Abstract
Body size has long been recognized to play a key role in shaping species interactions. For example, while small species thrive in a diversity of environments, they typically lose aggressive contests for resources with larger species. However, numerous examples exist of smaller species dominating larger species during aggressive interactions, suggesting that the evolution of traits can allow species to overcome the competitive disadvantage of small size. If these traits accumulate as lineages diverge, then the advantage of large size in interspecific aggressive interactions should decline with increased evolutionary distance. We tested this hypothesis using data on the outcomes of 23,362 aggressive interactions among 246 bird species pairs involving vultures at carcasses, hummingbirds at nectar sources, and antbirds and woodcreepers at army ant swarms. We found the advantage of large size declined as species became more evolutionarily divergent, and smaller species were more likely to dominate aggressive contests when interacting with more distantly-related species. These results appear to be caused by both the evolution of traits in smaller species that enhanced their abilities in aggressive contests, and the evolution of traits in larger species that were adaptive for other functions, but compromised their abilities to compete aggressively. Specific traits that may provide advantages to small species in aggressive interactions included well-developed leg musculature and talons, enhanced flight acceleration and maneuverability, novel fighting behaviors, and traits associated with aggression, such as testosterone and muscle development. Traits that may have hindered larger species in aggressive interactions included the evolution of morphologies for tree trunk foraging that compromised performance in aggressive contests away from trunks, and the evolution of migration. Overall, our results suggest that fundamental trade-offs, such as those associated with body size, are more likely to break down over evolutionary time, changing the rules that govern species interactions and structure ecological communities.
Introduction
Phylogenetic perspectives have changed the way we view ecological communities by incorporating evolutionary history into explanations of patterns of coexistence and resource use [1], [2]. Because closely-related species are more likely to share traits and be ecologically similar due to recent, shared ancestry, phylogenetic relationships may influence the degree to which species can coexist [1], [2]. One particular way that evolutionary relatedness can influence community structure is by altering the trade-offs that constrain species interactions and distributions [3], [4]. For example, small-sized animals benefit from reduced energetic and water requirements for survival and reproduction, reduced developmental times, reduced costs of locomotion, greater ability to quickly shed or absorb heat, greater maneuverability, and faster response time [5], [6]. However, small-sized animals typically lose aggressive contests for resources because larger animals generate greater force for a given acceleration, require a greater opposing force to overcome their inertia or change their momentum, and have greater muscle mass and strength, stronger defensive coverings, and larger and stronger traits used as weapons (e.g., teeth, bills, claws) [5], [7], [8]. Yet, the dominance of larger body size is not universal. Smaller species can overcome the advantages of large size in aggressive contests with the evolution of novel traits or trait values (e.g., weapons, enhanced maneuverability, or social behavior) that offset the disadvantage of small size (e.g., [9]–[11]). Such novel traits should accumulate over evolutionary time [3], [12], leading to the hypothesis that the advantage of large size in aggressive interactions should decline with evolutionary distance among the species.
Here, we test this hypothesis using data from 23,362 interactions among 246 species pairs, representing three phylogenetically and ecologically distinct groups of birds that have been studied extensively with respect to aggressive contests for shared food resources. These three groups are: 1) New World vultures (Accipitriformes: Cathartidae) and Old World vultures (Accipitriformes: Accipitridae) interacting at carcasses, 2) hummingbirds (Apodiformes: Trochilidae) interacting at nectar sources, and 3) antbirds (Passeriformes: Thamnophilidae) and woodcreepers (Passeriformes: Dendrocolaptidae) competing for invertebrates and small vertebrates fleeing from army ant swarms (Hymenoptera: Formicidae) [13].
Materials and Methods
Aggressive Interactions
We compiled published quantitative data on aggressive interactions between our focal species (vultures, hummingbirds, and woodcreepers and antbirds) and any other species of bird, but restricted our analysis to pairs of species with at least 6 interactions where each interaction was won by one of the two species (Figures 1–3; Tables S1, S2). We compiled data on aggressive interactions that were associated with a shared resource (following [14]), including (a) chases, where one species actively pursued the other species, (b) supplants and displacements, where one species actively flew at, lunged, pecked, or otherwise aggressively engaged another species, causing the other species to retreat, and (c) physical attacks, where one species fought with another species (e.g., pecking, grabbing, hitting with wings, pinning to ground), resulting in the losing species retreating from the altercation [14]. We included only observations where one species was a clear winner over the other, as described in the original reference. We excluded observations that could be viewed as defense of eggs, young, or nests because these interactions did not involve a shared resource, and because the fitness costs of losing offspring are higher for the parent species. We excluded interactions that involved more than one individual of each of two species. When possible, we excluded interactions involving young birds; however, details of age were often unavailable. We supplemented our interaction dataset with our own unpublished natural history observations of hummingbirds interacting at feeders in South America.
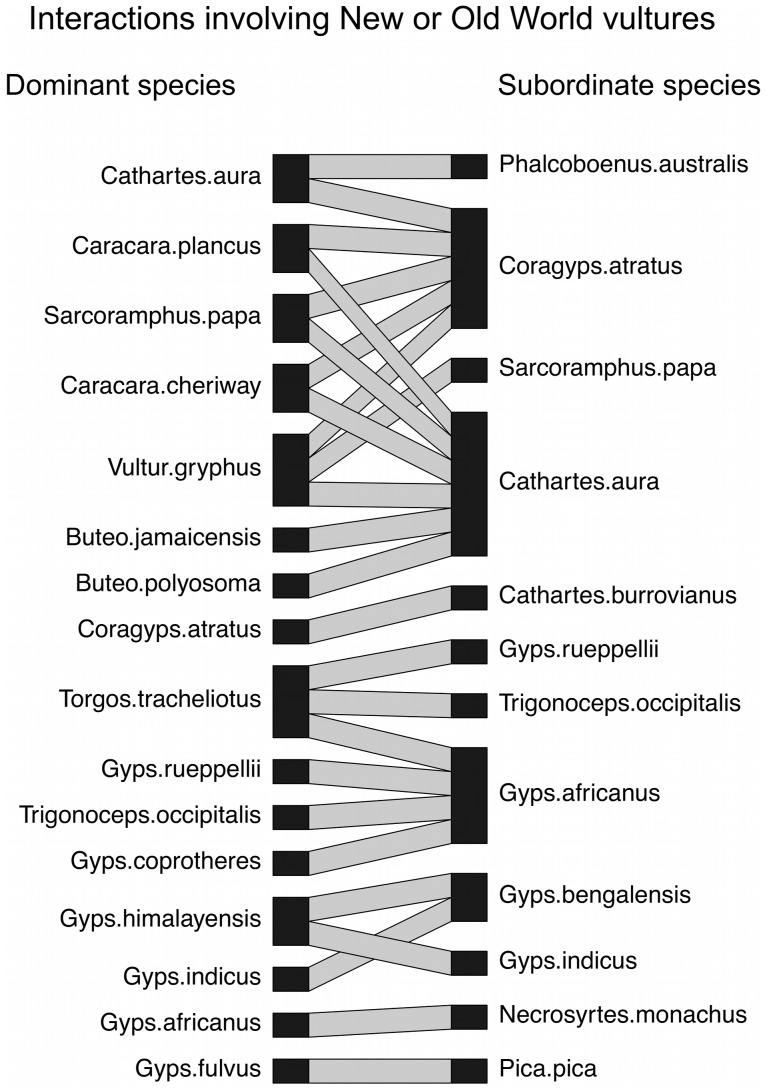
Figure 1. An interaction web for aggressive interactions involving New World vultures (Cathartidae) or Old World vultures (Accipitridae) at a carrion food source.
