Abstract
Background
Plant biomass consists primarily of carbohydrates derived from photosynthesis. Monitoring the assimilation of carbon via the Calvin-Benson cycle and its subsequent utilisation is fundamental to understanding plant growth. The use of stable and radioactive carbon isotopes, supplied to plants as CO2, allows the measurement of fluxes through the intermediates of primary photosynthetic metabolism, long-distance transport of sugars in the vasculature, and the synthesis of structural and storage components.
Results
Here we describe the design of a system for supplying isotopically labelled CO2 to single leaves of Arabidopsis thaliana. We demonstrate that the system works well using short pulses of 14CO2 and that it can be used to produce robust qualitative and quantitative data about carbon export from source leaves to the sink tissues, such as the developing leaves and the roots. Time course experiments show the dynamics of carbon partitioning between storage as starch, local production of biomass, and export of carbon to sink tissues.
Conclusion
This isotope labelling method is relatively simple to establish and inexpensive to perform. Our use of 14CO2 helps establish the temporal and spatial allocation of assimilated carbon during plant growth, delivering data complementary to those obtained in recent studies using 13CO2 and MS-based metabolomics techniques. However, we emphasise that this labelling device could also be used effectively in combination with 13CO2 and MS-based techniques.
Keywords: Arabidopsis, Photosynthesis, Carbohydrate metabolism, Assimilate partitioning, Phloem transport, Isotope labelling, Sink-source relationships
Background
Central carbon metabolism has been studied for many decades with the help of both radioactive (11C and 14C) and stable (13C) carbon isotopes. Each isotope offers advantages and disadvantages. The 11C isotope has a very short half-life of 20.334 minutes but emits high energy positrons upon decay. Therefore, it is well suited for in vivo studies of phloem transport, partitioning of carbon between alternative sinks, such as roots, leaves and seeds [1,2], and to calculate the rates of carbon transport and leakage [3]. Furthermore, the short half-life means that the same biological material can be labelled sequentially (e.g. before and after a treatment; [4,5]). However, due to its short-lived nature and high energy emissions, 11C has major drawbacks. It has to be produced at the site of the experiment, it is difficult to handle, and it is expensive. The long-lived radioactive 14C (half-life of 5,700 years), on the other hand, is relatively cheap and easy to use for labelling experiments. Labelled plant material can be fractionated and its low-energy beta particles measured using scintillation counters. This allows carbon allocation into different tissues and carbon partitioning into metabolic pools to be accurately quantified. Radiolabeled material is not usually analysed by mass spectrometry (MS), so measurement of carbon partitioning into specific metabolites is laborious. However, 13C labelling is highly amenable with techniques such as MS and nuclear magnetic resonance (NMR), which allow metabolite identification and can provide positional information for the isotope label [6,7]. Furthermore, elemental analysis of 13C labelled material allows measurements of carbon allocation towards sink tissues to be made [8-10].
Isotope labelling has been pivotal to some of the groundbreaking work that elucidated the pathways of the Calvin-Benson cycle and photorespiration [11,12]. Sucrose and starch were shown to be the main products of photoassimilation [13] and sucrose was identified as the major form in which carbon is transported to sink tissues such as seeds, flowers and roots [14]. Experiments on sugar beet and tobacco showed that young leaves are supported by mature ones with carbon reserves until they reach 30-60% of their final length, whereupon they themselves turn from sink into source leaves [15-17]. The movement of carbon towards the sink tissues follows a defined pattern, determined by the vascular connections [18,19].
Recently, 13C labelling has been used to analyse central carbon metabolism. In metabolic flux analyses, heterotrophic or mixothrophic cells are fed with positionally 13C labelled sugars (e.g. [6-13C]-glucose) until steady state is reached. Determination of the isotope pattern in several pathway-relevant metabolites allows metabolic fluxes to be calculated, based on an existing metabolic model [20-22]. Metabolic fluxes have also been measured in autotrophic tissues, though this is more difficult as 13C has to be supplied in the form of gaseous 13CO2. In this case, reaching a steady state is not informative, as all carbon atoms in a metabolite become labelled and flux calculations would be impossible. Therefore, time-course experiments in a non-stationary state are performed [23]. Thus far, only a limited number of metabolites and their isotope patterns can be measured. Nevertheless, 13CO2 labelling will certainly gain in importance in the future, and good tools for labelling will be necessary.
Labelling with 14CO2 has been used extensively in the last decades to study the allocation of photo-assimilated carbon in different species such as crops [24,25], trees [26-28] and grasses [29,30]. In most of these approaches, a setup containing polyethylene bags or chambers mounted over single leaves yielded valuable information of carbon allocation. In Arabidopsis, however, 14C feeding was used only rarely, which is surprising when considering the importance of this model species in plant biology today. When labelling was performed with Arabidopsis, it was generally on whole plants [31-34] or detached leaves [35,36]. Due to its very small, narrow leaves and rosette growth habit, Arabidopsis needs a special cuvette design for single leaf labelling experiments to avoid damage to the plant and to prevent contact of the isotopic label to other tissues.
In the present work, we introduce a cuvette for isotopic labelling of single leaves of intact Arabidopsis plants. The cuvette can be used for short-term isotopic feeding experiments with different carbon isotopes. It is easy to handle, allowing rapid attachment/removal and therefore a medium- to- high throughput, which we demonstrate with 14CO2 labelling. We show that 14CO2 labelling gives reliable information of carbon transport into sink tissues, such as developing leaves and roots, and broad information about carbon partitioning into major metabolite classes and biosynthetic end-products. It has the advantage of being very sensitive, which allows for short labelling pulses. The processing of the plant material as well as the data analysis are fast and straightforward and can be easily adapted by any lab. Therefore, despite the new emerging techniques using 11C and 13C labelling, we argue that 14C labelling, still has much potential to help us understand carbon metabolism in plants.
Results
Design of the single leaf labelling chamber and control experiments
We designed equipment for isotopic labelling experiments of individual leaves in-house (Figure 1). The equipment comprises a sealed reservoir chamber, in which the isotopically labelled carbon is released, connected via tubing to the leaf cuvette, which can be clamped over a single leaf. Isotopically labelled gas is circulated from the reservoir via the tubing to the cuvette in order to supply the labelled carbon to an individual leaf. In the tubing system are two valves that allow or prevent airflow between the reservoir chamber and the labelling cuvette. These valves allow leaves to be inserted and removed from the cuvette without loss of labelled CO2 from the reservoir chamber.
Figure 1.
