Abstract
Human speech and language underlie many aspects of social behavior and thus understanding their ultimate evolutionary function and proximate genetic and neural mechanisms is a fundamental goal in neuroscience. Mouse ultrasonic vocalizations have recently received enormous attention as possible models for human speech. This attention has raised the question of whether these vocalizations are learned and what roles they play in communication. In this review, we first discuss recent evidence that ultrasonic vocalizations are not learned. We then review current evidence addressing how adult vocalizations may communicate courtship, territorial and/or other information. While there is growing evidence for these signals to play important roles in communication, many important questions remain unanswered.
Keywords: vocal learning, courtship
Introduction
Many animals, including humans, live in complex social structures that are developed and maintained through the use of communication. Communication is the process by which an individual sends a signal that alters the receiver’s behavior. These signals are important for conveying diverse types of information, including membership in a group, social status, predator presence, fitness and willingness to mate. In addition, communication is important for facilitating cooperative behavior. This is particularly true in human language, which is an extreme example of the richness of communication signals that underlie social behavior. Because communication underlies so much of social behavior, understanding its underlying evolutionary, genetic and neural mechanisms is a fundamental scientific goal.
Communication can employ all sensory modalities, yet acoustic communication is one of the most ubiquitous and important across animal species. Many animals use a rich repertoire of vocalizations to communicate different types of information. In humans, speech and language are so fundamental for communication that speech and/or language disorders can have dramatic impact on the social behavior and well being of those afflicted and their families [reviewed in 1]. Thus, understanding the mechanisms underlying human speech and communication disorders is a key focus of current research in neuroscience.
Addressing the proximate mechanisms underlying the learning, production and processing of communication signals requires an animal model that can be manipulated in ways that are impossible or unethical in humans. Diverse rodent species use acoustic signals in social settings [2], but because of the power of genetics, mice (Mus musculus) have become one of the most important laboratory models for exploring the genetic and neural mechanisms underlying acoustic communication and associated disorders [3]. Mice emit a variety of vocalizations in different social contexts [reviewed in 4] and these change with particular social and genetic manipulations. For example, mutating particular genes related to autism spectrum disorder leads to altered vocalization behavior [5–9]. These studies provide intriguing evidence that mice can indeed be good models for understanding mechanisms of human communication and associated disorders. However, there is still some debate as to which areas of communication research will most benefit from studies using mice, and which areas may require other model systems. In this review, we first address the question of vocal learning in mice and then address the role of mouse vocalizations in communication.
Are mouse vocalizations learned?
A fundamental feature of human speech is that it is learned through imitation, one of several types of vocal learning [10]. Humans copy the sounds made by other individuals, underlying the cultural transmission of language and accents. There is evidence against imitative vocal learning in non-human primates [reviewed in 11] and some evidence for imitative vocal learning in a small number of non-human mammalian groups (see other chapters in this issue). On the other hand, thousands of species of songbirds exhibit imitative vocal learning of their songs [12–14]. While it has been known for several decades that male mice make ultrasonic vocalizations during social interactions [2,15,16], Holy and Guo [17] were the first to describe them as “song”. The use of this term catalyzed the study of whether those vocalizations are learned in a fashion similar to bird songs.
In evaluating the capacity for various species to learn their vocalizations, it is important to consider the types of experimental evidence that bear most strongly on this question. The strongest evidence that would support imitative learning comes from imitation of arbitrary sounds experienced earlier in life. Experimentally, this can be tested by rearing mice with foster parents of a different strain (cross-fostering) or co-housing adults of different strains. Two experiments have taken this approach, with conflicting results. Housing different strains of adult mice together led to small shifts in the frequency of some syllables emitted by C57Bl/6J mice toward those of a cagemate male of the B6D2F1/J [BxD] strain [18]. Considering that the shifts in frequency observed were small and could have occurred in response to aggressive or other social cues, this study does not provide convincing evidence for imitative vocal learning in mice. Kikusui et al. [19] cross-fostered two strains of mice with different vocal parameters and found that the mice reared in this fashion emitted vocalizations with acoustic parameters characteristic of their own strain. Thus, neither of these studies provided evidence for vocal imitation in mice.
