SUMMARY
We examined whether proximity to a major roadway and traffic density around the home during pregnancy are associated with risk of early life respiratory infection in a pre-birth cohort in the Boston area. We geocoded addresses for 1,263 mother-child pairs enrolled during the first trimester of pregnancy in Project Viva during 1999-2002. We calculated distance from home to nearest major roadway and traffic density in a 100 m buffer around the home. We defined respiratory infection as maternal report of >1 doctor-diagnosed pneumonia, bronchiolitis, croup or other respiratory infection from birth until the early childhood visit (median age 3.3). We used relative risk regression models adjusting for potential confounders to estimate associations between traffic exposures and risk of respiratory infection. Distance to roadway during pregnancy was associated with risk of respiratory infection. In fully adjusted models, relative risks (95% CI) for respiratory infection were: 1.30 (1.08, 1.55) for <100 m, 1.15 (0.93, 1.41) for 100 to <200 m, and 0.95 (0.84, 1.07) for 200 to <1000 m compared with living ≥1000 m away from a major roadway. Each interquartile range increase in distance to roadway was associated with an 8% (95% CI 0.87, 0.98) lower risk, and each interquartile range increase in traffic density was associated with a 5% (95% CI 0.98, 1.13) higher risk of respiratory infection. Our findings suggest that living close to a major roadway during pregnancy may predispose the developing lung to infection in early life.
Keywords: Epidemiology, Environmental Lung Disease, Air pollution, Traffic, Prenatal exposures, Respiratory Infection
INTRODUCTION
A number of epidemiologic studies have associated pre- and post-natal exposure to ambient air pollution with adverse respiratory outcomes. Post-natal exposures to air pollutants, including particulate matter (PM), ozone (O3), nitrogen dioxide (NO2), carbon monoxide (CO), and sulfur dioxide (SO2), have been associated with increased infant mortality, even in developed countries such as the United States, with the strongest associations for post-neonatal respiratory mortality1–4.
Although a number of studies report increased risks of respiratory symptoms in early life in association with air pollution exposure5,6, relatively few studies have specifically examined early life respiratory infection (the leading cause of infant morbidity) in relation to traffic-related emissions. Although some case control and emergency room visit studies suggest that exposure to traffic-related pollutants may increase risk of early life infection, the number of studies is small and these studies have relied on administrative data to assess health outcomes and regional monitors to estimate individual exposures7,8. Using GIS as a tool for estimating individual exposure in a well-characterized longitudinal birth cohort in the Boston area, we tested the hypothesis that greater pre- and post-natal exposure to traffic-related pollution increases risk of doctor-diagnosed respiratory infection in early childhood. We evaluated traffic-related pollution exposure based on the shortest distance from the home to a major roadway and traffic density around the home.
MATERIALS AND METHODS
Study Population
Study subjects were participants in Project Viva, a prospective, pre-birth cohort study that recruited women during early pregnancy from Harvard Pilgrim Medical Associates, a multi-specialty group practice in eastern Massachusetts. In our primary analysis, we included only participants with a recorded address at study enrollment and who had questionnaire data after birth indicating whether the child had a diagnosed respiratory infection by the early childhood visit (N = 1,263 participants, median age 3.3). Subjects were enrolled between April 1999 and July 2002. Detailed enrollment criteria have been described previously9. Briefly, pregnant women were approached at their initial prenatal visit. Exclusion criteria included multiple gestation, inability to answer questions in English, plans to move out of the area before delivery, and gestational age >22 weeks at initial prenatal clinic appointment. All mothers provided written consent for themselves and their child. This study was approved by the institutional review boards of Brigham and Women’s Hospital and Harvard Pilgrim Health Care.
Exposure Assessment
Each subject’s address at study enrollment and time of delivery was geocoded using ArcGIS (ESRI, Redlands, CA). U.S. neighborhood-level census data, including median household income and percent with at least a high school education, was assigned from the tract-level 2000 U.S. Census. Distance to the nearest A1 or A2 road (U.S. Census Features Class) was calculated as the distance to the nearest roadway from the home address at the time of study enrollment (1st trimester visit) and at the time of delivery. Based on published findings that traffic-related pollutants decay to background levels exponentially with distance from a freeway10,11, we examined associations with respiratory infection using categories of distance (<100 m, 100 to <200 m, 200 to <1000 m, and ≥1000 m) and the natural log of proximity to a major roadway. In our previous work, we have found that residential proximity to a major class A1 or A2 roadway using these distance categories and also the natural log of distance to roadway were associated with mortality in adults12.
