Summary
Aging is the primary risk factor for cognitive decline, an emerging health threat to aging societies worldwide. Whether anti-aging factors such as klotho can counteract cognitive decline is unknown. We show that a life span-extending variant of the human KLOTHO gene, KL-VS, is associated with enhanced cognition in heterozygous carriers. Because this allele increased klotho levels in serum, we analyzed transgenic mice with systemic overexpression of klotho. They performed better than controls in multiple tests of learning and memory. Elevating klotho in mice also enhanced long-term potentiation, a form of synaptic plasticity, and enriched synaptic GluN2B, an NMDA receptor subunit with key functions in learning and memory. Blockade of GluN2B abolished klotho-mediated effects. Surprisingly, klotho effects were evident also in young mice and did not correlate with age in humans, suggesting independence from the aging process. Augmenting klotho or its effects may enhance cognition at different life stages and counteract cognitive decline.
INTRODUCTION
The world’s population is aging rapidly and preserving brain health has emerged as a major biomedical challenge. Without novel interventions, over 80 million people worldwide will suffer from memory problems resulting from aging and age-related disease by 2040 (Prince et al., 2013). Since aging, a process amenable to change (Guarente and Kenyon, 2000), is the primary risk factor for failing cognition, regulators of aging might be harnessed for the treatment and prevention of cognitive decline.
Like aging, cognition is modifiable. Learning and memory depend on networks across brain regions, including the hippocampus and cortex (Ranganath and Ritchey, 2012; Wang and Morris, 2010), and involve coordinated activities of NMDA (Gladding and Raymond, 2011; Lee and Silva, 2009) and AMPA (Kerchner and Nicoll, 2008; Kessels and Malinow, 2009) type glutamate receptors (NMDARs and AMPARs). Importantly, NMDAR- and AMPAR-mediated functions are disrupted by aging (Henley and Wilkinson, 2013; Magnusson et al., 2010) and age-related neurodegenerative disease (Chang et al., 2012; Li et al., 2011).
Whether factors that prolong life can also prevent, delay or counteract neural dysfunction associated with aging and disease is a critical question with therapeutic implications. Klotho is an aging regulator that, when overexpressed, extends lifespan (Kurosu et al., 2005) and, when disrupted, accelerates aging phenotypes (Kuro-o et al., 1997). Higher klotho levels increase lifespan in mice (Kurosu et al., 2005) and nematodes (Chateau et al., 2010). In humans, a single allele of the KL-VS variant of the KLOTHO gene, which increases secreted klotho (Arking et al., 2002) and more strongly activates FGF23 signaling (Tucker Zhou et al., 2013) in cell culture, promotes longevity (Arking et al., 2005; Arking et al., 2002; Invidia et al., 2010) and diminishes age-related heart disease (Arking et al., 2005).
Klotho is a pleiotropic protein. Its transmembrane form (Shiraki-Iida et al., 1998) can be released by sheddases (Chen et al., 2007) and circulate throughout the body and brain (Imura et al., 2004; Kurosu et al., 2005). Klotho suppresses insulin (Kurosu et al., 2005) and wnt (Liu et al., 2007) signaling, regulates ion channel clustering (Chang et al., 2005) and transport (Imura et al., 2007), and promotes FGF23 function (Urakawa et al., 2006). Although it regulates aging-dependent pathways (Kurosu et al., 2005; Liu et al., 2007), klotho also supports physiologic functions that are aging-independent (Chang et al., 2005; Razzaque, 2009).
In mice, genetic klotho reduction during embryogenesis results in early postnatal death, hypomyelination (Chen et al., 2013), synaptic attrition (Shiozaki et al., 2008) and cognitive impairment (Nagai et al., 2003), suggesting that klotho is required for brain maturation. Because klotho circulates in serum and cerebrospinal fluid throughout life (Imura et al., 2004; Imura et al., 2007), and declines with aging (Duce et al., 2008; Semba et al., 2011; Semba et al., 2014), in parallel to the emergence of cognitive deficits, it is possible that klotho also fulfills important functions in the central nervous system at later life stages.
We therefore investigated whether klotho can impact physiological brain function, and more specifically, whether it can prevent or counteract cognitive decline in human aging. We demonstrate that the lifespan-extending variant of the KLOTHO gene, KL-VS, is associated with increased klotho levels in serum and enhanced cognition in aging people heterozygous for the allele in three cohorts and across multiple ages. We also analyzed transgenic mice with moderate systemic overexpression of klotho. Independent of age, these mice performed better in multiple tests of learning and memory than controls. Further investigation of potential underlying mechanisms revealed unexpected effects of klotho elevation on the functions of synapses and glutamate receptors.
RESULTS
KL-VS genetic variant of KLOTHO is associated with enhanced cognition in three independent human cohorts and in a meta-analysis
We first examined whether the KL-VS genetic variant of KLOTHO predicts healthy brain aging in humans in three independent populations, including the Hillblom Aging Study (Cohort 1), the Memory and Aging Project (Cohort 2), and the Normal Aging Cohort (Cohort 3) (Table S1). Collectively, the cohorts comprised 718 individuals (52-85 years of age), primarily Caucasian, without dementia or cognitive complaints, and with a Mini-Mental State Exam (MMSE) score of 28 or greater (see Tables S2, S3 for inclusion criteria and demographics). Twenty-six percent of individuals were heterozygous for the KL-VS allele, slightly above typical frequencies of 20-25% (Arking et al., 2002). Three percent were homozygous for KL-VS, a rare genotype that for unknown reasons is associated with decreased lifespan and detrimental effects (Arking et al., 2005; Arking et al., 2002; Deary et al., 2005); they were excluded from the study.
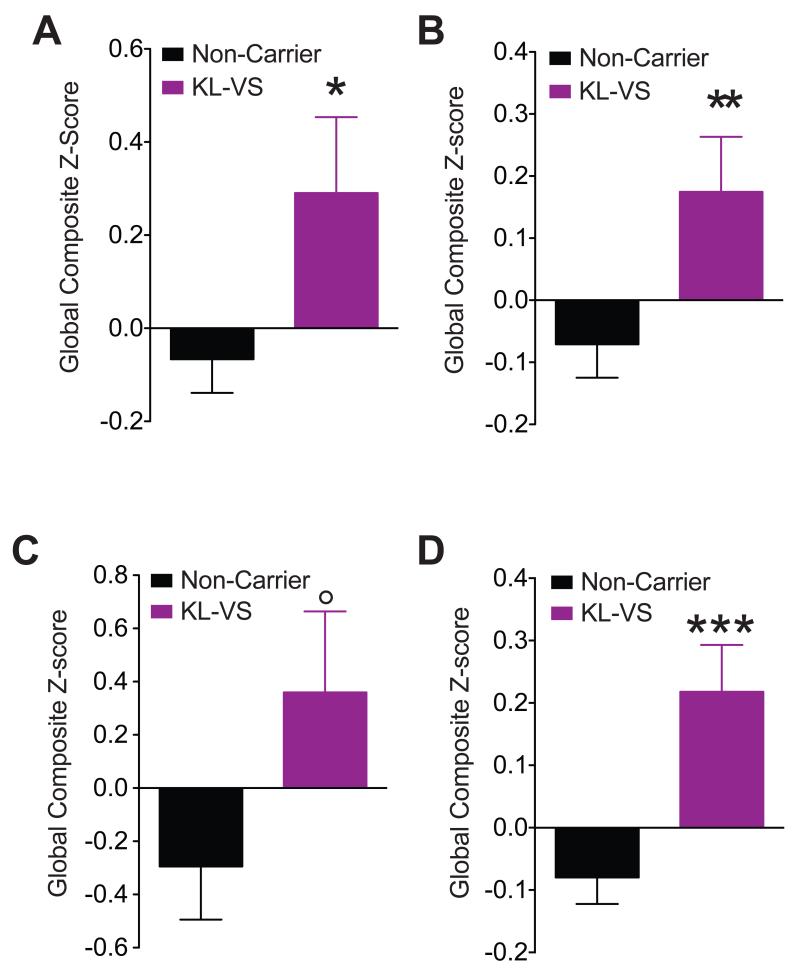
In each cohort cognitive abilities of heterozygous KL-VS carriers and non-carriers were analyzed using multiple neuropsychological tests (Table S4; Figure S1) and compiled into global composite Z-scores (Figure 1A-C; Figure S1). The scores represent a broad measure of cognition, including domains vulnerable to aging (Drag and Bieliauskas, 2010) and reflect the number of standard deviations from the global average. We used linear statistical models including KL-VS carrier status (0 or 1 allele), age, sex, and education, with or without APOEε4 as predictors for performance (Table S5). APOEε4, the main genetic risk factor for Alzheimer’s disease (AD) (Verghese et al., 2011), did not contribute significant variance (Table S5) and including “cohort” as a covariate in meta-analysis did not alter results (data not shown). KL-VS carriers scored higher than non-carriers in each cohort and in meta-analysis of all cohorts (Figure 1A–D; Table S5).
Figure 1. The KL-VS allele is associated with better cognitive performance in three independent aging populations without dementia and in a meta-analysis of the populations.