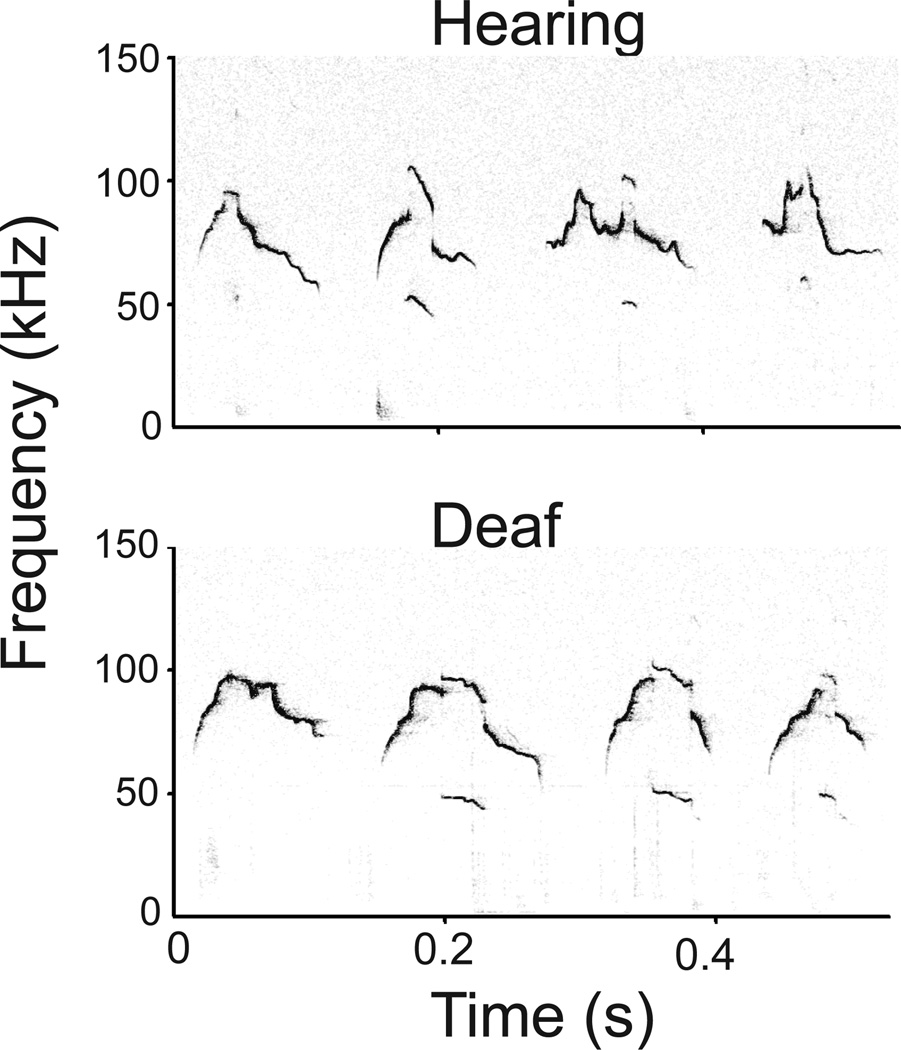
Because any imitation of acoustic behaviors would, by definition, require hearing, the strongest evidence against vocal learning would come from denying animals acoustic experience and finding that their vocalizations are normal. Two recent studies obtained this result in mice. Hammerschmidt et al., [20] showed that vocalizations emitted by mice lacking the gene for otoferlin, (a protein essential for synaptic transmission in hair cells and thus for hearing) were not acoustically different from those emitted by normal hearing mice. Mahrt et al. [21] also compared acoustic parameters of vocalizations emitted by deaf and normal hearing male mice. They used a strain of transgenic mice in which hair cells could be killed prior to the onset of hearing. Selectively preventing acoustic experience did not affect the acoustic features of different syllable types or the temporal features of sequences of vocalizations. Examples of vocalizations from hearing and deaf mice (Fig. 1) illustrate that hearing experience is not required for the production of normal vocalizations as adults.
Figure 1.