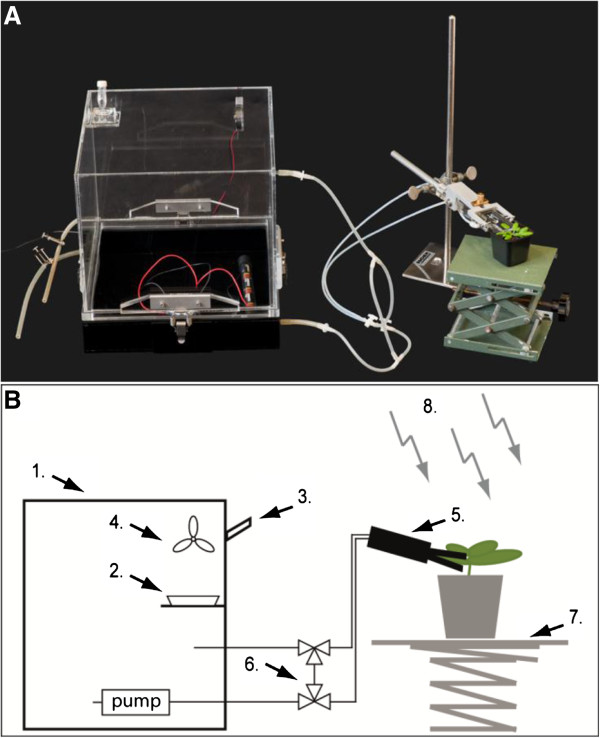
Chamber for isotopic labelling of individual leaves. (A) Picture of the single leaf labelling chamber. The labelling system comprises of a reservoir chamber (1) in which the isotopically labelled sodium-bicarbonate is placed within a petri dish (2). The bicarbonate is acidified by injecting an excess of lactic acid through a rubber seal (3) into the petri dish, releasing labelled CO2, which is homogenously dispersed within the chamber with a fan (4). The labelled air is pumped through a tubing system to the single leaf cuvette (5) or through a bypass (6) when exchanging leaves to be labelled. An adjustable table (7) is used to position the plants in relation to the external light source (8). (B) Scheme of the single leaf labelling chamber in use.
We first tested the equipment for gas-tightness. Only a sealed system will allow the accurate analysis of transport processes from a labelled leaf to unlabelled tissues – a leak would result in inadvertent fixation of isotopically labelled carbon by the unlabelled tissues. For this test, a mature leaf (leaf 8) still attached to the rosette was clamped into the leaf cuvette. Just before opening the valves to allow the flow of 14CO2-containing air to the leaf cuvette, the petiole of the leaf was cut to prevent phloem export. After 10 min labelling, the rosette and the cut leaf were harvested, rapidly dried, and analysed by autoradiography (Figure 2A). The cut leaf gave a strong signal on the film, but hardly any signal from the rosette was visible (Figure 2B). The experiment was repeated and the labelled leaf and the unlabelled rosette were harvested separately. The amount of 14C incorporated into each part was determined by liquid scintillation counting. In the labelled leaf, around 800,000 disintegrations per minute (dpm) were detected, compared with 1,200 dpm in the unlabelled rosette. This is 0.15% of the overall label found in the plant. Thus, if the cuvette is properly attached to the leaf, the amounts of 14CO2 escaping from the chamber for assimilation by other leaves is negligible.
Figure 2.
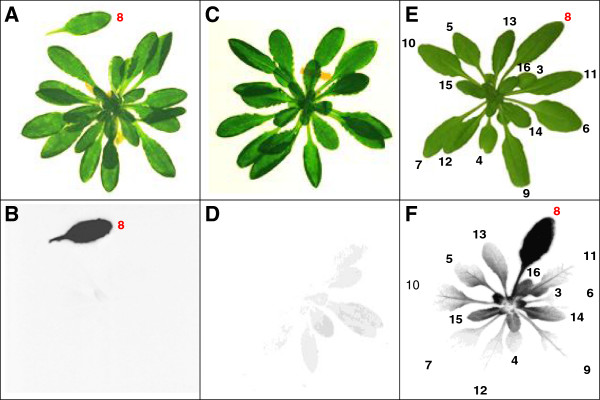
Testing of the single leaf labelling cuvette. Pictures (A, C, E) and autoradiograms (B, D, F) of Col-0 rosettes labelled with 14CO2. (A and B) Leaf 8 was introduced to the cuvette when still attached to the rosette. Just before labelling for 10 min with 14CO2, the petiole of leaf 8 was cut to prevent phloem export. (C and D) A rosette was placed for 60 min next to the leaf cuvette, which was being used to label other plants during a series of 5 pulse and chase experiments. (E and F) The 14C distribution pattern observed when the leaf cuvette is deliberately opened slightly during labelling in order to cause a leak. Leaf 8 was labelled for 5 min followed by a chase period of 1 h. The leaves are numbered according to the sequence of emergence where 1 is the first emerging true leaf after the cotyledons. Here, leaves 3 and upwards are indicated. Dried plant material was exposed to the film for 7 days.
The autoradiographs and the quantitative measurements show that labelling for 10 min is ample and can be even reduced (e.g. to 2–5 min). The extent of carbon uptake from the chamber was calculated to ensure that the labelled CO2 is not depleted. The released radioactivity (300 μCi) corresponds to 666 million dpm. After 10 min labelling 801,200 dpm were detected in the whole plant, corresponding to 0.12% of the total label. Many plants can therefore be labelled successively without a significant reduction of the CO2 content in the reservoir.
The labelling of the leaves (the pulse) and the subsequent chase period were performed in the same fume hood under fluorescent lights. In practice, small amounts of 14CO2 inevitably escape during the experiment, either as replicate plants are exchanged into the cuvette or due to the respiration of labelled plants. To evaluate the amount of available 14CO2 within the fume hood, a second control experiment was performed in which a plant was positioned for 60 min next to the leaf cuvette while a series of 5 other plants were labelled for complete pulse and chase cycles. Subsequently, the unlabelled plant was harvested and analysed by autoradiography. The autoradiogram showed a faint signal for the leaves orientated towards the labelled plants (Figure 2C and D). The experiment was repeated and the rosette was harvested to quantify the 14C incorporated into the tissue, using liquid scintillation counting. The control plant beside the cuvette contained less than 0.1% of the 14C detected in the average labelled plants. Thus, there is negligible accumulation of 14C in the fume hood during the experiments and assimilation of free 14CO2 during the chase period will not significantly influence the labelling pattern (especially as the plants were not left adjacent to the leaf cuvette or the reservoir).
To demonstrate the 14C distribution pattern resulting from a non-gas-tight chamber, a leak was deliberately introduced while labelling a single leaf. A mature leaf (leaf 8) was labelled with 14CO2 for a 5-min pulse in a leaky chamber, followed by a chase period of 1 h. After the chase, the plants were analysed by autoradiography (Figure 2E and F). A strong signal was seen for the labelled leaf, but signals were also observed from many of the other leaves, both younger and older than leaf 8. The pattern of 14C was weaker from tissues that were furthest from the leaf cuvette, but otherwise not dependent on the position of the leaves on the rosette. This distribution of label represents a combination of export from the labelled leaves and assimilation of leaked 14CO2 by tissues adjacent to the cuvette and differed from the patterns derived with a sealed chamber (see Figures 3, 4 and 5).
Figure 3.
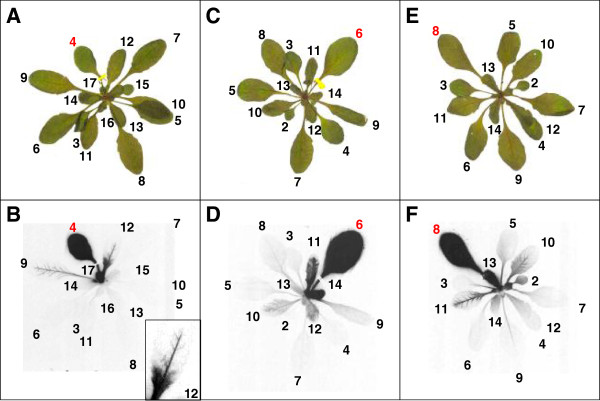
Autoradiograms visualizing carbon transport within an Arabidopsis rosette. Pictures (A, C, E) and autoradiograms (B, D, F) of Col-0 rosettes labelled with 14CO2. Leaf 4 (A and B), 6 (C and D) and 8 (E and F) were labelled for 5 min followed by a chase period of 1 h. A close-up of leaf 12 is shown in B. The leaves are numbered as described in Figure 2. Plant material was dried and exposed to the film for 10 days.