(A–D) Neuropsychological scores from tests spanning multiple cognitive domains (Table S4; Figure S1). Global composite Z-scores of 718 aging individuals (52–85 years of age) that were non-carriers (black; n=530) or carriers (purple; n=188) of a single KL-VS allele were obtained from three independent cohorts without cognitive impairments. In each cohort, an individual composite score was standardized and scaled to reflect performance as a measure of the number of standard deviations from the global average of that cohort (global composite Z-score). Higher scores indicate better cognitive performance. (A–C) Global composite Z-scores in (A) Cohort 1 (179 non-carriers, 41 carriers), (B) Cohort 2 (331 non-carriers, 135 carriers), (C) Cohort 3 (20 non-carriers, 12 carriers), and (D) Meta-analysis of the cohorts. All subjects had a MMSE score of 28 or greater and no dementia. Data were analyzed by linear models, accounting for effects of age, sex, and education and testing for effects due to KL-VS genotype. APOEε4 carrier status had no significant effects (Tables S5–S6). °p=0.06, *p<0.05, **p<0.01, ***p<0.001 vs Non-Carrier (linear regression t-test). See also Tables S2-S6 and Figure S1. Data are means ± SEM.
KL-VS is associated with better cognition independent of age, sex, and APOEε4 allele status
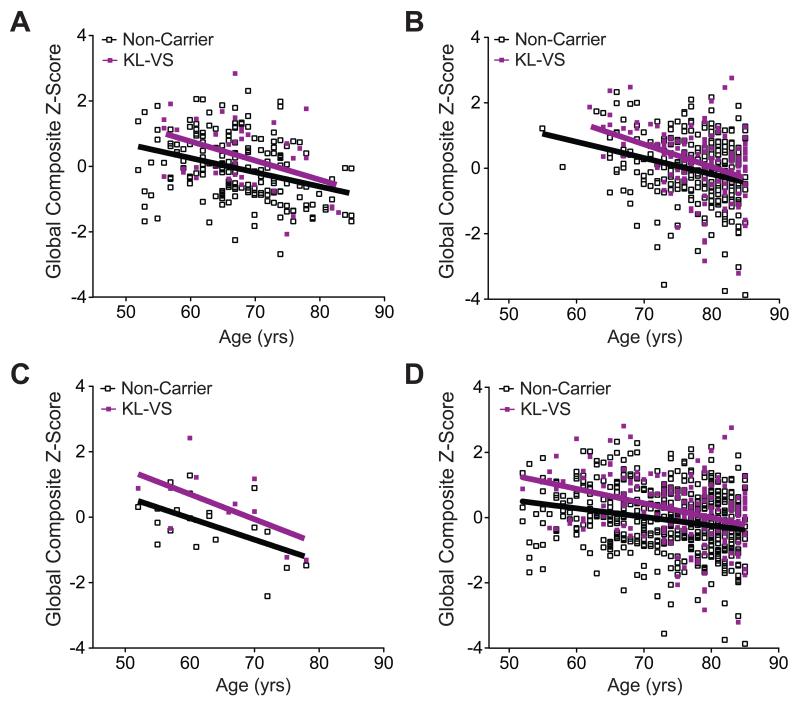
Meta-analysis showed that advancing age, male sex, and lower education decreased cognitive scores; KL-VS heterozygosity increased scores despite these effects (Table S5). Baseline cognition was not affected by APOEε4, consistent with previous findings (Yaffe et al., 1997), though detrimental effects of APOEε4 on cognitive aging might be revealed by longitudinal analysis (Deary et al., 2002; Yaffe et al., 1997). KL-VS did not differentially affect cognition by sex (Table S6), but there was a trend (Table S6) for its positive impact to decrease with advancing age (Figure 2 A-D).
Figure 2. KL-VS-associated cognitive enhancement is independent of age.
(A–C) Global composite Z-scores decreased as a function of age in non-carriers (empty squares) and carriers (purple squares) of the KL-VS allele in (A) Cohort 1, (B) Cohort 2, and (C) Cohort 3. (D) In meta-analysis of all cohorts, KL-VS-associated cognitive enhancement tended to decrease with advancing age (ANOVA, KL-VS:age interaction, p=0.10). Data were analyzed by linear models, accounting for effects of age, sex, and education and testing for effects due to an age by KL-VS interaction. APOEε4 carrier status had no significant effects on these measures. See also Tables S5 and S6. Data are means ± SEM.
KL-VS increases klotho levels in sera of humans
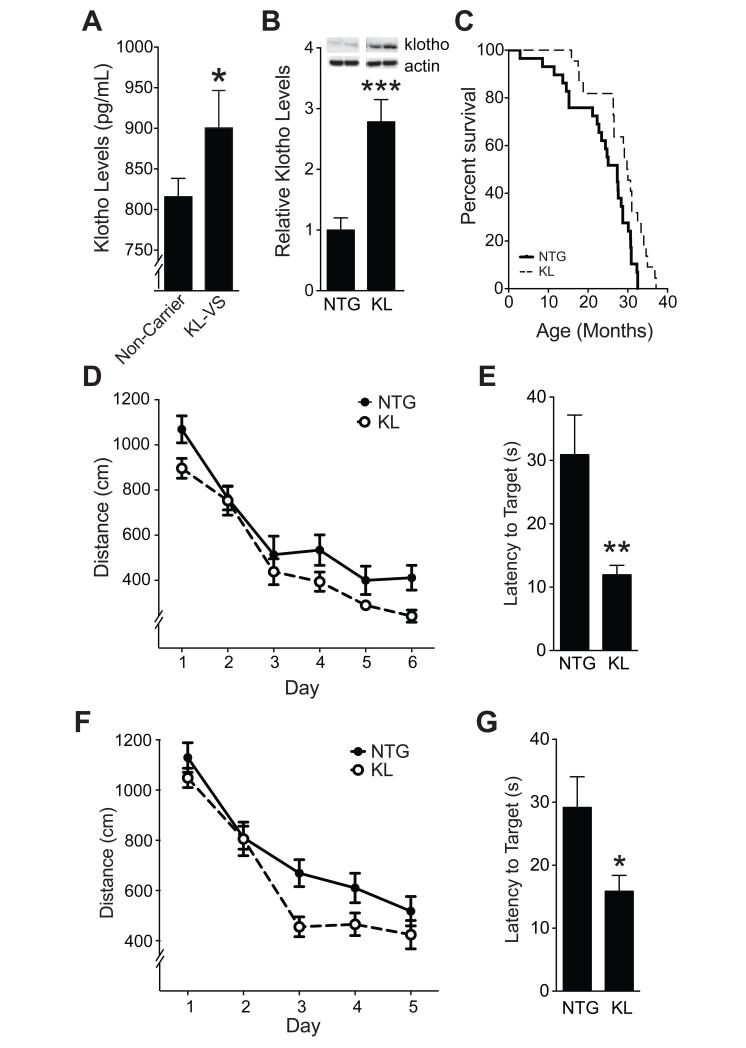
Based on these findings, we concluded that KL-VS enhances baseline cognition and hypothesized that it does so, in part, by increasing klotho levels or activity. To begin to test this, we measured fasting, morning levels of klotho in serum by ELISA in individuals from Cohort 1 with no or one KL-VS allele. Consistent with findings in cell culture (Arking et al., 2002), the KL-VS variant significantly increased levels of secreted klotho in the serum (Figure 3A and Table S7).
Figure 3. Elevation of systemic klotho levels occurs in human KL-VS carriers and enhances mouse survival, learning, and memory independent of age.
(A) Fasting morning serum klotho levels in individuals from Cohort 1 (55–85 years of age) that were non-carriers (n=118) or carriers (n=38) of a single KL-VS allele. Data were analyzed by a linear model, accounting for effects of age, sex, and education and testing for effects due to KL-VS genotype. APOEε4 carrier status had no effect (Table S7). (B) Hippocampal levels of klotho in NTG and KL mice (n=13–14 mice per genotype, age 3 months). Representative western blots for klotho and actin are shown above; images for each protein were from the same gel. (C) Kaplan-Meier curves show increased survival of heterozygous KL mice from line 46 (Kuro-o et al., 1997; Kurosu et al., 2005) compared to NTG littermates (n=22–29 mice per group, p<0.01 by log rank test). Proportional hazard testing revealed the KL effect was independent of age (p=0.76). (D-E) KL and NTG mice (n=8–9 mice per genotype) were tested in the Morris water maze at 10–12 months of age. (D) Spatial learning curves (platform hidden). Data represent the daily average of total distance traveled to the platform. Mixed model ANOVA: KL effect p<0.05. (E) Results of a probe trial (platform removed) 1 h after completion of hidden-platform training showing latency to reach the original platform location. (F–G) An independent cohort of mice (n=17–19 mice per genotype) was tested in the water maze at 4–7 months of age. (F) Spatial learning curves (platform hidden). Mixed model ANOVA: KL effect p<0.05. (G) Probe trial results. *p<0.05, **p<0.01, ***p<0.001 (t-test). See also Tables S7, S8 and Figure S2. Data are means ± SEM.