Example spectrograms of vocalizations emitted by hearing and deaf adult male mice during interactions with a female. The vocalizations produced by hearing and deaf mice are similar, both qualitatively, and quantitatively as demonstrated by Mahrt et al., (2013).
In contrast, Arriaga et al. [18] suggested that there is vocal learning in mice based on two results with deaf mice. They found that vocalizations from surgically-deafened adult mice gradually increased in frequency over eight months, and that vocalizations from congenitally deaf mice (due to caspase-3 gene knock-out) were somewhat altered. Each of these hints of vocal learning, however, comes with major issues of interpretation. Shifts in acoustic parameters with age or other types of learning such as operant conditioning occur in non-vocal learners [22] and do not provide evidence for imitative vocal learning. In addition, caspase-3 knockout animals have abnormal brain morphology [23,24], which could itself lead to altered vocalizations, independent of any possible vocal learning.
Overall, there is convincing evidence that mice are not imitative vocal learners. Consequently, mice are not a good model for studying the mechanisms of vocal learning. Their lack of learning, however, makes them a potentially strong model system for studying the genetic basis of vocal communication. Specifically, mice will provide insights into the circuitry underlying normal vocal production and processing. They may also shed light on the genetic basis of human communication disorders, for example, autism spectrum disorder [5,9,25]. A critical question that needs to be addressed to increase the utility of such studies is what roles mouse ultrasonic vocalizations play in communication.
What roles do ultrasonic vocalizations play in communication?
It is well documented that rodents emit a diverse repertoire of ultrasonic vocalizations in different social contexts. Pups emit a large number of ultrasonic vocalizations when isolated from the nest [26–31]. Because these isolation calls elicit retrieval behavior in the mother [33–35], it is clear that they are communication signals that serve a particular purpose. The communicative role of adult mouse ultrasonic vocalizations, however, is less clear. A number of hypotheses have been proposed, with varying degrees of support. The vocalizations emitted by female mice when they encounter an unknown female intruder and by socially isolated males when they encounter a male intruder may function in determining social hierarchy and/or territorial boundaries [36–39].
The ultrasonic vocalizations emitted by males in the presence of a female, or female pheromones in urine, have been recorded for decades and ascribed a role in courtship [2,15,17,40–42]. However, recently these male-emitted vocalizations have also been ascribed a territorial function [9,43] suggesting that these vocalizations may be important in a variety of different social contexts.
The evidence that male-emitted vocalizations are signals important for courtship comes from 1) patterns of emission by males and 2) behavioral responses of females. First, vocalizations emitted by males are more abundant in the presence of a female or female urine than in the presence of a male or male urine [42,44]. Moreover, there are changes in male-emitted vocalizations depending on the relative location of the male to the female [45–47]. In particular, when the male is close to or mounting the female, the number of vocalizations increases [40,48] and the syllable types change to include a higher proportion of complex, frequency-jump syllables [45,46]. These changes in the vocalizations during specific behaviors related to courtship may indicate a communicative function during courtship.
Second, phonotaxis experiments have shown that behavioral responses of females are affected by male vocalizations. Pomerantz et al. [49] showed that females spend more time with a vocalizing male than with a devocalized male. In addition, females preferentially approach a speaker playing male vocalizations rather than pup isolation calls or whistle-like sounds [50]. Females also preferentially move to a location to elicit playback of male vocalizations rather than a location eliciting silence [51]. In addition, wild female house mice (genus Mus) preferentially approach a speaker playing non-kin vocalizations rather than kin vocalizations [52]. In Neotropic singing mice (genus Scotinomys) females prefer male vocalizations that have fast temporal features [53]. Overall, the changes in male vocalizations in the presence of females, and the female behavioral responses to those sounds are consistent with the hypothesis that male mouse vocalizations are courtship signals.
In contrast to the idea that male mouse ultrasonic vocalizations are courtship signals, two recent studies have suggested that these vocalizations could be territorial signals. Using the standard intruder paradigm, Hammerschmidt et al., [54] found that the acoustic structure of vocalizations emitted by males in response to an intruder female were not significantly different from those emitted by females in response to a female intruder. Ey and colleagues [9] also found minimal acoustic differences in vocalizations emitted by male and female mice. These similarities suggest that the ultrasonic vocalizations emitted by males are used for more than just courtship and could have different meanings in different social contexts.
