Smad1 phosphatase only toward ligand-induced phosphorylation in the C-terminal SXS motif, but not the linker region.
FIGURE 2.
SCP4 specifically dephosphorylates the SXS motif of Smad1. A, SCP4 specifically dephosphorylates Smad1 at the C-terminal SXS motif (indicated by P-Smad1), but not the Ser-206 site in the linker region. Experiment was carried out as described for Fig. 1D. B, SCP4 dephosphorylates endogenous Smad1, but not endogenous PolII. HeLa stable cells expressing SCP4 under the control of the Tet-off promoter were used. Withdrawal of doxycycline (−Dox) induced expression of FLAG-SCP4. Exogenous SCP4 (FLAG-SCP4) and endogenous SCP4 (Endo-SCP4) are indicated. C, SCP4 does not dephosphorylate the SXS motif of Smad2. HEK293T cells were transfected with HA-Smad2 and a FLAG-tagged phosphatase. To stimulate Smad2 phosphorylation, constitutively active TGF-β type 1 receptor TβRI(T202D) was co-transfected (lanes 2–5). Total Smad2, P-Smad2, and phosphatase were detected by Western blotting.
Because SCP4 is structurally similar to FCP1, we examined the ability of SCP4 to dephosphorylate phospho-Ser-5 in the C-terminal domain of RNA polymerase II (P-PolII-Ser-5). For this purpose, Tet-off-regulated expression of SCP4 was generated in HeLa cells, and its effect on P-PolII-Ser-5 was determined. In the presence of Tet-derivative doxycycline (+Dox, when SCP4 was not expressed), BMP2 treatment resulted in a strong increase in the endogenous P-Smad1 level (Fig. 2B, lane 4). Removal of Dox (−Dox) induced expression of exogenous FLAG-SCP4 and profoundly reduced the level of P-Smad1 in response to BMP2 (Fig. 2B, lane 2 versus lane 4). On the contrary, the level of P-PolII-Ser-5 remained unchanged in the absence or presence of Dox. The results suggest that, although SCP4 is closely related to FCP/SCP1 that can dephosphorylate the P-PolII-Ser-2/Ser-5 (30), SCP4 may have a different substrate selectivity.
To test whether SCP4 affects the TGF-β signaling pathway, the effect of SCP4 on the level of Smad2 phosphorylation (P-Smad2) was determined. As shown in Fig. 2C, Smad2 phosphorylation was achieved by co-expression of TβRI(T202D), a constitutively active type I TGF-β receptor (Fig. 2C, lane 2). Overexpression of SCP1 or SCP4 had no effect on the phosphorylation level of Smad2 (Fig. 2C, lanes 3 and 4). In sharp contrast, PPM1A effectively dephosphorylated P-Smad2 (Fig. 2C, lane 5) as reported previously (19). Thus, SCP4 could not dephosphorylate the phosphorylated C-terminal SXS motif of TGF-β-specific Smad2 (Fig. 2C) and Smad3 (data not shown).
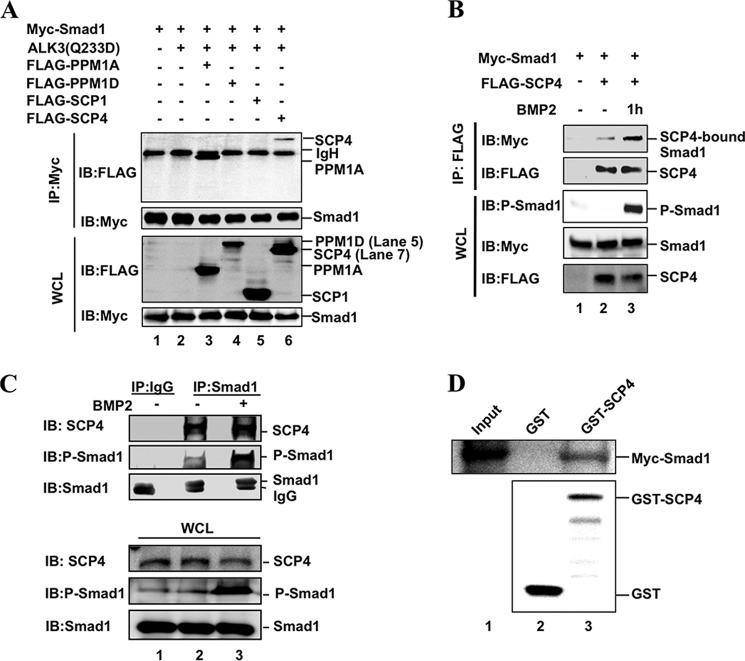
SCP4 Dephosphorylates Smad1 in Vitro
To further determine whether SCP4-mediated dephosphorylation of P-Smad1 is due to its direct interaction of P-Smad1 and to rule out the possibility that SCP4 may activate other phosphatases to dephosphorylate P-Smad1, an in vitro phosphatase assay was performed using immunopurified SCP4 and P-Smad1 proteins (Fig. 3A). P-Smad1 protein was purified by anti-Myc immunoprecipitation from HEK293T cells co-transfected with Myc-Smad1 and ALK3(Q233D). Separately, FLAG-SCP4 was immunopurified from HEK293T cells transfected with FLAG-SCP4. Purified SCP4 and P-Smad1 proteins were mixed in a phosphatase buffer, in which SCP4 acted as a phosphatase and P-Smad1 was its substrate (Fig. 3A). Results in Fig. 3B showed clearly that the P-Smad1 level induced by ALK3(Q233D) could be reduced by co-incubation with SCP4 (Fig. 3B, lane 4 compared with lane 2). We next determined whether dephosphorylation of Smad1 in vitro is due to the phosphatase activity of SCP4. As shown in Fig. 3C, only immunopurified SCP4, but not phosphatase-dead mutant SCP4DN, had a high efficiency toward dephosphorylation of Smad1, suggesting that SCP4 directly dephosphorylates BMP-specific R-Smads.