Lines connect species pairs for which we include data on aggressive interactions in this study. Dominant species (left column) were defined as species winning the majority of aggressive interactions with the subordinate species (right column).
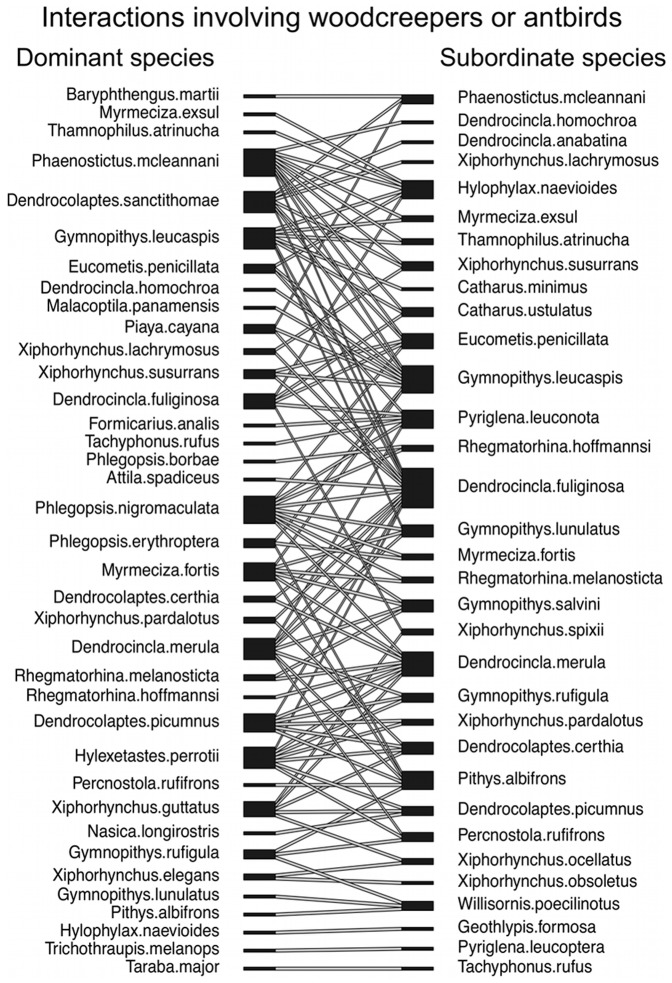
Figure 3. An interaction web for aggressive interactions involving antbirds (Thamnophilidae) or woodcreepers (Dendrocolaptidae) at army ant swarms, where they feed on prey flushed by the ants.
Lines connect species pairs for which we include data on aggressive interactions in this study. Dominant species (left column) were defined as species winning the majority of aggressive interactions with the subordinate species (right column). One species pair (Pyriglena leucoptera — Tachyphonus coronatus) was omitted from this figure because each species won the same number of interactions with the other.
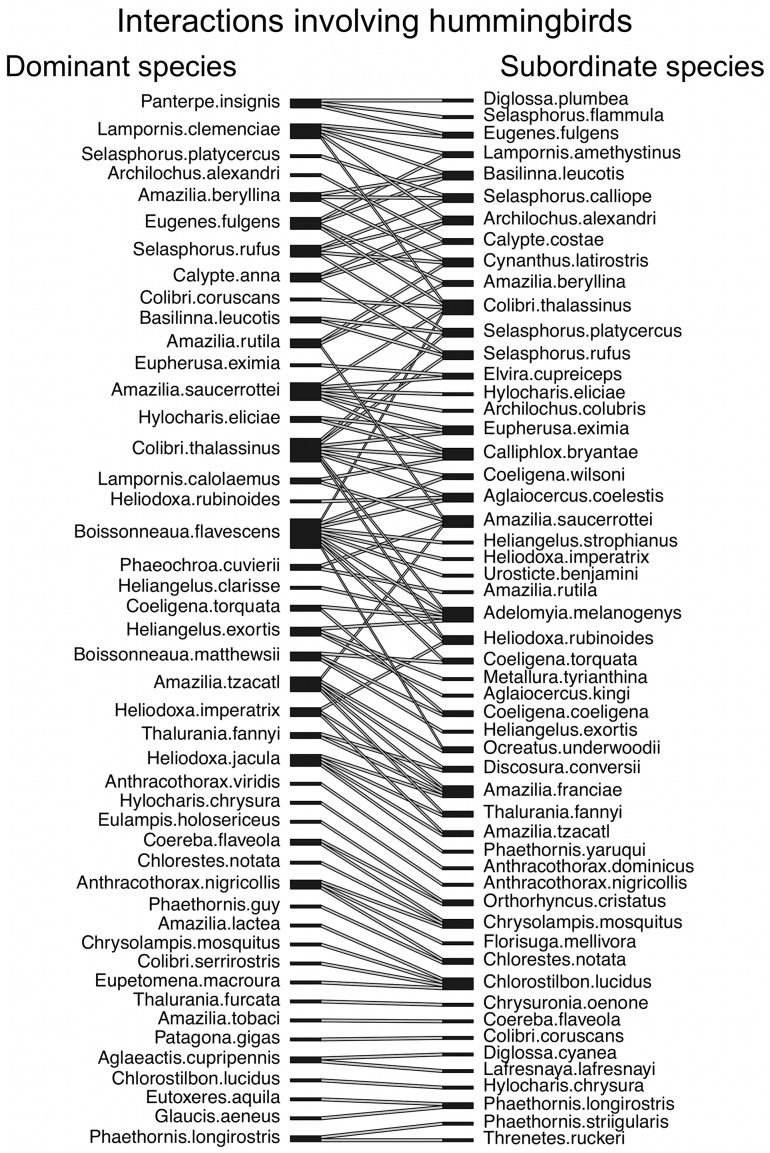
Figure 2. An interaction web for aggressive interactions involving hummingbirds (Trochilidae) at a nectar food source.
Lines connect species pairs for which we include data on aggressive interactions in this study. Dominant species (left column) were defined as species winning the majority of aggressive interactions with the subordinate species (right column). One species pair (Hylocharis chrysura — Thalurania furcata) was omitted from this figure because each species won the same number of interactions with the other.
Overall, we compiled data on 23,362 aggressive interactions among 246 species pairs (Tables S1, S2). In total, 145 different species were represented in our dataset, including 99 species as dominant (i.e., winning the majority of aggressive interactions with another species) and 99 species as subordinate (i.e., losing the majority of aggressive interactions with another species) (Figures 1–3). Twenty-seven pairs of interacting species were in the same genus, 157 pairs were in different genera but in the same family, and 62 pairs represented different taxonomic families. On average, each of the 145 species was represented in 3.4 pairwise interactions (range 1–18).
Body Mass
Whenever possible, we obtained average adult mass for each species in our study from the same studies that provided the interaction data. Otherwise, we obtained adult mass data from areas as geographically close as possible to the populations where the interaction data originated. We calculated the mean mass of each species as the mean of males (mean male mass) and females (mean female mass), if males and females had different masses. We provide the sources for mass data for each species pair in Table S2.
Genetic Distance
We compiled mitochondrial genetic sequence data from Genbank (accession numbers are provided in Text S1). Once aligned, we measured genetic distance between the sequences of focal species pairs using MEGA version 5.0 [15]. We calculated between-group mean Tamura-Nei genetic distances because this measure corrects for multiple substitutions at one site, incorporates differences in substitution rates between nucleotides, and does not assume equal nucleotide frequencies [16]. Species interactions lacking relevant genetic sequence data were excluded from our analysis.