It is currently unclear how the vocalizations signal different meaning in different social contexts. One possibility is that there are actual differences in vocalizations emitted in different contexts, but they have not yet been fully identified. Parsing vocalizations based on what the male mouse is doing (e.g. approaching versus mounting a female) has begun to identify differences in acoustic features that may otherwise have been missed [45,46]. Thus, it is vital to continue and extend this combined approach of acoustic and comprehensive behavioral analysis [55,56] in future studies. In addition, detailed examination of the temporal and sequencing aspects of vocalizations bouts could provide insight into how particular patterns of vocalizations have different meanings [57].
A second possibility is that other types of communication signals alter the meaning of a given vocalization. For example, chemosensory cues are used by mice to identify individuals and attract females [58–61], and it is possible that other types of sensory cues could modulate the meaning of particular vocalizations to a receiver. Understanding such multisensory communication will require expanded use of interdisciplinary approaches.
Avenues for Future Studies
There are still many unanswered questions about the communication function of mouse vocalizations. While there is mounting evidence that males use ultrasonic vocalizations for communication, each piece of evidence is correlative. To make a rigorous cause-and-effect link, there needs to be evidence that these vocalizations actually change the behavior of the individual receiving the signal. For example, a strong test of the role of vocalizations in courtship, though difficult, would involve assaying mating behavior in a large arena in which females have choice over with which male they mate. Another approach would be to explore the possibility of eliciting a behavioral response in female mice corresponding to the copulation solicitation display of birds [62,63], which allows direct assessment of which sounds are effective in promoting sexual receptivity.
Another unanswered question is which features of different vocalizations are attractive to females. Previous phonotaxis experiments have provided evidence for female preference for male-emitted vocalizations over silence or whistle-like artificial sounds [50,51] and for non-kin vocalizations over kin vocalizations [52]. An exciting avenue of future research would be to manipulate the features of the vocalizations (including timing and syllable sequences) to determine which parameters females prefer. It may also be important to explore different assays for female preference. For example, with phonotaxis experiments, it is not clear whether moving towards a sound indicates attraction to that sound or aversion to the other. One alternative approach could involve active playback, extending the approach used in [51], in which the animal actively makes one movement to trigger the playback of one sound and another movement to trigger playback of a different sound. The requirement for active participation of the animal would help to reduce possible alternative interpretations of results from phonotaxis experiments.
As existing technologies are applied to these problems in new combinations, and the power of genetics creates lines of mice with new traits, we anticipate accelerating progress toward understanding the evolutionary, genetic and neural mechanisms underlying acoustic communication and disorders of speech and language.
Highlights.
Mice do not show evidence of imitative vocal learning.
The lack of vocal learning makes the mouse an excellent model system for understanding the genetic basis of human communication disorders.
Mouse ultrasonic vocalizations likely play a role in both courtship and territorial interactions.