SCP4 Requires Both Regulatory and Catalytic Domains to Dephosphorylate Smad1
We next examined the domains of SCP4 responsible for Smad1 dephosphorylation. Based on its sequence features, two large deletions of SCP4 were generated. Although SCP4N (amino acids 1–262) harbors a potential regulatory domain, SCP4C (amino acids 263–466) possesses the FCP1-like catalytic phosphatase domain (Fig. 3D, bottom). Using artificial pNPP as substrate, SCP4C possessed high phosphatase activity toward pNPP substrate (data not shown), exhibiting its intrinsic phosphatase activity. As expected, SCP4N containing the regulatory domain had no phosphatase activity. However, overexpression of neither SCP4N nor SCP4C caused reduction in the level of P-Smad1 (Fig. 3D, lanes 4 and 5) when compared with wild-type full-length SCP4 (lane 3). Thus, despite its intrinsic phosphatase activity, SCP4C is insufficient to dephosphorylate P-Smad1, suggesting that SCP4 mediates Smad1 dephosphorylation dependent of its full structural integrity.
SCP4 Directly Interacts with Smad1
Catalytic activity of an enzyme toward its substrates requires a physical interaction between the enzyme and substrate. To test whether SCP4 binds to Smad1, we carried out a co-IP experiment. FLAG-tagged SCP4 or another phosphatase was co-transfected with Myc-Smad1 in HEK293T cells. Results from co-IP showed that anti-Myc (Smad1) IP could retrieve FLAG-PPM1A or FLAG-SCP4 (Fig. 4A, lanes 3 and 6), but not FLAG-PPM1D and SCP1 (Fig. 4A, lanes 4 and 5). This result is consistent with the observation that PPM1A and SCP4, but not PPM1D, act as Smad1/5/8 phosphatases (Fig. 1C). Furthermore, we examined the effect of C-terminal SXS phosphorylation of Smad1 in its interaction with SCP4. As shown in Fig. 4B, BMP2 treatment strongly induced P-Smad1 level, and concomitantly, the level of SCP4-bound Smad1 was increased (Fig. 4B, lane 3 compared with lane 2), which suggests that SCP4 binds to the phosphorylated Smad1.
FIGURE 4.
SCP4 physically interacts with Smad1. A, SCP4 interacts with Smad1 in vivo. HEK293T cells were co-transfected with Myc-Smad1, ALK3(Q233D), and a FLAG-tagged phosphatase. Smad1 was immunoprecipitated (IP) with anti-Myc antibody and then subjected to SDS-PAGE and Western blotting (IB) to detect Smad1-bound phosphatase. WCL, whole cell lysate. B, BMP2 enhances the interaction between SCP4 and Smad1. BMP2 treatment was to induce high phosphorylation of Smad1 for 1 h. Experiment was carried out as described for panel A. C, endogenous SCP4 and Smad1 were co-immunoprecipitated in C2C12 cells. Upon BMP2 treatment (1 h), C2C12 cells were harvested, and the lysates were immunoprecipitated with anti-Smad1 antibody or control IgG. The co-IP complexes and the inputs were analyzed by Western blotting with the indicated antibodies. D, direct interaction between SCP4 and Smad1 in vitro. GST-SCP4 fusion protein was expressed in E. coli and purified by glutathione beads. In vitro translated Myc-Smad1 was incubated with purified glutathione bead-bound GST protein or GST-SCP4. The retrieved complex was subjected to SDS-PAGE and Western blotting analysis.