We preferentially used the mitochondrial gene cytochrome b (cytb) to estimate genetic distance between pairs of interacting species because this gene appears to evolve in a clock-like fashion in birds [17]. However, too few hummingbird species had cytb sequences available, so we examined the mitochondrial gene NADH dehydrogenase subunit 2 (ND2) for hummingbirds. We aligned sequences of each gene with the same gene from the chicken (Gallus gallus) [18] using Clustal X [19], visually inspected the sequences using MacClade version 4.08 [20], and removed sequences that did not align with the chicken sequence. We included transitions and transversions, all codon positions, assumed uniform rates among sites and homogeneous patterns among lineages, and used pairwise deletion to address gaps or missing data [15]. We calculated genetic distances for each pair of interacting species when species were in the same taxonomic family. For species representing different families, we calculated the genetic distances incorporating all sequences of species in those families that were included in our study to improve the accuracy of our longer distance estimates.
Statistical Tests
We conducted all of our statistical tests in R [21]. We tested the hypothesis that the importance of large size in determining the outcome of aggressive contests declined with genetic distance between interacting species using a linear mixed-effects model with sqrt{ln[(wins by dominant species+1)/(wins by subordinate species+1)]} as the response variable, {(mass of dominant species−mass of subordinate species)/[(mass of dominant species+mass of subordinate species)/2]} and genetic distance as predictors within a saturated model, and group (vultures, hummingbirds, or antbirds/woodcreepers) as a random factor, with a Gaussian distribution using the R package nlme [22]. The dominant species was defined as the species that won the majority of aggressive contests. We also tested if smaller bird species were more likely to be dominant when interacting with a more distantly-related species using a generalized linear mixed model with the response variable equal to 0 if the larger species was dominant, and 1 if the smaller species was dominant, genetic distance and number of interactions between species as predictors in a saturated model, and group (vultures, hummingbirds, or antbirds/woodcreepers) as a random factor, with a binomial distribution using the R package lme4 [23]. In our generalized linear model, we standardized both genetic distance and the number of interactions between species prior to analysis by subtracting the mean and dividing by 2 standard deviations, using the rescale command in the R package arm [24]. Two species comparisons were excluded from analyses because each species won the same number of aggressive encounters, and thus we could not designate a dominant and subordinate species.
We used a mixed models approach with group (vultures, hummingbirds, woodcreepers/antbirds) as a random factor because interactions between species within vultures, hummingbirds, and woodcreepers/antbirds often lacked independence (e.g., one species interacted with more than one other species; Figures 1–3). In contrast, we had no overlap of species across our three groups. We also ran our analyses including either the dominant species or the subordinate species as a random factor, nested within group, to ensure that our results were not influenced by one or a few species that were small and dominant or large and subordinate. We did not run our analysis with both dominant and subordinate species as random factors because each interaction among species pairs was unique.
We first ran the saturated model with different random slopes and intercepts and chose the best model as the model with the lowest Akaike Information Criterion (AIC) value [25]. We then checked the fit of the best saturated model following [25]. If saturated models did not adequately fit the data (e.g., residuals were significantly different from normal for linear mixed-effects models), we either modeled heterogeneity and assessed new model performance using AIC values and improved model fit [25], or transformed dependent or independent variables to improve model fit. We then ran models that incorporated all possible combinations of predictor variables using the R package MuMIn [26], and compared the fit among models using AIC values adjusted for small sample sizes (AICc). We identified the best-fit model as the model with the lowest AICc score. We ran our generalized linear mixed models using both the R packages lme4 [23] and MASS [27] to ensure that our results were consistent across packages [25].
We ran two additional generalized linear models to test our hypothesis, with the same predictor and response variables as in the saturated linear mixed-effects and generalized linear mixed models, but with group (vultures, hummingbirds, antbirds/woodcreepers) entered as a predictor variable in a saturated model (without random effects). This model allowed us to test for variation in the effects of genetic distance and body mass on the outcome of aggressive interactions between our 3 focal groups. The model, however, assumes that interactions among species are independent — an assumption that was violated by some species interacting with multiple species within groups. We ran models incorporating all possible combinations of predictor variables and checked the fit of our models as in our previous models.
Number of Interactions among Species Pairs
The number of aggressive interactions over shared resources are expected to decline as species diverge over evolutionary time because more distantly-related species, on average, are ecologically more distinct [8], [28]. Small species could win more interactions with larger species when interactions are rare if they can bluff or if species are more inclined to back down when the other species is poorly known. We did not include the number of interactions among species pairs in our main linear mixed-effects model because the number of interactions was already incorporated into the dependent variable. We did, however, include the total number of interactions among species pairs in our generalized linear mixed model.
The total number of interactions in our generalized linear mixed model may be biased by the inclusion of different studies with different effort and sample sizes. Thus, we ran the analysis again including each study as a random factor nested within group (vultures, hummingbirds, woodcreepers/antbirds). By including study as a random factor, we could address if the number of interactions among species pairs within studies influenced our main results. We included only studies that had at least 2 species pairs, and included only species pairs with at least 6 interactions within a study. We again included the number of interactions and genetic distance as predictors in a saturated model, with the response variable equal to 0 if the larger species was dominant, and 1 if the smaller species was dominant, and a binomial distribution using the R package lme4 [23]. For all analyses, the number of interactions was transformed to improve model fit, as: n = ((log(log(number of interactions)))∧0.1).
Results
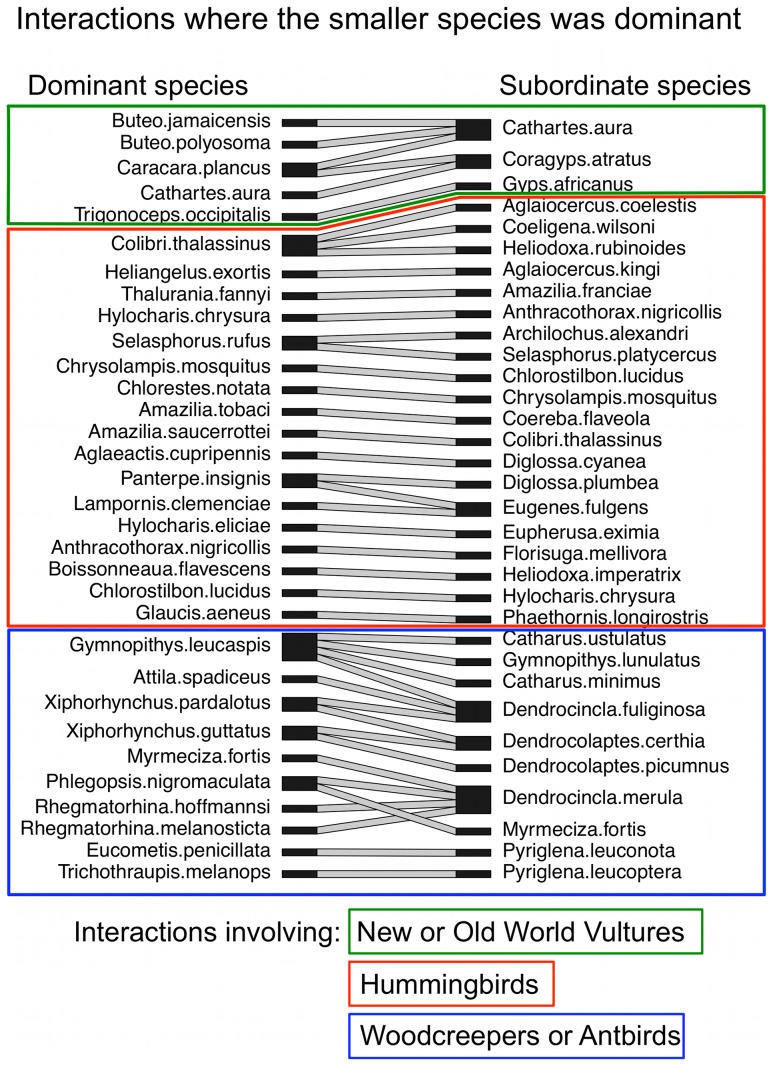
The larger species won the majority of aggressive interactions with the smaller species (i.e., was behaviorally dominant) in 201 of 246 species pairs (81.7%). Smaller species were behaviorally dominant in 43 species pairs (17.5%) involving 32 different small, dominant species, and 33 different large, subordinate species (Figure 4). Wins and losses were equal in only two species pairs. In some cases where smaller species were behaviorally dominant, differences in weight were small and perhaps insignificant. In 32 species pairs, however, the larger species was>5% heavier than the smaller, dominant species, while 25 species pairs involved larger species that were>10% heavier than the smaller, dominant species.
