Abstract
The antisaccade task is a widely used technique to measure failure of inhibition, an important cause of cognitive and clinical abnormalities found in schizophrenia. Although antisaccade performance, which reflects the ability to inhibit prepotent responses, is a putative schizophrenia endophenotype, researchers have not consistently reported the expected differences between first-degree relatives and comparison groups. Schizophrenia participants(n=219) from the large Consortium on the Genetics of Schizophrenia (COGS) sample (n=1078) demonstrated significant deficits on an overlap version of the antisaccade task compared to their first-degree relatives (n=443) and community comparison subjects (CCS; n=416). Although mean antisaccade performance of first-degree relatives was intermediate between schizophrenia participants and CCS, a linear mixed-effects model adjusting for group, site, age, and gender found no significant performance differences between the first-degree relatives and CCS. However, admixture analyses showed that two components best explained the distributions in all three groups, suggesting two distinct doses of an etiological factor. Given the significant heritability of antisaccade performance, the effects of a genetic polymorphism is one possible explanation of our results.
Descriptors: Oculomotor, Endophenotype, Antisaccade, Schizophrenia, Family
Impaired inhibitory function of the prefrontal cortex is well documented in schizophrenia and appears to play a significant role in schizophrenia-related cognitive and functional impairment (Daskalakis, Fitzgerald, & Christensen, 2007; Liu, Fitzgerald, Daigle, Chen, & Daskalakis, 2009; Volk & Lewis, 2002). Description of inhibitory function is therefore important for pathology, treatment, and genetic studies of schizophrenia. One way of assessing inhibitory processes is through carefully designed cognitive tasks such as the antisaccade task, which was first described by Hallett (1978). Although a number of cognitive processes may be important for the correct execution of an antisaccade (Hutton, 2008; McDowell, Dyckman, Austin,& Clementz, 2008), there is compelling evidence that inhibitory processes are active during the performance of antisaccade tasks (Nyffeler, Müri, Bucher-Ottiger, Pierrot-Deseilligny, Gaymard, & Rivaud-Pechoux, 2007; Reuter, Kaufmann, Bender, Pinkpank, & Kathmann, 2010). Behaviorally, the antisaccade task relies on the fact that, when presented with a visual stimulus, higher primates will reflexively make a saccade to focus on the stimulus. Unlike some reflexes, this reflex can be inhibited with conscious effort. To assess antisaccades systematically, subjects sit in a dark room and focus on a stimulus located in the center of a video monitor. This stimulus is extinguished, and an antisaccade cue is presented to the left or right. The subject is then instructed to look in the opposite direction.To perform the task correctly, the subject must be able to understand and remember task instructions, inhibit the reflexive saccade toward the cue, translate cue information into an internal representation of the contralateral position, and execute the antisaccade. More than 40 studies have found that schizophrenia patients perform significantly worse on this task than controls, and no studies have failed to replicate this result. This fact, combined with evidence that antisaccade performance depends on prefrontal cortex function (Ettinger, Ffytche, Kumari, Kathmann, Reuter, et al., 2008; Nyffeler et al., 2007; Pierrot-Deseilligny, Müri, Ploner, Gaymard, Demeret, & Rivaud-Pechoux, 2003), an area impacted by schizophrenia, makes the antisaccade task an ideal candidate schizophrenia endophenotype.
One potentially productive use of tasks that reflect a basic illness-related neurophysiological deficit, such as the antisaccade task, is as intermediate phenotypes for genetic studies. First described by Gottesman and Shields (1972), these intermediate phenotypes, which are often referred to as endophenotypes, are defined as traits that are stable over time (i.e., not related to fluctuations in illness severity), are more common among affected persons, cosegregate with the illness in families, and are also more common among biological relatives of persons with the illness. Ideally, endophenotypes are phenomenologically much simpler than the illness itself and hence much easier to quantify. The physiologically elemental nature of endophenotypes raises the hope that they will have a simpler genetic architecture than that of the illness itself and thus that the responsible genes will be easier to identify (Braff, Freedman, Schork, & Gottesman, 2007; Gould & Gottesman, 2006).
The antisaccade task has other characteristics of a high quality schizophrenia endophenotype (for review, see Hutton & Ettinger, 2006; Radant, Dobie, Calkins, Olincy, Braff, et al., 2007; Turetsky, Calkins, Light, Olincy, Radant, & Swerdlow, 2007), and a number of investigators have used it in this capacity (Greenwood, Braff, Light, Cadenhead, Calkins, et al., 2007; Kumari, Ettinger, Crawford, Zachariah, & Sharma, 2005; Myles-Worsley, Coon, McDowell, Brenner, Hoff, et al., 1999; Price, Michie, Johnston, Innes-Brown, Kent, et al., 2006). An important characteristic of an endophenotype is that the performance of unaffected first-degree relatives on pertinent tasks is distinct from the performance of the general population on those tasks (Berrettini, 2005; Braff et al., 2007; Gottesman & Gould, 2003). Meta-analytic results support a difference of moderate effect size in antisaccade performance between controls and first-degree relatives of schizophrenia patients, indicating poorer performance in relatives across studies (Calkins, Curtis, Iacono, & Grove, 2004; Calkins, Iacono, & Ones, 2008; Levy, Bowman, Abel, Krastoshevsky, Krause, & Mendell, 2008; Levy, O'Driscoll, Matthysse, Cook, Holzman, & Mendell, 2004). However, the interpretation of this difference has been debated. Some investigators have argued that subtle differences in inclusion-exclusion criteria between relatives and controls spuriously cause the differences between controls and first-degree relatives (Levy et al., 2004). On the other hand, evidence has also been presented showing that comparably screened relatives and controls still exhibit significant differences in performance (Calkins et al., 2004; Ettinger, Kumari, Crawford, Corr, Das, et al., 2004; Karoumi, Saoud, d'Amato, Rosenfeld, Denise, et al., 2001). These research issues clearly illustrate the importance of attending to the influence of participant selection factors on study outcome.
An effective method for assessing the nature of an endophenotype's genetic architecture is through admixture analysis. Admixture analysis determines whether two distinct doses of an important etiological factor are present as opposed to a continuous range of doses or a mixture of a large number of factors. Some traits, such as height, have a very high heritability yet nevertheless form a Gaussian distribution because many different genes have a significant impact (Lettre, 2009). On the other hand, traits with simpler genetic architecture are more likely to result in a population distribution that is clearly a mixture of subcomponent distributions (Friedlander, Kark, Sinnreich, Edwards, & Austin, 1999). If the admixture analysis suggests the presence of a two-dose etiological factor, that factor is not necessarily genetic; however, absent some obvious environmental factor, genetic polymorphisms are the most common dichotomous traits for explaining such a distribution (van Koolwijk, Healey, Hitchings, Mitchell, Sham, et al., 2009). Admixture analysis has been used successfully in the field of psychiatry to analyze age of onset of illness (Delorme, Golmard, Chabane, Millet, Krebs, et al., 2005; Schürhoff, Golmard, Szöke, Bellivier, Berthier, et al., 2004; Slama, Courtet, Golmard, Mathieu, Guillaume, et al., 2009) and smooth pursuit eye movements (Ross, Olincy, Mikulich, Radant, Harris, et al., 2002). Admixture analysis of putative endophenotypes, such as antisaccade performance, may provide clues as to the genetic architecture underlying these traits and hence be helpful with the overall analysis of schizophrenia genetics.
