Abstract
Viperid venoms often contain mixtures of Asp49 and Lys49 PLA2 myotoxin isoforms, relevant to development of myonecrosis. Given their difference in catalytic activity, mechanistic studies on each type require highly purified samples. Studies on Asp49 PLA2s have shown that enzyme inactivation using p-bromophenacyl bromide (p-BPB) drastically affects toxicity. However, based on the variable levels of residual toxicity observed in some studies, it has been suggested that effector mechanisms independent of catalysis may additionally be involved in the toxicity of these enzymes, possibly resembling those of the enzymatically inactive Lys49 myotoxins. A possibility that Lys49 isoforms could be present in Asp49 PLA2 preparations exists and, if undetected in previous studies, could explain the variable residual toxicity. This question is here addressed by using an enzyme preparation ascertained to be free of Lys49 myotoxins. In agreement with previous reports, inactivation of the catalytic activity of an Asp49 myotoxin preparation led to major inhibition of toxic effects in vitro and in vivo. The very low residual levels of myotoxicity (7%) and cytotoxicity (4%) observed can be attributed to the low, although detectable, enzyme remaining active after p-BPB treatment (2.7%), and would be difficult to reconcile with the proposed existence of additional catalytic-independent toxic mechanisms. These findings favor the concept that the effector mechanism of toxicity of Asp49 PLA2 myotoxins from viperids fundamentally relies on their ability to hydrolyze phospholipids, arguing against the proposal that membrane disruption may also be caused by additional mechanisms that are independent of catalysis.
Keywords: Snake venom, Asp49, Myotoxin, Phospholipase A2, Bothrops asper
Introduction
Phospholipases A2 (PLA2s) are abundant components of snake venoms, where they play major toxic roles in the immobilization and/or killing of prey. Among their diverse effects, these interfacial enzymes exert potent neurotoxicity and/or myotoxicity, and efforts to reveal their detailed mechanisms of action on neurons and skeletal muscle cells have long been pursued in toxinology (Kini, 1997; Gutiérrez & Lomonte, 2013). PLA2 enzymes found in Elapidae and Viperidae snake venoms conserve a common scaffold but present distinctive structural characteristics which allow their classification within groups I or II, respectively, of this large protein family (Arni & Ward, 1996; Montecucco, Gutiérrez & Lomonte, 2008). Since two different, non-toxic ancestral PLA2 genes evolved toward toxicity in elapids (group I) and viperids (group II) (Fry & Wüster, 2004), the acquisition of common toxic activities in both lineages represents a convergent evolutionary process (Lomonte & Gutiérrez, 2011; Lomonte & Rangel, 2012). As a consequence, mechanistic differences might exist in the mode by which group I and group II PLA2s exert their shared toxic effects. Furthermore, within the group II enzymes a subtype emerged which is typified by the substitution of the critical Asp49 residue in the catalytic center, most often by a Lys residue (Maraganore et al., 1984). Such Lys49 PLA2s are indeed “PLA2 homologues” or “PLA2-like” proteins because they are devoid of phospholipolytic activity, but are highly active as myotoxins. All Lys49 PLA2 homologues studied to date are basic proteins that induce skeletal muscle necrosis at the site of injection (Lomonte, Angulo & Calderón, 2003; Lomonte et al., 2009; Lomonte & Rangel, 2012), whereas not all Asp49 PLA2s from viperids are myotoxic. This effect is present almost exclusively in the basic but not the acidic enzymes, in spite of their shared catalytic activity (Lomonte & Gutiérrez, 2011).