Figure 4.
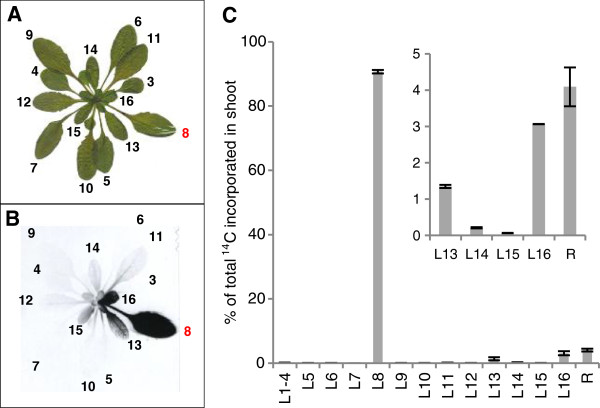
Qualitative and quantitative representation of carbon export within an Arabidopsis rosette. Picture (A) and autoradiogram (B) of a Col-0 rosette where leaf 8 was labelled with 14CO2, as described in Figure 2. The plant was exposed to the film for 9 days. (C) Quantitative analysis of the amount of 14C in different rosette leaves. Mean ± SE (n = 4). In this experiment, leaf 8 was labelled for 5 min, followed by a chase period of 1 h in air. The leaves were harvested separately for quantitative analysis. Leaf 1 (L1) is the first emerging leaf, L2 the next etc. R denotes the sample containing the leaves younger than leaf 16, the stem and meristem.
Figure 5.
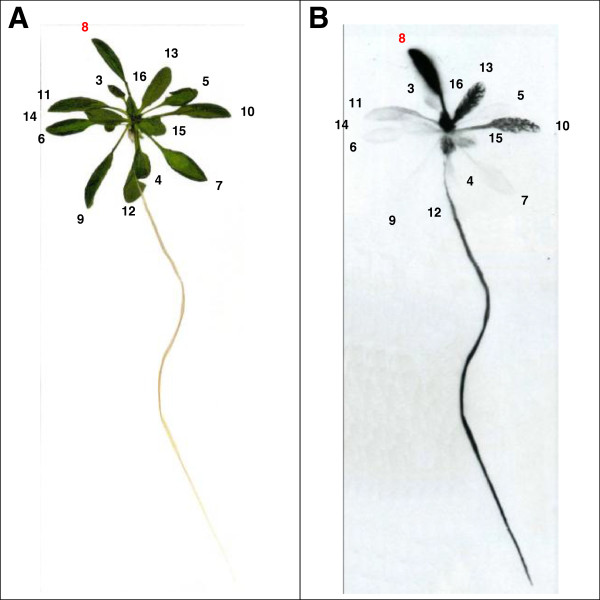
Autoradiogram visualizing carbon transport to sink tissues. Picture (A) and autoradiogram (B) of a Col-0 plant labelled with 14CO2. Leaf 8 was labelled for 5 min followed by a chase period of 1 h. The leaves are numbered as described in Figure 2. Plant material was exposed to the film for 13 days.
Analysing carbon transport processes with the single leaf labelling chamber
We used our system to illustrate the pattern of carbon allocation from source tissues to sink tissues in Arabidopsis qualitatively using autoradiograms and quantitatively by measuring the amount of incorporated radioactivity via liquid scintillation counting. The first experiment aimed to reveal if a defined pattern of carbon translocation can be seen in Arabidopsis. Different mature leaves were labelled with 14CO2 for 5 min, and the translocation of label into the rosette after a 1-h chase in air was analysed by autoradiography. Soil-grown, non-flowering plants with 17 to 19 leaves were used. Considering the first true emerging leaf as leaf 1, we chose leaves 4, 6 and 8 for labelling (Figure 3). All three labelled leaves showed a strong signal on the autoradiogram. Additionally, signals from other leaves were detectable. Labelling leaf 4 resulted in a strong signal in leaf 17 and weaker signals, mainly along the major veins, in leaves 9 and 12. In leaf 12, the base of the leaf was labelled strongly, but in the tip, only the major veins were labelled. When leaf 6 was labelled, a strong signal could be seen in leaves 14 and 11. Labelling leaf 8 led to a strong signal in leaves 13, 15 and 16, and accumulation of label along the major vein system in leaf 10 and 11. In each experiment, most of the other rosette leaves showed a weak background signal.
The export patterns in the three autoradiograms revealed that, in each case, carbon was predominantly exported to young, neighbouring leaves. Young leaves on the opposite side of the rosette did not import significant amounts of labelled carbon, nor did leaves older than the labelled leaf, irrespective of their rosette position. A similar pattern of carbon allocation has been described for several different species. The transport of carbon follows the vascular connections of the plant, which form along the orthostichies (i.e. between leaves that lie directly above each other; [19,37,38]). Very small developing leaves showed a strong signal evenly dispersed over the whole leaf blade, but larger developing leaves showed a signal mostly restricted to the veins. For several C-importing leaves an increase of signal towards the leaf base was seen. This gradient in carbon uptake is consistent with the idea that developing leaves undergo a basipetal sink-to-source transition, where the leaf tip matures first and stops importing carbon [15,39,40]. During this transition, the leaf tip exports carbon while the base still imports it. Gradually, as the leaf grows, there is a wave of maturation from tip to base until the whole leaf acts as a source tissue [41-43].
To quantify the carbon exported from a single leaf, leaf 8 was labelled as described above in several replicate plants. After the chase, the aerial parts of the plants were harvested for autoradiography (Figure 4A and B) or to quantify 14C in each leaf (Figure 4C). Of the total 14C incorporated during the pulse, 91% remained in the labelled leaf after the chase. Labelled carbon could also be detected in leaf 13 (1.3%), leaf 16 (3%) and the remaining part of the rosette containing all leaves smaller than leaf 16 and the shoot apex (4.1%). In all other harvested leaves, only 0.02% to 0.2% of the label could be measured, accounting for less than 0.8% of the assimilated carbon in total. These data confirmed the results gained from the autoradiograms (i.e. that most exported carbon was allocated to small developing leaves adjacent to the labelled leaf).
To monitor carbon translocation to the roots – another major carbon sink - plants were grown in hydroponic culture. Leaf 8 was labelled with 14CO2 as described above. Subsequently, autoradiograms were performed, revealing carbon transport on a whole plant level (Figure 5). The same pattern of carbon allocation could be seen as in the previous autoradiograms. Labelling leaf 8 leads to a strong signal in leaf 13 and leaf 16 and a signal in the veins of leaf 10. Additionally, the root gives a strong signal. This simultaneous transport towards the shoot apex and towards the roots of the plant was first reported decades ago [14,18,44]. However, it is still not known how the bidirectional transport is facilitated within the vascular system.