This study used data obtained from the Consortium on the Genetics of Schizophrenia (COGS) sample to examine antisaccade error rate, latency, and gain in a large group of schizophrenia participants, their first-degree relatives, and community comparison subjects (CCS). The COGS study is a seven-site, National Institute of Mental Health–funded project (Calkins, Dobie, Cadenhead, Olincy, Freedman, et al., 2007) that was designed to analyze the genetic architecture of multiple schizophrenia-related endophenotypes using a family-based linkage design. The large sample size, detailed characterization of COGS participants, and information about reliability and between-site effects (Radant et al., 2007) make the COGS study an ideal resource for teasing out the complex ascertainment and demographic issues that might influence between-group differences in antisaccade performance. These factors, especially the large sample size, also allowed us to perform a reliable admixture analysis.
Methods
Previous reports have described in detail both the general study design (Calkins et al., 2007) and the specific oculomotor methods (Radant et al., 2007) that were used in the COGS study. Radant et al. (2007) reported on 338 schizophrenia participants and CCS, which are a subset of the 1078 total subjects who participated in this study. All participants underwent a standardized diagnostic and clinical assessment protocol and a medical record review. The COGS enrolled families with at least one person diagnosed with schizophrenia and age-matched CCS. Families consisted of at least one schizophrenia proband, an unaffected sibling, and the parents of the proband. Schizophrenia participants, their relatives, and CCS were excluded for a history of electroconvulsive therapy (ECT) in the past 6 months, a positive drug or alcohol screen, a diagnosis of substance abuse disorder in the past 30 days or substance dependence in the past 6 months, or an estimated premorbid IQ of less than 70 as determined by the Wide Range Achievement Test-Third Edition (WRAT-3). We excluded participants with a history of ocular, neurological, or major systemic medical problems that could influence antisaccade performance. Additionally, we excluded CCS if they had a personal history of Cluster A Personality Disorder, a personal history of psychosis, or a family history of psychosis in first- or second-degree relatives. Schizophrenia participants all met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition diagnostic criteria for schizophrenia based on a bestestimate consensus diagnostic procedure, which included the Diagnostic Interview for Genetic studies (DIGS; Nurnberger, Blehar, Kaufmann, York-Cooler, Simpson, et al., 1994). Siblings (n=7) and parents (n=2) who were diagnosed with schizophrenia were excluded from this analysis.
In a large study such as the COGS, very strict exclusion criteria are impractical. To determine whether inclusion/exclusion criteria might have affected the primary results, a subset of participants who met very strict exclusion criteria were designated as “narrow” (see also Turetsky, Greenwood, Olincy, Radant, Braff, et al., 2008). By this definition, all narrow participants had no history of minor head injury, significant medical or neurological conditions, or substance abuse. Additionally, schizophrenia participants who were designated as narrow had no history of other Axis I disorders (except adjustment disorder) or ECT. Relatives who were designated as narrow had an IQ greater than 70 and no history of ECT, Axis I or cluster A personality disorder diagnoses, or treatment with psychotropic medications, and CCS who were designated as narrow had no history of nonpsychotic Axis I disorders or treatment with psychotropic medications. For each subject group, we analyzed antisaccade performance of the narrowly defined subjects and compared this with the group as whole.
Participation required approximately 10 h, spread over two days, and consisted of an interview, blood draw, and the measurement of neurophysiological and cognitive endophenotypes, including antisaccade performance. To permit participant acclimation to oculomotor testing, a prosaccade task was administered prior to the antisaccade tasks. The local institutional review board of each site approved the study, and all participants provided signed informed consent before commencing the study procedures.
The COGS investigators constructed an antisaccade task using a 200-ms overlap between central fixation and cue; antisaccade tasks constructed with these task parameters were previously reported to be sensitive to differences between firstdegree relatives and CCS (McDowell, Myles-Worsley, Coon, Byerley, Clementz, et al., 1999; Myles-Worsley et al., 1999). Participants viewed antisaccade stimuli on a video monitor in a dark room with their heads stabilized. The square stimuli subtended a visual angle of 0.35° and differed only by color (the cue stimulus was yellow; all other stimuli were blue). Each antisaccade trial consisted of 2400 to 3600 ms of the central fixation stimulus, 200 ms of overlap between the central fixation and cue stimulus, 800 ms of cue presentation alone, a 500-ms duration stimulus to indicate the location of a correct antisaccade (i.e., contralateral to the cue), and finally, a return to central fixation and the beginning of the next trial. Cue stimuli were presented pseudorandomly at four locations: 10° to the left, 10° to the right, 15° to the left, and 15° to the right. Participants performed three blocks of 20 antisaccade trials each, for a total of 60 antisaccade trials. Prior to commencing the antisaccade task, all participants established that they understood the task by pointing to the correct location during each phase of a quarter-speed practice version. Eye position was acquired at 500 Hz using infrared oculography (ASL 310 infrared system, Applied Science Laboratories, Bedford, MA). All oculomotor technicians were trained in person and met competency standards (for more details, see Calkins et al., 2007; Radant et al., 2007).
Oculomotor technicians performed a standardized calibration procedure by having participants focus at 0°, ±7.5°, and ±15° prior to each block of 20 antisaccade trials. Occasionally, calibration was so poor that subject data could not be reliably analyzed. To acclimate participants to the oculomotor laboratory, technicians had participants perform one block of a prosaccade task before the antisaccade task.
For each trial, custom software (Radant & Hommer, 1992; Ross, Harris, Olincy, Radant, Adler, & Freedman, 1998) identified and characterized the primary response saccade (correct versus error) or flagged trials that could not be analyzed due to prominent artifacts. This software has been used successfully for more than 15 years. Saccades have a characteristic waveform and are reliably characterized and distinguished from artifacts by the software. We required an initial acceleration of at least 2000°s –2, a minimum velocity of >30°/s, and epochs of near zero-velocity pre- and post-saccade to designate an eye movement as a true saccade. The first saccade of at least 3° amplitude beginning 80 ms after cue presentation was considered the “response” saccade. Saccades beginning after the onset of the stimulus indicating correct target position were not analyzed. Direction of the response saccade determined whether the participant made a correct or error saccade. Some trials were so contaminated by artifacts that a response saccade could not be identified. These trials were designated uninterpretable and were not included in determination of the error rate. Occasionally, entire 20-trial blocks were so contaminated by artifacts or poor calibration that they were discarded. Data from participants with less than 20 interpretable response saccades were discarded. After computerized analysis, at least one oculomotor specialist (ADR or SPM, each with a minimum of 5 years' experience), who was blinded to participant group, reviewed all of the tracings; tracings with unacceptable calibration or that were so contaminated by artifacts that the software could not reliably analyze them were discarded. Oculomotor specialists never changed the decision of the software but did identify blocks of data of such poor quality that they needed to be discarded. This decision was always made by the senior author (ADR).
A preliminary variable, which was designated “proportion of interpretable trials, ” was defined as the total number of antisaccade trials minus the tracings that could not be analyzed due to artifacts, divided by the total number of antisaccade trials. This variable reflected data quality rather than antisaccade performance. The primary outcome measure, “proportion correct, ” was defined as the number of correct antisaccade responses divided by the number of interpretable saccades. Thus, a participant who had 40 interpretable trials and made 10 correct responses would have a proportion correct of 0.25. Saccadic latency was defined as duration from the onset of the cue to the onset of the response and was calculated separately for correct and incorrect responses. Gain of correct antisaccades was defined as the saccadic amplitude divided by the distance between the central fixation point and the cue stimulus. Additionally, proportion correct was also calculated separately for 10° and 15° cue locations.