We also examined the interaction between endogenous SCP4 and Smad1 in C2C12 Cells. SCP4 could be retrieved by anti-Smad1 IP, but not by control IgG (Fig. 4C). After BMP2 treatment, Smad1-bound SCP4 was slightly increased (Fig. 4C, lane 3). Moreover, an in vitro GST pulldown assay showed that Smad1 was clearly retrieved by recombinant GST-SCP4 fusion protein, but not by GST protein alone (Fig. 4D), indicating a direct interaction between SCP4 and Smad1.
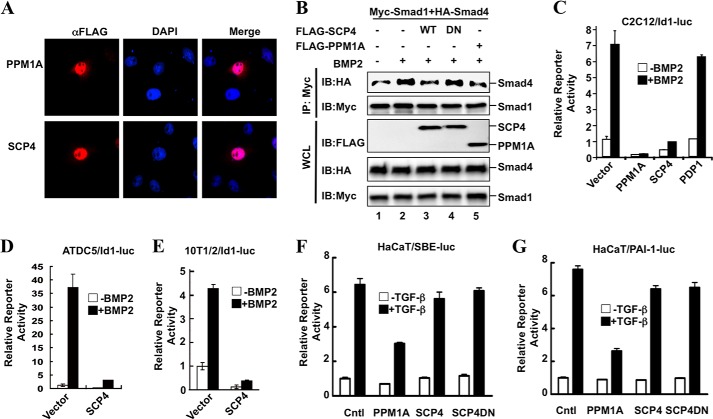
Ectopic Expression of SCP4 Specifically Attenuates BMP Signaling
Upon BMP ligand binding, Smad1 is phosphorylated, forms a complex with Smad4, and is then transported into the nucleus, where the Smad complex exerts transcriptional regulation. Thus, we determined where SCP4 dephosphorylates Smad1 and how SCP4 influences Smad signaling events. First, we detected the subcellular localization of SCP4. FLAG-SCP4 or FLAG-PPM1A was transfected into C2C12 cells and then analyzed by immunofluorescence. Similar to pan-Smad phosphatase PPM1A, SCP4 is localized in the nucleus (Fig. 5A), suggesting that SCP4-mediated dephosphorylation takes place in the nucleus. Next, we examined whether nuclear SCP4 affects the integrity of the Smad complex. As shown in Fig. 5B, BMP2 treatment induced the interaction between Smad1 and Smad4 (lane 2), and this interaction was significantly reduced in the presence of FLAG-SCP4 (lane 3). In contrast, SCP4DN mutant had no effect on the Smad1-Smad4 interaction (Fig. 5B, lane 4).
FIGURE 5.
Ectopic expression of SCP4 attenuates BMP signaling. A, SCP4 is localized in the nucleus. FLAG-PPM1A or FLAG-SCP4 was transfected into HeLa cells, and expressed proteins were detected by immunofluorescence with anti-FLAG antibodies. DNA was stained using DAPI dye. B, SCP4 reduces the Smad1-Smad4 complex formation. Myc-Smad1 and HA-Smad4 were co-transfected with WT SCP4 or SCP4 mutant SCP4DN in HEK293T cells. Smad1 was immunoprecipitated (IP) by anti-Myc antibodies, and the Smad1-bound Smad4 was detected by Western blotting (IB) with anti-HA antibodies. WCL, whole cell lysate. C, SCP4 inhibits BMP2-induced Id1-luc reporter activity. C2C12 cells were transfected with a phosphatase, together with Id1-luc reporter plasmid. BMP2 treatment and luciferase assays were done as described under “Experimental Procedures.” D, SCP4 inhibits BMP2-induced Id1-luc reporter expression in ATDC5 cells. Experiment was carried out as described for panel C. E, SCP4 inhibits BMP2-induced Id1-luc reporter expression in C3H10T1/2 cells. Experiment was carried out as described for panel C. F, SCP4 has no effect on TGF-β-induced synthetic SBE-luc reporter expression. HaCaT cells were transfected with SCP4, together with SBE-luc reporter plasmid, and then treated with 2 ng/ml TGF-β for 8 h. Experiment was carried out as described for panel C. Cntl, control. G, SCP4 has no effect on TGF-β-induced natural PAI-1-luc reporter expression in HaCaT cells. Experiment was carried out as described for panel F. Error bars in panels C–G indicate means ± S.E.