Acknowledgement
This work was supported by NSF IOS 1257768 to CVP and NIH R01 MH066128 to DJP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arlinger S. Negative consequences of uncorrected hearing loss--a review. Int J Audiol. 2003;42(Suppl 2):2S17–2S20. [PubMed] [Google Scholar]
- 2.Sales G, Pye D. Ultrasonic communication by animals. London: Chapman and Hall; New York: distributed in the U. S. by Halsted Press; 1974. [Google Scholar]
- 3.Fischer J, Hammerschmidt K. Ultrasonic vocalizations in mouse models for speech and socio-cognitive disorders: insights into the evolution of vocal communication. Genes Brain Behav. 2011;10:17–27. doi: 10.1111/j.1601-183X.2010.00610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Portfors CV. Types and functions of ultrasonic vocalizations in laboratory rats and mice. J Am Assoc Lab Anim Sci. 2007;46:28–34. [PubMed] [Google Scholar]
- 5.Scattoni ML, Gandhy SU, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS One. 2008;3:e3067. doi: 10.1371/journal.pone.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jamain S, Radyushkin K, Hammerschmidt K, Granon S, Boretius S, Varoqueaux F, Ramanantsoa N, Gallego J, Ronnenberg A, Winter D, et al. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc Natl Acad Sci U S A. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scattoni ML, Martire A, Cartocci G, Ferrante A, Ricceri L. Reduced social interaction, behavioural flexibility and BDNF signalling in the BTBR T+ tf/J strain, a mouse model of autism. Behav Brain Res. 2013;251:35–40. doi: 10.1016/j.bbr.2012.12.028. [DOI] [PubMed] [Google Scholar]
- 8.Wohr M, Silverman JL, Scattoni ML, Turner SM, Harris MJ, Saxena R, Crawley JN. Developmental delays and reduced pup ultrasonic vocalizations but normal sociability in mice lacking the postsynaptic cell adhesion protein neuroligin2. Behav Brain Res. 2013;251:50–64. doi: 10.1016/j.bbr.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ey E, Torquet N, Le Sourd AM, Leblond CS, Boeckers TM, Faure P, Bourgeron T. The Autism ProSAP1/Shank2 mouse model displays quantitative and structural abnormalities in ultrasonic vocalisations. Behav Brain Res. 2013;256:677–689. doi: 10.1016/j.bbr.2013.08.031.. This article characterized ultrasonic vocalizations of ProSAP1/Shank2−/− mice lacking a synaptic scaffold protein mutated in autism spectrum disorder. Alterations in vocalization behavior emerged only in adulthood and consisted of reduced rates of vocalizations, shorter sequences of syllables and a decrease in frequency. The results suggest this mouse model is a useful system for studying autism spectrum disorders.
- 10.Janik VM, Slater PJ. The different roles of social learning in vocal communication. Anim Behav. 2000;60:1–11. doi: 10.1006/anbe.2000.1410. [DOI] [PubMed] [Google Scholar]
- 11.Hammerschmidt K, Fischer J. Constraints in primate vocal production. In: Griebel UaO K, editor. The evolution of communication creativity: from fixed signals to contextual flexibility. The MIT Press; 2008. pp. 93–119. [Google Scholar]
- 12.Brenowitz EA, Beecher MD. Song learning in birds: diversity and plasticity, opportunities and challenges. Trends Neurosci. 2005;28:127–132. doi: 10.1016/j.tins.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Zeigler H, Marler P, editors. Behavioral neurobiology of birdsong. New York: New York Academy of Sciences; 2004. [Google Scholar]
- 14.Zeigler H, Marler P, editors. Neuroscience of birdsong. Cambridge, England, UK: Cambridge University Press; 2008. [Google Scholar]
- 15.Whitney G, Coble JR, Stockton MD, Tilson EF. Ultrasonic emissions: do they facilitate courtship of mice. J Comp Physiol Psychol. 1973;84:445–452. doi: 10.1037/h0034899. [DOI] [PubMed] [Google Scholar]
- 16.Nyby J, Dizinno G, Whitney G. Sexual dimorphism in ultrasonic vocalizations of mice (Mus musculus): gonadal hormone regulation. J Comp Physiol Psychol. 1977;91:1424–1431. doi: 10.1037/h0077411. [DOI] [PubMed] [Google Scholar]
- 17.Holy TE, Guo Z. Ultrasonic songs of male mice. PLoS Biol. 2005;3:e386. doi: 10.1371/journal.pbio.0030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arriaga G, Zhou EP, Jarvis ED. Of mice, birds, and men: the mouse ultrasonic song system has some features similar to humans and song-learning birds. PLoS One. 2012;7:e46610. doi: 10.1371/journal.pone.0046610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kikusui T, Nakanishi K, Nakagawa R, Nagasawa M, Mogi K, Okanoya K. Cross fostering experiments suggest that mice songs are innate. PLoS One. 2011;6:e17721. doi: 10.1371/journal.pone.0017721.. This is the first study to provide evidence that mice do not learn their vocalizations. Two strains of mice were cross-fostered, and the pups showed no evidence of learning features of the ultrasonic vocalizations of the foster parents.