Myotoxic PLA2s and PLA2 homologues present in viperid venoms have increasingly been studied due to their relevance in the development of skeletal muscle necrosis after envenomings, with serious clinical consequences such as tissue loss, dysfunction or amputation (Harris & Cullen, 1990; Gutiérrez & Ownby, 2003; Soares, Fontes & Giglio, 2004; Lomonte & Rangel, 2012). In order to understand the mechanisms involved in the pathological effects induced by group II myotoxic PLA2s, thorough protein purification is essential. The isolation of different variants of myotoxic PLA2s in viperid venoms is complicated by the fact they often contain a multiplicity of closely related isoforms that are variably expressed in the species (Faure & Bon, 1988; Lomonte & Kahan, 1988; Valiente et al., 1992) or in single individuals (Lomonte & Carmona, 1992). As a consequence, these venoms may contain complex mixtures of both Asp49 and Lys49 myotoxins, and given the fundamental difference in catalytic activity between them, mechanistic studies on each type require their efficient separation. Contamination of a Lys49 myotoxin preparation with Asp49 PLA2s can be straightforwardly detected by means of the catalytic activity of the latter (Scott et al., 1992; Cintra-Francischinelli et al., 2009). However, the converse situation poses a more difficult challenge, since there is no specific functional assay to detect the presence of Lys49 contaminants in preparations of Asp49 myotoxins.
Several Asp49 and Lys49 myotoxins have been isolated from the venom of Bothrops asper, the snake species causing the majority of envenomings in Central America (Angulo & Lomonte, 2009). A number of studies have demonstrated that Lys49 PLA2 homologues induce myonecrosis by a catalytic-independent mechanism, involving amino acids at their C-terminal region which affect the integrity of the sarcolemma (Lomonte et al., 1994; Núñez, Angulo & Lomonte, 2001; Chioato et al., 2002; Chioato & Ward, 2003; Lomonte, Angulo & Calderón, 2003; dos Santos et al., 2009; Lomonte & Rangel, 2012; Fernandes et al., 2013; Fernández et al., 2013). On the other hand, Asp49 PLA2s have been shown to depend on their catalytic activity to disrupt the integrity of this membrane (Lomonte & Gutiérrez, 2011). Nevertheless, some studies have reported that inhibition of PLA2 activity in Asp49 myotoxins may result in variable degrees of residual toxicity, and the possibility has been suggested that their overall toxic action may involve membrane-perturbing mechanisms additional to catalysis depending on molecular regions other than the catalytic site, in similarity with the Lys49 myotoxins (Díaz et al., 1991; Díaz-Oreiro & Gutiérrez, 1997; Andrião-Escarso et al., 2000; Soares et al., 2001a; Soares et al., 2001b; Soares & Giglio, 2003). An alternative explanation for the residual toxicity observed in some earlier studies after enzyme inactivation of Asp49 PLA2s, could be the presence of variable levels of undetected Lys49 isoforms. The present work addresses this possibility by reassessing the role of catalytic activity in the toxic effects of an Asp49 PLA2 myotoxin preparation from the venom of B. asper that was ascertained free of Lys49 myotoxin contaminants. After its inactivation by histidine modification with p-bromophenacyl bromide, its toxic actions in vivo (myotoxicity) and in vitro (cytotoxicity upon differentiated myotubes) were evaluated in comparison to the untreated toxin.
Methods
Isolation of Asp49 phospholipase A2 myotoxins
Venom of Bothrops asper, pooled from specimens collected in the Pacific versant of Costa Rica and kept at the serpentarium of Instituto Clodomiro Picado, was fractionated by cation-exchange chromatography on a CM-Sephadex C-25 column (20 × 2 cm) equilibrated with 0.05 M Tris, 0.1 M KCl, pH 7.0 buffer, at 0.4 mL/min (Lomonte & Gutiérrez, 1989). Protein elution was performed with a gradient toward 0.75 M KCl in the same buffer, and monitored at 280 nm with a Bio-Logic chromatograph (Bio-Rad). Peak 2 was collected, desalted by overnight dialysis, lyophilized, and further separated by reverse-phase high-performance liquid chromatography (RP-HPLC) on a C8 semipreparative column (250 × 10 mm) Elution was carried out at 2.5 mL/min with a gradient from water to acetonitrile, both containing 0.1% trifluoroacetic acid, in a model 1200 chromatograph (Agilent) monitored at 215 nm. The gradient toward acetonitrile stepped from 0 to 25% in 5 min, 25 to 50% in 22 min, 50 to 70% in 1 min, and was sustained at 70% for 2 min, for a total run time of 30 min. Fractions of interest were collected, dried in a vacuum centrifuge at 45 °C, and stored at −20 °C. Protein concentrations were estimated by measuring their absorbance at 280 nm in a Nanodrop (Thermo) reader.