After establishing the role of SCP4 in specifically dephosphorylating Smad1/5/8, we sought to know whether SCP4 could inhibit BMP-regulated Smad-dependent gene transcription in C2C12, a common mesenchymal cell line to study early osteoblast differentiation events. We first assessed BMP early response gene Id1, a sensitive BMP response gene that encodes a protein regulating cell proliferation and differentiation (31). Here, the Id1 promoter-driven luciferase reporter system was used to measure the BMP responses. As shown in Fig. 5C, BMP2 induced a sharp increase of the Id1-luc reporter expression. Notably, transient expression of SCP4 profoundly inhibited BMP-induced Id1-luc reporter activity (Fig. 5C). As reported previously (20), overexpression of PPM1A completely eliminated BMP2 signaling, whereas PDP1 had no effect on Id1-luc expression (Fig. 5C). SCP4 inhibited BMP-induced Id1-luc expression in a dose-dependent manner (data not shown). Using a chondrogenic cell line ATDC5 and mesenchymal cell line C3H10T1/2, similar results were obtained (Fig. 5, D and E).
To validate whether the inhibitory effect of SCP4 is restricted to BMP signaling, we also tested TGF-β-induced expression of SBE-luc and PAI-1-luc reporters in TGF-β-sensitive HaCaT cells. As shown in Fig. 5, F and G, unlike PPM1A, SCP4 exerted no effect on either SBE-luc or PAI-1-luc reporter activity, which further supports the notion that SCP4 does not dephosphorylate Smad2/3 (Fig. 2C).
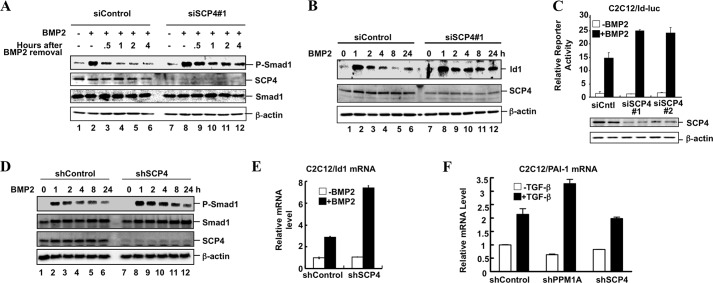
Knockdown of SCP4 Expression Prolongs Smad1 Phosphorylation and Enhances BMP Signaling
To determine the physiological functions of SCP4 in BMP signaling, we examined the effect of SCP4 knockdown in BMP-induced gene responses. About 70% knockdown of SCP4 could be achieved in C2C12 cells by two independent SCP4 siRNAs, siSCP4#1 and siSCP4#2 (Fig. 6, A–C). Upon BMP2 treatment for 1 h, the level of P-Smad1 markedly increased (Fig. 6A, lane 2), and gradually decreased after BMP2 withdrawal. Notably, both siSCP4s caused a much slower decrease in the P-Smad1 level (only siSCP4#1 is shown) than control siRNA (Fig. 6A). Knockdown of SCP4 could sustain induction of the Id1 protein (Fig. 6B). Accordingly, both siSCP4s enhanced BMP-induced Id1 promoter activity (Fig. 6C).
FIGURE 6.
Depletion of SCP4 prolongs Smad1 phosphorylation and enhances BMP-induced Id1 expression. A, knockdown of SCP4 sustains the level of Smad1 phosphorylation. C2C12 cells were transfected with SCP4 siRNA (siSCP4#1) or control siRNA (siControl), treated with BMP2 (50 ng/ml) for 1 h, washed with PBS twice, and then cultured with 0.2% FBS DMEM for 0.5, 1, 2, or 4 h. Whole cell lysates were subjected to Western blotting analysis with the indicated antibodies. B, knockdown of SCP4 prolongs BMP2-induced Id1 expression. C2C12 cells were transfected with SCP4 siRNA or control siRNA and treated with BMP2 (50 ng/ml) for 1, 2, 4, 8, or 24 h. Whole cell lysates were harvested for Western blot analysis with Id1, SCP4, and β-actin antibodies. C, knockdown of SCP4 enhances BMP2-induced Id1-luc reporter activity. C2C12 cells were transfected with control siRNA or SCP4 siRNA, together with Id1-luc reporter plasmid. Experiment was carried out as described for Fig. 5C. D, stable knockdown of SCP4 prolongs Smad1 phosphorylation. C2C12 cells stably expressing control shRNA or shSCP4 were treated with BMP2 (50 ng/ml) for 1, 2, 4, 8, or 24 h and then harvested for Western blotting analysis with SCP4, Smad1, P-Smad1, and β-actin antibodies. E, stable knockdown of SCP4 enhances BMP2-induced Id1 mRNA expression. C2C12 cells harboring control shRNA or shSCP4 were treated with BMP2 (50 ng/ml) for 4 h, and total RNA was extracted for qRT-PCR analysis. F, stable knockdown of SCP4 has no effect on TGF-β-induced PAI-1 mRNA expression. Error bars in panels C, E, and F indicate means ± S.E.