Figure 4. An interaction web for aggressive interactions where the smaller (lighter) species was dominant to the heavier species.
Each interacting species pair where the smaller species was dominant is connected by a line (n = 43 species pairs). Dominant species (left column) were defined as species winning the majority of aggressive interactions with the subordinate species (right column). Species pairs that include a vulture are indicated by a green box (top), hummingbird by a red box (middle), and antbird or woodcreeper by a blue box (bottom).
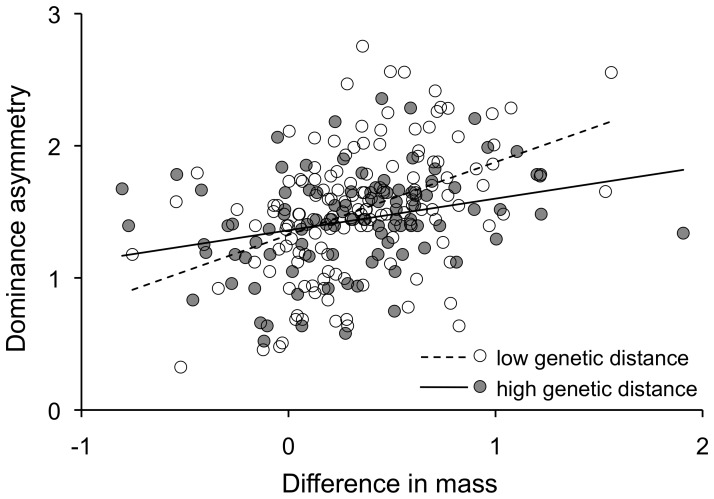
The advantage of large size in aggressive contests was greatest among closely-related species and declined with increasing genetic distance (Figure 5; Tables 1,2; see Figure S1 for a 3-dimensional plot that includes difference in mass, genetic distance and dominance asymmetry). Similarly, smaller species were more likely to be dominant in aggressive interactions with distantly-related species (Figure 6; Tables 3,4). For example, among interacting species in the same genus, the smaller species was dominant in only 2 of 27 species pairs (7.4%), whereas among interacting species in different taxonomic families, the smaller species was dominant in 18 of 62 species pairs (29.0%). The decline in the importance of large size in aggressive interactions with increased genetic distance was evident in each of the three independent groups and did not differ significantly between them (Tables 5,6).
Figure 5. Relatively heavier bird species were more likely to win aggressive contests for resources (positive slope), but the advantage of large size in aggressive contests declined with genetic distance (shallower slope for high genetic distance).
Dominance asymmetry (y-axis) = sqrt {ln ((wins by dominant species+1)/(wins by subordinate species+1))}. Difference in mass (x-axis) = (mass of dominant species−mass of subordinate species)/(average mass of dominant and subordinate species). Genetic distance groups are split by the midpoint value for the dataset (low = 0.006–0.179; high = 0.180–0.352).
Table 1. Comparison of model fit for different linear mixed-effects models that include all possible combinations of predictor variables.
| Model ranking | Intercept1 | Difference in mass | Genetic distance | Interaction between difference in mass and genetic distance | df | logLik | AICc | delta AICc | weight |
| 1 | 1.17 | 0.84 | 0.91 | −2.46 | 6 | −120.5 | 253.3 | 0.00 | 0.882 |
| 2 | 1.35 | 0.37 | — | — | 4 | −124.9 | 258.0 | 4.72 | 0.083 |
| 3 | 1.31 | 0.37 | 0.24 | — | 5 | −124.8 | 259.8 | 6.49 | 0.034 |
| 4 | 1.47 | —2 | — | — | 3 | −140.5 | 287.1 | 33.85 | 0.000 |
| 5 | 1.45 | — | 0.14 | — | 4 | −140.5 | 289.1 | 35.83 | 0.000 |
Models tested the prediction that the advantage of large size in aggressive contests for resources declines with genetic distance among interacting bird species (n = 244 species pairs).
numbers for predictor variables are effect sizes.
long dash (—) indicates that the predictor variable was absent from the model.
Table 2. Results from the best-fit (lowest AICc) linear mixed-effects model testing the prediction that the advantage of large size in aggressive contests for resources declines with genetic distance among interacting bird species (n = 244 species pairs).
| Fixed effects1 | Estimate | SE | t | df | P |
| Intercept | 1.17 | 0.10 | 11.91 | 238 | <0.0001 |
| Difference in mass2 | 0.84 | 0.17 | 4.91 | 238 | <0.0001 |
| Genetic distance | 0.91 | 0.48 | 1.89 | 238 | 0.0598 |
| Difference in mass2×Genetic distance | −2.46 | 0.84 | −2.94 | 238 | 0.0036 |
dependent = sqrt{ln[(wins by dominant species+1)/(wins by subordinate species+1)]}; taxonomic group included as a random effect.
(mass of dominant species−mass of subordinate species)/(average mass of dominant and subordinate species).
Figure 6. Smaller (lighter) bird species were more likely to be dominant (i.e., win the majority of aggressive contests for resources) when interacting with a more distantly-related species (greater genetic distance between species).
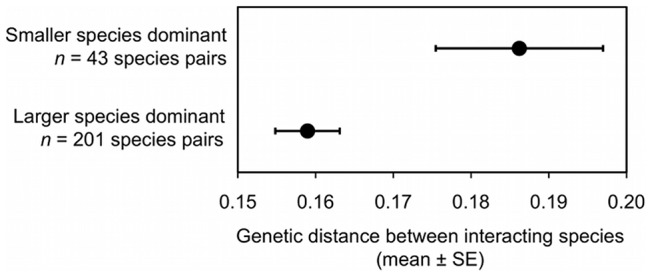
Data are from aggressive interactions involving vultures at carcasses, hummingbirds at nectar sources, and woodcreepers and antbirds at army ant swarms.