Traffic density was examined in separate models from distance to roadway and was calculated for the 100 m buffer zone around each home address as the number of vehicles x road length (km) per day at home address at the time of study enrollment (1st trimester visit) and at the time of delivery. Daily traffic counts for each road were measured or estimated in 2002 for each road in the state by the Massachusetts Department of Transportation. Road length in the 100 m buffer was calculated using GIS software. We examined the log of traffic density in association with risk of respiratory infection, consistent with previous studies of traffic density and health outcomes13. There were 14 observations with an estimated traffic density of 0 that were excluded from the analysis.
Questionnaire and Interview Data
Questionnaire and/or interview data were obtained from the mother at study enrollment during the first prenatal visit, by questionnaire when the child reached 6 months, 1, and 2 years of age, and by interview at the early childhood visit (median age 3.3). Information was obtained about the child’s health (including diagnosis of respiratory infection), demographics, smoking, breastfeeding, and the home environment. Each child was categorized as having had an acute respiratory infection if there was at least one parental report of a diagnosis of bronchiolitis, pneumonia, bronchitis, croup or other respiratory infection since the child’s birth (at age 1) or last birthday (at ages 2 and 3) by a health care professional (e.g., doctor, physician assistant or nurse practitioner) on an annual questionnaire at age 1 or 2 or the early childhood interview (median age 3.3). The diagnosis of respiratory infection did not include colds, ears infections or sinusitis, which were asked about separately on the questionnaire and interview. Participants who reported a diagnosis of acute respiratory infection at least once were included in the primary analysis even if one or more questionnaires or interviews were missing on other years. Participants who did not report a respiratory infection diagnosis and who had missing outcome data on at least one annual questionnaire or interview were excluded from the primary analysis.
Statistical Analysis
The associations between exposures (proximity to major roadway and traffic density during the first trimester) and respiratory infection (a binary outcome) were analyzed using relative risk regression, adjusting for potential confounders that were selected a priori based on known associations with air pollution and/or risk of respiratory infection. We directly estimated relative risk rather than odds of respiratory infection because the outcome was common (affecting 53.4% of participants) and therefore the odds ratio would not closely approximate relative risk of infection. Models were fit using the PROC GENMOD procedure using SAS 9.2 software with a log link, Poisson distribution and robust standard errors (SAS Institute, Cary, NC). This has been shown to be a consistent estimator of the relative risk of a binary outcome, using robust standard errors to account for the fact that the data are not Poisson distributed14,15. We first ran parsimonious models adjusting only for maternal race/ethnicity, infant sex, season of birth, and time. The fully adjusted model adjusted for the these covariates and also maternal education, smoking during pregnancy, months of any breastfeeding, smokers in the household after birth, presence of at least one child under age 12 in the household, census tract income (quartile), census tract education, gestational age, birth weight for gestational age z-score (based on US nationality data16) and childcare attendance. We used sine and cosine functions of the date of birth to estimate the amplitude and phase of the seasonal cycle. We adjusted for time as a continuous linear variable using the date of the last menstrual period.
We performed several sensitivity analyses on our data. To account for potential bias arising from excluding participants in whom it was unknown (due to missing outcome data) whether at least 1 respiratory infection was diagnosed by the early childhood visit, we performed a sensitivity analysis including only those participants who completed all 3 questionnaires/interviews at ages 1, 2 and 3 (N = 1,137 participants compared to 1,263 in the primary analysis) and a last-observation-carried-forward sensitivity analysis assigning the last reported respiratory infection status for those with missing outcome data (N = 1,369 participants). Since 10% of the cohort moved between the first trimester and the time of birth, we examined the associations of distance to roadway and traffic density with respiratory infection using the home address at the time of delivery instead of the first trimester visit. We excluded gestational age and birth weight for gestational age from our models in a sensitivity analysis to evaluate whether the observed associations with respiratory infection might be mediated by pre-term birth and intra-uterine growth restriction, as a number of studies have found associations between air pollution exposure and both gestational age and birth weight17,18. We evaluated the linearity of the relationships between the natural log of distance to roadway and traffic density with risk of respiratory infection using restricted cubic splines with knots at the 5, 27.5, 50, 72.5, and 95th percentiles of the distribution19 and compared the fit of these models to the linear models using likelihood ratio tests.
We investigated whether associations between exposure to traffic-related pollution and risk of respiratory infection varied according to infant sex, preterm birth (<37 weeks, 7.0% of the cohort), maternal smoking during pregnancy (9.7% of the cohort) and census tract income quartile. We tested for statistical interaction of associations of the natural log of distance to roadway and traffic density with risk of respiratory infection by these characteristics using cross-product terms and determined statistical significance from the p-value from the Wald test of the cross-product.