Between-group differences in demographic variables were assessed with either one-way ANOVA (for continuous variables) or chi-square analyses (for dichotomous variables). Post hoc comparisons were based on Fisher's least significant difference test. The proportion of interpretable saccades, proportion of correct saccades, saccade gain, saccade latencies, and effects of cue distance from the center were analyzed separately with linear mixed-effects models (Pinheiro & Bates, 2000) in which family membership served as a random effect to account for the relatedness of observations among family members. The initial model for proportion of interpretable response saccades included the potential effects of group, site, and group × site interaction. The initial model for proportion of correct antisaccades, saccade gain, and saccade latencies included the potential effects of group, site, age, gender, smoking status, and parental education (maximum grade level of mother and father), as well as group × site, group × age, and group × smoking interactions. The final model for each variable contained only those covariates with a statistically significant (p<.05) contribution to the model. Parental education and smoking status contained missing values that were imputed based on regressing parental education on age, site, and cohort (parent, schizophrenia participant/siblings, CCS) and regressing smoking status on age, site, and group (Little & Rubin, 2002). Effect size was computed as the difference in group means adjusted for other variables in the model and then divided by the estimated population standard deviation. Because the distribution of proportion correct antisaccades is skewed by nature, analyses of this measure were repeated after arcsine transformation.
The admixture analysis was performed with the publicly available NOCOM program (see linkage.rockefeller.edu; Ott, 1979). This program uses an iterative, least likelihood algorithm to determine how many theoretical Gaussian components are present in a distribution. The program requires seeding with initial starting values; for each analysis, we checked a large range of initial values, almost all of which yielded the same solutions. A common standard deviation was used for all components, as recommended on the NOCOM web site. Because arcsine-transformed data were used, no skew parameter was included in the model. To avoid the spurious influence of significant covariates, the residuals of the arcsine-transformed data were used for admixture analyses. Maximum likelihood estimates were determined for one to four distributions. Note that the G2 statistic (2[ln(maximum likelihood 1) – ln (maximum likelihood 2)]) used to determine significance is only approximately chi-squared (linkage.rockefeller.edu); therefore, our confidence in significant but high p values is limited.
All other analyses were performed using S-PLUS version 8 (Insightful Corporation, Palo Alto, CA). Because the distribution of proportion correct antisaccades is skewed by nature, analyses of these measures were repeated after arcsine transformation.
Results
Valid oculomotor data were obtained on 219 schizophrenia participants, 293 siblings, 150 parents, and 416 CCS (Table 1). Data from 16 subjects were invalid due to poor technical quality (i.e., 1094 total subjects completed the antisaccade task). Schizophrenia participants were significantly more likely to smoke and to be male than siblings, parents, and CCS. The parental education of the schizophrenia participants was slightly, but significantly, higher than that of the CCS. Handedness did not vary among groups. Most of the schizophrenia participants and a small proportion of the relatives were on antipsychotic medications. The Global Assessment of Functioning (GAF) score of CCS was significantly higher than that of the first-degree relatives and schizophrenia participants, and the GAF score of first-degree relatives was significantly higher than that of the schizophrenia participants. The Scale for the Assessment of Negative Symptoms (Andreasen, 1983) scores of the schizophrenia participants were 9.7 ± 5.8, and the Scale for the Assessment of Positive Symptoms (Andreasen, 1984) scores of the schizophrenia participants were 6.2 ± 4.2, which together indicated a moderate degree of current illness.
Table 1. Demographic Data for Schizophrenia Participants, Their First-Degree Relatives, and Community Comparison Subjects.
| Schizophrenia participants (S) | Parents (P) | Siblings (SB) | Community comparison subjects (C) | Test for group differencese | Post hoc comparisonsf | |
|---|---|---|---|---|---|---|
| Number of participants | 219 | 150 | 293 | 416 | ||
| Age (years)a | 34.4 (11.1) | 55.6 (4.9) | 36.3 (11.7) | 36.2 (12.6) |
F(3,1074) =134.5 p <.0001 |
P> S, SB, C |
| % Male | 73 | 39 | 42 | 42 | chi-squared (3) =69.5 p <.0001 |
S> P, SB, C |
| Parent education (years)a,b,c | 15.7 (3.3) 2 NAs |
12.9 (3.6) 5 NAs |
15.1 (3.1) 24 NAs |
F(2,751) P=36.1 p<.0001 |
S> C> P | |
| % Right-handed | 87 | 93 | 89 | 89 | chi-squared (3) = 4.1 p= .25 |
|
| % Smokersb | 45 | 9 1 NA |
15 | 13 | chi-squared (3) = 114.2 p<.0001 |
S> P, SB, C |
| % on Antipsychoticsb | 94 1 NA |
3 3 NAs |
0 4 NAs |
0 8 NAs |
chi-squared (3) = 960.0 p<.0001 |
S>P, SB, C |
| GAFd scorea | 45.8 (12.6) 12 NAs |
78.8 (13.6) 9 NAs |
80.4 (11.3) 10 NAs |
83.7 (9.4) 21 NAs |
F(3,1022) = 574.0 p<.0001 |
C>P, SB > S |
| % Narrow | 55 | 59 | 52 | 73 | chi-squared (3) =38.0 p<.0001 |
C> S, P, SB |
Mean (± SD).
NA indicates number of missing values.
Maximum value of parents' education level when both available; otherwise based on the parent for whom information is available. NA refers to the case when education is missing for both parents.
Global Assessment of Functioning.
One-way ANOVA for continuous variables; chi-squared test for categorical variables.
Age post hoc comparisons based on Tamhane's method to account for unequal variances; all other post hoc comparisons based on Fisher's LSD.
All participants tolerated testing well. Occasionally an artifact contaminated the epoch of the saccade response, precluding the identification of an interpretable response saccade. Schizophrenia participants had a significantly lower proportion of interpretable saccades than the first-degree relatives or CCS (Table 2). Although there was a main effect of site on interpretable saccades, the range in the proportion of interpretable saccades was small across sites (from 0.93 to 0.97), reflecting excellent data quality at all sites; also, the group × site interaction was not significant (see also Radant et al., 2007).
Table 2. Antisaccade Performance for Schizophrenia Participants, Their First-Degree Relatives, and Community Comparison Subjects.
| Schizophrenia participants (S) | First-degree relatives (R) | Community comparison subjects (C) | Test for differencesd | Post hoc comparisonse | ||
|---|---|---|---|---|---|---|
| Number of participants | 219 | 443 | 416 | |||
| Proportion interpretablea | 0.92 (0.12) | 0.96 (0.09) | 0.96 (0.09) | Group | F(2,404) =13.6, p<.0001 | S< R, C |
| Site | F(6,665) = 4.0, p=.0007 | |||||
| Proportion correcta | 0.60 (0.26) | 0.79 (0.20) | 0.82 (0.16) | Group | F(2,401) =116.0, p<.0001 | S< R, C |
| Site | F(6,665) =5.1, p<.0001 | |||||
| Age | F(1,401) = 30.0, p<.0001 | |||||
| Sex | F(1,401) =4.8, p= .03 | |||||
| Proportion correct 10°a | 0.57 (0.27) | 0.75 (0.22) | 0.79 (0.17) | Degree | F(1,1077) = 352.3, p<.0001 | 10°<15° |
| Proportion correct 15°a | 0.64 (0.27) | 0.82 (0.20) | 0.84 (0.16) | |||
| Latency to correct (ms)a,b,f | 425 (99) 2 NAs | 401 (72) 1 NA | 392 (70) | Group × Age | F(2,394) =5.1, p=.007 | |
| Group × Smoker | F(2,394)=3.4, p=.03 | |||||
| Site | F(6,664) =3.1, p=.005 | |||||
| Sex | F(1,394) = 19.3, p<.0001 | |||||
| Parent Ed | F(1,394) = 14.4, p= .0002 | |||||
| Latency to incorrect (ms)a,c,f | 243 (70) | 255 (74) | 256 (72) | Group × Age | F(2,393) =4.4, p=.01 | |
| Site | F(6,660) =1.9, p=.08 | |||||
| Sex | F(1,393) =4.7, p=.03 | |||||
| Gain of correcta | 0.88 (0.20) | 0.96 (0.15) | 0.95 (0.16) | Group | F(2,402) = 21.2, p<.0001 | S< R, C |
| Site | F(6,664) =2.5, p=.02 | |||||
Mean (± SD).