Homogeneity evaluation
The Asp49 PLA2 preparation was evaluated by three analytical methods. (a) A sample (40 µg) of the fraction was run by RP-HPLC on a C4 column at analytical scale (4.6 × 150 mm), eluted at 1 mL/min with an acetonitrile gradient (0% at 5 min, 0 to 70% in 45 min, and 70% for 10 min, for a total run time of 60 min) and monitored at 215 nm; (b) the fraction was analyzed by nano-electrospray mass spectrometry (nESI-MS), by directly infusing the sample (2 µg/10 µL), loaded into a metal-coated capillary tip (Proxeon), in a Q-Trap 3200 instrument (Applied Biosystems) operated in positive multicharge enhanced mode using an ionization voltage of 1,200–1,300 V. Spectra were acquired in the 700–1,700 m/z range and deconvoluted with the BioAnalyst v.1.5 software (ABSciex); (c) the fraction (200 pmoles) was subjected to ten cycles of N-terminal sequencing by automated Edman degradation in a model PPSQ33A instrument (Shimadzu Biotech). In all three analytical techniques, a sample of Lys49 myotoxin (B. asper myotoxin II; Lomonte & Gutiérrez, 1989), was included for comparison.
Asp49 phospholipase A2 inactivation with p-bromophenacyl bromide
Three mg of the Asp49 myotoxin were dissolved in 1 mL of 0.1 M Tris, 0.7 mM EDTA, pH 8.0 buffer. Then, 125 µL of p-bromophenacyl bromide (p-BPB; 1.5 mg/mL in ethanol; Sigma) were added and incubated at room temperature (20–25 °C) for 24 h (Díaz-Oreiro & Gutiérrez, 1997). Excess reagent and salts were eliminated by RP-HPLC on a semi-preparative C8 column, as described above. The protein was collected and finally dried by vacuum centrifugation at 45 °C. To serve as a control in all assays, a parallel sample of the Asp49 PLA2 was subjected to all steps of this procedure, but omitting the p-BPB reagent during the 24 h incubation.
Phospholipase A2 activity
PLA2 activity was assayed on the synthetic monodisperse substrate 4-nitro-3-octanoyloxybenzoic acid (NOBA; Holzer & Mackessy, 1996). Various amounts of p-BPB-treated or control myotoxin, dissolved in 25 µL of 10 mM Tris, 0.1 M NaCl, 10 mM CaCl2, pH 8.0 buffer, were mixed with 200 µL of this buffer and 25 µL of NOBA (1 mg/mL in acetonitrile), and incubated at 37 °C for 60 min (Mora-Obando et al., 2014). The reaction product was colorimetrically quantified at 405 nm in a microplate reader (Thermo). Wells containing all reagents, except the enzyme, were used as a blank. All samples were assayed in triplicate wells.
Cytotoxic activity
Cytotoxic activity was assayed on the murine myogenic cell line C2C12 (ATCC-CRL1772) (Lomonte et al., 1999). Various amounts of p-BPB-treated or control myotoxin, dissolved in 150 µL of assay medium (Dulbecco’s modified Eagle’s medium with 1% fetal calf serum [DMEM, 1% FCS]), were added to wells of a 96-well plate where the myogenic cells had grown until confluency and then differentiated to myotubes for 4–6 days in assay medium at 37 °C and 7% CO2. The medium was removed and, immediately, the toxins dissolved in a fresh assay medium were added. After 3 h of incubation at 37 °C, an aliquot of cell supernatants (60 µL) was collected from each well and the released lactate dehydrogenase (LDH) activity was quantified by a UV kinetic assay (LDH-BR Cromatest; Linear Chemicals). Controls for 0 and 100% cytotoxicity consisted of assay medium and 0.1% Triton X-100 diluted in assay medium, respectively. All samples were assayed in triplicate wells.