Table 3. Comparison of model fit for different generalized linear mixed models (binomial distribution) that include all possible combinations of predictor variables.
| Model ranking | Intercept1 | Genetic Distance | Number of interactions | Interaction between genetic distance and number of interactions | df | logLik | AICc | delta AICc | weight |
| 1 | −1.60 | 0.89 | — | — | 3 | −110.1 | 226.4 | 0.00 | 0.587 |
| 2 | −1.61 | 0.91 | 0.07 | — | 4 | −110.1 | 228.4 | 2.02 | 0.213 |
| 3 | −1.58 | 0.96 | 0.00 | 0.76 | 5 | −109.6 | 229.4 | 3.03 | 0.129 |
| 4 | −1.54 | —2 | — | — | 2 | −113.6 | 231.3 | 4.88 | 0.051 |
| 5 | −1.54 | — | −0.10 | — | 3 | −113.6 | 233.2 | 6.84 | 0.019 |
Models tested the prediction that smaller bird species were more likely to win aggressive contests for resources when interacting with a more distantly-related species (n = 244 species pairs).
numbers for predictor variables are effect sizes; genetic distance and number of interactions were standardized prior to analysis by subtracting the mean and dividing by 2 standard deviations.
long dash (—) indicates that the predictor variable was absent from the model.
Table 4. Results from the best-fit (lowest AICc) generalized linear mixed model (binomial distribution) testing the prediction that smaller bird species were more likely to win aggressive contests for resources when interacting with a more distantly-related species (n = 244 species pairs).
| Fixed effects1 | Estimate | SE | z | P |
| Intercept | −1.60 | 0.18 | −9.08 | <0.0001 |
| Genetic distance2 | 0.89 | 0.35 | 2.59 | 0.0098 |
dependent = 0 if larger species was dominant, 1 if smaller species was dominant; taxonomic group included as a random effect.
standardized prior to analysis by subtracting the mean and dividing by 2 standard deviations.
Table 5. Comparison of model fit for different generalized linear models, with taxonomic group entered as a predictor that include all possible combinations of predictor variables.
| Model ranking | Intercept1 | Taxonomic group | Difference in mass | Genetic distance | Interaction between group and difference in mass | Interaction between group and genetic distance | Interaction between difference in mass and genetic distance | Interaction between group, genetic distance and mass | df | logLik | AICc | delta AICc | weight |
| 1 | 1.08 | +2 | 0.85 | 0.85 | — | — | −2.53 | — | 7 | −117.2 | 249.0 | 0.00 | 0.387 |
| 2 | 0.79 | + | 0.93 | 3.26 | — | + | −2.92 | — | 9 | −115.3 | 249.3 | 0.35 | 0.325 |
| 3 | 1.11 | + | 0.78 | 0.93 | + | — | −2.88 | — | 9 | −116.4 | 251.6 | 2.65 | 0.103 |
| 4 | 0.82 | + | 0.87 | 3.35 | + | + | −3.32 | — | 11 | −114.5 | 252.1 | 3.11 | 0.082 |
| 5 | 1.27 | + | 0.37 | — | — | — | — | — | 5 | −121.9 | 254.0 | 5.07 | 0.031 |
| 6 | 1.17 | —3 | 0.85 | 0.93 | — | — | −2.44 | — | 5 | −122.1 | 254.5 | 5.59 | 0.024 |
| 7 | 0.65 | + | 1.19 | 4.51 | + | + | −5.35 | + | 13 | −113.6 | 254.8 | 5.87 | 0.021 |
| 8 | 1.25 | + | 0.37 | 0.19 | — | — | — | — | 6 | −121.8 | 255.9 | 6.99 | 0.012 |
| 9 | 1.28 | + | 0.32 | — | + | — | — | — | 7 | −121.4 | 257.3 | 8.34 | 0.006 |
| 10 | 1.10 | + | 0.37 | 1.50 | — | + | — | — | 8 | −120.8 | 258.3 | 9.31 | 0.004 |
| 11 | 1.35 | — | 0.39 | — | — | — | — | — | 3 | −126.4 | 259.0 | 10.03 | 0.003 |
| 12 | 1.26 | + | 0.33 | 0.17 | + | — | — | — | 8 | −121.3 | 259.3 | 10.32 | 0.002 |
| 13 | 1.31 | — | 0.39 | 0.23 | — | — | — | — | 4 | −126.3 | 260.7 | 11.79 | 0.001 |
| 14 | 1.12 | + | 0.34 | 1.46 | + | + | — | — | 10 | −120.4 | 261.7 | 12.76 | 0.001 |
| 15 | 1.41 | + | — | — | — | — | — | — | 4 | −137.2 | 282.5 | 33.59 | 0.000 |
| 16 | 1.40 | + | — | 0.12 | — | — | — | — | 5 | −137.2 | 284.6 | 35.62 | 0.000 |
| 17 | 1.29 | + | — | 1.09 | — | + | — | — | 7 | −136.6 | 287.8 | 38.81 | 0.000 |
| 18 | 1.48 | — | — | — | — | — | — | — | 2 | −143.5 | 291.1 | 42.19 | 0.000 |
| 19 | 1.47 | — | — | 0.09 | — | — | — | — | 3 | −143.5 | 293.2 | 44.20 | 0.000 |
Models tested the prediction that the advantage of large size in aggressive contests for resources declines with genetic distance among interacting bird species (n = 244 species pairs).
numbers for predictor variables are effect sizes.
plus sign (+) indicates that the predictor variable (factor) was included in the model.
long dash (—) indicates that the predictor variable was absent from the model.
Table 6. Results from the best-fit (lowest AICc) generalized linear model, with taxonomic group included as a predictor variable, testing the prediction that the advantage of large size in aggressive contests for resources declines with genetic distance among interacting bird species (n = 244 species pairs).
| Fixed effects1 | Estimate | SE | t | df | P |
| Intercept2 | 1.08 | 0.11 | 9.70 | 243,238 | <0.0001 |
| Taxonomic group (hummingbirds) | 0.05 | 0.09 | 0.58 | 243,238 | 0.56 |
| Taxonomic group (antbirds/woodcreepers) | 0.20 | 0.09 | 2.21 | 243,238 | 0.028 |
| Difference in mass3 | 0.85 | 0.17 | 4.93 | 243,238 | <0.0001 |
| Genetic distance | 0.85 | 0.49 | 1.76 | 243,238 | 0.08 |
| Difference in mass3×Genetic distance | −2.53 | 0.84 | −3.01 | 243,238 | 0.0029 |
dependent = sqrt{ln[(wins by dominant species+1)/(wins by subordinate species+1)]}.
intercept value for taxonomic group = vultures.
(mass of dominant species−mass of subordinate species)/(average mass of dominant and subordinate species).
The decline in the advantage of large size with increased genetic distance was not caused by variation in the number of interactions among species pairs (overall number of interactions: Tables 3,4; number of interactions within a study: Tables S3,S4). The number of interactions among species (overall, or within a study) also did not predict when small bird species would be dominant in aggressive interactions (overall number of interactions: Tables 3,4; number of interactions within a study: Tables S3,S4).
Including species (either the dominant species, or the subordinate species) as a random factor, nested within group, yielded similar results for both linear mixed-effects models (Table S5) and generalized linear mixed models (Table S6), with the interaction between genetic distance and body mass significant in all analyses. We also found similar results in linear mixed-effects models with interactions between Gyps fulvus and Pica pica (an outlier point) excluded from the dataset (interaction between genetic distance and body mass in our best-fit model, estimate = −2.21±0.86SE, t = −2.58 df = 237, p = 0.011; Figure S2).