Final model includes group, site, age, sex, smoker, parent education, group × age, and group × smoker.
Final model includes group, site, age, sex, and group × age.
Marginal conditional F-test based on linear mixed-effect model.
Based on Fisher's LSD from linear mixed-effect model.
Test for main effect of group is not applicable due to significant interaction terms that include group.
For all of the linear mixed-effects models, the results that were based on omitting participants with missing covariate values were similar to the results that were based on imputing missing covariate values; we used imputation for all analyses. Also, the analysis of arcsine-transformed data (for proportion correct) and raw data yielded nearly identical results; only analyses of raw proportion-correct data are reported.
The linear mixed-effects model indicated significant differences between the three groups in the main outcome variable, the proportion of correctly performed antisaccades (Table 2). Schizophrenia participants performed significantly worse than the other two groups. Although the mean of first-degree relatives fell between that of the schizophrenia participants and CCS, post hoc analyses indicated that the CCS and first-degree relatives did not differ significantly. The effect sizes (difference in the means of the residuals) were 1.18 for the schizophrenia-CCS difference, 1.10 for the schizophrenia-relative difference, and 0.09 for the CCS-relative difference.
The linear mixed-effects model also indicated that males performed slightly but significantly better than females in the main outcome variable. Performance of all three groups declined significantly with age. Some authors have argued that analysis of covariance can spuriously obscure real findings (Miller & Chapman, 2001). To insure this did not occur with our results with respect to age, we compared the antisaccade performance of younger CCS (<50 years old) to that of siblings and the antisaccade performance of older CCS (>49 years old) to that of the parents. In both age cohorts, there was no significant difference in antisaccade performance (younger CCS versus siblings: proportion correct 0.83 ± 0.14, 0.81 ± 0.20, respectively, p= .085; older CCS versus parents: proportion correct 0.76 ± 0.19, 0.74 ± 0.20, respectively, p=.52).
There was a main effect of site but no significant site × group interaction. All participants performed significantly better when presented with a 15° cue rather than a 10° cue; the magnitude of this effect was similar in all groups (i.e., there was no significant group × degree interaction effect).
Admixture analysis was performed separately for each group and for the entire participant pool. Because antisaccade performance is a proportion, it has a naturally skewed distribution, a circumstance that is problematic for admixture analysis; our data were therefore arcsine-transformed prior to analysis. After accounting for group, site, gender, and smoking status, we used the residuals of the arcsine-transformed data for admixture analyses (Figure 1).
Figure 1.
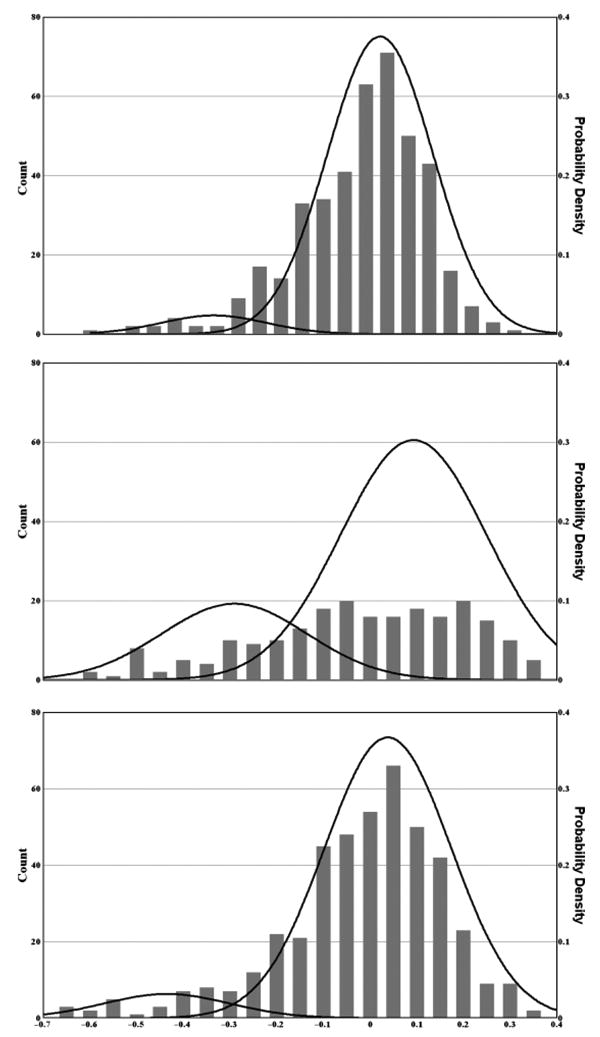
Admixture analyses of antisaccade data for the CCS (top), schizophrenia participants (middle), and the first-degree relatives of schizophrenia participants (bottom). To account for the effects of significant main factors and the naturally skewed distribution of antisaccade performance measures, residuals of arcsine-transformed data were used for these analyses. A minimum likelihood algorithm (Ott, 1979) was used to identify the underlying components. Two-component solutions were significantly better than one-component solutions for all three groups.
Two components accounted for the distributions significantly better than one component for all three groups (p<10–6, p<10–6, and p<.005 for the CCS, relatives, and schizophrenia participants, respectively). Although three components were significantly better than two components for the CCS and schizophrenia participants but not the relatives (p<.01, p=.48, and p=.054 for the CCS, schizophrenia participants, and relatives, respectively), the significance of three-component solutions as compared to two-component solutions was much less than the difference between the one- and two-component solutions. No four-component solution was superior to any three-component solution. The intersection between the Gaussian curves was used to define the point of rarity between the two components. This point was – .026 for the CCS, – 0.29 for the relatives, – 0.18 for the schizophrenia participants, and – 0.28 for the participant pool as a whole. Therefore, a threshold of – 0.28 was used to define participants as having good versus bad antisaccade performance. This corresponds to approximately 0.53 proportion correct in raw antisaccade performance. Twenty-six of 219 schizophrenia participants (11.9%), 33 of 443 relatives (7.9%), and 10 of 416 CCS (3.8%) had poor antisaccade performance, a statistically significant difference (chi square = 22.8, df=2, p<.0001). The difference between the relatives and CCS was significant (chi square = 11.5, df=1, p<.001), whereas the difference between schizophrenia participants and relatives was marginally significant (chi square = 3.53, df = 1, p= .06).
There was no significant main effect of group on latency to correct antisaccades (Table 2). Male sex and higher parental education were both significantly associated with shorter latencies to correct antisaccades. There was a significant interaction between group and smoking status: being a smoker negatively impacted the correct antisaccade latency of schizophrenia participants more than the other two groups. Similarly, there was an interaction between group and age such that the correct antisaccade latency of schizophrenia participants increased more significantly with age than that of the other two groups. Finally, antisaccade gain was significantly lower in the schizophrenia participants than the other two groups.
Some experts (Levy et al., 2004) have argued that asymmetric inclusion/exclusion criteria have caused spurious differences between controls and first-degree relatives. To address this important issue, we reanalyzed the data, using only subjects from all three groups that met narrow criteria for study enrollment. This distinction was made in the exact same way as reported in Turetsky et al. (2007). The proportion of correct antisaccades for all narrow subjects was almost identical to that from the participant pool as a whole (0.753 ± 0.215 versus 0.761 ± 0.220). Unexpectedly, schizophrenia participants meeting narrow criteria performed worse than the average schizophrenia participant (0.604 ± 0.259 and 0.570 ± 0.260 proportion correct, respectively, for all schizophrenia participants versus narrow schizophrenia participants). Antisaccade performance of CCS and relatives who met narrow criteria was nearly identical to the performance of these groups as a whole (0.817 ± 0.157 and 0.820 ± 0.160 proportion correct, respectively, for all CCS versus narrow CCS; 0.780 ± .202 and 0.797 ± 0.200 proportion correct, respectively, for all relatives versus narrow relatives).