Time course of carbon export to the root and the shoot
In the previous experiments, a 1-h chase was applied before analysing the plant tissue. To visualize the dynamics of carbon export from a source leaf into sink leaves and the root, time course experiments were carried out using hydroponically-grown plants. Leaf 8 (of plants with 16 to 20 leaves) was labelled with 14CO2 for 5 min in the middle of the light period, followed by a chase in air varying from 0 to 360 min. The labelled leaf, the unlabelled leaves and the root were harvested separately after each chase period and the 14C in each sample was determined (Figure 6).
Figure 6.
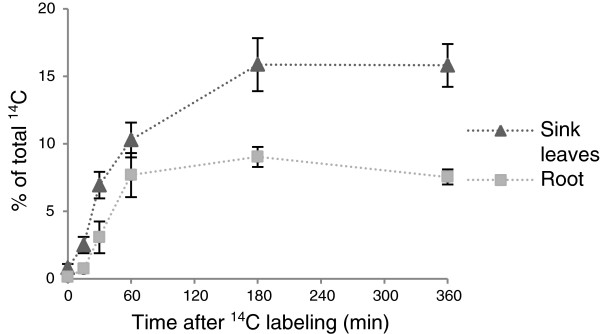
Export of 14C to the root and rosette leaves. Leaf 8 of Col-0 plants was labelled for 5 min at 6 h into the light period with 14CO2, followed by chase periods of 0, 15, 30, 60, 180 or 360 min. At each time point, the labelled leaf, the unlabelled (sink) leaves and the root were harvested separately, and the incorporation of 14C was determined. The sum of the label in the labelled leaf, the sink leaves and the root is set to 100%. Mean ± SE (n = 5).
Immediately after the pulse, export into the sink leaves was observed. One percent of the labelled carbon was detected in the unlabelled leaves. After a 15-min chase, 2.5% of the label was detected in the sink leaves. The amount of export increased rapidly during the first hour of the chase, after which the rate of export was slower, reaching a value of 16% after 180 min. The export values remained stable thereafter. Export of label to the roots was slightly delayed compared with export into rosette leaves. A significant amount of labelled carbon (3%) could only be detected after 30 min. The amount of imported carbon increased in the following 30 min up to 7.5% (after 1 h chase) and remained at around 7.5% - 9% thereafter. The release of CO2 from the plants was not measured during the chase period, but certainly labelled carbon was lost during the course of the experiment due to respiration, particularly from heterotrophic tissues like the root. This means that our values are slight underestimations of the exported amount of carbon. Zeeman and ap Rees [31] detected that 3% of the total label assimilated during a 1-h pulse was respired from wild-type roots during a five hour chase period.
Time course of carbon partitioning into major metabolic pools
The previous experiments demonstrated the translocation of carbon from source to sinks. However, isotopic labelling can also be used to analyse the partitioning of carbon into the different major metabolite pools and biosynthetic end-products. Therefore, we fractionated our plant material into soluble and insoluble compounds after 14C labelling. Soluble compounds were further separated by ion-exchange chromatography into neutral, acidic and basic compounds. The neutral fraction is enriched in sugars, like sucrose, glucose and fructose. The acidic fraction is enriched in organic acids and sugar phosphates, and the basic fraction is enriched in amino acids. The insoluble fraction was further processed by specific enzymatic digestions, allowing the amount of carbon introduced into starch, proteins and other insoluble compounds (i.e. cell wall material) to be determined. The proportion of assimilated 14C in each fraction was measured using liquid scintillation counting. These data reveal the amount of assimilated carbon utilized in different biosynthetic pathways.
To assess carbon partitioning within source tissues, a time course experiment was performed using soil-grown plants. Leaf 8 was labelled for 5 min followed by chase periods between 0 and 180 min. At given times, the labelled leaves were harvested and the amount of carbon partitioned to the water-soluble compounds, ethanol-soluble compounds and insoluble compounds was determined (Table 1). After the pulse, most 14C was found in the water-soluble fraction, with the basic, acidic and neutral fractions all containing significant amounts of label. The amount of 14C in acidic and basic compounds decreased markedly after the 15-min chase, indicative of a rapid turnover of the metabolic intermediates. A fast incorporation of carbon and high turnover rate can be expected for intermediates of the Calvin-Benson cycle (found in the acidic fraction) and for photorespiratory intermediates (such as serine and glycine in the basic fraction). Label in the basic compounds continued to decrease after the initial rapid drop during the first 15 min of the chase period. From the 25% of label just after the pulse, only 5% remained in this pool after a 180-min chase period. Some amino acids may be used for protein synthesis and an increase in label in protein was observed during the time course. However, most of the label is probably cycled into other pools as photorespiration and photosynthesis continue throughout the chase. Consistent with this, after the rapid decrease of label in the acidic fraction from 0 to 15 min chase (most likely representing turnover of sugar phosphates from the Calvin-Benson cycle), the amount of label increased again from 14% to 23% after 180 min chase. This increase probably represents the synthesis of organic acids (e.g. malate and fumarate) that are stored in the vacuole. A peak of labelled carbon incorporated into the neutral fraction appeared after the 15 min chase. The amount of label decreased thereafter from 22% to 7%. The peak after 15 min most likely represents labelled sucrose, which is then either metabolized within the tissue or exported to sink tissues.
Table 1.
Time course of carbon partitioning into the major carbon compound classes in the labelled leaf
|
% of
14
C recovered in each fraction related to the total amount per tissue |
|||||||
|---|---|---|---|---|---|---|---|
| After pulse | 15 min chase | 30 min chase | 60 min chase | 180 min chase | |||
|
Labeled leaf
1
|
100 |
100 |
100 |
100 |
100 |
||
| |
Soluble |
77.0 ± 2.2 |
58.4 ± 3.5 |
59.3 ± 2.1 |
46.2 ± 3.4 |
44.7 ± 2.4 |
|
| |
|
Neutral |
14.6 ± 0.9 |
23.1 ± 1.2 |
22.8 ± 1.9 |
11.6 ± 2.0 |
7.3 ± 0.9 |
| |
Acidic |
27.7 ± 0.9 |
15.1 ± 0.7 |
17.0 ± 1.8 |
20.0 ± 1.5 |
25.9 ± 1.8 |
|
| |
Basic |
24.9 ± 1.4 |
14.3 ± 1.2 |
11.3 ± 1.3 |
8.2 ± 0.5 |
5.4 ± 0.5 |
|
| |
Insoluble |
17.3 ± 2.0 |
30.5 ± 4.3 |
36.6 ± 1.9 |
42.5 ± 4.6 |
45.1 ± 4.7 |
|
| |
|
Starch |
16.6 ± 1.1 |
27.7 ± 2.4 |
29.3 ± 2.2 |
34.4 ± 3.1 |
37.2 ± 3.2 |
| |
Protein |
0.8 ± 0.2 |
3.1 ± 0.7 |
3.1 ± 0.5 |
4.6 ± 0.5 |
4.2 ± 0.5 |
|
| |
Others |
0.3 ± 0.1 |
2.0 ± 0.6 |
2.3 ± 0.3 |
3.8 ± 0.2 |
3.7 ± 0.4 |
|
| EtOH soluble | 1.7 ± 0.1 | 4.0 ± 0.6 | 4.1 ± 0.4 | 5.6 ± 0.2 | 5.7 ± 0.3 | ||
1Leaf 8 of Col-0 plants was labelled for 5 min with 14CO2, 6 h into the light, followed by a chase period of 0, 15, 30, 60 or 180 min. After the chase, the labelled leaf was harvested and the incorporation of 14C was determined. The sum of the label in the labelled leaf is set to 100%. The relative amount of 14C incorporated into the different compound classes (as a percentage of the total label in the leaf) is shown. Mean ± SE (n ≥ 5).