To demonstrate the effect of stable depletion of SCP4, we established a C2C12 cell line expressing shRNA against SCP4 (shSCP4). As shown in Fig. 6D, expression of SCP4 was drastically reduced in shSCP4 cells (i.e. stable depletion of SCP4). It is also apparent that stable SCP4 knockdown enabled a stronger induction of P-Smad1 by BMP2, and further sustained the P-Smad1 level (Fig. 6D). Consequently, BMP2 induced a higher increase in the level of Id1 mRNA, with 7-fold increase in shSCP4 cells when compared with 2.8-fold increase in the control cells (Fig. 6E). However, SCP4 depletion has no effect in TGF-β-mediated responses such as induction of PAI-1 mRNA (Fig. 6F). These results suggest that SCP4 knockdown enabled cells to prolong the BMP2-induced response.
SCP4 Blocks BMP-induced Osteoblastic Differentiation
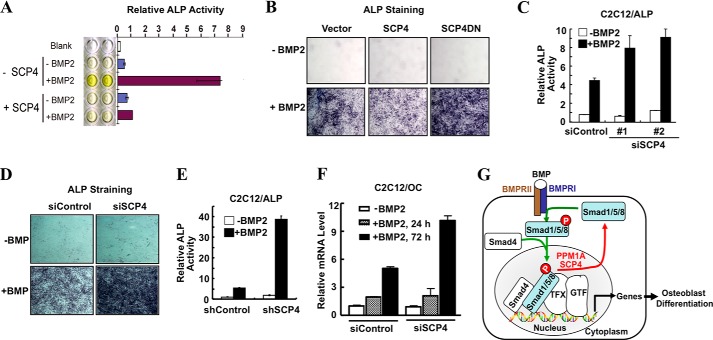
BMPs play an important role in osteoblast differentiation from progenitor cells (32). To investigate the role of SCP4 in BMP physiological responses, we first tested the effect of SCP4 on the BMP-induced osteoblast-like differentiation in C2C12 cells, as assessed by the expression of osteoblastic marker genes, such as ALP and osteocalcin (OC) (33). As shown in Fig. 7A, BMP2 stimulation markedly increased the ALP expression in C2C12 cells, and ectopic expression of SCP4 abolished the ALP expression. ALP staining further confirmed that SCP4, but not its phosphatase-dead mutant SCP4DN, inhibited BMP-induced ALP expression (Fig. 7B). Conversely, knockdown of SCP4 could enhance BMP-induced ALP expression as assessed by using pNPP assay (Fig. 7C) and ALP staining (Fig. 7D). Stable knockdown of SCP4, indicated by shSCP4, yielded an even more profound induction of ALP (Fig. 7E). Furthermore, we measured the mRNA level of late osteoblastic marker osteocalcin. In comparison with control C2C12 cells that responded to BMP2 to express osteocalcin, knockdown of SCP4 enabled cells to express a significantly higher level of osteocalcin mRNA (Fig. 7F). Taken together, our results suggest that SCP4 attenuates BMP signaling and inhibits osteoblast-like differentiation.
FIGURE 7.