- 20. Hammerschmidt K, Reisinger E, Westekemper K, Ehrenreich L, Strenzke N, Fischer J. Mice do not require auditory input for the normal development of their ultrasonic vocalizations. BMC Neurosci. 2012;13:40. doi: 10.1186/1471-2202-13-40.. This study examined the pup and adult vocalizations from mice lacking the otoferlin gene, which encodes for an essential component of synaptic transmission from hair cells. These mutant mice were deaf, but their vocalizations did not differ from those of wild-type or heterozygous littermates.
- 21. Mahrt EJ, Perkel DJ, Tong L, Rubel EW, Portfors CV. Engineered deafness reveals that mouse courtship vocalizations do not require auditory experience. J Neurosci. 2013;33:5573–5583. doi: 10.1523/JNEUROSCI.5054-12.2013.. This study shows that mice do not learn their vocalizations through imitation. Mice were engineered to express the diphtheria toxin receptor in hair cells, allowing the mice to be deafened before the onset of hearing through a systemic injection of diphtheria toxin. When adult male mice that had been raised with no auditory experience through this method were placed in the presence of a female, their vocalizations had similar acoustics, timing and sequencing as their siblings with normal hearing.
- 22.Ey E, Pfefferle D, Fischer J. Do age- and sex-related variations reliably reflect body size in non-human primate vocalizations? A review. Primates. 2007;48:253–267. doi: 10.1007/s10329-006-0033-y. [DOI] [PubMed] [Google Scholar]
- 23.Kuida K, Zheng TS, Na S, Kuan C, Yang D, Karasuyama H, Rakic P, Flavell RA. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature. 1996;384:368–372. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi K, Kamiya K, Urase K, Suga M, Takizawa T, Mori H, Yoshikawa Y, Ichimura K, Kuida K, Momoi T. Caspase-3-deficiency induces hyperplasia of supporting cells and degeneration of sensory cells resulting in the hearing loss. Brain Res. 2001;894:359–367. doi: 10.1016/s0006-8993(01)02123-0. [DOI] [PubMed] [Google Scholar]
- 25.Schmeisser MJ, Ey E, Wegener S, Bockmann J, Stempel AV, Kuebler A, Janssen AL, Udvardi PT, Shiban E, Spilker C, et al. Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature. 2012;486:256–260. doi: 10.1038/nature11015. [DOI] [PubMed] [Google Scholar]
- 26.Brudzynski SM, Kehoe P, Callahan M. Sonographic structure of isolation-induced ultrasonic calls of rat pups. Dev Psychobiol. 1999;34:195–204. doi: 10.1002/(sici)1098-2302(199904)34:3<195::aid-dev4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 27.Liu RC, Miller KD, Merzenich MM, Schreiner CE. Acoustic variability and distinguishability among mouse ultrasound vocalizations. J Acoust Soc Am. 2003;114:3412–3422. doi: 10.1121/1.1623787. [DOI] [PubMed] [Google Scholar]
- 28.Hashimoto H, Moritani N, Aoki-Komori S, Tanaka M, Saito T. Comparison of ultrasonic vocalizations emitted by rodent pups. Exp Anim. 2004;53:409–416. doi: 10.1538/expanim.53.409. [DOI] [PubMed] [Google Scholar]
- 29.D'Amato FR, Scalera E, Sarli C, Moles A. Pups call, mothers rush: does maternal responsiveness affect the amount of ultrasonic vocalizations in mouse pups? Behav Genet. 2005;35:103–112. doi: 10.1007/s10519-004-0860-9. [DOI] [PubMed] [Google Scholar]