Elevation of klotho promotes longevity and enhances cognition in mice in an age-independent manner
To test whether systemic increases in klotho levels enhance cognition, we analyzed heterozygous klotho (KL) transgenic mice that overexpress mouse klotho in plasma (Kurosu et al., 2005) and throughout the body and brain (Kuro-o et al., 1997), including the hippocampus (Figure 3B), which is critical to learning and memory. Klotho overexpression improved survival in mice, independent of age or time by proportional hazard analysis (Figure 3C). Similar to our human findings, these data suggest that klotho can engage mechanisms that do not depend on aging per se.
We tested spatial learning and memory in 10-12-month-old (“middle-aged”) mice in the Morris water maze. KL mice performed better than non-transgenic (NTG) controls (Figure 3D). After hidden platform training, the platform was removed and spatial memory retention was assessed in a probe trial. KL mice reached the original platform location 2.5 times faster than NTG mice (Figure 3E). Klotho elevation also improved spatial learning and memory in 4-7-month-old (“young”) mice (Figure 3F, G). Effects were independent of sex (Table S8). Of note, KL and NTG mice swam at equal speeds (Figure S2A, B) and located a cued platform equally well (Figure S2C, D). Thus, elevation of klotho enhances spatial learning and memory independent of sex and across different age groups. Because the effect of klotho on young mice was surprising and such mice are readily available and most suitable for electrophysiological analysis, we focused subsequent studies on this age group.
Klotho elevation improves working and fear memory without altering other behaviors in young mice
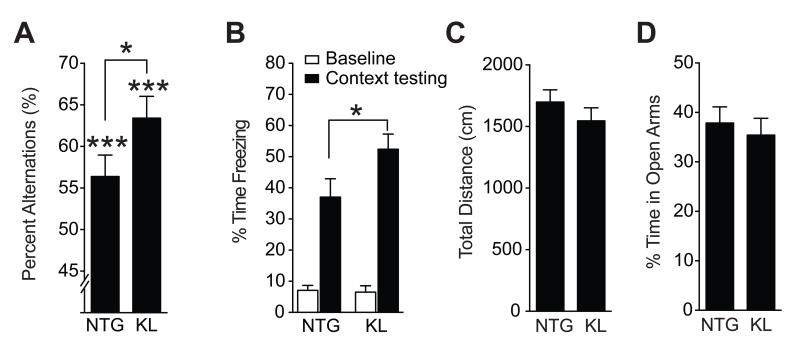
We tested whether elevation of klotho in mice enhances cognition in young mice (3–4 and 4–7 months of age) in other cognitive tasks. In the Y-maze, KL mice showed more alternations than NTG controls (Figure 4A), an indication of superior working memory. In a fear conditioning paradigm, KL mice showed better contextual memory than NTG mice (Figure 4B), but similar cued recall (Figure S3A, B). In contrast, klotho elevation did not affect exploratory and anxiety-related behaviors in the open field (Figure 4C) or elevated plus maze (Figure 4D). Thus, elevation of klotho enhances learning and memory in a range of tasks without altering other behaviors.
Figure 4. Klotho elevation also improves working and context memory without altering other behaviors in young mice.
(A) Percent alternations among arms by 3–4-month-old mice during exploration of a Y-maze (n=8–10 mice per genotype). (B) Percent time 6-month-old mice spent freezing at baseline and 24 h after context training in a fear conditioning task (n=6–7 male mice per genotype). (C) Movements during exploration of an open field (n=13–14 mice per genotype; p=0.30 by two-tailed t-test). (D) Percent time spent exploring the open arms of an elevated plus maze (n=14–15 mice per genotype; p=0.60 by two-tailed t-test). *p<0.05, ***p<0.001 vs chance or as indicated by bracket (t-test). See also Figure S3. Data are means ± SEM.
Klotho elevation increases synaptic levels of the GluN2B subunit of NMDARs in mouse hippocampus and cortex
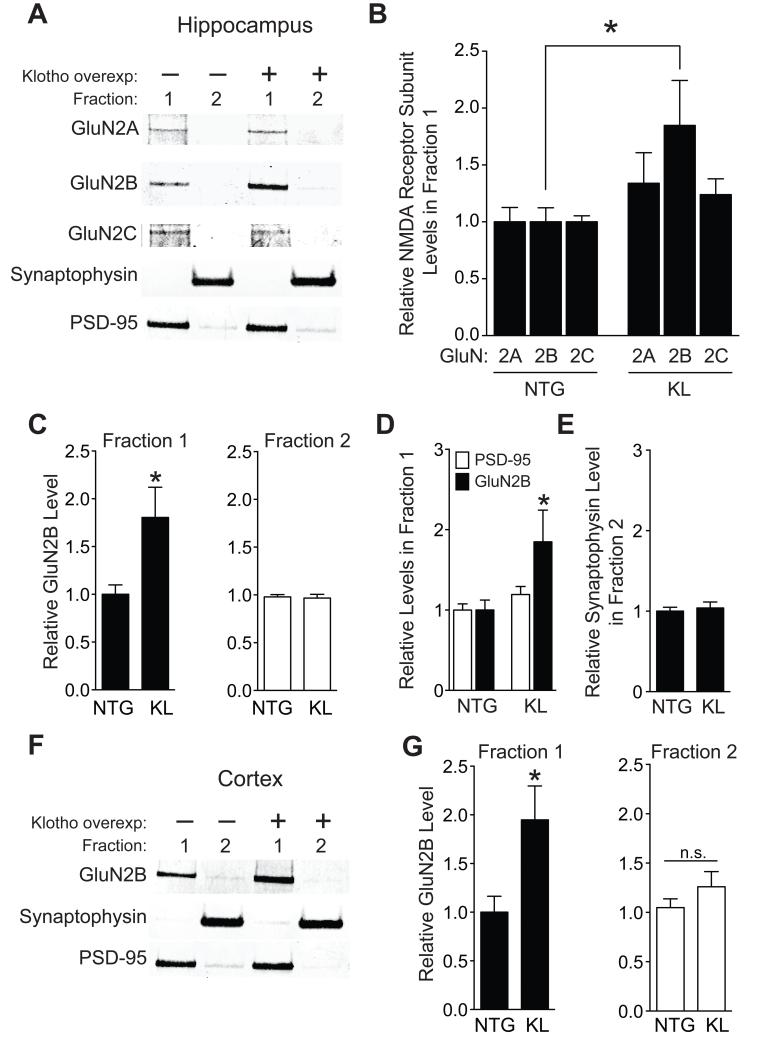
To further explore the mechanism underlying beneficial effects of klotho on cognition, we turned our attention to NMDARs and AMPARs, whose functions are essential for learning and memory (Anggono and Huganir, 2012; Nakazawa et al., 2004). An initial screen of their subunit expression in hippocampus revealed that klotho elevation specifically increased total protein levels of the NMDAR subunit GluN2B (Figure S4A–E), without altering its mRNA (Figure S4F). Interestingly, transgenic overexpression of GluN2B in mice (Cao et al., 2007; Tang et al., 1999) and rats (Wang et al., 2009) enhances cognition, whereas dysfunction of this subunit in humans and mice contributes to cognitive decline in aging (Piggott et al., 1992; Zhao et al., 2009) and AD (Ittner et al., 2010; Li et al., 2011; Sze et al., 2001). In light of these findings and because the enhancement of learning and memory in KL mice resembled that in GluN2B-overexpressing mice, we investigated whether klotho modulates the GluN2B protein at synapses in the hippocampus and frontal cortex, regions directly involved in cognitive functions. Klotho elevation nearly doubled GluN2B, but not GluN2A or GluN2C, levels in post-synaptic density (PSD) fractions isolated from the mouse hippocampus (Figure 5A-C). It did so without altering pre-synaptic (synaptophysin) and post-synaptic (PSD-95) markers in membrane fractions (Figure 5A,D,E). Furthermore, klotho-mediated increases in GluN2B extended to PSD-enriched fractions of the frontal cortex (Figure 5F,G). Collectively, these data suggest that klotho elevates synaptic GluN2B in a subunit-specific manner through post-transcriptional mechanisms.
Figure 5. Klotho overexpression enhances synaptic GluN2B levels in the hippocampus and cortex.
(A-G) Synaptic membrane Fraction 1 (PSD-enriched) and Fraction 2 (non-PSD enriched) isolated from hippocampus or cortex of NTG and KL mice (n=15-18 mice per genotype, age 3-4 months). (A) Representative western blots of hippocampal fractions. (B-E) Quantitation of (B) GluN2A, GluN2B, and GluN2C levels in hippocampal fraction 1, (C) GluN2B in hippocampal fractions 1 (left) and 2 (right), (D) PSD-95 and GluN2B and (E) synpatophysin levels. (F) Representative western blots of cortical fractions isolated from frontal, motor and somatosensory regions (Bregma 0-3.5 mm). (G) Quantitation of GluN2B levels in cortical Fractions 1 (left) and 2 (right). For each fraction, protein levels are relative to NTG levels, arbitrarily defined as 1.0. *p<0.05 vs NTG (t-test). See also Figure S4. Data are means ± SEM.