SCP4 inhibits BMP-induced osteoblast-like differentiation. A, SCP4 inhibits BMP2-induced ALP activity in C2C12 cells. C2C12 cells were transfected with or without FLAG-SCP4 and treated with BMP2 (50 ng/ml) for 40 h. ALP activity was measured by absorbance at 405 nm after adding substrate pNPP. B, overexpression of SCP4 modulates BMP2-induced ALP expression. C2C12 cells stably expressing SCP4 or SCP4DN were cultured for 40 h with or without BMP2 (50 ng/ml) and then stained for ALP as described under “Experimental Procedures.” C, knockdown of SCP4 enhances BMP-induced ALP expression. C2C12 cells were transfected with siSCP4 or control siRNA. Cells were treated with BMP2 (50 ng/ml) for 40 h. ALP activity was measured by absorbance at 405 nm after adding substrate pNPP. D, knockdown of SCP4 accelerates BMP2-induced ALP expression. C2C12 cells were transfected with SCP4 siRNA or control siRNA. BMP2 (50 ng/ml) treatment and ALP staining were done as described for panel B. E, enhanced expression of ALP activity was assessed in stable knockdown cell line. F, enhanced expression of osteocalcin mRNA in SCP4 knockdown cells was assessed by qRT-PCR. G, a working model of SCP4 as a nuclear Smad phosphatase. SCP4 dephosphorylates Smad1/5/8, thereby turning off Smad-mediated transcriptional activation in the BMP signaling pathway. TFX and GTF represent Smad cofactors and general transcription factors, respectively. Error bars in panels C, E, and F indicate means ± S.E.
DISCUSSION
Smad1/5/8 are critical ligand-activated transcription factors in the BMP signaling pathway, and their activities are controlled through reversible phosphorylation at the C-terminal SXS motif (29). In this study, we report the identification and characterization of a novel phosphatase SCP4 for Smad1/5/8. It has recently been reported that BMP signaling is negatively regulated by a few phosphatases, including pan-Smad phosphatase PPM1A (19, 20). The observation that PPM1A depletion did not completely prevent the decline in the P-Smad1/5/8 level suggests the presence of additional phosphatases. Subsequent systematic screening identified SCP4 as another Smad phosphatase. Like PPM1A, SCP4 is a ubiquitously expressed nuclear phosphatase (Figs. 5A and 7G). In catalyzing Smad1/5/8 dephosphorylation, PPM1A and SCP4 may play complementary roles. Depletion of either one can somewhat enhance the P-Smad1 level. Double depletion of PPM1A and SCP4 in C2C12 restores P-Smad1 to a much higher level.
Distinct properties of SCP4 underscore its importance in its specific role in regulating BMP signaling. First, SCP4 specifically targets Smad1/5/8 in the BMP pathway, but not Smad2/3 in the TGF-β pathway, whereas PPM1A is capable of dephosphorylating all R-Smads (19, 20). Because both TGF-β and BMP signaling can regulate early embryonic development, often in an opposing manner, we can envision that regulation of SCP4 levels or activity can only influence BMP-induced, but not TGF-β-induced, responses. Indeed, SCP4 negatively shuts off BMP-induced signaling and osteoblastic-like differentiation in a phosphatase-dependent manner, whereas it has no effects on TGF-β signaling. However, SCP4 is not a negative feedback product of the BMP signaling pathway. Both SCP4 and PPM1A are not transcriptionally regulated by BMP2 or TGF-β1 (supplemental Fig. 1). Further investigations on the regulation of SCP4 are required to characterize the physiological functions of SCP4.
Second, SCP4 has no effect on BMP-induced phosphorylation of the linker region of Smad1, particularly Ser-206. The siblings of SCP4, i.e. small CTD phosphatases such as SCP1/2/3, can target the linker region of all R-Smads for dephosphorylation (24, 25). Our finding that SCP4 differs from SCP1/2/3 suggests the different roles of these closely related SCPs in TGF-β superfamily signaling. This finding is significant as the linker phosphorylation often inhibits BMP/TGF-β signaling. Thus, the selectivity of SCP4 on the SXS motif of Smad1/5/8 ensures its negative effect on BMP signaling.
Third, SCP4 does not dephosphorylate the Ser-5 in the C-terminal domain of RNA polymerase II, although it is structurally similar to the PolII phosphatase FCP1. Accordingly, we have not observed any effects of overexpression or depletion of SCP4 on general transcription such as that of housekeeping genes GAPDH and β-actin. These results further support the notion that SCP4 is a nuclear phosphatase selectively dephosphorylating the SXS motif of Smad1/5/8 in the BMP pathway (Fig. 7G). Indeed, through blocking BMP signaling, SCP4 can inhibit osteoblast-like differentiation. Despite the limited studies of SCP4, our identification of SCP4 as a new Smad phosphatase not only helps us understand how the BMP signaling pathway is sophisticatedly regulated through reversible phosphorylation of Smad proteins, but also opens up new ways of understanding the functions and mechanisms of SCP4 in physiological and pathophysiological settings.
Supplementary Material
Acknowledgments
We thank David Luskutoff for p800(PAI-1)-Luc, Peter ten Dijke for Id1-Luc, and Bert Vogelstein for SBE-luc. We are grateful to the laboratory members for helpful discussion and technical assistance.