Myotoxic activity
Myotoxic activity was assayed in groups of five CD-1 mice of 18–20 g of body weight. These in vivo assays followed protocols approved by the Institutional Committee for the Use and Care of Animals (CICUA; 132-13), University of Costa Rica. Mice were housed in cages in groups of 4–6, and provided food and water ad libitum. A fixed amount of the toxins (50 µg), dissolved in 50 µL of phosphate-buffered saline (PBS; 0.12 M NaCl, 0.04 M sodium phosphate buffer, pH 7.2), was injected intramuscularly into the gastrocnemius (Lomonte & Gutiérrez, 1989). A control group of mice received an identical injection of PBS only. After 3 h, blood was collected from the tip of the tail into a heparinized capillary and centrifuged. The plasma creatine kinase (CK) activity, expressed in U/L, was determined using a UV kinetic assay (CK-Nac; Biocon Diagnostik).
Statistical analysis
Statistical significance of mean values comparisons was determined by the Student’s t-test, at p < 0.05, using the GraphPad Instat v.3 software.
Results and Discussion
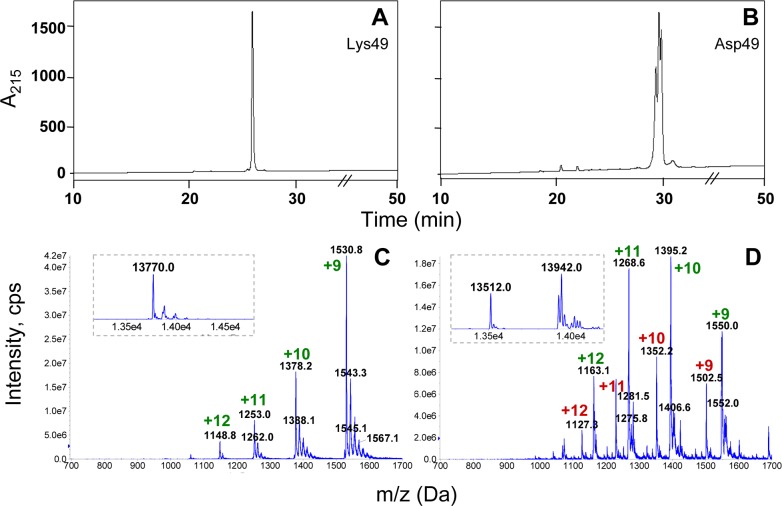
Fractionation of B. asper (Pacific region of Costa Rica) venom on CM-Sephadex at pH 7.0 separated the basic proteins from the acidic components eluting in the flow-through peak (Fig. 1A). Peak 1 corresponded to the metalloproteinase BaP1 described by Gutiérrez et al. (1995), whereas peaks 2–4 contained basic PLA2s or PLA2 homologues, in similarity with the profile of B. asper venom from specimens of the Caribbean region (Gutiérrez, Ownby & Odell, 1984; Lomonte & Gutiérrez, 1989). Although the resolution obtained in the Asp49-rich region of the Pacific venom chromatogram was slightly lower in comparison to the Caribbean venom, the final yield of Asp49 PLA2 was found to be higher with the former venom, which was therefore selected for further purification. Peak 2 (Fig. 1A) presented high PLA2 activity (data not shown) and was subsequently fractionated by semi-preparative C8 RP-HPLC, resulting in two major peaks (Fig. 1B). The peak eluting at ∼19 min was devoid of PLA2 activity, corresponding to Lys49 myotoxins, whereas the larger peak eluting at ∼24 min showed PLA2 activity, corresponding to Asp49 myotoxins.