RESULTS
Participant characteristics stratified by distance to nearest A1/A2 roadway are summarized in Table 1. The mothers were predominantly of white race (75% in total), with a higher proportion of white mothers (79%) living furthest from a major roadway. The mothers were overall well-educated: 73% were college graduates. Maternal smoking during pregnancy was rare in all groups, though smoking during pregnancy was slightly more common in the group living closest to a major roadway (12.5% vs 8.5 to 10.1% in the other groups). The average duration of any breastfeeding was more than 6 months for mother-infant pairs in all distance to roadway categories. Fewer than 20% of households had a smoker after the birth of the child in all distance categories. Most households (>70%) had least one other child under the age of 12 during the study follow-up period. The average median census tract income was $59,329 overall and was similar in all distance to roadway categories ($55,989 to $60,800). Study participants lived in neighborhoods where, on average, nearly 90% of adults had completed at least a high school education, according to the 2000 U.S. Census. A higher proportion of female than male infants lived close to major roadways compared to living further away. On average, the children were born at term, mean (SD) gestational age was 39.5 (1.9), with a normal birth weight for gestational age: mean (SD) z-score of birth weight for gestational age was 0.23 (0.94). Most children attended childcare during the study follow-up period. Childcare attendance was highest in the group living closest to a major roadway (69.0% vs 57.5 to 61.8% in the other groups). The prevalence of any doctor-diagnosed respiratory infection was highest in the groups closest to a major roadway (65.9% and 57.7% among those in the two closest categories compared to 50.0% and 53.4% in the groups further away).
Table 1.
Participant Characteristics by Distance to Major Roadway at Enrollment Home Address Data from 1,263 mother-infant pairs participating in Project Viva.
| <100m | 100 - <200m | 200 - <1,000m | ≥1,000m | |
|---|---|---|---|---|
| (n=88) | (n=78) | (n=422) | (n=675) | |
|
|
||||
| Mean (SD) or % | ||||
| Mother | ||||
| Maternal race/ethnicity | ||||
| White | 63.6 | 71.8 | 72.1 | 79.0 |
| African American | 13.6 | 16.7 | 12.1 | 8.9 |
| Hispanic | 5.7 | 6.4 | 5.5 | 4.8 |
| Other | 17.0 | 5.1 | 10.2 | 7.3 |
| College grad | 73.9 | 78.2 | 73.8 | 71.8 |
| Smoking during pregnancy | 12.5 | 9.1 | 8.5 | 10.1 |
| Duration of any breastfeeding (months) | 6.2 (4.4) | 7.2 (4.5) | 6.9 (4.5) | 6.0 (4.6) |
| Household | ||||
| ETS at home after birth* | 18.2 | 16.7 | 13.0 | 16.3 |
| More than 1 child under 12 years | 79.6 | 71.8 | 77.1 | 81.0 |
| Census tract median annual income ($) | 57,267 (24,930) | 55,989 (24,697) | 58,038 (20,671) | 60,800 (19,374) |
| Census tract % no high school | 11.9 (11.6) | 14.1 (12.7) | 13.3 (9.8) | 11.5 (7.7) |
| Child | ||||
| Female child | 56.8 | 53.9 | 47.4 | 47.1 |
| Gestational age (weeks) | 39.4 (1.8) | 39.5 (1.8) | 39.5 (1.9) | 39.5 (1.8) |
| Z value BW for GA† (units) | 0.02 (0.81) | 0.34 (0.85) | 0.18 (0.93) | 0.28 (0.98) |
| Season of birth | ||||
| Winter | 22.7 | 19.2 | 25.6 | 26.8 |
| Spring | 21.6 | 24.4 | 26.3 | 27.3 |
| Summer | 28.4 | 30.8 | 27.0 | 24.9 |
| Fall | 27.3 | 25.6 | 21.1 | 21.0 |
| Childcare attendance | 69.0 | 61.8 | 60.4 | 57.5 |
| Respiratory infection | 65.9 | 57.7 | 50.0 | 53.4 |
ETS = environmental tobacco smoke, defined here as any smoker living in the household
BW for GA = birthweight for gestational age
The median, interquartile range (IQR) and range of distance to roadway and traffic density based on address at enrollment are provided in Table 2. The median distance to a major roadway was just over 1 km, but there was a wide range of distances with some families living only a few meters from a census feature class A1 or A2 road and others living several km away. The median traffic density, 895 vehicle-km/day, was consistent with an urban setting and more than twice as high as the median traffic density in a study conducted in the metropolitan area of Worcester, Massachusetts (369 vehicle-km/day)13.