This research was partly supported by grants from the Ministry of Science and Technology (2012CB966600); the National Natural Science Foundation of China (31090360); the National Institutes of Health (R01GM63773, R01AR053591, and R01CA108454 to X.-H. F., and R01DK073932 to X. L.); Project 111 (B13026); Project 985; and the Fundamental Research Funds for the Central Universities.

This article contains supplemental Fig. 1.
- BMP
- bone morphogenetic protein
- CTD
- C-terminal domain
- R-Smad
- receptor-regulated Smad
- PDP
- pyruvate dehydrogenase phosphatase
- PPM1A
- protein phosphatase 1A
- ALP
- alkaline phosphatase
- pNPP
- p-nitrophenyl phosphate
- PolII
- RNA polymerase II
- TβRI
- TGF-β type II receptor
- P-Smad1
- phospho-Smad1
- IP
- immunoprecipitation
- qRT-PCR
- quantitative real-time RT-PCR
- Dox
- doxycycline
- luc
- luciferase.
REFERENCES
- 1. Derynck R., Miyazono K. (2008) The TGF-β Family, Cold Spring Harbor Press, Cold Spring Harbor, NY: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bandyopadhyay A., Yadav P. S., Prashar P. (2013) BMP signaling in development and diseases: a pharmacological perspective. Biochem. Pharmacol. 85, 857–864 [DOI] [PubMed] [Google Scholar]
- 3. Zhang K., Li L., Huang C., Shen C., Tan F., Xia C., Liu P., Rossant J., Jing N. (2010) Distinct functions of BMP4 during different stages of mouse ES cell neural commitment. Development 137, 2095–2105 [DOI] [PubMed] [Google Scholar]
- 4. Wu M. Y., Hill C. S. (2009) TGF-β superfamily signaling in embryonic development and homeostasis. Dev. Cell 16, 329–343 [DOI] [PubMed] [Google Scholar]
- 5. Waite K. A., Eng C. (2003) From developmental disorder to heritable cancer: it's all in the BMP/TGF-β family. Nat. Rev. Genet. 4, 763–773 [DOI] [PubMed] [Google Scholar]
- 6. Ying Q. L., Nichols J., Chambers I., Smith A. (2003) BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell 115, 281–292 [DOI] [PubMed] [Google Scholar]
- 7. Ying Q. L., Wray J., Nichols J., Batlle-Morera L., Doble B., Woodgett J., Cohen P., Smith A. (2008) The ground state of embryonic stem cell self-renewal. Nature 453, 519–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu X., Shi W., Cao X. (2007) Multiplicity of BMP signaling in skeletal development. Ann. N.Y. Acad. Sci. 1116, 29–49 [DOI] [PubMed] [Google Scholar]
- 9. Singbrant S., Karlsson G., Ehinger M., Olsson K., Jaako P., Miharada K., Stadtfeld M., Graf T., Karlsson S. (2010) Canonical BMP signaling is dispensable for hematopoietic stem cell function in both adult and fetal liver hematopoiesis, but essential to preserve colon architecture. Blood 115, 4689–4698 [DOI] [PubMed] [Google Scholar]
- 10. Feng X.-H., Derynck R. (2005) Specificity and versatility in TGF-β signaling through Smads. Annu. Rev. Cell. Dev. Biol. 21, 659–693 [DOI] [PubMed] [Google Scholar]
- 11. Shi Y., Massagué J. (2003) Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 113, 685–700 [DOI] [PubMed] [Google Scholar]
- 12. Massague J. (2003) Integration of Smad and MAPK pathways: a link and a linker revisited. Gene. Dev. 17, 2993–2997 [DOI] [PubMed] [Google Scholar]
- 13. Liu F., Matsuura I. (2005) Inhibition of Smad antiproliferative function by CDK phosphorylation. Cell Cycle 4, 63–66 [DOI] [PubMed] [Google Scholar]
- 14. Derynck R., Zhang Y. E. (2003) Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 425, 577–584 [DOI] [PubMed] [Google Scholar]
- 15. Inman G. J., Nicolás F. J., Hill C. S. (2002) Nucleocytoplasmic shuttling of Smads 2, 3, and 4 permits sensing of TGF-β receptor activity. Mol. Cell 10, 283–294 [DOI] [PubMed] [Google Scholar]
- 16. Xu L., Kang Y., Cöl S., Massagué J. (2002) Smad2 nucleocytoplasmic shuttling by nucleoporins CAN/Nup214 and Nup153 feeds TGFβ signaling complexes in the cytoplasm and nucleus. Mol. Cell 10, 271–282 [DOI] [PubMed] [Google Scholar]