- 30.Ehret G. Infant rodent ultrasounds -- a gate to the understanding of sound communication. Behav Genet. 2005;35:19–29. doi: 10.1007/s10519-004-0853-8. [DOI] [PubMed] [Google Scholar]
- 31.Sewell GD. Ultrasonic communication in rodents. Nature. 1970;227:410. doi: 10.1038/227410a0. [DOI] [PubMed] [Google Scholar]
- 32.Koch M, Ehret G. Estradiol and parental experience, but not prolactin are necessary for ultrasound recognition and pup-retrieving in the mouse. Physiol Behav. 1989;45:771–776. doi: 10.1016/0031-9384(89)90293-x. [DOI] [PubMed] [Google Scholar]
- 33.Ehret G, Haack B. Categorical perception of mouse pup ultrasound by lactating females. Naturwissenschaften. 1981;68:208–209. doi: 10.1007/BF01047208. [DOI] [PubMed] [Google Scholar]
- 34.Ehret G, Haack B. Ultrasound recognition in house mce - key-stimulus configuration and recognition mechanism. J Comp Physiol. 1982;148:245–251. [Google Scholar]
- 35.Ehret G, Haack B. Motivation and arousal influence sound-induced maternal pup-retrieving behavior in lactating house mice. Z Tierpsychol. 1984;65:25–39. [Google Scholar]
- 36.Maggio JC, Whitney G. Ultrasonic vocalizing by adult female mice (Mus musculus) J Comp Psychol. 1985;99:420–436. [PubMed] [Google Scholar]
- 37.D'Amato FR, Moles A. Ultrasonic vocalizations as an index of social memory in female mice. Behav Neurosci. 2001;115:834–840. doi: 10.1037//0735-7044.115.4.834. [DOI] [PubMed] [Google Scholar]
- 38.Chabout J, Serreau P, Ey E, Bellier L, Aubin T, Bourgeron T, Granon S. Adult male mice emit context-specific ultrasonic vocalizations that are modulated by prior isolation or group rearing environment. PLoS One. 2012;7:e29401. doi: 10.1371/journal.pone.0029401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moles A, Costantini F, Garbugino L, Zanettini C, D'Amato FR. Ultrasonic vocalizations emitted during dyadic interactions in female mice: a possible index of sociability? Behav Brain Res. 2007;182:223–230. doi: 10.1016/j.bbr.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 40.Sales GD. Ultrasound and aggressive behaviour in rats and other small mammals. Anim Behav. 1972;20:88–100. doi: 10.1016/s0003-3472(72)80177-5. [DOI] [PubMed] [Google Scholar]
- 41.Dizinno G, Whitney G, Nyby J. Ultrasonic vocalizations by male mice (Mus musculus) to female sex pheromone: experimental determinants. Behav Biol. 1978;22:104–113. [Google Scholar]
- 42.Nyby J, Wysocki CJ, Whitney G, Dizinno G, Schneider J. Elicitation of male mouse (Mus musculus) ultrasonic vocalizations I. Urinary cues. J Comp Physiol Psychol. 1979;93:957–975. [Google Scholar]
- 43.Hammerschmidt K, Jurgens U. Acoustical correlates of affective prosody. J Voice. 2007;21:531–540. doi: 10.1016/j.jvoice.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 44.Guo Z, Holy TE. Sex selectivity of mouse ultrasonic songs. Chem Senses. 2007;32:463–473. doi: 10.1093/chemse/bjm015. [DOI] [PubMed] [Google Scholar]
- 45. Hanson JL, Hurley LM. Female presence and estrous state influence mouse ultrasonic courtship vocalizations. PLoS One. 2012;7:e40782. doi: 10.1371/journal.pone.0040782.. This article reported that male mice change the pattern of vocalizations they emit depending on how they are interacting with the female. Males emit syllable types with higher levels of complexity during mounting than during other types of interactions.