Table 2.
Distributions of Distance to Roadway and Traffic Density at Study Enrollment Data from 1,263 mother-infant pairs participating in Project Viva.
| Exposure | Median | IQR* | Range |
|---|---|---|---|
| Distance to Major Roadway (m) | 1,090 | 1,956 | 9.6 - 12,941 |
| Traffic Density (vehicle-km/day) | 895 | 1,485 | 0.4 - 16,916 |
IQR = interquartile range
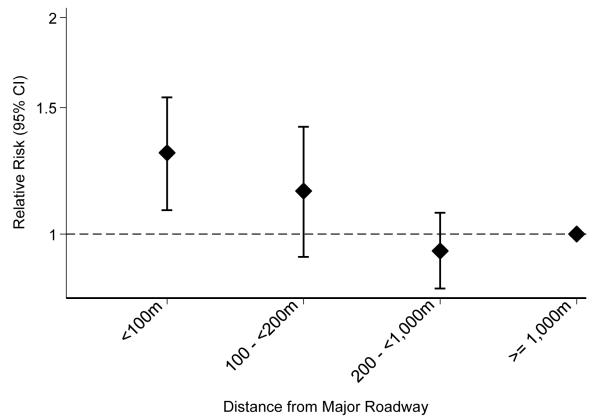
Of the 1,263 children followed, 675 (53.4%) had at least one doctor-diagnosed respiratory infection by the early childhood visit. Figure 1 shows the fully adjusted relative risk of respiratory infection by distance to roadway category during the first trimester. There was a 30% higher risk (95% CI 1.08, 1.55) of respiratory infection among those living <100 m from a major roadway compared to the reference group living >1000 m from a major road. There was a somewhat elevated risk among those living between 100 and 200 m (RR 1.15, 95% CI 0.93, 1.41), though confidence intervals include the null.
Figure 1. Distance to Major Roadway and Relative Risk of Respiratory Infection by Early Childhood*.
Data from 1,263 mother-infant pairs participating in Project Viva.
* Adjusted for maternal race/ethnicity, maternal education, smoking during pregnancy, breastfeeding, smokers in household after birth, other children <12y in household, census tract income (quartile), census tract education, infant sex, gestational age, birth weight for gestational age z-score, season of birth, childcare attendance, and time
Table 3 shows the relative risk of respiratory infection by distance to roadway and by traffic density during the first trimester, each modeled continuously and log transformed. Results of the parsimonious and fully adjusted models for each exposure are shown. The point estimates and confidence intervals for the full and parsimonious models for each exposure were nearly the same. There was a negative linear relationship of distance to roadway with respiratory infection: each IQR increase in distance to roadway was associated with an 8% reduction (95% CI 0.87, 0.98) in risk of respiratory infection in the fully adjusted model. Each IQR increase in traffic density was associated with a 5% higher (95% CI 0.98, 1.13) risk of respiratory infection in the fully adjusted model, though the confidence interval for this association includes the null.
Table 3.
Exposure to Major Roadways, Traffic Density and Risk of Respiratory Infection by Early Childhood Data from 1,263 mother-infant pairs participating in Project Viva.
| Parsimonious Model* | Fully Adjusted Model† | |||
|---|---|---|---|---|
| Exposure | Relative Risk‡ |
95% Confidence Interval |
Relative Risk† |
95% Confidence Interval |
| Log of Distance to Major Roadway (m) |
0.93 | (0.87, 0.98) | 0.92 | (0.87, 0.98) |
| Log of Traffic Density (vehiclekm/ day) |
1.06 | (0.99, 1.13) | 1.05 | (0.98, 1.13) |
Adjusted for maternal race/ethnicity, infant sex, season of birth, and time
Adjusted for maternal race/ethnicity, maternal education, smoking during pregnancy, breastfeeding, smokers in household after birth, other children <12y in household, census tract income (quartile), census tract education, infant sex, gestational age, birth weight for gestational age z-score, season of birth, childcare attendance, and time
Relative risk scaled per IQR increase in exposure
In sensitivity analyses, distance to roadway and traffic density had very similar associations with risk of respiratory infection in the complete case analysis (N = 1,137) and last-observation-carried-forward analysis (N = 1,369) as the primary analysis (N = 1,263). Neither the point estimates nor the confidence intervals were materially changed in either sensitivity analysis. We found that distance to roadway and traffic density using birth address instead of the first trimester address (which were different addresses for 10% of the cohort who moved during pregnancy) had nearly identical associations with risk of respiratory infection. We found that risk of respiratory infection was 1.31 (95% CI 1.08, 1.60) times higher for those living <100 m compared to those living >1000 m from a major roadway at the time of birth. The relative risk was 1.04 (95% CI 0.97, 1.11) per IQR increase in traffic density using birth address. Excluding gestational age and birth weight for gestational age from the models did not change the observed associations with distance to roadway and traffic density. In our sensitivity analysis examining non-linear relationships between each exposure and risk of respiratory infection, we constructed restricted cubic splines but did not observe any statistically significant evidence of departures from linearity (p=0.49 for distance to roadway, p=0.49 for traffic density).