Discussion
Small animals flourish in many ecological settings, but they often suffer the recurrent cost of losing aggressive contests for resources with larger animals [5]–[8]. At the same time, numerous examples exist of smaller species dominating larger species (e.g., [9]–[11]), but the conditions leading to small species dominating larger species are poorly understood. Here we tested the hypothesis that the disadvantage of being small in aggressive interactions could be overcome over evolutionary time through the accumulation of novel traits that can counteract the advantages of being large. We found support for this hypothesis: larger species were dominant over smaller species during aggressive interactions for shared resources, but the advantage of a larger body size declined with increased evolutionary distance in vultures, hummingbirds, and antbirds/woodcreepers (Figure 5; Tables 1,2). Similarly, small species were more likely to win the majority of aggressive contests when they interacted with more distantly-related species (Figure 6; Tables 3,4). These results suggest that body size and phylogenetic distance jointly shape the outcome of aggressive interactions in birds, which in turn may influence the structure of bird communities.
While our results are consistent with the predictions of our hypothesis, our results are also consistent with three alternative hypotheses. Distantly-related species are, on average, less likely to share preferred resources [8], [28], which could explain the patterns observed here, if (i) large species were less willing to invest in aggressive encounters over less preferred resources with distantly-related smaller species, (ii) if the costs of losing an aggressive encounter are lower over less preferred resources, or (iii) if the context of aggressive interactions (e.g., age, experience, condition, hunger level, time of arrival or colonization) shift as resource preferences diverge, allowing some distantly-related species to win interactions that they would normally lose (e.g., [29]–[31]). If ecological similarity declines with genetic distance [8], [28], then these alternatives predict that behaviorally dominant species should lose a larger proportion of interactions with distantly-related subordinates, independent of differences in size. Our results did not support this prediction: while genetic distance was an important predictor of the outcome of aggressive interactions outside of its interaction with body size (Tables 1,2,5,6), dominant species won a greater proportion of their interactions with more distantly-related subordinate species, opposite to the predicted pattern. Detailed data on resource use also suggests that larger species did not lose aggressive contests over less preferred food (Text S2, Table S7), contrary to the predictions of (i) and (ii). While our data do not rule out the contribution of these alternative hypotheses to the decline in the importance of size in aggressive encounters among more distantly-related species, they suggest that these alternative hypotheses cannot explain the pattern by themselves.
What traits offset the importance of size in aggressive interactions?
No study to date has quantified a general set of traits which predictably offset the advantages of large size in aggressive interactions. However, upon reviewing the focal studies used to generate the data in this study (Text S3), in addition to other studies, we find a diverse set of traits that play a key role in offsetting the importance of size in aggressive interactions among birds. These traits include the evolution of well-developed leg musculature and talons (raptors [32]), adaptations that enhance flight acceleration (hummingbirds [33], [34]) and maneuverability (hummingbirds [33]–[37], woodcreepers [38]–[40]), novel fighting behaviors (woodcreepers [38]–[40], grouse [41]), and traits directly associated with aggression, such as testosterone and muscle development (hummingbirds [29], [42], [43], woodcreepers [44], blackbirds [45], [46]), The evolution of social behavior, where smaller individuals perform coordinated attacks on individuals of larger species, may shift the outcomes of aggressive interactions among species (e.g., mammalian carnivores [8]), although we excluded interactions involving multiple individuals of each species in our study. In addition, intraspecific clustering, leading to high densities of subordinate species, can overwhelm individuals of dominant species, leading to dominants reducing their territory size or abandoning resources altogether (vultures [47], [48], hummingbirds [29], [49], blackbirds [45], [46]). In these cases, social coordination among individuals is unnecessary — simply a high density of subordinate relative to dominant individuals may increase the costs of aggressive defense of a resource for dominants. In all of these cases, the evolution of novel traits (or trait values) in smaller species were thought to allow them to overcome the costs of small size in aggressive contests with larger species.
Focal studies of species interactions also revealed cases where adaptations for other functions compromised the ability of large species to compete aggressively with smaller species — a mechanism that we did not predict. Traits that may have hindered larger species in aggressive interactions included the evolution of specialized morphologies for tree trunk foraging in woodcreepers [50] that in turn compromised their performance in aggressive contests away from trunks [44], [51], and the evolution of migratory behavior, that compromised performance in aggressive contests with resident species. In the case of woodcreepers, the specialized adaptations for tree climbing [50] enhanced their performance in aggressive contests with some woodcreeper species on tree trunks [38]–[40], but compromised their performance in aggressive interactions with antbirds, which typically occur on small saplings and branches [44], [50]–[52]. In the case of migration, a fundamental trade-off appears to constrain the ability of species to excel at both migration and performance in aggressive interactions simultaneously (Text S3), resulting in smaller resident species dominating larger migrant species across many different environments [14], [53]–[55]
Overall, observations of smaller species dominating larger species suggest that diverse adaptations in both the smaller and larger species may offset the disadvantage of small size in aggressive contests among species. While the evolution of novel traits in small species could lead to counter-adaptations in large species, and thus an evolutionary arms race, subordinate species (large or small) may reduce costs of aggressive interactions from dominant species in a myriad of ways, such as by using alternative resources (e.g., [47]), shifting resource use in space and time (e.g., [13], [56]), or even by mimicking dangerous species (e.g., mimicry of bumblebees by some subordinate hummingbirds; [33], [42]). Trade-offs and other evolutionary constraints are also likely to limit evolutionary arms races — most traits involved in interspecific aggression are used for other functions as well, and changes to these traits could influence organismal performance in many different ways. Most of our focal species also interacted with many species simultaneously (e.g., [43], [56]), creating diverse sources of selection that can influence patterns of co-evolution among species [57].
Overcoming trade-offs over evolutionary time
Overall, our results suggest that the phylogenetic relationships among species can influence the rules that govern their interactions. Closely-related species typically share more traits in common [28], intensifying the importance of fundamental trade-offs that constrain their interactions and relative distributions. Thus, any advantages to being small must be balanced against the fitness cost of coexisting with larger, closely-related species [7], [58]. Over evolutionary time, however, novel traits may significantly alter the costs associated with trade-offs [3], creating new ecological opportunities and different patterns of community organization. Thus, a phylogenetic perspective of community ecology is important, not just for understanding causal processes or rules that structure communities [1], [2], but also for understanding when and how these rules can be broken over evolutionary time. These dynamic interactions provide an example of why phylogenetic perspectives are invaluable for our understanding of the structure and function of ecological communities [1], [2].
Supporting Information
A 3-dimensional plot illustrating the relationships between dominance asymmetry, difference in mass, and genetic distance among the focal species pairs in our study. Dominance asymmetry = sqrt {ln ((wins by dominant species+1)/(wins by subordinate species+1))}. Difference in mass = (mass of dominant species−mass of subordinate species)/(average mass of dominant and subordinate species). Genetic distance is the Tamura-Nei genetic distance between interacting species for mtDNA.
(TIF)
Relatively heavier bird species were more likely to win aggressive contests for resources (positive slope), but the advantage of large size in aggressive contests declined with genetic distance (shallower slope for high genetic distance). Dominance asymmetry (y-axis) = sqrt {ln ((wins by dominant species+1)/(wins by subordinate species+1))}. Difference in mass (x-axis) = (mass of dominant species−mass of subordinate species)/(average mass of dominant and subordinate species). Genetic distance groups are split by the midpoint value for the dataset (low = 0.006–0.179; high = 0.180–0.352). Figure S2 is identical to Figure 5, except that an outlier point (interaction between Gyps fulvus and Pica pica) has been removed.
(TIF)
Dataset used in our study.