Discussion
In this large sample from the COGS, the antisaccade performance of schizophrenia participants was impaired compared to both CCS and unaffected first-degree relatives. The main findings of this study are similar to previous findings that the antisaccade performance of schizophrenia participants is inferior to that of controls and that the antisaccade performance of first-degree relatives of schizophrenia participants is intermediate between schizophrenia participants and controls (Clementz, McDowell, & Zisook, 1994; Curtis, Calkins, Grove, Feil, & Iacono, 2001). The control-relative difference reached statistical significance in some, but not all, of these studies (for review of this issue, see Calkins et al., 2004; Levy et al., 2004). Our study was adequately powered to detect small but meaningful between-group differences and to detect the effects of parental education, GAF scores, smoking, site, and sex. Furthermore, admixture analyses indicated that two Gaussian components fit the data much better than one in all three groups. Post hoc analysis showed that the sample sizes accrued in this study yielded 90% power to detect a difference of less than 0.05 in the proportion of correct antisaccades between the relatives and CCS.
Within groups, the performance of medically and psychiatrically healthy non-schizophrenia participants who met our narrow, strict exclusion criteria was nearly identical to the performance of all subjects. Thus, factors such as substance abuse and comorbid nonpsychotic psychiatric illness had no effect on antisaccade performance. This suggests that antisaccade deficits are relatively specific for schizophrenia pathology. Thus, the differences between first-degree relatives and controls that have been reported in previous studies are likely due to factors related to schizophrenia rather than subtle between-group differences in inclusion/exclusion criteria (Calkins et al., 2004).
Admixture analyses of the antisaccade data of CCS, relatives, and schizophrenia participants in the current study resulted in two components for each group. The proportion of subjects in the poorer performing component (i.e., below the point of rarity) was greatest among the schizophrenia participants and least among the CCS. Three-component solutions were slightly more significant than two-component solutions, but given the uncertainty about determining the significance of the test statistic at marginal p values and the fact that inspection of the distributions did not lend face validity to three-component solutions, we had little confidence in the three-component solutions.
The highly significant advantage in the explanatory power of two-component versus one-component solutions implies that two distinct doses of some important etiological factor influence the antisaccade performance of our participants. Furthermore, the proportion of participants below the threshold identified by admixture analysis increased significantly from the CCS to the relatives and from the relatives to the schizophrenia participants. Although many factors might explain this pattern of results, one possibility is a single major gene effect with a frequency of the deleterious allele that is highest among schizophrenia participants, intermediate among relatives, and lowest among CCS. This finding, combined with the significant heritability of antisaccade performance (h2 = .42) that has been previously reported in the COGS sample (Greenwood et al., 2007) and in healthy twins (h2 = .57; Malone & Iacono, 2002), supports a genetic influence on antisaccade performance. Other putative schizophrenia endophenotypes evidence a similar pattern: for example, a polymorphism in the alpha-7 subunit of the nicotinic receptor is associated with magnitude of suppression of the P50 waveform of the auditory evoked potential (Freedman, Olincy, Ross, Waldo, Stevens, et al., 2003) in the presence of a priming stimulus.
In addition to our findings concerning the overall differences in antisaccade performance between schizophrenia participants, their first-degree relatives, and CCS, our study also provides insight into secondary attributes of oculomotor performance in these groups. For example, unlike some studies (Boudet, Bocca, Chabot, Delamillieure, Brazo, et al., 2005; Calkins et al., 2004; Crawford, Sharma, Puri, Murray, Berridge, & Lewis, 1998; Curtis, Calkins, Grove, et al., 2001), but not all studies (Olincy, Ross, Young, & Freedman, 1997), we found a significant correlation between antisaccade performance and age (despite our exclusion of participants over the age of 65). Therefore, age was included in the mixed-effects model that was used in this study. Notably, if age was not included as a covariate in the model, proportion correct was significantly lower in the first-degree relatives (which included both siblings and parents) than in the CCS.
Due to methodological differences between studies, the impact of target excursion size on antisaccade performance is not well understood. In our study, all participants, regardless of group, performed better with the 15° excursion than the 10° excursion, but McDowell et al. (1999) used the same two excursions and found that between-group differences were accentuated using the 15° excursion as compared to the 10° excursion. Other studies have used different target excursions (Curtis, Calkins, Grove, et al., 2001) and as many as five different excursion distances. A mix of target excursions may be important in preventing the use of predictive strategies by participants, whereas excursion size may be less important. Studies designed to focus specifically on this issue — for example, studies using a large range of target excursions — would be required to illuminate the reasons for this.
Saccadic gain was lower among schizophrenia participants in our study. Normal saccadic gain, with respect to an antisaccade, requires accurate perception of a cue stimulus, generation of a sensorimotor representation of that location in the opposite hemifield, and generation of an accurate saccade based on the representation (Barash & Zhang, 2006; Everling & Fischer, 1998). Correctly matching cue amplitude with antisaccade amplitude (saccadic gain) likely reflects the quality of the sensorimotor transformation. Although antisaccade performance itself most likely depends on dorsolateral prefrontal cortex areas (Hutton & Ettinger, 2006; Nyffeler et al., 2007), abnormalities of antisaccade amplitude in schizophrenia may reflect function of the parietal and supplementary oculomotor cortex (McDowell et al., 2008; Moon, Barton, Mikulski, Polli, Cain, et al., 2007). The significantly lower saccadic gain among schizophrenia participants is consistent with the results of most other studies that have examined this issue; however, the relatives in this study performed no differently than the CCS. Relatively few studies have examined antisaccade gain in relatives, and the results thus far have been conflicting (Ettinger et al., 2004; Levy et al., 2008), rendering its status as a candidate endophenotype unclear. Although antisaccade performance itself probably depends on dorsolateral prefrontal cortex areas (Hutton & Ettinger, 2006; Nyffeler et al., 2007), saccadic gain may reflect function of the parietal and supplementary oculomotor cortex.
In addition to deficits in inhibitory processing, there is significant evidence for impairment in other aspects of attention in schizophrenia, such as reaction time (Luck & Gold, 2008). Haraldsson, Ettinger, Magnusdottir, Sigmundsson, Sigurdsson, et al. (2008) have identified possible anatomic and neurochemical substrates of reaction time that are relevant to schizophrenia. With respect to the antisaccade task, the latency (i.e., reaction time) of correctly performed antisaccades reflects both basic reaction time and the extra processing time required to inhibit a prepotent response (Hutton & Ettinger, 2006). Therefore, we anticipated longer latencies in our schizophrenia participants than our relatives and CCS. However, we found that, although the latency to correct antisaccades of schizophrenia participants was longer than that of the other two groups, the difference fell short of significance. This finding differs from the results of a number of previous studies (e.g., Curtis, Calkins, Grove, et al., 2001; Myles-Worsley et al., 1999) that have identified significantly increased latency to correct responses in schizophrenia patients. Moreover, recent meta-analyses of a small number of family studies indicate longer latencies to correct responses in schizophrenia relatives, with small mean effect sizes ranging from .33 (Levy et al., 2008) to .39 (Calkins et al., 2008). A possible explanation for the unexpected finding in our study may be that our latency data had very high variances, making proof of statistical significance difficult. Also, many of these other studies used the no-overlap version of the task, and perhaps the overlap version is less sensitive to between-group differences in latency.