The observed associations of distance to roadway and traffic density with respiratory infection did not differ according to infant sex, preterm birth, maternal smoking during pregnancy or census tract income quartile (p-values for interaction terms ranged from 0.10 to 0.75).
DISCUSSION
In this pre-birth cohort study in the Boston area, we found that living near a major roadway during pregnancy or at the time of birth was associated with a higher risk of doctor-diagnosed respiratory infection by early childhood. There was a borderline significant higher risk of infection associated with greater traffic density around the home.
Results of a modest number of studies suggest that long-term exposure to traffic-related outdoor air pollution may increase risk of early life respiratory infection. A case-control study in Southern California found increased risk of hospitalization for infant bronchiolitis with exposure to particulate matter less than 2.5 microns in diameter (PM2.5) the month prior to hospitalization and over the infant’s lifetime8. In a large cohort study in the Netherlands that modeled long-term air pollutant concentrations at the home address using air pollution measurements and GIS traffic data, lifetime exposure to PM2.5, soot and NO2 were all associated with increased risk of doctor-diagnosed flu/severe colds and ENT infections by age 220. A study in Munich that also estimated individual air pollutant exposure using GIS variables and exposure measurements found an increased risk of sneezing and rhinitis at ages 1 and 2 in association with PM2.5 and increased risk of dry cough or bronchitis in association with NO 212. No associations with respiratory infections were found in that study. This group also examined proximity to roadways and found an increased risk of wheeze, but not infection. A Swiss study measuring pollution exposures inside bedrooms and outside the homes of children aged 0 to 5 found that exposures to NO2 and total suspended particles were associated with episodes of coughing, upper respiratory infections, and episodes of breathing difficulty22. Karr and colleagues examined modeled air pollution exposure and distance to roadway at home address in a nested case-control study in British Columbia and found increased risk of clinical encounters for bronchiolitis in association with NO2 (OR 1.12, 95% CI 1.09, 1.16 per interquartile range increase) and proximity to major roadways (OR 1.06, 95% CI 0.97, 1.17 for living <50m from a highway), but not PM 23 2.5. Karr and colleagues also examined community-level exposure to PM2.5 and infant hospitalization for RSV bronchiolitis in the relatively unpolluted region of Puget Sound, Washington State and found only non-significant trends in association with chronic PM2.5 exposure (OR 1.14, 95% CI 0.88, 1.46 per 10 mcg/m3 increase in lifetime exposure) and living <150 m from a highway (OR 1.17, 95% CI 0.95, 1.44)24. These studies suggest that chronic exposure to traffic-related air pollution may modestly increase risk of lower and possibly upper early life respiratory infection.
Very few studies have examined exposure traffic-related pollution in the prenatal period in association with respiratory infection, despite a relatively large literature demonstrating that prenatal tobacco exposure increases risk of infection25. A recent study measured personal prenatal PM2.5 exposure in a pre-birth cohort and found an increased odds for recurrent broncho-pulmonary infections (five or more spells of doctor-diagnosed bronchitis and/or pneumonia) by age 7 in association with prenatal PM 26 2.5. A Spanish cohort study found that prenatal and first year exposures to NO2 (a marker of traffic-related pollution) estimated by land-use regression models were associated with lower respiratory infections during the first 12-18 months of life (RR 1.08, 95% CI 0.97, 1.21 per interquartile range increase in prenatal NO2) 27.