Klotho elevation increases NMDAR-dependent gene expression and synaptic plasticity
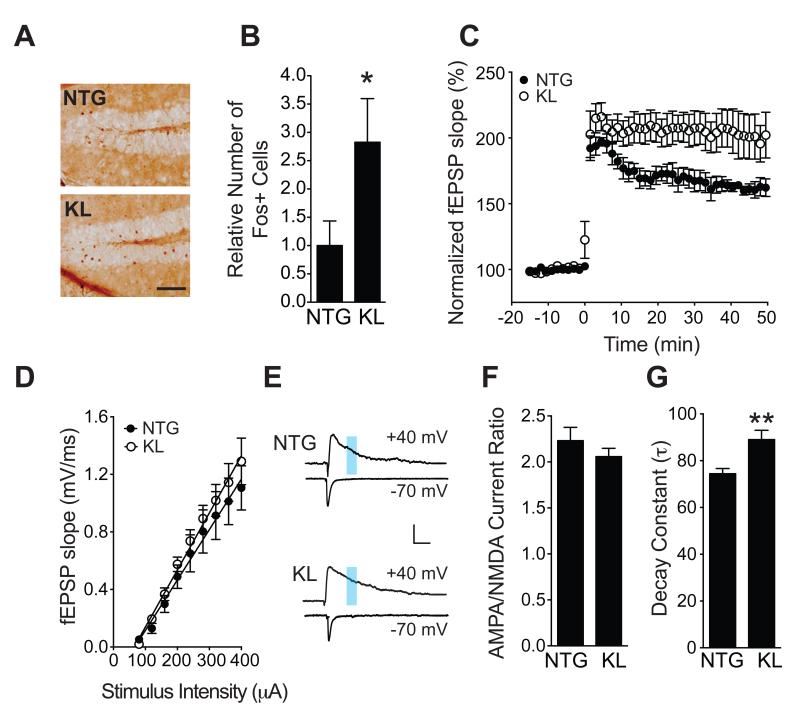
We next analyzed effects of klotho elevation on NMDAR-dependent gene expression and synaptic plasticity. We first examined Fos, an immediate early gene involved in memory consolidation (Kubik et al., 2007) increased by NMDAR activation (Bading et al., 1993). We measured FOS-positive cells in the granular layer of the hippocampal dentate gyrus in 3-4-month-old KL and NTG mice after hidden-platform training and a probe trial in the water maze, tasks that engage the dentate gyrus and other regions of the hippocampus and cortex (Wang and Morris, 2010). Consistent with findings in mice at 10-12 and 4-7 months of age (Figure 3D-G), KL mice also showed better learning and memory in the water maze at 3-4 months (data not shown). In the same cohort, FOS expression was more prominent in KL mice than NTG controls following the probe trial (Figure 6A, B), indicating that enhanced cognition in KL mice was associated with increased NMDAR-dependent gene expression.
Figure 6. Klotho overexpression enhances NMDAR-, but not AMPAR-, dependent functions.
(A, B) FOS expression in the dentate gyrus of NTG and KL mice immediately following a probe trial in the water maze. (A) Staining with antibodies to FOS revealed more immunoreactive granule cells in the dentate gyrus of KL (bottom) than NTG (top) mice. Scale bar: 100 μm. (B) Quantitation of FOS-positive granule cells (n=7 mice per genotype, age 3-4 months). The mean level in NTG controls was arbitrarily defined as 1.0. (C) Field excitatory postsynaptic potential (fEPSP) recordings from acute hippocampal slices of 3.5-4.5-month-old NTG and KL mice. LTP induction and decay in the dentate gyrus were monitored for 45-50 minutes following theta burst stimulation of the medial perforant pathway. Mixed model ANOVA: KL vs NTG genotype by time effect p<0.01. Number of slices/mice: NTG 4/4, KL 8/6. (D) AMPAR-mediated basal synaptic transmission in acute hippocampal slices of 3.5-4.5-month-old mice at the medial perforant path to dentate granule cell synapse. Number of slices/mice: NTG 5/3, KL 9/5. (E-G) Isolated NMDAR and AMPAR EPSCs measured by whole-cell patch-clamp recordings from dentate granule cells in acute hippocampal slices of 3–4-month-old mice. (E) Representative traces of evoked EPSCs at +40 mV or −70 mV. NMDAR–mediated EPSCs were quantitated between 80–100 ms after stimulation (blue shading) in the top traces and AMPAR–mediated EPSCs at the nadir of the bottom traces. Scale: 100 pA, 50 ms. (F) Quantitation of AMPAR/NMDAR EPSC ratios. Number of slices/mice: NTG 10/3, KL 10/3. p=0.32 (t-test). (G) Decay constant (τ) of isolated NMDAR EPSCs in the presence of NBQX (10 μM). Number of slices/mice: NTG 7/3, KL 5/3. *p<0.05, **p<0.01 (t-test). See also Figure S5. Data are means ± SEM.
NMDAR activation is also critical for long-term potentiation (LTP), a form of synaptic plasticity thought to underlie learning and memory (Morris et al., 1986; Nakazawa et al., 2004). To assess whether klotho enhances synaptic plasticity, we measured LTP in acute hippocampal slices at the medial perforant path to granule cell synapse, which is mostly mediated by NMDARs (Nguyen and Kandel, 1996). Elevation of klotho enhanced hippocampal LTP (Figure 6C) without changing AMPAR-mediated basal synaptic strength (Figures 6D and S5), as determined by field EPSP and whole–cell EPSC recordings.
Klotho elevation increases the GluN2B portion of NMDAR currents
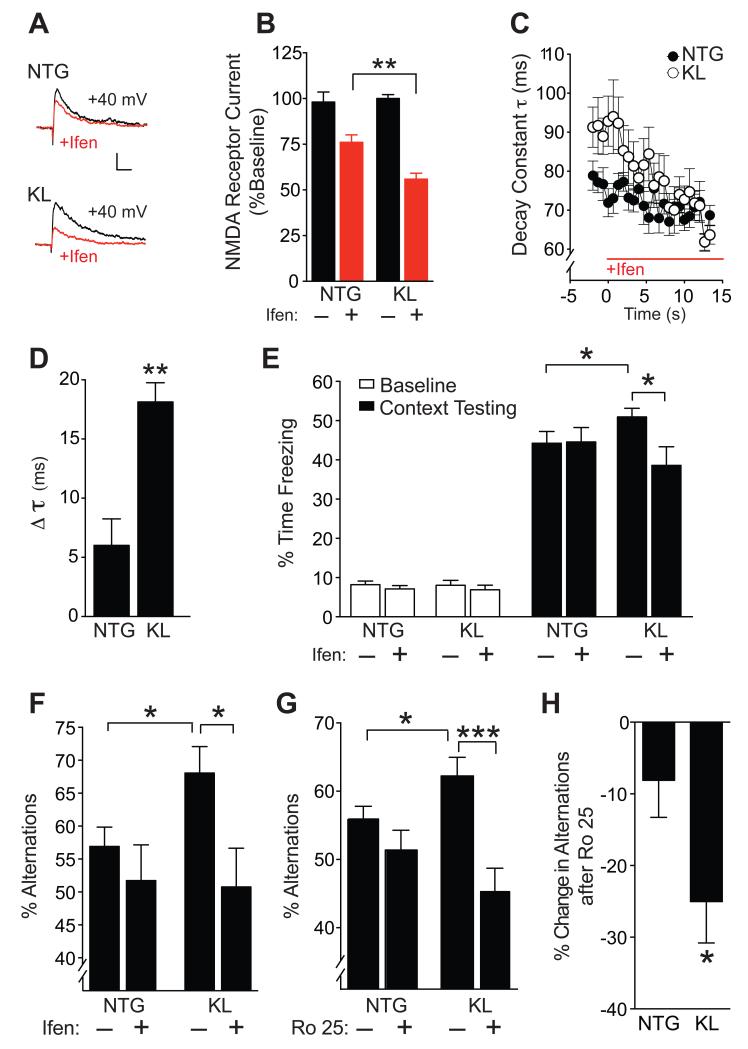
We observed that klotho-mediated enrichment of synaptic GluN2B positively correlated with GluN1 levels (Figure S6A, B), suggesting an increase in functional GluN2B–containing NMDARs. To assess whether overexpression of klotho increases NMDAR currents, we measured the amplitude and kinetics of synaptic NMDAR-mediated currents by whole-cell patch-clamp recordings from dentate granule cells following perforant path stimulation. Because AMPA transmission did not differ between NTG and KL slices (Figures 6D and S5), the amplitudes of NMDAR currents were normalized to AMPAR currents, revealing similar amplitudes in NTG and KL slices (Figure 6E, F). However, NMDAR currents decayed more slowly in KL slices than NTG slices, reflected by an increased decay constant (Figure 6G). Because NMDARs containing GluN2B deactivate slower than those containing GluN2A (Wang et al., 2008), the increased decay time of currents in KL slices suggests that GluN2B-containing NMDARs contributed more to currents in KL slices than in NTG slices. Consistent with this interpretation, treatment of slices with the GluN2B-specific antagonist ifenprodil (Mony et al., 2009) reduced NMDAR current amplitudes (Figure 7A, B) and decay constants (Figure 7C, D) more in KL slices than in NTG slices. Thus, klotho elevation probably increases the GluN2B component of synaptic NMDAR activity.