(TXT)
Comparison of model fit for different generalized linear mixed models (binomial distribution) that include all possible combinations of predictor variables, controlling for variation in sample sizes of interactions across studies. Models tested the prediction that smaller bird species were more likely to win aggressive contests for resources when interacting with a more distantly-related species.
(CSV)
Results from the best-fit (lowest AICc) generalized linear mixed model (binomial distribution), controlling for variation in sample sizes of interactions across studies. The model tests the prediction that smaller bird species were more likely to win aggressive contests for resources when interacting with a more distantly-related species.
(CSV)
Results from the best-fit (lowest AICc) linear mixed-effects model, with dominant or subordinate species included as a random effect. The model tested the prediction that the advantage of large size in aggressive contests for resources declines with genetic distance among interacting bird species (n = 244 species pairs).
(CSV)
Results from the best-fit (lowest AICc) generalized linear mixed model (binomial distribution), with dominant or subordinate species included as a random effect. The model tested the prediction that smaller bird species were more likely to win aggressive contests for resources when interacting with a more distantly-related species (n = 244 species pairs).
(CSV)
Relative diet specialization among species pairs where the smaller species was dominant (i.e., won the majority of aggressive interactions). Focal diets were the food source over which aggressive interactions occurred: carrion for interactions involving vultures, nectar for interactions involving hummingbirds, and prey flushed by army ant swarms for interactions involving woodcreepers and antbirds.
(CSV)
Genbank accession numbers for genetic sequences used in our study.
(DOCX)
Patterns of resource use and preference in cases where small species dominated large species in aggressive contests.
(DOCX)
A review of traits that have been proposed to explain why small species dominate large species of birds, focusing on specific cases in all three of our taxonomic groups.
(DOCX)
Acknowledgments
We thank F. Bonier and an anonymous reviewer for helpful comments on earlier drafts of this manuscript.
Funding Statement
The authors' work was funded by the Natural Sciences and Engineering Research Council of Canada (Discovery Grant RGPIN/355519-2008; www.nserc-crsng.gc.ca), a Baillie Family Chair Endowment, and a Good Family Fellowship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Web CO, Ackerly DD, McPeek MA, Donoghue MJ (2002) Phylogenies and community ecology. Annual Review of Ecology and Systematics 33: 475–505. [Google Scholar]
- 2. Cavender-Bares J, Kozak KH, Fine PVA, Kembel SW (2009) The merging of community ecology and phylogenetic biology. Ecology Letters 12: 693–715. [DOI] [PubMed] [Google Scholar]
- 3. Lenski RE (1988) Experimental studies of pleiotropy and epistasis in Escherichia coli. II. Compensation for maladaptive effects associated with resistance to virus T4. Evolution 42: 433–440. [DOI] [PubMed] [Google Scholar]
- 4.Martin PR (2014) Trade-offs and biological diversity: integrative answers to ecological questions. In Integrative organismal biology (eds. Martin LB, Ghalambor CK, Woods HA), pp. 291–308. John Wiley and Sons, Inc., New York, New York, USA. [Google Scholar]
- 5.Peters RH (1983) The ecological implications of body size. Cambridge University Press, Cambridge, UK. [Google Scholar]
- 6.Bonner JT (2006) Why size matters. Princeton University Press, Princeton, New Jersey, USA. [Google Scholar]
- 7. Morse DH (1974) Niche breadth as a function of social dominance. American Naturalist 108: 818–830. [Google Scholar]
- 8. Donadio E, Buskirk SW (2006) Diet, morphology, and interspecific killing in Carnivora. American Naturalist 167: 524–536. [DOI] [PubMed] [Google Scholar]
- 9. Burger J (1978) Competition between Cattle Egrets and native North American herons, egrets, and ibises. Condor 80: 15–23. [Google Scholar]
- 10. Gill FB, Mack AL, Ray RT (1982) Competition between hermit hummingbirds Phaethorninae and insects for nectar in a Costa Rican rain forest. Ibis 124: 44–49. [Google Scholar]
- 11. Hino T (2005) Resident males of small species dominate immigrants of large species in heterospecific, winter bird flocks. Ornithological Science 4: 89–94. [Google Scholar]
- 12.Harvey PH, Pagel MD (1991) The comparative method in evolutionary biology. Oxford University Press, Oxford, UK. [Google Scholar]
- 13. Willis EO, Oniki Y (1978) Birds and army ants. Annual Review of Ecology and Systematics 9: 243–263. [Google Scholar]
- 14. Freshwater C, Ghalambor CK, Martin PR (2014) Repeated patterns of trait divergence between closely related dominant and subordinate bird species. Ecology 95: 2334–2345. [DOI] [PubMed] [Google Scholar]
- 15. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al. (2011) MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biology and Evolution 10: 512–526. [DOI] [PubMed] [Google Scholar]
- 17. Weir JT, Schluter D (2008) Calibrating the avian molecular clock. Molecular Ecology 17: 2321–2328. [DOI] [PubMed] [Google Scholar]
- 18. Desjardins P, Morais R (1990) Sequence and gene organization of the chicken mitochondrial genome. Journal of Molecular Biology 212: 599–634. [DOI] [PubMed] [Google Scholar]
- 19. Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, et al. (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23: 2947–2948. [DOI] [PubMed] [Google Scholar]
- 20.Maddison DR, Maddison WP (2005) MacClade, version 4.08. Sinauer Associates, Sunderland, Massachusetts, USA. [Google Scholar]
- 21.R Core Team 2014 R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org/. Accessed 2014. [Google Scholar]
- 22.Pinheiro J, Bates D, DebRoy S, Sarkar D, R Development Core Team (2012) nlme: linear and nonlinear mixed effects models. R package version 3.1-103.
- 23.Bates D, Maechler M, Bolker B (2011) lme4: Linear mixed-effects models using S4 classes. R package version 0.999375-42.
- 24.Gelman A, Su Y-S, Yajima M, Hill J, Pittau MG, et al. (2014) arm: Data analysis using regression and multilevel/hierarchical models. R package version 1.7-03.
- 25.Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York, New York, USA. [Google Scholar]
- 26.Bartoń K (2013) MuMIn: Multi-model inference. R package version 1.9.5.
- 27.Ripley B, Hornik K, Gebhardt A, Firth D (2012) Support functions and datasets to support Venables and Ripley's ‘Modern applied statistics with S’ (4th edition, 2002). R package version 7.3.18.