Figure 1. Isolation of myotoxic phospholipases A2 from the venom of Bothrops asper (Pacific versant of Costa Rica).
(A) Venom (200 mg) was applied to a CM-Sephadex C-25 column (20 × 2 cm) equilibrated with 0.05 M Tris, 0.1 M KCl, pH 7.0 and, after elution of unbound proteins, a linear gradient (G; dotted line) toward 0.05 M Tris, 0.75 M KCl, pH 7.0 buffer was developed. Proteins from peak 2 (horizontal green line) were pooled and further separated by (B) reverse-phase HPLC on a C8 semipreparative column (250 × 10 mm). Proteins were eluted at 2.5 mL/min with a gradient from water to acetonitrile (dotted line) in the presence of 0.1% trifluoroacetic acid. The second major peak (∼24 min), corresponding to Asp49 phospholipases A2, was collected and subjected to further analyses.
In order to ascertain that the Asp49 PLA2 preparation was devoid of Lys49 proteins, several analyses were conducted. First, the sample was subjected to analytical RP-HPLC using a C4 column with a longer elution gradient. In similarity with the semi-preparative C8 separation, this technique efficiently separated the Lys49 from the Asp49 proteins with a difference in retention times between them of nearly 3 min (Figs. 2A and 2B). The higher resolution of this technique evidenced that the Asp49 PLA2 preparation contained several peaks with very close retention times, which could not be adequately resolved (Fig. 2B). In contrast, the Lys49 protein eluted as a sharp single peak (Fig. 2A). nESI-MS analyses confirmed these observations, revealing the presence of at least two main molecular masses of 13,512 ± 2 and 13,942 ± 2 Da in the Asp49 preparation (Fig. 2D), while the Lys49 myotoxin presented a main mass of 13,770 ± 3 Da (Fig. 2C). The latter mass is in agreement with that expected for myotoxin II from the Caribbean versant of Costa Rica (UniProt accession; P24605; Mav =13773), if the Leu/Phe ambiguity reported for its position 114 (Francis et al., 1991) is considered as Phe (the sequence P24605 in databanks shows Leu at this position).
Figure 2. Analysis of the Asp49 phospholipase A2 preparation obtained after cation-exchange chromatography and semi-preparative RP-HPLC on C8.
A Lys49 myotoxin control (A) and the Asp49 peak shown in Fig. 1 (B) were subjected to analytical RP-HPLC on a C4 column, using a 60 min gradient (see Materials and Methods). For clarity, the gradient line is omitted and the time scale is magnified in the region of interest. The same samples were analyzed by nano-electrospray mass spectrometry in (C) and (D). Insets within dotted lines show the corresponding deconvolutions of the multicharged ion series and calculation of the isotope-averaged observed molecular masses of these samples.
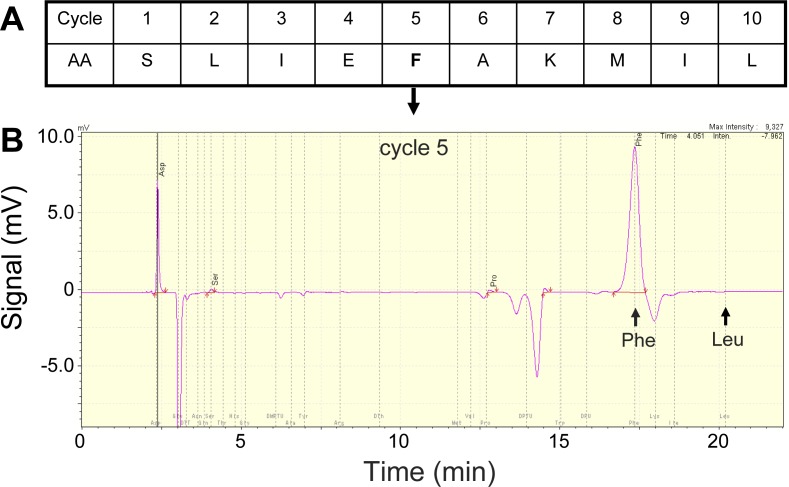