While 80% of the label was found in the water-soluble fraction right after the labelling pulse, 17% were found in insoluble compounds (mainly starch). Carbon was very rapidly partitioned into starch, as half of the label detected in starch after 180 min chase was already found in this pool after the 5-min pulse. The amount of label in starch increased from 17% to 27% after the 15-min chase period, followed by a further increase to 37% over the rest of the chase. In the other insoluble compounds, like proteins and cell wall material, hardly any label could be detected immediately after the labelling experiment, but the amount of label increased over the first hour of the chase. After a further two hours chase, only minor changes were seen. Carbon partitioning into ethanol-soluble compounds (representing lipids, pigments and waxes) increased from 1.7% immediately after labelling to 5.1% after 1-h chase. No more 14C was introduced into the ethanol-soluble compounds in the subsequent two hours. The pattern is similar to that of other end-products like proteins and cell wall material. This reveals that approximately half of the assimilated carbon is either used immediately for storage (in the form of starch) or it is channelled to its final destination within the first hour after fixation. For the longer chase periods, it should be noted that the percentage values might be slight overestimates, as carbon export into the roots was not monitored in this experiment. As carbon is exported during the chase, less label is retained in the rosette, so the percentage of 14C in the fractions of the labelled leaf increases even if the absolute amount of 14C is constant.
Discussion
Here, we have shown that our labelling chambers are air-tight, fast to attach and to remove, and therefore ideal for labelling experiments with single Arabidopsis leaves. During the experimental procedure only minimal amounts of labelled CO2 were released from the system, probably while attaching and removing the chamber, causing negligible incorporation of 14C by unlabelled leaves.
Feeding labelled carbon to individual leaves of intact plants has the advantage that carbon transport and carbon partitioning (in the specific source tissue) can be monitored simultaneously. It allowed us to determine, qualitatively as well as quantitatively, the amount of assimilated carbon which is transported form source leaves to sink leaves and other sink tissue such as the root in parallel. A defined pattern of carbon allocation from a mature leaf could be seen. A similar pattern, which is determined by the vascular system, was observed for other plants like tobacco [18,19]. Following the vascular connections, carbon is transported from a mature leaf upwards to the leaves n + 3, n + 5, n + 8 and n + 10 in tobacco plants. For Arabidopsis, two vascular models have been described which either suggest connections from one leaf to the leaves n + 2 and n + 3 (from leaf 4 onwards; [45]) or to the leaves n + 5 and n + 8 [46]. In the majority of autoradiograms performed, we could see carbon import into leaves n + 5, n + 8 and, if already emerged, in leaf n + 13 (which is linked to the fed leaf via leaf n + 5 and n + 8). This supports the vascular model proposed by Kang et al. [46]. However, we occasionally observed carbon import into the leaves n + 2 and n + 3 (Figures 3, 4 and 5) which could be explained by stochastic irregularities during the formation of vascular connections.
A simultaneous transport from mature leaves towards developing leaves and towards the root was seen in the autoradiographs as well as in the quantitative measurements. It suggests that, in certain regions of the vasculature, bidirectional phloem transport occurs. Bidirectional transport within the phloem is long-known, but it is still unclear how it occurs [14,18,44]. According to our current understanding of the phloem, simultaneous transport within one sieve tube cannot occur. Therefore, a more complex phloem structure with separate cell files facilitating acropetal and basipetal transport seems likely [47,48]. The labelling system presented in this work could help gain new insights into carbon transport and a better understanding of phloem structure/function.
The quantitative export data revealed that about 25% of the assimilated carbon is exported from the labelled source leaves within the same photoperiod, most of it already within the first hour after fixation. Higher export rates were previously described for sugar beet, where 24% of carbon was exported to sink leaves after a 1-h chase period [16]. In Curcurbita pepo, 50% of label from a single leaf was found to be exported after a 2-h chase period, with 16% allocated to the roots [15,49]. Export processes in the dark have not been measured in this study, but are likely to be significant. Experiments with whole Arabidopsis rosettes by Zeeman and ap Rees [31], using similar conditions to those described here (i.e. labelling in the middle of the photoperiod), revealed 6.9% export to the root by the end of the day, comparable to our results. However, by the end of the subsequent night, export from the rosette to the root had increased to 22.7%. This night-time export is driven by carbon released from starch during the night, significantly increasing the amount of carbon allocated to sinks during the whole day-night cycle. The time course analysis of the labelled leaf also showed that, as well as storage compounds like starch, carbon flows via intermediary metabolite pools towards the synthesis of end products like cell wall and proteins. These data suggest that leaf 8, despite being one of the most mature leaves on the rosette, is still growing, so not all assimilated carbon will be destined for export. This is consistent with sensitive growth analysis data which reveal a continuation of Arabidopsis leaf expansion for up to 3 weeks [50].
While the flow through acidic and basic fractions was very rapid after the labelling pulse, carbon flow through neutral compounds (e.g. sucrose, glucose and fructose) was slower and peaked well after the end of the labelling - between 15 to 30 min into the chase. Carbon incorporation into starch was fast, with the majority of carbon incorporation during the pulse period and the following 15-min chase. These data are broadly in line with data recently obtained in a detailed study using 13C labelling [51], where the degree of label saturation in the different metabolic intermediates followed quite different kinetics during one hour of labelling (presumably depending on both fluxes and intermediate pool sizes). In that study, sucrose labelling was relatively slow, with glucose and fructose labelling slower still. However, ADPglucose - the precursor for starch biosynthesis - was rapidly labelled. These data are also consistent with the dynamics we observed in label export from the leaf, which approached completion an hour after the pulse. It should be emphasized that, compared with the fairly crude separation of compound classes presented here, 13C labelling coupled with mass spectrometry gives excellent resolution in terms of the degree of labelling of intermediates over time. However, even with today’s technology it is still a major undertaking to measure metabolite fluxes with 13C time-course labelling.
Conclusions
We have shown the design and functionality of a system for single-leaf isotopic labelling in Arabidopsis suitable for both 13CO2 and 14CO2. Although not widely used in the field of plant physiology, 14C-labeling remains a valuable tool. The method is inexpensive and can be introduced easily in the laboratory. Moreover, our study shows that short labelling times are sufficient to obtain robust data, allowing for a medium to high throughput. The method is ideal for measuring carbon export into sink regions, as we recently demonstrated for a mutant affected in callose synthesis in the phloem [52], as well as carbon partitioning within tissues. With sufficient accumulation of radioactivity in the sample, partitioning of fixed carbon in the labelled tissues, as well as imported carbon in unlabelled tissues, can be analysed in further detail. For example, it would be possible to analyse partitioning of assimilated carbon as well as imported carbon in a growing leaf. It would also be possible to study carbon partitioning in response to changing environmental conditions or to observe altered partitioning patterns in any of the vast number of Arabidopsis mutant lines available today. Thus, we suggest that by giving insight into whole-plant carbon allocation, 14CO2 feeding experiments can help to elucidate how carbon metabolism is controlled and provide valuable data for models of plant growth and biomass production.