- 28. Violle C, Nemergut DR, Pu Z, Jiang L (2011) Phylogenetic limiting similarity and competitive exclusion. Ecology Letters 14: 782–787. [DOI] [PubMed] [Google Scholar]
- 29. Stiles FG (1973) Food supply and the annual cycle of the Anna Hummingbird. University of California Publications in Zoology 97: 1–109. [Google Scholar]
- 30. Cole BJ (1983) Assembly of mangrove ant communities: patterns of geographical distribution. Journal of Animal Ecology 52: 339–347. [Google Scholar]
- 31. Tanner CJ, Adler FR (2009) To fight or not to fight: context-dependent interspecific aggression in competing ants. Animal Behaviour 77: 297–305. [Google Scholar]
- 32.del Hoyo J, Elliott A, Sargatal J, eds. (1994) Handbook of the birds of the world. Volume 2. Lynx Edicions, Barcelona, Spain. [Google Scholar]
- 33. Feinsinger P, Chaplin SB (1975) On the relationship between wing disc loading and foraging strategy in hummingbirds. American Naturalist 109: 217–224. [Google Scholar]
- 34. Altshuler DL (2006) Flight performance and competitive displacement of hummingbirds across elevational gradients. American Naturalist 167: 216–229. [DOI] [PubMed] [Google Scholar]
- 35. Feinsinger P, Colwell RK, Terborgh J, Chaplin SB (1979) Elevation and the morphology, flight energetics, and foraging ecology of tropical hummingbirds. American Naturalist 113: 481–497. [Google Scholar]
- 36. Kodric-Brown A, Brown JH (1978) Influence of economics, interspecific competition, and sexual dimorphism on territoriality of migrant Rufous Hummingbirds. Ecology 59: 285–296. [Google Scholar]
- 37.del Hoyo J, Elliott A, Sargatal J, eds. (1999) Handbook of the birds of the world. Volume 5. Lynx Edicions, Barcelona, Spain. [Google Scholar]
- 38. Willis EO (1982) The behavior of Black-banded Woodcreepers (Dendocolaptes picumnus). Condor 84: 272–285. [Google Scholar]
- 39. Willis EO (1983) Cis-Andean Xiphorhynchus and relatives (Aves, Dendrocolaptidae) as army ant followers. Revista Brasileira de Biologia 43: 133–142. [Google Scholar]
- 40. Willis EO (1992) Comportamento e ecologia do arapaçu-barrado Dendrocolaptes certhia (Aves, Dendrocolaptidae). Boletim do Museu Paraense Emílio Goeldi, Série Zoologia 8: 151–216. [Google Scholar]
- 41. Sharp WM (1957) Social and range dominance in gallinaceous birds — pheasants and prairie grouse. Journal of Wildlife Management 21: 242–244. [Google Scholar]
- 42. Feinsinger P, Colwell RK (1978) Community organization among neotropical nectar-feeding birds. American Zoologist 18: 779–795. [Google Scholar]
- 43. Feinsinger P, Swarm LA, Wolfe JA (1985) Nectar-feeding birds on Trinidad and Tobago: comparison of diverse and depauperate guilds. Ecological Monographs 55: 1–28. [Google Scholar]
- 44. Willis EO (1972) The behavior of Plain-brown Woodcreepers, Dendrocincla fuliginosa . Wilson Bulletin 84: 377–420. [Google Scholar]
- 45. Orians GH (1961) The ecology of blackbird (Agelaius) social systems. Ecological Monographs 31: 285–312. [Google Scholar]
- 46. Orians GH, Collier G (1963) Competition and blackbird social systems. Evolution 17: 449–459. [Google Scholar]
- 47.König C (1983) Interspecific and intraspecific competition for food among Old World vultures. In Vulture biology and management (Wilbur SR, Jackson JA, eds.), pp. 153–171. University of California Press, Berkeley, California, USA. [Google Scholar]
- 48. Wallace MP, Temple SA (1987) Competitive interactions within and between species in a guild of avian scavengers. Auk 104: 290–295. [Google Scholar]
- 49. Lyon DL (1976) A montane hummingbird territorial system in Oaxaca, Mexico. Wilson Bulletin 88: 280–299.. [Google Scholar]
- 50.del Hoyo J, Elliott A, Christie DA, eds. (2003) Handbook of the birds of the world. Volume 8. Lynx Edicions, Barcelona, Spain. [Google Scholar]
- 51. Willis EO (1979) Behavior and ecology of two forms of White-chinned Woodcreepers (Dendrocincla merula, Dendrocolaptidae) in Amazonia. Papéis Avulsos de Zoologia 33: 27–66. [Google Scholar]
- 52. Willis EO (1967) The behavior of Bicolored Antbirds. University of California Publications in Zoology 79: 1–132. [Google Scholar]
- 53. Willis EO (1966) The role of migrant birds at swarms of army ants. Living Bird 5: 187–231. [Google Scholar]
- 54.DesGranges J-L, Grant PR (1980) Migrant hummingbirds' accommodation into tropical communities. In Migrant birds in the Neotropics: ecology, behavior, distribution, and conservation (eds. Keast A, Morton ES), pp. 395–409. Smithsonian Institution, Washington, DC, USA. [Google Scholar]
- 55. Leisler B (1992) Habitat selection and coexistence of migrants and Afrotropical residents. Ibis 134: S77–S82. [Google Scholar]
- 56. Feinsinger P (1976) Organization of a tropical guild of nectarivorous birds. Ecological Monographs 46: 257–291. [Google Scholar]
- 57.Thompson JN (2005) The geographic mosaic of coevolution. University of Chicago Press, Chicago, Illinois, USA. [Google Scholar]
- 58. Brown JH, Maurer BA (1986) Body size, ecological dominance and Cope's rule. Nature 324: 248–250. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A 3-dimensional plot illustrating the relationships between dominance asymmetry, difference in mass, and genetic distance among the focal species pairs in our study. Dominance asymmetry = sqrt {ln ((wins by dominant species+1)/(wins by subordinate species+1))}. Difference in mass = (mass of dominant species−mass of subordinate species)/(average mass of dominant and subordinate species). Genetic distance is the Tamura-Nei genetic distance between interacting species for mtDNA.
(TIF)
Relatively heavier bird species were more likely to win aggressive contests for resources (positive slope), but the advantage of large size in aggressive contests declined with genetic distance (shallower slope for high genetic distance). Dominance asymmetry (y-axis) = sqrt {ln ((wins by dominant species+1)/(wins by subordinate species+1))}. Difference in mass (x-axis) = (mass of dominant species−mass of subordinate species)/(average mass of dominant and subordinate species). Genetic distance groups are split by the midpoint value for the dataset (low = 0.006–0.179; high = 0.180–0.352). Figure S2 is identical to Figure 5, except that an outlier point (interaction between Gyps fulvus and Pica pica) has been removed.
(TIF)
Dataset used in our study.
(TXT)
Comparison of model fit for different generalized linear mixed models (binomial distribution) that include all possible combinations of predictor variables, controlling for variation in sample sizes of interactions across studies. Models tested the prediction that smaller bird species were more likely to win aggressive contests for resources when interacting with a more distantly-related species.
(CSV)
Results from the best-fit (lowest AICc) generalized linear mixed model (binomial distribution), controlling for variation in sample sizes of interactions across studies. The model tests the prediction that smaller bird species were more likely to win aggressive contests for resources when interacting with a more distantly-related species.
(CSV)
Results from the best-fit (lowest AICc) linear mixed-effects model, with dominant or subordinate species included as a random effect. The model tested the prediction that the advantage of large size in aggressive contests for resources declines with genetic distance among interacting bird species (n = 244 species pairs).
(CSV)
Results from the best-fit (lowest AICc) generalized linear mixed model (binomial distribution), with dominant or subordinate species included as a random effect. The model tested the prediction that smaller bird species were more likely to win aggressive contests for resources when interacting with a more distantly-related species (n = 244 species pairs).
(CSV)
Relative diet specialization among species pairs where the smaller species was dominant (i.e., won the majority of aggressive interactions). Focal diets were the food source over which aggressive interactions occurred: carrion for interactions involving vultures, nectar for interactions involving hummingbirds, and prey flushed by army ant swarms for interactions involving woodcreepers and antbirds.
(CSV)
Genbank accession numbers for genetic sequences used in our study.
(DOCX)
Patterns of resource use and preference in cases where small species dominated large species in aggressive contests.
(DOCX)
A review of traits that have been proposed to explain why small species dominate large species of birds, focusing on specific cases in all three of our taxonomic groups.
(DOCX)