We cannot conclude from the results of our study whether the pre- or early post-natal traffic exposures were responsible for the association between distance to roadway and respiratory infection, since the majority of mothers did not move during pregnancy. The abovementioned Spanish cohort study also observed very high correlation between pre- and post-natal NO2 exposure levels, making it difficult to determine which exposure is responsible for the observed association with respiratory infection27. A study of pre- and post-natal traffic-related PM2.5 exposure in mice found that exposure during both the pre- and postnatal periods impaired lung growth, but not either exposure alone28. Similarly, a study of pre- and early post-natal tobacco exposure in rats found marked airway sensitivity alterations, decrease compliance and a larger number of neuroendocrine cells in the lungs of rats who were exposed to tobacco both in utero and during the post-natal period but not in those who were only exposed pre- or post-natally29. These studies suggest that critical windows of lung growth and development likely include both fetal and early post-natal periods and that both pre- and early post-natal air pollution exposures are harmful to the developing lung.
Animal exposure studies have identified both anatomic/mechanical and immunological mechanisms by which pre-natal and early post-natal air pollution exposure may increase susceptibility of the respiratory system to infection. PM2.5 and tobacco exposure have been found to impair pre- and post-natal development of tracheobronchial tree28,30. Post-natal diesel exposure has been found to attenuate the lung’s immune response to viral respiratory infection and to augment the inflammatory response, which likely result in a worse course of illness31,32. Perinatal tobacco exposure has also been found to alter the inflammatory response to pulmonary infection33.
There are a number of limitations to our study. This investigation included a population of predominantly white, well-educated women and their children residing in the Boston area; generalizability to other groups may be limited. We did not have information on frequency or severity of infection. Further, there was no confirmation of respiratory infections, for example by medical record review or viral testing. We used distance to roadway and estimates of traffic density as proxies for long-term traffic-related ambient air pollution exposure, which may not reflect actual pollution exposures. Although we did not find evidence that risk of respiratory infection in association with distance to roadway and traffic density differed by infant sex, preterm birth, maternal smoking during pregnancy and census tract income quartile, we had limited power to test for effect modification by preterm birth (7.0% less than 37 weeks) and maternal smoking during pregnancy (9.7%) and cannot conclude with confidence that these subgroups are not more susceptible to traffic-related air pollution. Lastly, as this was an observational study, we cannot infer causality from the associations observed.
This study also has a number of strengths. While most studies have focused on short-term air pollution exposure and hospitalization for respiratory infection using administrative data, we analyzed associations between long-term traffic-related pollution during both the preand early post-natal periods in a well-characterized urban pre-birth cohort. We used GIS to estimate exposures at the child’s home address and were able to account for changing addresses over time through sensitivity analyses. Our results were adjusted for a robust list of potential confounders and predictors of respiratory infection, including measures of income from census tract data, personal education, detailed smoking history, and other details about the child’s environment. Comparison of our parsimonious and full model results (which were essentially identical), suggests that there was very little confounding of the associations of distance to roadway and traffic density with respiratory infection by these potential confounders. Although our outcome of interest (doctor-diagnosed respiratory infection) required access to healthcare, healthcare access was an unlikely confounder or limitation of this study, as this cohort had excellent access to healthcare with 99.8% of the study cohort reporting a current (verified) relationship with a pediatrician at age 3.
In conclusion, we found that proximity to a major roadway during pregnancy and early life was associated with higher risk of doctor-diagnosed respiratory infection by early childhood. These findings have potential clinical implications for children at high risk for respiratory infection who live in urban environments or near highways, and also policy implications regarding traffic pollution control and urban planning to protect the respiratory health of children. To inform policies aimed at reducing the most harmful pollutants, there is a need for longitudinal research on long-term pre-natal and early life air pollution exposures to identify which traffic-related exposures contribute to early life respiratory infection risk.
Acknowledgments
Sources of Financial Assistance: NIH/NHLBI (5T32HL007374), NIH/NIEHS (1F32ES023352-01, P30ES000002), NIH P01-ES009825, NICHD K24 HD069408 and US EPA (R832416, RD834798). This publication’s contents are solely the responsibility of the grantee and do not necessarily represent the official views of the US EPA. Further, US EPA does not endorse the purchase of any commercial products or services mentioned in the publication.
Footnotes
Location of Research: Dr. Rice conducted the data analysis and drafted the manuscript at the Cardiovascular Epidemiology Research Unit, Beth Israel Deaconess Medical Center. All research team meetings took place at the Harvard School of Public Health. The Project Viva cohort study was conducted through Harvard Pilgrim Health Care.
This paper was presented as an oral presentation at the 2013 American Thoracic Society meeting in Philadelphia.
Conflicts of Interest: The authors have no potential conflicts of interest to declare.