Since both the semi-preparative and analytical RP-HPLC procedures were able to resolve Lys49 from Asp49 proteins of this venom, it was assumed that the heterogeneity observed in the latter preparation would correspond only to Asp49 isoforms (free of Lys49 contamination). In order to evaluate this assumption, the Asp49 PLA2 sample was subjected to ten cycles of automated N-terminal sequencing. The resulting sequence showed single amino acid signals in all cycles but, most importantly, showed no traces of Leu in the fifth cycle (Fig. 3). Since this amino acid position is conserved in all known Lys49 myotoxins, this result confirmed that the RP-HPLC yielded an Asp49 PLA2 preparation that, despite containing isoforms possibly due to microheterogeneity, should be free from any contaminating Lys49 PLA2 homologues. The short N-terminal sequence obtained (Fig. 3) matches with 10/10 identity the Asp49 PLA2 myotoxin reported by Kaiser et al. (1990), which has been variably described in the literature as B. asper myotoxin I or myotoxin III (UniProt accession P20474). This is the only basic Asp49 PLA2 myotoxin isolated from B. asper venom that has been sequenced, but it should be noted that it was reported as containing ambiguous amino acid residues in at least three positions near its C-terminus (Kaiser et al., 1990), evidencing the difficulties in resolving such closely related isoforms during isolation. Due to this complex microheterogeneity of isoforms, we made no attempts to assign the masses of the purified Asp49 protein preparation (13,512 ± 2 and 13,942 ± 2 Da) to any variable combination of the Asp49 myotoxin sequence previously reported (Kaiser et al., 1990), but instead refer to these proteins only as Asp49 PLA2 myotoxins.
Figure 3. N-terminal amino acid sequencing of the Asp49 phospholipase A2 N-terminal amino acid sequencing of the Asp49 phospholipase A2 peak shown in Fig. 1 (A; first ten residues), and snapshot of the fifth cycle chromatogram (B).
As expected for this type of enzyme, this cycle yields a strong signal corresponding to phenylalanine (Phe), while the retention time corresponding to leucine (Leu), a conserved position in Lys49 myotoxins, shows no traces of this amino acid residue.
The final Asp49 PLA2 preparation was subjected to histidine modification using p-BPB, aiming to reassess earlier studies on the role of enzyme activity in its toxic activities by ruling out the possibility of the presence of Lys49 contamination. The difference with previous studies (Díaz et al., 1991; Bultrón, Thelestam & Gutiérrez, 1993; Díaz-Oreiro & Gutiérrez, 1997) is that Asp49 PLA2 myotoxin isolation was earlier accomplished only by cation-exchange chromatography without the further use of HPLC and screening by sensitive analytical techniques such as mass spectrometry. Therefore the possible presence of undetected levels of Lys49 myotoxins remained open, in contrast to the present work.