Figure 7. Treatment with GluN2B selective antagonists blocks klotho effects on NMDAR currents and cognition.
(A,B) Representative traces (A) and quantitation (B) of isolated NMDAR EPSCs in the presence of NBQX (10 μm) at baseline (black) and following perfusion with ifenprodil (Ifen., 3 μM) (red) in the same slices. Number of slices/mice: NTG 6/3, KL 5/3. Two-way repeated measures ANOVA: ifenprodil effect p<0.0001, ifenprodil by KL interaction p<0.05. (C) Time course of NMDAR EPSC decay constant following ifenprodil perfusion in each genotype. Mixed model ANOVA: p<0.0001 for KL vs NTG genotype by time effect. (D) Change in decay constant (τ) between 0-10 min after initiation of ifenprodil treatment in NTG and KL slices. Number of slices/number of mice: NTG 7/3, KL 5/3. (E-F) Mice (n=8-19 per group) received a single i.p. injection of vehicle (−) or ifenprodil (5 mg/kg) 10 min before training in a fear conditioning paradigm or testing in the Y-maze. (E) Percent time mice (age 5-7 months) spent freezing at baseline and 24 h after context training in a fear conditioning task. Two-way ANOVA: ifenprodil by KL interaction p<0.05. (F) Percent alternations among Y-maze arms that mice (age 10-12 months) showed during 3 min exploration. Two-way ANOVA: ifenprodil by KL interaction p<0.09. (G-H) Mice (n=13-19 per group, age 3-5 months) received a single i.p. injection of vehicle (−) or Ro 25-6981 (Ro 25; 5 mg/kg) 30 min before testing in Y-maze. (G) Percent alternations among Y-maze arms. Two-way ANOVA: Ro 25 by KL interaction p<0.05. (H) Percent decrease in alternations following Ro 25 treatment in NTG and KL mice. *p<0.05, **p<0.01, ***p<0.001 vs. NTG or as indicated by brackets by t-test (B, D, H) or Bonferroni-Holm test (E, F, G). See also Figure S6. Data are means ± SEM.
Acute inhibition of GluN2B blocks klotho-mediated enhancement of learning and memory
We tested whether blocking GluN2B-containing NMDARs modulates effects of klotho elevation on context and working memory. We specifically tested if acute, transient, and broad blockade of GluN2B affects klotho-mediated enhancement of cognition without disrupting baseline cognitive functions. To this end, we used low doses of pharmacological GluN2B antagonists that minimally affect NTG mice (Mathur et al., 2009; Sotres-Bayon et al., 2007). Young mice were injected intraperitoneally (i.p.) with ifenprodil (5 mg/kg) or vehicle before training in fear conditioning and then tested one day later. Untreated KL mice showed better hippocampus-dependent contextual fear memory than untreated NTG mice (Figure 7E), consistent with their superior performance in other cognitive tasks (Figures 3D-G; 4A, B), enhanced hippocampal LTP (Figure 6C), and increased GluN2B function (Figure 7A, B). At the low dose used, ifenprodil did not affect fear conditioning in NTG controls (Figure 7E), consistent with previous results (Kojima et al., 2005; Sotres-Bayon et al., 2007). In KL mice, ifenprodil blocked enhancement of context learning and memory (Fig. 7E). At a higher dose, and with a lower number of shocks, ifenprodil suppressed learning and memory also in NTG mice (Figure S6C).
To assess effects of ifenprodil during another life stage and in another behavioral task, we injected middle-aged mice i.p. with ifenprodil or vehicle and tested them in the Y-maze, which engages frontal cortical circuits. Untreated KL mice showed better working memory than NTG mice, as measured by percent alternations between maze arms (Figure 7F). In KL mice, ifenprodil blocked the enhancement of working memory (Figure 7F). Thus, at a low dose, ifenprodil blocked klotho-mediated cognitive enhancement in young and middle-aged mice in two independent cognitive measures.
To further test and validate the role of GluN2B in klotho-mediated cognitive enhancement, we used Ro 25-6981 (Ro 25), a second-generation NDMAR blocker with 3000-fold specificity to GluN2B relative to other subunits (Paoletti and Neyton, 2007). Young mice were injected i.p. with a low dose of Ro 25 (5 mg/kg) (Mathur et al., 2009) or vehicle and tested in the Y-maze. As expected, vehicle-treated, but not Ro 25-treated, KL mice showed better working memory than NTG controls (Figure 7G, H). All together, these results are consistent with the synaptic enrichment of GluN2B in KL mice (Figure 5B) and the greater susceptibility of KL slices to ifenprodil-induced suppression of NMDAR currents (Figure 7A-D).
DISCUSSION
Our genetic and neuropsychological data in humans combined with molecular, electrophysiological, pharmacological, and behavioral studies in transgenic mice reveal a novel role for the life extension factor klotho in enhancing cognition. In three independent human populations, the longevity-promoting KL-VS variant of the KLOTHO gene was associated with enhanced cognition in heterozygous individuals across all ages examined. As predicted based on cell culture studies (Arking et al., 2002), KL-VS increased klotho levels in the sera of heterozygous individuals. In mice, systemic overexpression of klotho improved learning and memory in multiple cognitive tests and this effect was independent of age. Klotho elevation enhanced NMDAR-related functions, including FOS expression following a learning and memory task and LTP in the dentate gyrus. It also resulted in post-synaptic enrichment of the NMDAR subunit GluN2B in the hippocampus and cortex, brain regions central to networks supporting cognition. Acute blockade of GluN2B abolished klotho-mediated effects on hippocampal NMDAR currents and on learning and memory. Taken together, these data support the hypothesis that, in addition to extending lifespan, klotho exerts beneficial effects on cognitive and synaptic functions through mechanisms that involve regulation of NMDARs and are uncoupled from aging per se.
Klotho and Better Cognitive Status: Effect Size, Relevance and Caveats
Because cognition is a highly valued and central manifestation of brain function that diminishes with aging and disease, the potential to enhance it – even slightly – is of great relevance to the human condition. In human studies, we captured the magnitude of group differences in cognition between KL-VS carriers and non-carriers, by calculating an “effect size” with the widely used Cohen’s d method (Ray and Shadish, 1996) to estimate the biologic significance of our findings. In studies of human cognition and behavior, an effect size of 0.25 on a scale of 0-1 is broadly considered clinically significant (Rockwood, 2004; Smith et al., 2006). Our findings demonstrate an average Cohen’s d score of 0.34 across three populations. This effect size exceeds that of APOEε4, the most robust known genetic modifier of cognition in aging, calculated at 0.27 from longitudinal cognitive decline (Deary et al., 2002) or at 0.13-0.25 from select baseline scores in comparable aging individuals (Brown et al., 2011). It also exceeds effect sizes of FDA-approved treatments for Alzheimer’s disease, calculated between 0.15-0.28 (Rockwood, 2004; Smith et al., 2006). Determining if this effect translates into advantages in daily life or greater reserve against neurodegenerative diseases are important objectives that require further study.
Since our findings were replicated in three independent human cohorts in three geographic regions, it is likely that our inferences are suitable at a population level, particularly since the populations included community-dwelling individuals. However, potential limitations in extrapolating our data worldwide include that most participants were Caucasian and that our studies were limited to the United States. Thus, it is possible that more diverse genetic or environmental influences could alter or mask the effect in other populations.
To our knowledge, the KL-VS variant has not been identified as a significant modulator in GWAS studies of human cognition in aging (De Jager et al., 2012; Luciano et al., 2011; Seshadri et al., 2007), including in a study of populations overlapping with the current study (De Jager et al., 2012). However, our hypothesis-driven query of a population previously studied by GWAS (De Jager et al., 2012) did, in fact, reveal a significant association of KL-VS heterozygosity with improved baseline cognition. Potential reasons for this include: 1) previous outcome measures focused on cognitive decline (De Jager et al., 2012), which KL-VS did not prevent, and 2) benefits of KL-VS are limited to the heterozygous state and, thus, may be missed by conventional GWAS methodologies that assess additive effects of the minor allele.
Indeed, KL-VS homozygosity eliminates advantages in lifespan and health (Arking et al., 2005; Arking et al., 2002; Invidia et al., 2010; Majumdar et al., 2010), and similarly eliminated cognitive advantage in our discovery cohort (data not shown). The reasons for the paradoxical dose effect of the minor allele are unknown. Possibilities include adverse effects of high klotho levels maintained over decades in homozygous carriers or more complex dysregulation of klotho expression by the variant. For example, it is possible that the variant actually reduces klotho production, resulting in abnormally low levels of klotho in homozygotes, but causes a compensatory increase in expression of the wildtype allele in heterozygotes. Additional studies are needed to investigate these possibilities.
KL-VS homozygosity also decreased cognition in a previous study (Deary et al., 2005), consistent with our findings. However, the previous study did not detect differences in cognitive performance of KL-VS heterozygous individuals and non-carriers. The reasons for this discrepancy are unknown. It is worth noting, though, that we replicated our findings in three independent cohorts and that the current study focused on tests without maximum scores to increase the sensitivity of detecting cognitive enhancement.