Methods
Isotopic labelling chambers
A reservoir chamber and a single leaf labelling cuvette were designed and constructed in-house. All chambers are made of Plexiglas with EPDM foam rubber to seal between the different components. The reservoir chamber comprises a lower part made of black Plexiglas, a lid made of transparent Plexiglas, and has an internal volume of 17.3 litres. The chamber is closed via four clamps, sealing with a foam gasket between the upper and lower parts. In the lid, a holder and a fan are mounted. A petri dish containing the isotopically labelled sodium bicarbonate is placed on the holder and acidified with lactic acid to liberate isotopically labelled CO2, which is distributed by the fan. The chamber has two inlets and two outlets which can be used to connect it to other devices (i.e. the leaf cuvette), or sealed if not used. The leaf cuvette is 7 cm long and 3 cm wide and was constructed to label individual leaves of an Arabidopsis plant (see Figure 1). With the leaf cuvette, leaves with a minimum length of approximately 2 cm and a maximum length of 6.5 cm can be labelled. The cuvette consists of a lower and an upper part made of transparent Plexiglas which can be clamped over single leaves. The Plexiglas parts are surrounded by foam gasket to enable an air-tight sealing, yet avoid damage of the leaf petiole. In the lower part of the cuvette is an inlet and an outlet for connecting it to the reservoir chamber containing the isotopically labelled gas. The inlet and outlet feed into opposite ends of the leaf cuvette. A small battery driven pump (Nitto Kohki, Germany) within the reservoir chamber provides an even air-flow (250 ml/min) through the leaf cuvette (Figure 1). Labelling times of 5 to 10 min were typically used, meaning that many replicate plants can be labelled within one experiment. A bypass with three-way taps allows the cuvette to be isolated from the reservoir chamber while replicate plants are exchanged. Further design information on the apparatus is available on request. The entire apparatus was placed inside a fume hood, illuminated with fluorescent bulbs similar to those in the growth chambers used for plant cultivation.
Plant growth conditions
For soil grown plants, Arabidopsis thaliana seeds were sown on Einheitserde Typ ED 73. Sown seeds were stratified for three days at 4°C before being transferred to a Percival AR95 growth cabinet with a 12-h photoperiod (light intensity, 150 μmol quanta m-2 s-1; temperature, 20°C; relative humidity, 65%). For hydroponic culture, seeds were sown in 1.5-ml microcentrifuge tubes filled with 0.65% (w/v) plant agar. The bottom of the tubes was cut off and the tubes were placed in a rack with the cut ends immersed in nutrient solution [53]. The seeds were stratified grown as specified above. The nutrient solution was renewed weekly.
14CO2 pulse-chase labelling
Labelling experiments on single leaves were performed using the same light conditions specified above. By acidification of sodium 14C-bicarbonate (Hartmann Analytic GmbH, Germany; supplied with a specific activity of 59.5 mCi/mmol), 14CO2 was released in the sealed reservoir chamber. This increased the CO2 content in the chamber by only 5–10 ppm. The leaf cuvette was clamped onto an individual mature leaf of the plant. After a ‘pulse’ period (as specified, but typically 5 to 10 min), the cuvette was isolated from the reservoir, opened, the leaf removed, and the plant kept in normal air for a chase period (as specified, but typically 60 min).
Autoradiography
To visualize transport of carbon within the Arabidopsis rosette, autoradiograms were taken after 14CO2 labelling of single leaves. Following the chase period, either the rosette was removed from the root (soil-grown plants) or the whole plant was taken (hydroponically-grown plants) and placed between four layers of filter paper. The plant material was immediately dried at 80°C in a gel drier for 20 min. After drying, the filter paper was exchanged and the plants were further flattened under a weight for 1 week. Afterwards, the plant material was wrapped in transparent cling film and exposed to a Kodak BioMax MR film (as indicated, but typically for 1 week).
Extraction and fractionation of 14CO2 labelled plant tissue
Liquid scintillation counting was used to determine the 14C incorporated into different metabolic fractions. Plant material was harvested and quenched in boiling 80% (v/v) ethanol. The tissue was homogenized using an all-glass homogenizer and extracted in successive 10 min incubations in 80% (v/v) ethanol, 50% (v/v) ethanol, 20% (v/v) ethanol, water, and finally 80% (v/v) ethanol. Between each extraction step the insoluble cell debris was removed by centrifugation. The supernatants were combined and dried in vacuo. The dry residue was dissolved in 2 ml water, giving the water-soluble fraction. Any water-insoluble residue was dissolved in 1 ml 98% (v/v) ethanol and referred to as the ethanol-soluble fraction. The insoluble cell debris was resuspended in 1 ml water. The total 14C in the insoluble fraction was determined by dissolving an aliquot in tissue solubilizer (NCS, GE Healthcare) for 16 h at 23°C. The sum of the label detected in the water-soluble, the ethanol-soluble and the insoluble fractions from all parts of the plant gave the total incorporated 14C. The sum of the label in the unlabelled leaves and the root (where analysed) gave the amount of 14C exported from the labelled leaf.
To measure the 14C incorporated into starch, the insoluble material was digested with amyloglucosidase and α-amylase (from Aspergillus niger and pig pancreas, respectively; Roche) for 16 h at 37°C. The incorporation of 14C into proteins was determined by protease digestion (Protease Type XIV from Streptomyces griseus, Sigma-Aldrich) for 16 h at 37°C. The amount of 14C in the remaining insoluble material (primarily cell wall material) was determined by subtracting the 14C in starch and protein from the overall 14C in the insoluble fraction. The water-soluble fraction was further separated into neutral, acidic, and basic compounds by ion exchange chromatography on sequential columns of a cation exchanger (Dowex 50WX4) and an anion exchanger (Dowex1X8) as described in Zeeman and ap Rees [21]. The resulting neutral fraction was enriched for sugars such as sucrose, glucose and fructose, the acidic fraction was enriched for organic acids and sugar phosphates, and the basic fraction was enriched for amino acids.
The incorporation of 14C into the different metabolic pools was determined by measuring an aliquot of the respective fraction via liquid scintillation counting. Therefore, the aliquot was adjusted to 1 ml with water and 4 ml scintillation cocktail were added. For the ethanol, starch and acidic fractions, Ultima Gold LLT Scintillation Cocktail was used, for all other fractions Ultima Gold Scintillation Cocktail was used (both from Perkin Elmer, Switzerland). After an incubation period of 30 min in the dark, the sample was measured with a LS1801 scintillation counter (Beckmann, Switzerland).
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SZ and KK conceived the project. SZ, KK and PF designed and built the apparatus. KK and AM carried out the experiments. KK and SZ drafted the manuscript. All authors read and approved the final manuscript.
Contributor Information
Katharina Kölling, Email: koellink@ethz.ch.
Antonia Müller, Email: anmuelle@student.ethz.ch.
Patrick Flütsch, Email: patrick.fluetsch@ipw.agrl.ethz.ch.
Samuel C Zeeman, Email: szeeman@ethz.ch.