We also noted a more rapid decay in saccadic latency with age in schizophrenia participants than in relatives and CCS. This finding has not been reported before, perhaps because a large sample is required to detect this effect. Our study included 1078 participants, which is, to our knowledge, much larger than any other study of antisaccade performance in these specific groups. Previous studies suggest that the effects of age on cognition differ for schizophrenia patients and healthy study participants or patients with other neuropsychiatric illnesses (Brodaty, Sachdev, Koschera, Monk, & Cullen, 2003; Friedman, Harvey, Coleman, Moriarty, Bowie, et al., 2001). Kirkpatrick, Messias, Harvey, Fernandez-Egea, and Bowie (2007) have argued that many manifestations of schizophrenia are attributable to an abnormal aging process. However, little research has been done specifically investigating whether aging affects basic neurocognitive abilities such as reaction time differently in schizophrenia participants than controls. Given the putative neurodevelopmental antecedents of schizophrenia (Rapoport, Addington, Frangou, & Psych, 2005; Weinberger, 1996) and the unique forms of dementia that are sometimes associated with schizophrenia (de Vries, Honer, Kemp, & McKenna, 2001; Friedman et al., 2001), this interesting observation is worthy of further exploration.
The major limitation of this study relates to our ascertainment strategy, which may have biased families and relatives in the direction of less psychopathology. The overall COGS research design necessitated enrollment of families who were functional enough to participate jointly in a comprehensive research protocol, and this requirement may have unintentionally led to the exclusion of families with estranged or more severely affected probands; likewise, these excluded families may also have included more relatives with overt schizophrenia-spectrum disorders. Consistent with this, the higher level of parental education in the schizophrenia participants than in the CCS suggests that schizophrenia families in our study were less ill than the average schizophrenia family. Moreover, even in COGS families, the healthiest relatives may have been more likely to participate in endophenotype testing than siblings with a diathesis toward schizophrenia. Indeed, 38% of siblings refused to participate in the COGS study, and their unwillingness to participate might be partially explained by an increased frequency of subclinical schizotypal traits in these siblings. Also, because the COGS research design required that at least one sibling was unaffected (Braff, Greenwood, Swerdlow, Light, Schork, et al., 2008; Calkins et al., 2007), families in which all siblings were affected, a condition that is likely related to a higher genetic diathesis for schizophrenia, were by definition excluded from the COGS study. In our study, we enrolled only 13 families with two affected persons and just 1 family with three affected persons. Another related limitation is that there was a significant main effect of site, suggesting across-site differences in recruitment or data collection. Despite the significant main effect, the absolute differences between sites were quite small. We have discussed this issue in detail in previous reports (Radant et al., 2007; Calkins et al., 2007).
No other study of antisaccade performance in relatives of schizophrenia patients has used such exacting requirements for family structure. Two recent meta-analyses (Calkins et al., 2004; Levy et al., 2004) found that the average antisaccade performance of first-degree relatives was approximately 67% (range 55% to 83%) correct. In contrast, relatives in the COGS study performed 78.5% of antisaccades correctly, suggesting that they were not as impaired in antisaccade performance as “average” first-degree relatives of schizophrenia patients. Thus, ascertainment biases secondary to our recruitment strategy may have biased both schizophrenia participants and their relatives to be higher functioning, less neurophysiologically impacted by genetic diatheses toward schizophrenia and, hence, less prone to poor antisaccade performance. If it were possible to test relatives from a truly random sample of families with at least one member diagnosed with schizophrenia, a significant difference might emerge between relatives and CCS. However, the obstacles encountered in the COGS recruitment would be unavoidable in any similarly designed, family-based genetic study of schizophrenia.
Another potential limitation of our study is our decision to use a 200-ms overlap between fixation and cue rather than the more widely used no-overlap version. We selected the overlap paradigm because it has yielded highly significant relative-control differences (McDowell et al., 1999) with effect sizes between relatives and controls that were considerably larger than in studies using the no-overlap version (Clementz et al., 1994; Curtis, Calkins, & Iacono, 2001; Karoumi et al., 2001). The choice of the overlap version is supported by the fact that we found a highly significant heritability using the overlap task (Greenwood et al., 2007). However, the no-overlap version of the task may have some advantages: in contrast to the overlap version of the task, with the no-overlap when the antisaccade cue is presented, no stimulus is present and visual attention is not actively engaged. Suppression of a prepotent response, which is required for correct performance on the antisaccade task, may be more difficult during a task situation where attention is not engaged (Klein, Brügner, Foerster, Müller, & Schweickhardt, 2000) than in a task situation where attention is engaged (i.e., during the overlap version). In support of this suggestion, Curtis, Calkins, and Iacono (2001) administered two versions of the antisaccade task (an overlap and a no-overlap version) to schizophrenia patients, their biological relatives, and controls, and they found that the no-overlap task produced greater differences between relatives and controls. More reflexive errors were committed by all groups during the no-overlap task than during the overlap task, and the schizophrenia and relative groups had disproportionately more errors. Thus, it is unclear which type of antisaccade task is most sensitive to schizophrenia-related psychopathology. Unfortunately, it was not possible for us to systematically compare the overlap antisaccade task version to the no-overlap antisaccade task version in the large and time-consuming COGS study.
Our study confirms the impairment of schizophrenia participants in performing the antisaccade task. Although schizophrenia relatives and CCS did not differ significantly in antisaccade performance, admixture analysis showed that antisaccade distributions were best explained by two components. Given the significant heritability of antisaccade performance in the COGS sample, one explanation of this is a major gene effect on antisaccade performance. We also identify the importance of age, target excursion, and demographics as well as task parameters such as the presence of overlap in analyzing antisaccade performance. Future studies should focus on further genetic analysis and further exploration of the genetic architecture of antisaccade performance with careful attention to task- and participant-related variables that might influence the ability to detect between-group differences in antisaccade performance.
Acknowledgments
This material is based upon work supported (or supported in part) by the Office of Research and Development Medical Research Service (or) Health Services R&D Service, Department of Veterans Affairs. This study was supported by NIMH grants R01 MH65571, R01 MH65588, R01 MH65562, R01 MH65707, R01 MH65554, R01 MH65578, and R01 MH65558.