Klotho-Mediated Cognitive Enhancement is Independent of Aging
In light of klotho’s role in aging, we were surprised that transgenic mice with global overexpression of klotho showed better learning and memory than NTG controls at all ages examined, including at 3 months, following entry into early adulthood. Furthermore, KL-VS-associated increases in serum klotho levels did not prevent aging-related cognitive decline in humans. Instead, the KL-VS variant enhanced cognitive functions over a wide age range, though there was a trend toward decreased effects at the most advanced ages.
In our opinion, a plausible interpretation of these findings is that the KL-VS variant, similar to global overexpression of klotho in transgenic mice, modulates baseline cognition and that this effect diminishes when the age-associated decline in secreted klotho levels (Duce et al., 2008; Semba et al., 2011) crosses a critical threshold. Longitudinal studies over a broader age span are needed to test this hypothesis. Although klotho elevation does not slow down cognitive aging per se, its age-independent cognition-enhancing effects could increase “cognitive reserve” and thereby augment one’s ability to counter adverse effects of aging or related diseases, at least for a while.
Klotho and Mechanisms of Learning and Memory
In humans and mice, klotho effects were observed in cognitive tests that interrogate functions of diverse networks that include, but are not limited to, hippocampus and frontal cortex (Frankland and Bontempi, 2005; Wang and Morris, 2010). Klotho enhanced learning and memory in the water maze across several ages (Table S1). We conducted mechanistic studies in young mice because they are more readily available than older mice, yield high-quality electrophysiological recordings, and allowed us to address one of the most novel findings of our studies – that klotho enhances cognition even in the young life-stage. We focused on the hippocampus because of its well-characterized electrophysiological properties of learning and memory circuits.
Klotho elevation increased neuronal FOS expression during memory retrieval in the water maze, as well as LTP and the decay time of NMDAR currents. Collectively, these findings suggest a causal role for NMDARs and their GluN2B subtype in klotho–mediated cognitive enhancement.
The subunit composition of NMDARs dictates their functional properties (Yashiro and Philpot, 2008). Since GluN2B-containing NMDARs deactivate slower than GluN2A-containing NMDARs (Wang et al., 2008), the increased decay time of NMDAR currents in hippocampal slices from KL mice suggests that klotho elevation augments the contribution of GluN2B-containing NMDARs to NMDAR currents, a possibility that may extend to other brain regions. Consistent with this hypothesis, treatment of slices with the GluN2B-specific antagonist ifenprodil preferentially reduced NMDAR current amplitudes and decay constants in hippocampal slices from KL mice. We conclude that klotho elevation directly or indirectly increases the GluN2B component of synaptic NMDAR activity, which in turn facilitates induction of LTP (Foster et al., 2010; Yashiro and Philpot, 2008), a potential substrate of learning and memory. In support of this conclusion, blockade of GluN2B abolished klotho-mediated increases in learning and memory. Because GluN2B is also involved in long-term depression, homeostatic plasticity, and metaplasticity (Brigman et al., 2010; Liu et al., 2004; Yang et al., 2012), it will be interesting to determine if and how klotho alters other forms of synaptic plasticity.
Although our initial screen of receptor subtype expression revealed that klotho increased total hippocampal protein levels of GluN2B, but not of other NMDAR or AMPAR subtypes, this does not exclude the possibility that klotho modulates other receptors in sub-cellular or sub-membrane compartments or under disease conditions. Furthermore, cognition and its underlying substrates are complex. Therefore, other klotho-related mechanisms may also contribute to enhanced cognition, including regulation or signaling of other ion channels (Imura et al., 2007), insulin (Chen et al., 2007; Kurosu et al., 2005), wnt (Liu et al., 2007), or FGF23 (Urakawa et al., 2006) in neuronal or non-neuronal cells. Klotho-mediated enrichment of GluN2B could intersect with one or more of these pathways.
Klotho elevated total and synaptic GluN2B protein levels in a subunit-specific manner without altering pre- and post-synaptic markers. How klotho elevates total levels of GluN2B protein and enriches GluN2B within synapses, directly or indirectly, remains to be determined, but may involve stabilizing mRNA, increasing translation, affecting posttranslational modification, regulating recycling, trafficking, or some combination thereof. It also remains to be determined whether the effects of klotho elevation on GluN2B are mediated by the transmembrane or secreted form of klotho.
Our findings suggest that the KL-VS variant promotes cognition by increasing levels of secreted klotho. KL-VS carriers had higher serum klotho than non-carriers – and in both groups, higher klotho levels correlated or trended to correlate with better cognitive function on tests such as semantic fluency, category fluency, and modified trails (p=0.04-0.17, linear regression t-tests, Cohort 1, n=153). Furthermore, elevating klotho levels in another species, mice, also enhanced cognition. Nonetheless, it is important to note that the KL-VS variant may also exert other effects such as altering klotho activities (Abraham et al., 2012; Arking et al., 2002; Tucker Zhou et al., 2013). Additional studies are needed to further explore the pleiotropic functions of this lifespan-extending and cognition-enhancing factor. Strategies that increase the level (Abraham et al., 2012; King et al., 2012) or activity of klotho or simulate its functions may improve cognition at different life stages and, possibly, even under pathological circumstances.
EXPERIMENTAL PROCEDURES
Cohorts
Several cohorts of humans and mice were utilized (Table S1).
Human studies
Subjects were genotyped for the KL-VS variant. Cognitive data were collected blinded to genotype. Neuropsychological testing (Figure S1) spanned multiple cognitive domains (Table S4). Serum was analyzed for soluble α-klotho levels by ELISA (Immuno-Biological Laboratories, Takasaki, Japan) (Yamazaki et al., 2010).
Mice, cognition, and behavior
NTG C57BL/6 mice were crossed with hemizygous KL transgenic mice (Line 46) (Kuro-o et al., 1997), which express mouse klotho under the EF-1α promoter. All studies were conducted in a blinded manner on age-matched and sex-balanced littermate offspring, unless indicated otherwise. Mice were tested in the Morris water maze, Y-maze, fear conditioning apparatus, elevated plus maze, and open field, as described (Cisse et al., 2011; Harris et al., 2010).
Protein analyses
Separation of synaptic membrane fractions (Goebel-Goody et al., 2009; Li et al., 2011) was performed as described with minor modifications. Western blot analyses (Palop et al., 2003) and immunohistochemistry (Masliah et al., 2011) were performed as described. Protein input, antibody dilutions, and other details are described in Supplemental Experimental Procedures.
Electrophysiology
LTP was induced and measured from acute hippocampal slices using methods as described (Cisse et al., 2011) with minor modifications. AMPAR/NMDAR EPSC ratios and spontaneous EPSCs were recorded from dentate granule cells as described in detail in the Supplemental Experimental Procedures.
Statistical Analyses
Experimenters were blinded to the genotypes of humans and the genotypes and treatment of mice. Statistical analyses were performed using GraphPad Prism (5.0) for t-tests, log-rank tests, and repeated measures ANOVA. R (nlme package) was used for mixed model ANOVAs, post-hoc tests, principle component analysis, linear models, and power analyses.
See Supplemental Experimental Procedures for further details on all experimental methods.
Supplementary Material
Highlights.