REFERENCES
- 1.Loomis D, Castillejos M, Gold DR, McDonnell W, Borja-Aburto VH. Air pollution and infant mortality in Mexico City. Epidemiology. 1999;10:118–123. [PubMed] [Google Scholar]
- 2.Bobak M, Leon DA. Air pollution and infant mortality in the Czech Republic, 1986-88. Lancet. 1992;340:1010–1014. doi: 10.1016/0140-6736(92)93017-h. [DOI] [PubMed] [Google Scholar]
- 3.Woodruff TJ, Darrow LA, Parker JD. Air pollution and postneonatal infant mortality in the United States, 1999-2002. Environ Health Perspect. 2008;116:110–115. doi: 10.1289/ehp.10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saldiva PH, Lichtenfels AJ, Paiva PS, Barone IA, Martins MA, Massad E, Pereira JC, Xavier VP, Singer JM, Bohm GM. Association between air pollution and mortality due to respiratory diseases in children in Sao Paulo, Brazil: a preliminary report. Environ Res. 1994;65:218–225. doi: 10.1006/enrs.1994.1033. [DOI] [PubMed] [Google Scholar]
- 5.Nordling E, Berglind N, Melen E, Emenius G, Hallberg J, Nyberg F, Pershagen G, Svartengren M, Wickman M, Bellander T. Traffic-related air pollution and childhood respiratory symptoms, function and allergies. Epidemiology. 2008;19:401–408. doi: 10.1097/EDE.0b013e31816a1ce3. [DOI] [PubMed] [Google Scholar]
- 6.Ryan PH, Bernstein DI, Lockey J, Reponen T, Levin L, Grinshpun S, Villareal M, Hershey GK, Burkle J, LeMasters G. Exposure to traffic-related particles and endotoxin during infancy is associated with wheezing at age 3 years. Am J Respir Crit Care Med. 2009;180:1068–1075. doi: 10.1164/rccm.200808-1307OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Segala C, Poizeau D, Mesbah M, Willems S, Maidenberg M. Winter air pollution and infant bronchiolitis in Paris. Environ Res. 2008;106:96–100. doi: 10.1016/j.envres.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Karr C, Lumley T, Schreuder A, Davis R, Larson T, Ritz B, Kaufman J. Effects of subchronic and chronic exposure to ambient air pollutants on infant bronchiolitis. Am J Epidemiol. 2007;165:553–560. doi: 10.1093/aje/kwk032. [DOI] [PubMed] [Google Scholar]
- 9.Gillman MW, Rich-Edwards JW, Rifas-Shiman SL, Lieberman ES, Kleinman KP, Lipshultz SE. Maternal age and other predictors of newborn blood pressure. J Pediatr. 2004;144:240–245. doi: 10.1016/j.jpeds.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 10.Zhu Y, Hinds WC, Kim S, Sioutas C. Concentration and size distribution of ultrafine particles near a major highway. J Air Waste Manag Assoc. 2002;52:1032–42. doi: 10.1080/10473289.2002.10470842. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Y, Levy JI. Factors influencing the spatial extent of mobile source air pollution impacts: a meta-analysis. BMC Public Health. 2007;7:89. doi: 10.1186/1471-2458-7-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenbloom JI, Wilker EH, Mukamal KJ, Schwartz J, Mittleman MA. Residential proximity to major roadway and 10-year all-cause mortality after myocardial infarction. Circulation. 2012;125:2197–2203. doi: 10.1161/CIRCULATIONAHA.111.085811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tonne C, Melly S, Mittleman M, Coull B, Goldberg R, Schwartz J. A case-control analysis of exposure to traffic and acute myocardial infarction. Environ Health Perspect. 2007;115:53–7. doi: 10.1289/ehp.9587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 15.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 16.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parker JD, Woodruff TJ, Basu R, Schoendorf KC. Air pollution and birth weight among term infants in California. Pediatrics. 2005;115:121–8. doi: 10.1542/peds.2004-0889. [DOI] [PubMed] [Google Scholar]
- 18.Stieb DM, Chen L, Eshoul M, Judek S. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ Res. 2012;117:100–11. doi: 10.1016/j.envres.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Harrell FE. Regression Modeling Strategies with Applications to Linear Models, Logistic Regression and Survival Analysis. Springer-Verlag; New York: 2001. [Google Scholar]
- 20.Brauer M, Hoek G, Van Vliet P, Meliefste K, Fischer PH, Wijga A, Koopman LP, Neijens HJ, Gerritsen J, Kerkhof M, Heinrich J, Bellander T, Brunekreef B. Air pollution from traffic and the development of respiratory infections and asthmatic and allergic symptoms in children. Am J Respir Crit Care Med. 2002;166:1092–1098. doi: 10.1164/rccm.200108-007OC. [DOI] [PubMed] [Google Scholar]