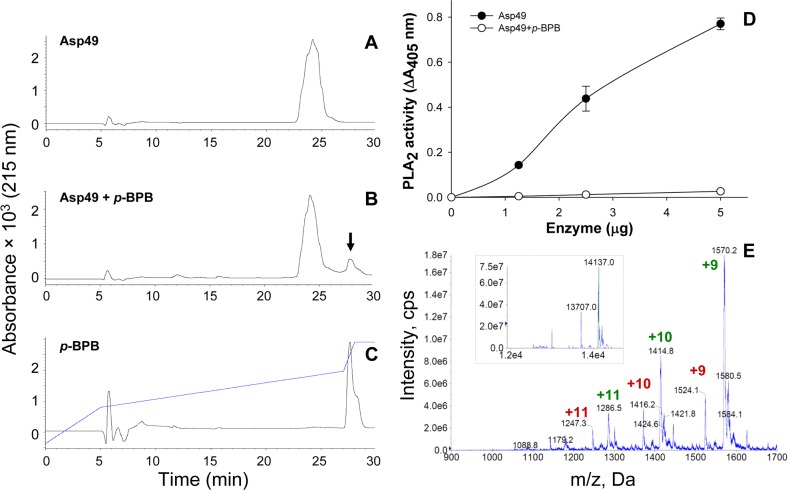
As shown in Figs. 4A–4C, the p-BPB-modified Asp49 PLA2 did not alter its retention time in comparison to the control toxin, and was readily separated from the excess reagent by the C8 RP-HPLC procedure. The covalent incorporation of a single molecule of p-BPB was confirmed by nESI-MS (Fig. 4E), where an increase in molecular mass of 195 ± 3 Da (0.02% instrumental error) was observed in comparison to the unmodified protein (Fig. 2D), in agreement with the theoretically expected mass increase of 198 Da as a result of this reaction. This covalent modification should correspond to the single His48 residue of the protein (consensus numbering), which inactivates the catalytic mechanism of PLA2s (Volwerk, Pieterson & de Haas, 1974). Inactivation of catalysis was confirmed (Fig. 4D), although the use of a sensitive synthetic substrate allowed the detection of a low level of residual PLA2 activity at the highest protein concentration tested, corresponding to ∼2.7% of the unmodified enzyme. The difference between this result and previous studies where PLA2 activity was reported to be undetectable (0%) after 24 h of reaction with p-BPB, using similar protocols, may reside in the different sensitivities of the enzymatic assays used.
Figure 4. Modification of the Asp49 phospholipase A2 by p-bromophenacyl bromide (p-BPB).
(A) unmodified enzyme control; (B) p-BPB-treated enzyme; and (C) p-BPB reagent control. The three samples were separated by RP-HPLC in a semi-preparative C8 column as in Fig. 1B. (D) Comparison of the phospholipase A2 (PLA2) activities of the control Asp49 enzyme and the p-BPB-treated enzyme on the synthetic monodisperse substrate 4-nitro-3-octanoyloxybenzoic acid. Each point represents mean ± SD of three replicates. (E) Nano-electrospray mass spectrometry confirmation of the covalent incorporation of a single molecule of p-BPB in the modified Asp49 myotoxin. The observed isotope-averaged masses of the modified Asp49 preparation show an increase of 195 ± 3 Da in comparison to the masses of untreated proteins (Fig. 2D).
The p-BPB-modified Asp49 PLA2 was tested for myotoxicity in mice (Fig. 5A) and for in vitro cytotoxicity upon differentiated C2C12 myotubes (Fig. 5B), in comparison to the unmodified toxin control. Results showed that inactivation of catalytic activity led to a major inhibition of both toxic effects, in agreement with the previous observations of Díaz-Oreiro & Gutiérrez (1997). The very low residual levels of toxicity observed can be attributed to the low, but detectable amounts of enzyme that remained catalytically active after p-BPB treatment. Some studies have reported variable levels of residual toxicity for Asp49 myotoxins under conditions that abrogated PLA2 activity by using p-BPB (Table 1). For example, residual toxicities of 30%, 14% and 16%, were observed in studies with B. asper myotoxin III, crotoxin B, and piratoxin III, respectively after p-BPB modification (Table 1). These observations have led to the suggestion that viperid Asp49 PLA2 myotoxins might possess mechanisms additional to their catalytic activity, to exert toxicity (Díaz et al., 1991; Bultrón, Thelestam & Gutiérrez, 1993; Díaz-Oreiro & Gutiérrez, 1997; Andrião-Escarso et al., 2000; Soares et al., 2001a; Soares et al., 2001b). The present results strengthen the concept that the effector mechanism of toxicity of viperid Asp49 PLA2 myotoxins, i.e., the capacity to disrupt the integrity of plasma membrane, essentially relies on their ability to hydrolyze phospholipids on the membrane of target cells (Fernández et al., 2013). On