References
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) Iowa City, IA: The University of Iowa; 1983. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) Iowa City, IA: The University of Iowa; 1984. [Google Scholar]
- Barash S, Zhang M. Switching of sensorimotor transformations: Antisaccades and parietal cortex. Novartis Foundation Symposium. 2006;270:59–71. [PubMed] [Google Scholar]
- Berrettini WH. Genetic bases for endophenotypes in psychiatric disorders. Dialogues in Clinical Neuroscience. 2005;7:95–101. doi: 10.31887/DCNS.2005.7.2/wberrettini. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudet C, Bocca ML, Chabot B, Delamillieure P, Brazo P, Denise P, et al. Are eye movement abnormalities indicators of genetic vulnerability to schizophrenia? European Psychiatry. 2005;20:339–345. doi: 10.1016/j.eurpsy.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Braff DL, Greenwood TA, Swerdlow NR, Light GA, Schork NJ The Investigators of the Consortium on the Genetics of Schizophrenia. Advances in endophenotyping schizophrenia. World Psychiatry. 2008;7:11–18. doi: 10.1002/j.2051-5545.2008.tb00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL, Freedman R, Schork NJ, Gottesman II. Deconstructing schizophrenia: An overview of the use of endophenotypes in order to understand a complex disorder. Schizophrenia Bulletin. 2007;33:21–32. doi: 10.1093/schbul/sbl049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodaty H, Sachdev P, Koschera A, Monk D, Cullen B. Long-term outcome of late-onset schizophrenia: 5-year follow-up study. British Journal of Psychiatry. 2003;1:213–219. doi: 10.1192/bjp.183.3.213. [DOI] [PubMed] [Google Scholar]
- Calkins ME, Curtis CE, Iacono WG, Grove WM. Antisaccade performance is impaired in medically and psychiatrically healthy biological relatives of schizophrenia patients. Schizophrenia Research. 2004;71:167–178. doi: 10.1016/j.schres.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Calkins ME, Dobie DJ, Cadenhead KS, Olincy A, Freedman R, Green MF, et al. The Consortium on the Genetics of Endophenotypes in Schizophrenia: Model recruitment, assessment, and endophenotyping methods for a multisite collaboration. Schizophrenia Bulletin. 2007;33:33–48. doi: 10.1093/schbul/sbl044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins ME, Iacono WG, Ones DS. Eye movement dysfunction in first-degree relatives of patients with schizophrenia: A meta-analytic evaluation of candidate endophenotypes. Brain and Cognition. 2008;68:436–461. doi: 10.1016/j.bandc.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementz BA, McDowell JE, Zisook S. Saccadic system functioning among schizophrenia patients and their first-degree biological relatives. Journal of Abnormal Psychology. 1994;103:277–287. [PubMed] [Google Scholar]
- Crawford TJ, Sharma T, Puri BK, Murray RM, Berridge DM, Lewis SW. Saccadic eye movements in families multiply affected with schizophrenia: The Maudsley Family Study. American Journal of Psychiatry. 1998;155:1703–1710. doi: 10.1176/ajp.155.12.1703. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Calkins ME, Grove WM, Feil KJ, Iacono WG. Saccadic disinhibition in patients with acute and remitted schizophrenia and their first-degree biological relatives. American Journal of Psychiatry. 2001;158:100–106. doi: 10.1176/appi.ajp.158.1.100. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Calkins ME, Iacono WG. Saccadic disinhibition in schizophrenia patients and their first-degree biological relatives: A parametric study of the effects of increasing inhibitory load. Experimental Brain Research. 2001;137:228–236. doi: 10.1007/s002210000635. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Fitzgerald PB, Christensen BK. The role of cortical inhibition in the pathophysiology and treatment of schizophrenia. Brain Research Reviews. 2007;56:427–442. doi: 10.1016/j.brainresrev.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Delorme R, Golmard JL, Chabane N, Millet B, Krebs MO, Mouren-Simeoni MC, et al. Admixture analysis of age at onset in obsessive-compulsive disorder. Psychological Medicine. 2005;35:237–243. doi: 10.1017/s0033291704003253. [DOI] [PubMed] [Google Scholar]
- de Vries PJ, Honer WG, Kemp PM, McKenna PJ. Dementia as a complication of schizophrenia. Journal of Neurology, Neurosurgery, and Psychiatry. 2001;70:588–596. doi: 10.1136/jnnp.70.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger U, Ffytche DH, Kumari V, Kathmann N, Reuter B, Zelaya F, et al. Decomposing the neural correlates of antisaccade eye movements using event-related FMRI. Cerebral Cortex. 2008;18:1148–1159. doi: 10.1093/cercor/bhm147. [DOI] [PubMed] [Google Scholar]
- Ettinger U, Kumari V, Crawford TJ, Corr PJ, Das M, Zachariah E, et al. Smooth pursuit and antisaccade eye movements in siblings discordant for schizophrenia. Journal of Psychiatric Research. 2004;38:177–184. doi: 10.1016/s0022-3956(03)00105-5. [DOI] [PubMed] [Google Scholar]
- Everling S, Fischer B. The antisaccade: A review of basic research and clinical studies. Neuropsychologia. 1998;36:885–899. doi: 10.1016/s0028-3932(98)00020-7. [DOI] [PubMed] [Google Scholar]
- Freedman R, Olincy A, Ross RG, Waldo MC, Stevens KE, Adler LE, et al. The genetics of sensory gating deficits in schizophrenia. Current Psychiatry Reports. 2003;5:155–161. doi: 10.1007/s11920-003-0032-2. [DOI] [PubMed] [Google Scholar]
- Friedlander Y, Kark JD, Sinnreich R, Edwards KL, Austin MA. Inheritance of LDL peak particle diameter: Results from a segregation analysis in Israeli families. Genetic Epidemiology. 1999;16:382–396. doi: 10.1002/(SICI)1098-2272(1999)16:4<382::AID-GEPI5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Friedman JI, Harvey PD, Coleman T, Moriarty PJ, Bowie C, Parrella M, et al. Six-year follow-up study of cognitive and functional status across the lifespan in schizophrenia: A comparison with Alzheimer's disease and normal aging. American Journal of Psychiatry. 2001;158:1441–1448. doi: 10.1176/appi.ajp.158.9.1441. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Shields J. Schizophrenia and genetics: A twin study vantage point. New York: Academic Press; 1972. [Google Scholar]
- Gould TD, Gottesman II. Psychiatric endophenotypes and the development of valid animal models. Genes Brain and Behavior. 2006;5:113–119. doi: 10.1111/j.1601-183X.2005.00186.x. [DOI] [PubMed] [Google Scholar]
- Greenwood TA, Braff DL, Light GA, Cadenhead KS, Calkins ME, Dobie DJ, et al. Initial heritability analyses of endophenotypic measures for schizophrenia: The consortium on the genetics of schizophrenia. Archives of General Psychiatry. 2007;64:1242–1250. doi: 10.1001/archpsyc.64.11.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett PE. Primary and secondary saccades to goals defined by instructions. Vision Research. 1978;18:1279–1296. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- Haraldsson HM, Ettinger U, Magnusdottir B, Sigmundsson T, Sigurdsson E, Petursson H. Eye movement deficits in schizophrenia: Investigation of a genetically homogenous Icelandic sample. European Archives of Psychiatry and Clinical Neuroscience. 2008;258:373–383. doi: 10.1007/s00406-008-0806-y. [DOI] [PubMed] [Google Scholar]
- Hutton SB. Cognitive control of saccadic eye movements. Brain and Cognition. 2008;68:327–340. doi: 10.1016/j.bandc.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Hutton SB, Ettinger U. The antisaccade task as a research tool in psychopathology: A critical review. Psychophysiology. 2006;43:302–313. doi: 10.1111/j.1469-8986.2006.00403.x. [DOI] [PubMed] [Google Scholar]
- Karoumi B, Saoud M, d'Amato T, Rosenfeld F, Denise P, Gutknecht C, et al. Poor performance in smooth pursuit and antisaccadic eye-movement tasks in healthy siblings of patients with schizophrenia. Psychiatry Research. 2001;101:209–219. doi: 10.1016/s0165-1781(01)00227-x. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Messias E, Harvey PD, Fernandez-Egea E, Bowie CR. Is schizophrenia a syndrome of accelerated aging? Schizophrenia Bulletin. 2007;34:1024–1032. doi: 10.1093/schbul/sbm140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein CH, Brügner G, Foerster F, Müller W, Schweickhardt A. The gap effect in pro-saccades and anti-saccades in psychometric schizotypes. Biological Psychology. 2000;55:25–39. doi: 10.1016/s0301-0511(00)00062-4. [DOI] [PubMed] [Google Scholar]