- 21.Morgenstern V, Zutavern A, Cyrys J, Brockow I, Gehring U, Koletzko S, Bauer CP, Reinhardt D, Wichmann HE, Heinrich J. Respiratory health and individual estimated exposure to traffic-related air pollutants in a cohort of young children. Occup Environ Med. 2007;64:8–16. doi: 10.1136/oem.2006.028241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braun-Fahrländer C, Ackermann-Liebrich U, Schwartz J, Gnehm HP, Rutishauser M, Wanner HU. Air pollution and respiratory symptoms in preschool children. Am Rev Respir Dis. 1992;145:42–7. doi: 10.1164/ajrccm/145.1.42. [DOI] [PubMed] [Google Scholar]
- 23.Karr CJ, Demers PA, Koehoorn MW, Lencar CC, Tamburic L, Brauer M. Influence of ambient air pollutant sources on clinical encounters for infant bronchiolitis. Am J Respir Crit Care Med. 2009;180:995–1001. doi: 10.1164/rccm.200901-0117OC. [DOI] [PubMed] [Google Scholar]
- 24.Karr CJ, Rudra CB, Miller KA, Gould TR, Larson T, Sathyanarayana S, Koenig JQ. Infant exposure to fine particulate matter and traffic and risk of hospitalization for RSV bronchiolitis in a region with lower ambient air pollution. Environ Res. 2009;109:321–327. doi: 10.1016/j.envres.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones LL, Hashim A, McKeever T, Cook DG, Britton J, Leonardi-Bee J. Parental and household smoking and the increased risk of bronchitis, bronchiolitis and other lower respiratory infections in infancy: systematic review and meta-analysis. Respir Res. 2011;12:5. doi: 10.1186/1465-9921-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jedrychowski WA, Perera FP, Spengler JD, Mroz E, Stigter L, Flak E, Majewska R, Klimaszewska-Rembiasz M, Jacek R. Intrauterine exposure to fine particulate matter as a risk factor for increased susceptibility to acute broncho-pulmonary infections in early childhood. Int J Hyg Environ Health. 2013;216:395–401. doi: 10.1016/j.ijheh.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aguilera I, Pedersen M, Garcia-Esteban R, Ballester F, Basterrechea M, Esplugues A, Fernández-Somoano A, Lertxundi A, Tardón A, Sunyer J. Early-life exposure to outdoor air pollution and respiratory health, ear infections, and eczema in infants from the INMA study. Environ Health Perspect. 2013;121:387–92. doi: 10.1289/ehp.1205281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mauad T, Rivero DHRF, de Oliveira RC, Lichtenfels AJ, de FC, Guimarães ET, de Andre PA, Kasahara DI, Bueno HM, de S, Saldiva PHN. Chronic exposure to ambient levels of urban particles affects mouse lung development. Am J Respir Crit Care Med. 2008;178:721–8. doi: 10.1164/rccm.200803-436OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joad JP, Ji C, Kott KS, Bric JM, Pinkerton KE. In utero and postnatal effects of sidestream cigarette smoke exposure on lung function, hyperresponsiveness, and neuroendocrine cells in rats. Toxicol Appl Pharmacol. 1995;132:63–71. doi: 10.1006/taap.1995.1087. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Pinkerton KE. Detrimental effects of tobacco smoke exposure during development on postnatal lung function and asthma. Birth Defects Res C Embryo Today. 2008;84:54–60. doi: 10.1002/bdrc.20114. [DOI] [PubMed] [Google Scholar]
- 31.Harrod KS, Jaramillo RJ, Rosenberger CL, Wang S-Z, Berger JA, McDonald JD, Reed MD. Increased susceptibility to RSV infection by exposure to inhaled diesel engine emissions. Am J Respir Cell Mol Biol. 2003;28:451–63. doi: 10.1165/rcmb.2002-0100OC. [DOI] [PubMed] [Google Scholar]
- 32.Castranova V, Ma JY, Yang HM, Antonini JM, Butterworth L, Barger MW, Roberts J, Ma JK. Effect of exposure to diesel exhaust particles on the susceptibility of the lung to infection. Environ Health Perspect. 2001;109(Suppl):609–12. doi: 10.1289/ehp.01109s4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Claude JA, Grimm A, Savage HP, Pinkerton KE. Perinatal exposure to environmental tobacco smoke (ETS) enhances susceptibility to viral and secondary bacterial infections. Int J Environ Res Public Health. 2012;9:3954–64. doi: 10.3390/ijerph9113954. [DOI] [PMC free article] [PubMed] [Google Scholar]