- 17. Reguly T., Wrana J. L. (2003) In or out? The dynamics of Smad nucleocytoplasmic shuttling. Trends Cell Biol. 13, 216–220 [DOI] [PubMed] [Google Scholar]
- 18. Xu L., Massagué J. (2004) Nucleocytoplasmic shuttling of signal transducers. Nat. Rev. Mol. Cell Biol. 5, 209–219 [DOI] [PubMed] [Google Scholar]
- 19. Lin X., Duan X., Liang Y. Y., Su Y., Wrighton K. H., Long J., Hu M., Davis C. M., Wang J., Brunicardi F. C., Shi Y., Chen Y. G., Meng A., Feng X.-H. (2006) PPM1A functions as a Smad phosphatase to terminate TGFβ signaling. Cell 125, 915–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Duan X., Liang Y. Y., Feng X.-H., Lin X. (2006) Protein serine/threonine phosphatase PPM1A dephosphorylates Smad1 in the bone morphogenetic protein signaling pathway. J. Biol. Chem. 281, 36526–36532 [DOI] [PubMed] [Google Scholar]
- 21. Chen H. B., Shen J., Ip Y. T., Xu L. (2006) Identification of phosphatases for Smad in the BMP/DPP pathway. Gene. Dev. 20, 648–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Knockaert M., Sapkota G., Alarcón C., Massagué J., Brivanlou A. H. (2006) Unique players in the BMP pathway: small C-terminal domain phosphatases dephosphorylate Smad1 to attenuate BMP signaling. Proc. Natl. Acad. Sci. U.S.A. 103, 11940–11945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yu J., He X., Chen Y. G., Hao Y., Yang S., Wang L., Pan L., Tang H. (2013) Myotubularin-related protein 4 (MTMR4) attenuates BMP/Dpp signaling by dephosphorylation of Smad proteins. J. Biol. Chem. 288, 79–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sapkota G., Knockaert M., Alarcón C., Montalvo E., Brivanlou A. H., Massagué J. (2006) Dephosphorylation of the linker regions of Smad1 and Smad2/3 by small C-terminal domain phosphatases has distinct outcomes for bone morphogenetic protein and transforming growth factor-β pathways. J. Biol. Chem. 281, 40412–40419 [DOI] [PubMed] [Google Scholar]
- 25. Wrighton K. H., Willis D., Long J., Liu F., Lin X., Feng X.-H. (2006) Small C-terminal domain phosphatases dephosphorylate the regulatory linker regions of Smad2 and Smad3 to enhance transforming growth factor-β signaling. J. Biol. Chem. 281, 38365–38375 [DOI] [PubMed] [Google Scholar]
- 26. Dai F., Lin X., Chang C., Feng X.-H. (2009) Nuclear export of Smad2 and Smad3 by RanBP3 facilitates termination of TGF-β signaling. Dev. Cell 16, 345–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yu P. B., Hong C. C., Sachidanandan C., Babitt J. L., Deng D. Y., Hoyng S. A., Lin H. Y., Bloch K. D., Peterson R. T. (2008) Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat. Chem. Biol. 4, 33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shen T., Sun C., Zhang Z., Xu N., Duan X., Feng X.-H., Lin X. (2014) Specific control of BMP signaling and mesenchymal differentiation by cytoplasmic phosphatase PPM1H. Cell Res. 24, 727–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wrighton K. H., Lin X., Feng X.-H. (2009) Phospho-control of TGF-β superfamily signaling. Cell Res. 19, 8–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang Y., Kim Y., Genoud N., Gao J., Kelly J. W., Pfaff S. L., Gill G. N., Dixon J. E., Noel J. P. (2006) Determinants for dephosphorylation of the RNA polymerase II C-terminal domain by Scp1. Mol. Cell 24, 759–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Korchynskyi O., ten Dijke P. (2002) Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. J. Biol. Chem. 277, 4883–4891 [DOI] [PubMed] [Google Scholar]
- 32. Katagiri T., Yamaguchi A., Komaki M., Abe E., Takahashi N., Ikeda T., Rosen V., Wozney J. M., Fujisawa-Sehara A., Suda T. (1994) Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J. Cell Biol. 127, 1755–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Włodarski K. H. (1990) Properties and origin of osteoblasts. Clin. Orthop. Relat. Res. 252, 276–293 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.