- 46.Wang H, Liang S, Burgdorf J, Wess J, Yeomans J. Ultrasonic vocalizations induced by sex and amphetamine in M2, M4, M5 muscarinic and D2 dopamine receptor knockout mice. PLoS One. 2008;3:e1893. doi: 10.1371/journal.pone.0001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang M, Loureiro D, Kalikhman D, Crawley JN. Male mice emit distinct ultrasonic vocalizations when the female leaves the social interaction arena. Front Behav Neurosci. 2013;7:159. doi: 10.3389/fnbeh.2013.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nyby J. Ultrasonic vocalizations during sex behavior of male house mice (Mus musculus): a description. Behav Neural Biol. 1983;39:128–134. doi: 10.1016/s0163-1047(83)90722-7. [DOI] [PubMed] [Google Scholar]
- 49.Pomerantz SM, Nunez AA, Bean NJ. Female behavior is affected by male ultrasonic vocalizations in house mice. Physiol Behav. 1983;31:91–96. doi: 10.1016/0031-9384(83)90101-4. [DOI] [PubMed] [Google Scholar]
- 50.Hammerschmidt K, Radyushkin K, Ehrenreich H, Fischer J. Female mice respond to male ultrasonic 'songs' with approach behaviour. Biol Lett. 2009;5:589–592. doi: 10.1098/rsbl.2009.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shepard KN, Liu RC. Experience restores innate female preference for male ultrasonic vocalizations. Genes Brain Behav. 2011;10:28–34. doi: 10.1111/j.1601-183X.2010.00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Musolf K, Hoffmann F, Penn D. Ultrasonic courtship vocalizations in wild house mice, Mus musculus musculus. Anim Behav. 2010;79:757–764. [Google Scholar]
- 53.Pasch B, George AS, Hamlin HJ, Guillette LJ, Jr, Phelps SM. Androgens modulate song effort and aggression in Neotropical singing mice. Horm Behav. 2011;59:90–97. doi: 10.1016/j.yhbeh.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 54. Hammerschmidt K, Radyushkin K, Ehrenreich H, Fischer J. The structure and usage of female and male mouse ultrasonic vocalizations reveal only minor differences. PLoS One. 2012;7:e41133. doi: 10.1371/journal.pone.0041133.. This study suggests that vocalizations emitted by males may serve a territorial function rather than solely a courtship function. Using a standard intruder paradigm, the vocalizations emitted by male mice in the presence of a female were not significantly different than those emitted by a female mouse towards another female.
- 55. de Chaumont F, Coura RD, Serreau P, Cressant A, Chabout J, Granon S, Olivo-Marin JC. Computerized video analysis of social interactions in mice. Nat Methods. 2012;9:410–417. doi: 10.1038/nmeth.1924.. This article describes a mouse-tracking method and software that provides position, orientation, distance and speed of the body parts of two interacting mice. The program allows for comprehensive and un-biased monitoring of the behavioral states of two mice in close proximity.
- 56.Ohayon S, Avni O, Taylor AL, Perona P, Roian Egnor SE. Automated multi-day tracking of marked mice for the analysis of social behaviour. J Neurosci Methods. 2013;219:10–19. doi: 10.1016/j.jneumeth.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Templeton CN, Greene E, Davis K. Allometry of alarm calls: black-capped chickadees encode information about predator size. Science. 2005;308:1934–1937. doi: 10.1126/science.1108841. [DOI] [PubMed] [Google Scholar]
- 58.Bowers JM, Alexander BK. Mice: individual recognition by olfactory cues. Science. 1967;158:1208–1210. doi: 10.1126/science.158.3805.1208. [DOI] [PubMed] [Google Scholar]
- 59.Penn D, Potts WK. Untrained mice discriminate MHC-determined odors. Physiol Behav. 1998;64:235–243. doi: 10.1016/s0031-9384(98)00052-3. [DOI] [PubMed] [Google Scholar]
- 60.Binns KE, Brennan PA. Changes in electrophysiological activity in the accessory olfactory bulb and medial amygdala associated with mate recognition in mice. Eur J Neurosci. 2005;21:2529–2537. doi: 10.1111/j.1460-9568.2005.04090.x. [DOI] [PubMed] [Google Scholar]
- 61.Arakawa H, Blanchard DC, Arakawa K, Dunlap C, Blanchard RJ. Scent marking behavior as an odorant communication in mice. Neurosci Biobehav Rev. 2008;32:1236–1248. doi: 10.1016/j.neubiorev.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.King A, West M. Species identification in the North American Cowbird: Appropriate responses to abnormal song. Science. 1977;195:1002–1004. doi: 10.1126/science.841321. [DOI] [PubMed] [Google Scholar]
- 63.Brenowitz EA. Altered perception of species-specific song by female birds after lesions of a forebrain nucleus. Science. 1991;251:303–305. doi: 10.1126/science.1987645. [DOI] [PubMed] [Google Scholar]