Acknowledgements
This work was funded by the SystemsX.ch grant “Plant Growth in a Changing Environment” and the EU FP7 contract 245143 (TiMet). We are grateful to Wilhelm Gruissem for his advice.
References
- Moorby J, Evans NTS, Ebert M. Translocation of 11C-labelled photosynthate in soybean. J Exp Bot. 1963;14:210–220. doi: 10.1093/jxb/14.2.210. [DOI] [Google Scholar]
- Minchin PEH, Thorpe MR, Farrar JF. A simple mechanistic model of phloem transport which explains sink priority. J Exp Bot. 1993;44:947–955. doi: 10.1093/jxb/44.5.947. [DOI] [Google Scholar]
- Gould N, Thorpe MR, Pritchard J, Christeller JT, Williams LE, Roeb G, Schurr U, Minchin PEH. AtSUC2 has a role for sucrose retrieval along the phloem pathway: Evidence from carbon-11 tracer studies. Plant Sci. 2012;188:97–101. doi: 10.1016/j.plantsci.2011.12.018. [DOI] [PubMed] [Google Scholar]
- Schwachtje J, Minchin PEH, Jahnke S, Van Dongen JT, Schittko U, Baldwin IT. SNF1-related kinases allow plants to tolerate herbivory by allocating carbon to roots. Proc Natl Acad Sci USA. 2006;103:12935–12940. doi: 10.1073/pnas.0602316103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babst BA, Karve AA, Judt T. Radio-metabolite analysis of carbon-11 biochemical partitioning to non-structural carbohydrates for integrated metabolism and transport studies. Plant Cell Physiol. 2013;54:1016–1025. doi: 10.1093/pcp/pct045. [DOI] [PubMed] [Google Scholar]
- Krishnan P, Kruger NJ, Ratcliffe RG. Metabolite fingerprinting and profiling in plants using NMR. J Exp Bot. 2005;56:255–265. doi: 10.1093/jxb/eri010. [DOI] [PubMed] [Google Scholar]
- Huege J, Sulpice R, Gibon Y, Lisec J, Koehl K, Kopka J. GC-EI-TOF-MS analysis of in vivo carbon-partitioning into soluble metabolite pools of higher plants by monitoring isotope dilution after 13CO2 labelling. Phytochemistry. 2007;68:2258–2272. doi: 10.1016/j.phytochem.2007.03.026. [DOI] [PubMed] [Google Scholar]
- Cliquet JB, Deléens E, Bousser A, Martin M, Lescure JC, Prioul JL, Mariotti A, Morot-Gaudry JF. Estimation of carbon and nitrogen allocation during stalk elongation by C-13 and N-15 tracing in Zea mays L. Plant Physiol. 1990;92:79–87. doi: 10.1104/pp.92.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Améziane R, Deléens E, Noctor G, Morot-Gaudry JF, Limami MA. Stage of development is an important determinant in the effect of nitrate on photoassimilate (13C) partitioning in chicory (Cichorium intybus) J Exp Bot. 1997;48:25–33. doi: 10.1093/jxb/48.1.25. [DOI] [Google Scholar]
- Dyckmans J, Flessa H, Shangguan Z, Beese F. A dual C-13 and N-15 long term labelling technique to investigate uptake and translocation of C and N in beech (Fagus sylvatica L.) Isotopes Environ Health Stud. 2000;36:63–78. doi: 10.1080/10256010008032933. [DOI] [PubMed] [Google Scholar]
- Calvin M. The photosynthetic carbon cycle. J Chem Soc. 1956. pp. 1895–1915.
- Schaefer J, Kier LD, Stejskal EO. Characterization of photorespiration in intact leaves using 13carbon dioxide labeling. Plant Physiol. 1980;65:254–259. doi: 10.1104/pp.65.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon LP, Aronoff S. Metabolism of soybean leaves. IV. Translocation from soybean leaves. Arch Biochem Biophys. 1952;36:383–398. doi: 10.1016/0003-9861(52)90424-4. [DOI] [PubMed] [Google Scholar]
- Aronoff S. Translocation from soybean leaves II. Plant Physiol. 1955;30:184–186. doi: 10.1104/pp.30.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon R, Webb JA. Leaf development and phloem transport in Cucurbita pepo: transition from import to export. Planta. 1973;113:179–191. doi: 10.1007/BF00388202. [DOI] [PubMed] [Google Scholar]
- Fellows RJ, Geiger DR. Structural and physiological changes in sugar beet leaves during sink to source conversion. Plant Physiol. 1974;54:877–885. doi: 10.1104/pp.54.6.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon R. The sink-source transition in leaves. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:119–138. doi: 10.1146/annurev.pp.40.060189.001003. [DOI] [Google Scholar]
- Jones H, Martin RV, Porter HK. Translocation of 14carbon in tobacco following assimilation of 14carbon dioxide by a single leaf. Ann Bot. 1959;23:493–508. [Google Scholar]
- Joy KW. Translocation in sugar beet. I. Assimilation of 14CO2 and distribution of materials from leaves. J Exp Bot. 1964;15:485–494. doi: 10.1093/jxb/15.3.485. [DOI] [Google Scholar]
- Schwender J, Goffman F, Ohlrogge JB, Shachar-Hill Y. Rubisco without the Calvin cycle improves the carbon efficiency of developing green seeds. Nature. 2004;432:779–782. doi: 10.1038/nature03145. [DOI] [PubMed] [Google Scholar]
- Alonso AP, Raymond P, Hernould M, Rondeau-Mouro C, De Graaf A, Chourey P, Lahaye M, Shachar-Hill Y, Rolin D, Dieuaide-Noubhani M. A metabolic flux analysis to study the role of sucrose synthase in the regulation of the carbon partitioning in central metabolism in maize root tips. Metab Eng. 2007;9:419–432. doi: 10.1016/j.ymben.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Williams TCR, Miguet L, Masakapalli SK, Kruger NJ, Sweetlove LJ, Ratcliffe RG. Metabolic network fluxes in heterotrophic Arabidopsis cells: Stability of the flux distribution under different oxygenation conditions. Plant Physiol. 2008;148:704–718. doi: 10.1104/pp.108.125195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JD, Shastri AA, Stephanopoulos G, Morgan JA. Mapping photoautotrophic metabolism with isotopically nonstationary C-13 flux analysis. Metab Eng. 2011;13:656–665. doi: 10.1016/j.ymben.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho LC, Shaw AF. Carbon economy and translocation of C-14 in leaflets of the seventh leaf of tomato during leaf expansion. Ann Bot. 1977;41:833–848. [Google Scholar]
- Farrar SC, Farrar JF. Carbon fluxes in leaf blades of barley. New Phytol. 1985;100:271–283. doi: 10.1111/j.1469-8137.1985.tb02778.x. [DOI] [Google Scholar]
- Dickson RE, Larson PR. Incorporation of 14C-photosynthate into major chemical fractions of source and sink leaves of cottonwood. Plant Physiol. 1975;56:185–193. doi: 10.1104/pp.56.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieleski RL, Redgwell RJ. Sorbitol versus sucrose as photosynthesis and translocation products in developing apricot leaves. Aust J Plant Physiol. 1985;12:657–668. doi: 10.1071/PP9850657. [DOI] [Google Scholar]