KLOTHO variant elevates klotho levels and is associated with enhanced human cognition
Elevation of klotho in mice enhances normal cognition, independent of age
Klotho elevation leads to greater synaptic GluN2B (NMDAR subunit) levels and plasticity
GluN2B blockade abolishes klotho-mediated effects on NMDAR functions and cognition
Acknowledgements
We thank X. Wang, C. Wang, and W. Guo for technical assistance; J. Palop, N. Devidze, B. Djukic, P. Taneja, A. Gazzaley, and S. Hauser for discussions; A. Karydas, G. Klein, P. Siddarth, and N. Patel for assistance accessing genetic, cognitive, or biospecimen databases; M. Dela Cruz for administrative support; and M. Kelley, A. Moreno, and T. Singh for graphics. Primary support for human data analyses and mouse studies was provided by NIH Grants NS065780 and AG022074 (L.M.), AG034531 (D.B.D.); gifts from the S.D. Bechtel, Jr. (L.M.) and Coulter-Weeks (D.B.D.) Foundations; a MetLife Foundation Award (L.M.) and American Federation for Aging Award (D.B.D.); and NIH RR18938-01 to the Gladstone Institutes. Additional support, including for assembly and characterization of human cohorts, was provided by NIH Grants AG00001 (C.R.A.), AG18440 and AG010435 (E.M.), AG019712 (M.K.), P50AG23501 and AG19724 (B.L.M.), Hillblom Aging Network (B.L.M.), AG032289 (J.K.), AG025831 and RR00865 (G.W.S.), and AG15819 and AG17917 (D.A.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham CR, Chen C, Cuny GD, Glicksman MA, Zeldich E. Small-molecule Klotho enhancers as novel treatment of neurodegeneration. Future Med Chem. 2012;4:1671–1679. doi: 10.4155/fmc.12.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anggono V, Huganir RL. Regulation of AMPA receptor trafficking and synaptic plasticity. Curr Opin Neurobiol. 2012;22:461–469. doi: 10.1016/j.conb.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arking DE, Atzmon G, Arking A, Barzilai N, Dietz HC. Association between a functional variant of the KLOTHO gene and high-density lipoprotein cholesterol, blood pressure, stroke, and longevity. Circ Res. 2005;96:412–418. doi: 10.1161/01.RES.0000157171.04054.30. [DOI] [PubMed] [Google Scholar]
- Arking DE, Krebsova A, Macek M, Sr., Macek M, Jr., Arking A, Mian IS, Fried L, Hamosh A, Dey S, McIntosh I, et al. Association of human aging with a functional variant of klotho. Proc Natl Acad Sci USA. 2002;99:856–861. doi: 10.1073/pnas.022484299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bading H, Ginty DD, Greenberg ME. Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science. 1993;260:181–186. doi: 10.1126/science.8097060. [DOI] [PubMed] [Google Scholar]
- Brigman JL, Wright T, Talani G, Prasad-Mulcare S, Jinde S, Seabold GK, Mathur P, Davis MI, Bock R, Gustin RM, et al. Loss of GluN2B-containing NMDA receptors in CA1 hippocampus and cortex impairs long-term depression, reduces dendritic spine density, and disrupts learning. J Neurosci. 2010;30:4590–4600. doi: 10.1523/JNEUROSCI.0640-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JA, Terashima KH, Burggren AC, Ercoli LM, Miller KJ, Small GW, Bookheimer SY. Brain network local interconnectivity loss in aging APOE-4 allele carriers. Proc Natl Acad Sci USA. 2011;108:20760–20765. doi: 10.1073/pnas.1109038108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Cui Z, Feng R, Tang YP, Qin Z, Mei B, Tsien JZ. Maintenance of superior learning and memory function in NR2B transgenic mice during ageing. Eur J Neurosci. 2007;25:1815–1822. doi: 10.1111/j.1460-9568.2007.05431.x. [DOI] [PubMed] [Google Scholar]
- Chang PK, Verbich D, McKinney RA. AMPA receptors as drug targets in neurological disease--advantages, caveats, and future outlook. Eur J Neurosci. 2012;35:1908–1916. doi: 10.1111/j.1460-9568.2012.08165.x. [DOI] [PubMed] [Google Scholar]
- Chang Q, Hoefs S, van der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG. The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science. 2005;310:490–493. doi: 10.1126/science.1114245. [DOI] [PubMed] [Google Scholar]
- Chateau MT, Araiz C, Descamps S, Galas S. Klotho interferes with a novel FGF-signalling pathway and insulin/Igf-like signalling to improve longevity and stress resistance in Caenorhabditis elegans. Aging. 2010;2:567–581. doi: 10.18632/aging.100195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci USA. 2007;104:19796–19801. doi: 10.1073/pnas.0709805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CD, Sloane JA, Li H, Aytan N, Giannaris EL, Zeldich E, Hinman JD, Dedeoglu A, Rosene DL, Bansal R, et al. The antiaging protein Klotho enhances oligodendrocyte maturation and myelination of the CNS. J Neurosci. 2013;33:1927–1939. doi: 10.1523/JNEUROSCI.2080-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisse M, Halabisky B, Harris JA, Devidze N, Dubal D, Lotz G, Kim DH, Hamto T, Ho K, Yu G-Q, et al. Reversing EphB2 depletion rescues cognitive functions in Alzheimer model. Nature. 2011;469:47–52. doi: 10.1038/nature09635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jager PL, Shulman JM, Chibnik LB, Keenan BT, Raj T, Wilson RS, Yu L, Leurgans SE, Tran D, Aubin C, et al. A genome-wide scan for common variants affecting the rate of age-related cognitive decline. Neurobiol Aging. 2012;33:1017, e1011–1015. doi: 10.1016/j.neurobiolaging.2011.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Harris SE, Fox HC, Hayward C, Wright AF, Starr JM, Whalley LJ. KLOTHO genotype and cognitive ability in childhood and old age in the same individuals. Neurosci Lett. 2005;378:22–27. doi: 10.1016/j.neulet.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Whiteman MC, Pattie A, Starr JM, Hayward C, Wright AF, Carothers A, Whalley LJ. Cognitive change and the APOE epsilon 4 allele. Nature. 2002;418:932. doi: 10.1038/418932a. [DOI] [PubMed] [Google Scholar]
- Drag LL, Bieliauskas LA. Contemporary review 2009: Cognitive aging. J Geriatr Psychiatry Neurol. 2010;23:75–93. doi: 10.1177/0891988709358590. [DOI] [PubMed] [Google Scholar]
- Duce JA, Podvin S, Hollander W, Kipling D, Rosene DL, Abraham CR. Gene profile analysis implicates Klotho as an important contributor to aging changes in brain white matter of the rhesus monkey. Glia. 2008;56:106–117. doi: 10.1002/glia.20593. [DOI] [PubMed] [Google Scholar]
- Foster KA, McLaughlin N, Edbauer D, Phillips M, Bolton A, Constantine-Paton M, Sheng M. Distinct roles of NR2A and NR2B cytoplasmic tails in long-term potentiation. J Neurosci. 2010;30:2676–2685. doi: 10.1523/JNEUROSCI.4022-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Gladding CM, Raymond LA. Mechanisms underlying NMDA receptor synaptic/extrasynaptic distribution and function. Mol Cell Neurosci. 2011;48:308–320. doi: 10.1016/j.mcn.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Goebel-Goody SM, Davies KD, Alvestad Linger RM, Freund RK, Browning MD. Phospho-regulation of synaptic and extrasynaptic N-methyl-d-aspartate receptors in adult hippocampal slices. Neuroscience. 2009;158:1446–1459. doi: 10.1016/j.neuroscience.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408:255–262. doi: 10.1038/35041700. [DOI] [PubMed] [Google Scholar]
- Harris JA, Devidze N, Verret L, Ho K, Hamto T, Lo I, Yu G-Q, Palop JJ, Masliah E, Mucke L. Transsynaptic progression of amyloid-β-induced neuronal dysfunction within the entorhinal-hippocampal network. Neuron. 2010;68:428–441. doi: 10.1016/j.neuron.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley JM, Wilkinson KA. AMPA receptor trafficking and the mechanisms underlying synaptic plasticity and cognitive aging. Dialogues Clin Neurosci. 2013;15:11–27. doi: 10.31887/DCNS.2013.15.1/jhenley. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, Fujimori T, Nabeshima Y. Secreted Klotho protein in sera and CSF: Implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004;565:143–147. doi: 10.1016/j.febslet.2004.03.090. [DOI] [PubMed] [Google Scholar]
- Imura A, Tsuji Y, Murata M, Maeda R, Kubota K, Iwano A, Obuse C, Togashi K, Tominaga M, Kita N, et al. Science. 2007;316:1615–1618. doi: 10.1126/science.1135901. [DOI] [PubMed] [Google Scholar]
- Invidia L, Salvioli S, Altilia S, Pierini M, Panourgia MP, Monti D, De Rango F, Passarino G, Franceschi C. The frequency of Klotho KL-VS polymorphism in a large Italian population, from young subjects to centenarians, suggests the presence of specific time windows for its effect. Biogerontology. 2010;11:67–73. doi: 10.1007/s10522-009-9229-z. [DOI] [PubMed] [Google Scholar]
- Ittner LM, Ke YD, Delerue F, Bi M, Gladbach A, van Eersel J, Wolfing H, Chieng BC, Christie MJ, Napier IA, et al. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer’s disease mouse models. Cell. 2010;142:387–397. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- Kerchner GA, Nicoll RA. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci. 2008;9:813–825. doi: 10.1038/nrn2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King GD, Chen C, Huang MM, Zeldich E, Brazee PL, Schuman ER, Robin M, Cuny GD, Glicksman MA, Abraham CR. Identification of novel small molecules that elevate Klotho expression. Biochem J. 2012;441:453–461. doi: 10.1042/BJ20101909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima N, Sakamoto T, Endo S, Niki H. Impairment of conditioned freezing to tone, but not to context, in Fyn-transgenic mice: relationship to NMDA receptor subunit 2B function. Eur J Neurosci. 2005;21:1359–1369. doi: 10.1111/j.1460-9568.2005.03955.x. [DOI] [PubMed] [Google Scholar]
- Kubik S, Miyashita T, Guzowski JF. Using immediate-early genes to map hippocampal subregional functions. Learn Mem. 2007;14:758–770. doi: 10.1101/lm.698107. [DOI] [PubMed] [Google Scholar]
- Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Silva AJ. The molecular and cellular biology of enhanced cognition. Nat Rev Neurosci. 2009;10:126–140. doi: 10.1038/nrn2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Jin M, Koeglsperger T, Shepardson NE, Shankar GM, Selkoe DJ. Soluble Aβ oligomers inhibit long-term potentiation through a mechanism involving excessive activation of extrasynaptic NR2B-containing NMDA receptors. J Neurosci. 2011;31:6627–6638. doi: 10.1523/JNEUROSCI.0203-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira II, Schimel D, Kuo CJ, et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–806. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- Luciano M, Hansell NK, Lahti J, Davies G, Medland SE, Raikkonen K, Tenesa A, Widen E, McGhee KA, Palotie A, et al. Whole genome association scan for genetic polymorphisms influencing information processing speed. Biol Psychol. 2011;86:193–202. doi: 10.1016/j.biopsycho.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson KR, Brim BL, Das SR. Selective vulnerabilities of N-methyl-D-aspartate (NMDA) receptors during brain aging. Front Aging Neurosci. 2010;2:11. doi: 10.3389/fnagi.2010.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar V, Nagaraja D, Christopher R. Association of the functional KL-VS variant of Klotho gene with early-onset ischemic stroke. Biochem Biophys Res Commun. 2010;403:412–416. doi: 10.1016/j.bbrc.2010.11.045. [DOI] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Mante M, Crews L, Spencer B, Adame A, Patrick C, Trejo M, Ubhi K, Rohn TT, et al. Passive immunization reduces behavioral and neuropathological deficits in an alpha-synuclein transgenic model of Lewy body disease. PLoS One. 2011;6:e19338. doi: 10.1371/journal.pone.0019338. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mathur P, Graybeal C, Feyder M, Davis MI, Holmes A. Fear memory impairing effects of systemic treatment with the NMDA NR2B subunit antagonist, Ro 25-6981, in mice: Attenuation with ageing. Pharmacol Biochem Behav. 2009;91:453–460. doi: 10.1016/j.pbb.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mony L, Kew JN, Gunthorpe MJ, Paoletti P. Allosteric modulators of NR2B-containing NMDA receptors: Molecular mechanisms and therapeutic potential. Br J Pharmacol. 2009;157:1301–1317. doi: 10.1111/j.1476-5381.2009.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Nagai T, Yamada K, Kim HC, Kim YS, Noda Y, Imura A, Nabeshima Y, Nabeshima T. Cognition impairment in the genetic model of aging klotho gene mutant mice: A role of oxidative stress. FASEB J. 2003;17:50–52. doi: 10.1096/fj.02-0448fje. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, McHugh TJ, Wilson MA, Tonegawa S. NMDA receptors, place cells and hippocampal spatial memory. Nat Rev Neurosci. 2004;5:361–372. doi: 10.1038/nrn1385. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Kandel ER. A macromolecular synthesis-dependent late phase of long-term potentiation requiring cAMP in the medial perforant pathway of rat hippocampal slices. J Neurosci. 1996;16:3189–3198. doi: 10.1523/JNEUROSCI.16-10-03189.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Jones B, Kekonius L, Chin J, Yu G-Q, Raber J, Masliah E, Mucke L. Neuronal depletion of calcium-dependent proteins in the dentate gyrus is tightly linked to Alzheimer’s disease-related cognitive deficits. Proc Natl Acad Sci USA. 2003;100:9572–9577. doi: 10.1073/pnas.1133381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P, Neyton J. NMDA receptor subunits: Function and pharmacology. Curr Opin Pharmacol. 2007;7:39–47. doi: 10.1016/j.coph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Piggott MA, Perry EK, Perry RH, Court JA. [3H]MK-801 binding to the NMDA receptor complex, and its modulation in human frontal cortex during development and aging. Brain Res. 1992;588:277–286. doi: 10.1016/0006-8993(92)91586-4. [DOI] [PubMed] [Google Scholar]
- Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri C. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimers Dement. 2013;9:63–75 e62. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Ritchey M. Two cortical systems for memory-guided behaviour. Nat Rev Neurosci. 2012;13:713–726. doi: 10.1038/nrn3338. [DOI] [PubMed] [Google Scholar]
- Ray JW, Shadish WR. How interchangeable are different estimators of effect size? J Consult Clin Psychol. 1996;64:1316–1325. doi: 10.1037//0022-006x.64.6.1316. [DOI] [PubMed] [Google Scholar]
- Razzaque MS. The FGF23-Klotho axis: Endocrine regulation of phosphate homeostasis. Nat Rev Endocrinol. 2009;5:611–619. doi: 10.1038/nrendo.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwood K. Size of the treatment effect on cognition of cholinesterase inhibition in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2004;75:677–685. doi: 10.1136/jnnp.2003.029074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba RD, Cappola AR, Sun K, Bandinelli S, Dalal M, Crasto C, Guralnik JM, Ferrucci L. Plasma klotho and mortality risk in older community-dwelling adults. J Gerontol A Biol Sci Med Sci. 2011;66:794–800. doi: 10.1093/gerona/glr058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba RD, Moghekar AR, Hu J, Sun K, Turner R, Ferrucci L, O’Brien R. Klotho in the cerebrospinal fluid of adults with and without Alzheimer’s disease. Neuroscience letters. 2014;558:37–40. doi: 10.1016/j.neulet.2013.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri S, DeStefano AL, Au R, Massaro JM, Beiser AS, Kelly-Hayes M, Kase CS, D’Agostino RB, Sr., Decarli C, Atwood LD, et al. Genetic correlates of brain aging on MRI and cognitive test measures: A genome-wide association and linkage analysis in the Framingham Study. BMC Med Genet. 2007;8(Suppl 1):S15. doi: 10.1186/1471-2350-8-S1-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozaki M, Yoshimura K, Shibata M, Koike M, Matsuura N, Uchiyama Y, Gotow T. Morphological and biochemical signs of age-related neurodegenerative changes in klotho mutant mice. Neuroscience. 2008;152:924–941. doi: 10.1016/j.neuroscience.2008.01.032. [DOI] [PubMed] [Google Scholar]
- Shiraki-Iida T, Aizawa H, Matsumura Y, Sekine S, Iida A, Anazawa H, Nagai R, Kuro-o M, Nabeshima Y. Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett. 1998;424:6–10. doi: 10.1016/s0014-5793(98)00127-6. [DOI] [PubMed] [Google Scholar]
- Smith M, Wells J, Borrie M. Treatment effect size of memantine therapy in Alzheimer disease and vascular dementia. Alzheimer Dis Assoc Disord. 2006;20:133–137. doi: 10.1097/00002093-200607000-00002. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE, LeDoux JE. Acquisition of fear extinction requires activation of NR2B-containing NMDA receptors in the lateral amygdala. Neuropsychopharmacology. 2007;32:1929–1940. doi: 10.1038/sj.npp.1301316. [DOI] [PubMed] [Google Scholar]
- Sze C, Bi H, Kleinschmidt-DeMasters BK, Filley CM, Martin LJ. N-Methyl-D-aspartate receptor subunit proteins and their phosphorylation status are altered selectively in Alzheimer’s disease. J Neurol Sci. 2001;182:151–159. doi: 10.1016/s0022-510x(00)00467-6. [DOI] [PubMed] [Google Scholar]
- Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- Tucker Zhou TB, King GD, Chen C, Abraham CR. Biochemical and functional characterization of the Klotho-VS polymorphism implicated in aging and disease risk. J Biol Chem. 2013;288:36302–36311. doi: 10.1074/jbc.M113.490052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol. 2011;10:241–252. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Cui Z, Zeng Q, Kuang H, Wang LP, Tsien JZ, Cao X. Genetic enhancement of memory and long-term potentiation but not CA1 long-term depression in NR2B transgenic rats. PLoS One. 2009;4:e7486. doi: 10.1371/journal.pone.0007486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Stradtman GG, 3rd, Wang XJ, Gao WJ. A specialized NMDA receptor function in layer 5 recurrent microcircuitry of the adult rat prefrontal cortex. Proc Natl Acad Sci USA. 2008;105:16791–16796. doi: 10.1073/pnas.0804318105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SH, Morris RG. Hippocampal-neocortical interactions in memory formation, consolidation, and reconsolidation. Annu Rev Psychol. 2010;61:49–79. C41–44. doi: 10.1146/annurev.psych.093008.100523. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Cauley J, Sands L, Browner W. Apolipoprotein E phenotype and cognitive decline in a prospective study of elderly community women. Arch Neurol. 1997;54:1110–1114. doi: 10.1001/archneur.1997.00550210044011. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Imura A, Urakawa I, Shimada T, Murakami J, Aono Y, Hasegawa H, Yamashita T, Nakatani K, Saito Y, et al. Establishment of sandwich ELISA for soluble alpha-Klotho measurement: Age-dependent change of soluble alpha-Klotho levels in healthy subjects. Biochem Biophys Res Commun. 2010;398:513–518. doi: 10.1016/j.bbrc.2010.06.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Trepanier C, Sidhu B, Xie YF, Li H, Lei G, Salter MW, Orser BA, Nakazawa T, Yamamoto T, et al. Metaplasticity gated through differential regulation of GluN2A versus GluN2B receptors by Src family kinases. EMBO J. 2012;31:805–816. doi: 10.1038/emboj.2011.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashiro K, Philpot BD. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology. 2008;55:1081–1094. doi: 10.1016/j.neuropharm.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Rosenke R, Kronemann D, Brim B, Das SR, Dunah AW, Magnusson KR. The effects of aging on N-methyl-D-aspartate receptor subunits in the synaptic membrane and relationships to long-term spatial memory. Neuroscience. 2009;162:933–945. doi: 10.1016/j.neuroscience.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.