- Kumari V, Ettinger U, Crawford TJ, Zachariah E, Sharma T. Lack of association between prepulse inhibition and antisaccadic deficits in chronic schizophrenia: Implications for identification of schizophrenia endophenotypes. Journal of Psychiatric Research. 2005;39:227–240. doi: 10.1016/j.jpsychires.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Lettre G. Genetic regulation of adult stature. Current Opinion in Pediatrics. 2009;21:515–522. doi: 10.1097/MOP.0b013e32832c6dce. [DOI] [PubMed] [Google Scholar]
- Levy DL, O'Driscoll G, Matthysse S, Cook SR, Holzman PS, Mendell NR. Antisaccade performance in biological relatives of schizophrenia patients: A meta-analysis. Schizophrenia Research. 2004;71:113–125. doi: 10.1016/j.schres.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Levy DL, Bowman EA, Abel L, Krastoshevsky O, Krause V, Mendell NR. Does performance on the standard antisaccade task meet the co-familiality criterion for an endophenotype? Brain and Cognition. 2008;68:462–475. doi: 10.1016/j.bandc.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little RJA, Rubin DB. Statistical analysis with missing data. New York: John Wiley & Sons; 2002. [Google Scholar]
- Liu SK, Fitzgerald PB, Daigle M, Chen R, Daskalakis ZJ. The relationship between cortical inhibition, antipsychotic treatment, and the symptoms of schizophrenia. Biological Psychiatry. 2009;65:503–509. doi: 10.1016/j.biopsych.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Gold JM. The construct of attention in schizophrenia. Biological Psychiatry. 2008;64:34–39. doi: 10.1016/j.biopsych.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone SM, Iacono WG. Error rate on the antisaccade task: Heritability and developmental change in performance among preadolescent and late-adolescent female twin youth. Psychophysiology. 2002;39:664–673. [PubMed] [Google Scholar]
- McDowell JE, Dyckman KA, Austin BP, Clementz BA. Neurophysiology and neuroanatomy of reflexive and volitional saccades: Evidence from studies of humans. Brain and Cognition. 2008;68:255–270. doi: 10.1016/j.bandc.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell JE, Myles-Worsley M, Coon H, Byerley W, Clementz BA. Measuring liability for schizophrenia using optimized antisaccade stimulus parameters. Psychophysiology. 1999;36:138–141. doi: 10.1017/s0048577299980836. [DOI] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. Journal of Abnormal Psychology. 2001;110:40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Moon SY, Barton JJ, Mikulski S, Polli FE, Cain MS, Vangel M, et al. Where left becomes right: A magnetoencephalographic study of sensorimotor transformation for antisaccades. NeuroImage. 2007;36:1313–1323. doi: 10.1016/j.neuroimage.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myles-Worsley M, Coon H, McDowell J, Brenner C, Hoff M, Lind B, et al. Linkage of a composite inhibitory phenotype to a chromosome 22q locus in eight Utah families. American Journal of Medical Genetics. 1999;88:544–550. [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Archives of General Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- Nyffeler T, Müri RM, Bucher-Ottiger Y, Pierrot-Deseilligny C, Gaymard B, Rivaud-Pechoux S. Inhibitory control of the human dorsolateral prefrontal cortex during the anti-saccade paradigm: A transcranial magnetic stimulation study. European Journal of Neuroscience. 2007;26:1381–1385. doi: 10.1111/j.1460-9568.2007.05758.x. [DOI] [PubMed] [Google Scholar]
- Olincy A, Ross RG, Young DA, Freedman R. Age diminishes performance on an antisaccade eye movement task. Neurobiology of Aging. 1997;18:483–489. doi: 10.1016/s0197-4580(97)00109-7. [DOI] [PubMed] [Google Scholar]
- Ott J. Detection of rare major genes in lipid levels. Human Genetics. 1979;51:79–91. doi: 10.1007/BF00278296. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Müri RM, Ploner CJ, Gaymard B, Demeret S, Rivaud-Pechoux S. Decisional role of the dorsolateral prefrontal cortex in ocular motor behaviour. Brain. 2003;126:1460–1473. doi: 10.1093/brain/awg148. [DOI] [PubMed] [Google Scholar]
- Pinheiro JC, Bates DM. Mixed-effects models in S and S-PLUS. New York: Springer-Verlag; 2000. [Google Scholar]
- Price GW, Michie PT, Johnston J, Innes-Brown H, Kent A, Clissa P, et al. A multivariate electrophysiological endophenotype, from a unitary cohort, shows greater research utility than any single feature in the Western Australian family study of schizophrenia. Biological Psychiatry. 2006;60:1–10. doi: 10.1016/j.biopsych.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Radant AD, Dobie DJ, Calkins ME, Olincy A, Braff DL, Cadenhead KS, et al. Successful multi-site measurement of antisaccade performance deficits in schizophrenia. Schizophrenia Research. 2007;89:320–329. doi: 10.1016/j.schres.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Radant AD, Hommer DW. A quantitative analysis of saccades and smooth pursuit during visual pursuit tracking: A comparison of schizophrenics with normals and substance abusing controls. Schizophrenia Research. 1992;6:225–235. doi: 10.1016/0920-9964(92)90005-p. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Addington AM, Frangou S, Psych MR. The neurodevelopmental model of schizophrenia: Update 2005. Molecular Psychiatry. 2005;10:434–449. doi: 10.1038/sj.mp.4001642. [DOI] [PubMed] [Google Scholar]
- Reuter B, Kaufmann C, Bender J, Pinkpank T, Kathmann N. Distinct neural correlates for volitional generation and inhibition of saccades. Journal of Cognitive Neuroscience. 2010;22:728–738. doi: 10.1162/jocn.2009.21235. [DOI] [PubMed] [Google Scholar]
- Ross RG, Harris JG, Olincy A, Radant A, Adler LE, Freedman R. Familial transmission of two independent saccadic abnormalities in schizophrenia. Schizophrenia Research. 1998;30:59–70. doi: 10.1016/s0920-9964(97)00133-3. [DOI] [PubMed] [Google Scholar]
- Ross RG, Olincy A, Mikulich SK, Radant AD, Harris JG, Waldo M, et al. Admixture analysis of smooth pursuit eye movements in probands with schizophrenia and their relatives suggests gain and leading saccades are potential endophenotypes. Psychophysiology. 2002;39:809–819. doi: 10.1111/1469-8986.3960809. [DOI] [PubMed] [Google Scholar]
- Schürhoff F, Golmard JL, Szöke A, Bellivier F, Berthier A, Méary A, et al. Admixture analysis of age at onset in schizophrenia. Schizophrenia Research. 2004;71:35–41. doi: 10.1016/j.schres.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Slama F, Courtet P, Golmard JL, Mathieu F, Guillaume S, Yon L, et al. Admixture analysis of age at first suicide attempt. Journal of Psychiatric Research. 2009;43:895–900. doi: 10.1016/j.jpsychires.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Calkins ME, Light GA, Olincy A, Radant AD, Swerdlow NR. Neurophysiological endophenotypes of schizophrenia: The viability of selected candidate measures. Schizophrenia Bulletin. 2007;33:69–94. doi: 10.1093/schbul/sbl060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turetsky BI, Greenwood TA, Olincy A, Radant AD, Braff DL, Cadenhead KS, et al. Abnormal auditory N100 amplitude: A heritable endophenotype in first-degree relatives of schizophrenia probands. Biological Psychiatry. 2008;64:1051–1059. doi: 10.1016/j.biopsych.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Koolwijk LM, Healey PR, Hitchings RA, Mitchell P, Sham PC, McGuffin P, et al. Major genetic effects in glaucoma: Commingling analysis of optic disc parameters in an older Australian population. Investigative Ophthalmology and Visual Science. 2009;50:5275–5280. doi: 10.1167/iovs.08-3065. [DOI] [PubMed] [Google Scholar]
- Volk DW, Lewis DA. Impaired prefrontal inhibition in schizophrenia: Relevance for cognitive dysfunction. Physiology and Behavior. 2002;77:501–505. doi: 10.1016/s0031-9384(02)00936-8. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. On the plausibility of “the neurodevelopmental hypothesis” of schizophrenia. Neuropsychopharmacology. 1996;14:1S–11S. doi: 10.1016/0893-133X(95)00199-N. [DOI] [PubMed] [Google Scholar]