- Pumpanen JS, Heinonsalo J, Rasilo T, Hurme KR, Ilvesniemi H. Carbon balance and allocation of assimilated CO2 in Scots pine, Norway spruce, and Silver birch seedlings determined with gas exchange measurements and C-14 pulse labelling. Trees-Struct Funct. 2009;23:611–621. doi: 10.1007/s00468-008-0306-8. [DOI] [Google Scholar]
- Atkinson CJ, Farrar JF. Allocation of photosynthetically-fixed carbon in Festuca ovina L. and Nardus stricta L. New Phytol. 1983;95:519–531. doi: 10.1111/j.1469-8137.1983.tb03517.x. [DOI] [Google Scholar]
- DaCosta M, Huang BR. Changes in carbon partitioning and accumulation patterns during drought and recovery for colonial bentgrass, creeping bentgrass, and velvet bentgrass. J Am Soc Hortic Sci. 2006;131:484–490. [Google Scholar]
- Zeeman SC, Ap Rees T. Changes in carbohydrate metabolism and assimilate export in starch-excess mutants of Arabidopsis. Plant Cell Environ. 1999;22:1445–1453. doi: 10.1046/j.1365-3040.1999.00503.x. [DOI] [Google Scholar]
- Sun JD, Okita TW, Edwards GE. Modification of carbon partitioning, photosynthetic capacity, and O2 sensitivity in arabidopsis plants with low ADP-glucose pyrophosphorylase activity. Plant Physiol. 1999;119:267–276. doi: 10.1104/pp.119.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draborg H, Villadsen D, Nielsen TH. Transgenic Arabidopsis plants with decreased activity of frustose-6-phosphate,2-kinase/fructose-2,6-bisphosphatase have altered carbon partitioning. Plant Physiol. 2001;126:750–758. doi: 10.1104/pp.126.2.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundmark M, Cavaco AM, Trevanion S, Hurry V. Carbon partitioning and export in transgenic Arabidopsis thaliana with altered capacity for sucrose synthesis grown at low temperature: a role for metabolite transporters. Plant Cell and Environ. 2006;29:1703–1714. doi: 10.1111/j.1365-3040.2006.01543.x. [DOI] [PubMed] [Google Scholar]
- Schneider A, Häusler RE, Kolukisaoglu Ü, Kunze R, van der Graaff E, Schwacke R, Catoni E, Desimone M, Flügge UI. An Arabidopsis thaliana knock-out mutant of the chloroplast triose phosphate/phosphate translocator is severely compromised only when starch synthesis, but not starch mobilisation is abolished. Plant J. 2002;32:685–699. doi: 10.1046/j.1365-313X.2002.01460.x. [DOI] [PubMed] [Google Scholar]
- Nielsen TH. Intermediary glucan structures formed during starch granule biosynthesis are enriched in short side chains, a dynamic pulse labeling approach. J Biol Chem. 2002;277:20249–20255. doi: 10.1074/jbc.M201866200. [DOI] [PubMed] [Google Scholar]
- Larson PR, Dickson RE. Distribution of imported C-14 in developing leaves of eastern cottonwood according to phyllotaxy. Planta. 1973;111:95–112. doi: 10.1007/BF00386270. [DOI] [PubMed] [Google Scholar]
- Watson MA, Casper BB. Morphogenetic constraints on patterns of carbon distribution in plants. Annu Rev Ecol Syst. 1984;15:233–258. doi: 10.1146/annurev.es.15.110184.001313. [DOI] [Google Scholar]
- Avery GS. Structure and development of the tobacco leaf. Am J Bot. 1933;20:565–592. doi: 10.2307/2436259. [DOI] [Google Scholar]
- Jones H, Eagles JE. Translocation of 14carbon within and between leaves. Ann Bot. 1962;26:505–511. [Google Scholar]
- Donnelly PM, Bonetta D, Tsukaya H, Dengler RE, Dengler NG. Cell cycling and cell enlargement in developing leaves of Arabidopsis. Dev Biol. 1999;215:407–419. doi: 10.1006/dbio.1999.9443. [DOI] [PubMed] [Google Scholar]
- Andriankaja M, Dhondt S, De Bodt S, Vanhaeren H, Coppens F, De Milde L, Mühlenbock P, Skirycz A, Gonzalez N, Beemster GTS, Inzé D. Exit from proliferation during leaf development in Arabidopsis thaliana: a not-so-gradual process. Dev Cell. 2012;22:64–78. doi: 10.1016/j.devcel.2011.11.011. [DOI] [PubMed] [Google Scholar]
- Fitzgibbon J, Beck M, Zhou J, Faulkner C, Robatzek S, Oparka K. A developmental framework for complex plasmodesmata formation revealed by large-scale imaging of the Arabidopsis leaf epidermis. Plant Cell. 2013;25:57–70. doi: 10.1105/tpc.112.105890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crafts AS. The mechanism of translocation: Methods of study with C14-labeled 2,4-D. Hilgardia. 1956;26:287–334. [Google Scholar]
- Busse JS, Evert RF. Vascular differentiation and transition in the seedling of Arabidopsis thaliana (Brassicaceae) Int J Plant Sci. 1999;160:241–251. doi: 10.1086/314117. [DOI] [Google Scholar]
- Kang J, Tang J, Donnelly P, Dengler N. Primary vascular pattern and expression of ATHB-8 in shoots of Arabidopsis. New Phytol. 2003;158:443–454. doi: 10.1046/j.1469-8137.2003.00769.x. [DOI] [PubMed] [Google Scholar]
- Fritz E. Microautoradiographic investigations on bidirectional translocation in phloem of Vicia faba. Planta. 1973;112:169–179. doi: 10.1007/BF00388587. [DOI] [PubMed] [Google Scholar]
- Haritatos E, Ayre BG, Turgeon R. Identification of phloem involved in assimilate loading in leaves by the activity of the galactinol synthase promoter. Plant Physiol. 2000;123:929–937. doi: 10.1104/pp.123.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb JA, Gorham PR. Translocation of photosynthetically assimilated C14 in straight-necked squash. Plant Physiol. 1964;39:663–672. doi: 10.1104/pp.39.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baerenfaller K, Massonnet C, Walsh S, Baginsky S, Bühlmann P, Hennig L, Hirsch-Hoffmann M, Howell KA, Kahlau S, Radziejwoski A. et al. Systems-based analysis of Arabidopsis leaf growth reveals adaptation to water deficit. Mol Syst Biol. 2012;8:606. doi: 10.1038/msb.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szecowka M, Heise R, Tohge T, Nunes-Nesi A, Vosloh D, Huege J, Feil R, Lunn J, Nikoloski Z, Stitt M. et al. Metabolic fluxes in an iIluminated Arabidopsis rosette. Plant Cell. 2013;25:694–714. doi: 10.1105/tpc.112.106989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt DH, Koelling K, Graf A, Pike M, Calder G, Findlay K, Zeeman SC, Smith AM. Callose synthase GSL7 Is necessary for normal phloem transport and inflorescence growth in Arabidopsis. Plant Physiol. 2011;155:328–341. doi: 10.1104/pp.110.166330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibeaut DM, Hulett J, Cramer GR, Seemann JR. Maximal biomass of Arabidopsis thaliana using a simple, low-maintenance hydroponic method and favorable environmental conditions. Plant Physiol. 1997;115:317–319. doi: 10.1104/pp.115.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]