the other hand, p-BPB, besides blocking the catalytic center of Asp49 PLA2s, may induce subtle conformational changes at distant sites. Such changes have been shown by crystallography in the bovine pancreatic PLA2 (Renetseder et al., 1988) and the non-myotoxic, acidic PLA2 BthA-I from Bothrops jararacussu venom (Magro et al., 2005), although not in the case of the acidic APLA2 from Agkistrodon halys venom (Zhao et al., 1998). Moreover, conformational changes induced by p-BPB have also been demonstrated for two Lys49 myotoxins, PrTX-I from B. pirajai (Marchi-Salvador et al., 2009) and BthTX-I from B. jararacussu (Fernandes et al., 2010). These myotoxins, in spite of being enzymatically inactive, suffer a reduction in myotoxicity of nearly 50% when modified by p-BPB, which has been possibly attributed to the conformational changes caused by the alkylation of their absolutely conserved His48 (Díaz et al., 1993; Andrião-Escarso et al., 2000; Soares et al., 2000; Marchi-Salvador et al., 2009). In contrast, as shown by the present results and some previous studies (Table 1), the Asp49 myotoxins behave differently since their toxicity is essentially lost after His48 alkylation, concomitantly with the loss of catalytic activity. Since conformational changes induced by His48 alkylation do not completely abrogate toxicity in the case of Lys49 myotoxins, but leave a residual effect as high as 50%, the complete loss of toxicity in p-BPB-treated Asp49 myotoxins would be hard to reconcile with the proposal that mechanisms independent of catalysis (in a manner analogous to the catalytic-independent membrane-damaging action of Lys49 myotoxins) may also be responsible for muscle necrosis. In line with this concept, it has been shown that synthetic peptides from the C-terminal region of Asp49 PLA2 myotoxins do not display toxicity, in contrast to equivalent peptides from Lys49 myotoxins which reproduce the catalytic-independent toxic effect of their parent molecules (Núñez, Angulo & Lomonte, 2001; Lomonte, Angulo & Santamaría, 2003; Cintra-Francischinelli et al., 2010).
Figure 5. Comparison of the myotoxic (A) and cytotoxic (B) activities of untreated and p-BPB-treated Asp49 myotoxins.
Bars in (A) represent mean ± SD of five mice. Values corresponding to p-BPB-modified enzyme are very low, although significantly higher (*; p < 0.05) in comparison to the phosphate-buffered saline (PBS) in (A) or culture medium in (B). Bars represent mean ± SD of five replicates in (A) or three replicates in (B).
Table 1. Summary of studies.
The inactivation of group II Asp49 phospholipases A2 (PLA2s) from snake venoms by p-bromophenacyl bromide (p-BPB) and its consequences for myotoxicity in vivo or cytotoxicity in vitro have been analyzed.
| Asp49 PLA2 | Residual PLA2 activity (%) |
Residual toxicity (%), assay |
Reference |
|---|---|---|---|
| Crotalus d. terrificus crotoxin-B | 15 | very low myotoxicityb | Gopalakrishnakone et al. (1984) |
| Bothrops asper myotoxin III | 3 | 30, cytotoxicity | Bultrón, Thelestam & Gutiérrez (1993) |
| Bothrops asper myotoxin III | 0 | 4, myotoxicity | Díaz-Oreiro & Gutiérrez (1997) |
| Lachesis muta LM-PLA2 | 0a | 0, myotoxicity | Fuly et al. (2000) |
| Bothrops jararacussu BthTX-II | 0.05 | 1, cytotoxicity | Andrião-Escarso et al. (2000) |
| 0.5, myotoxicity | |||
| Crotalus d. terrificus crotoxin-B | 0 | 14.1, myotoxicity | Soares et al. (2001a) |
| 5.9a | |||
| Bothrops jararacussu BthTX-II | 0 | 5, myotoxicity | Soares & Giglio (2003) |
| 1, cytotoxicity | |||
| Bothrops pirajai PrTX-III | 0 | 16, myotoxicity | Soares et al. (2001b) |
| 5, cytotoxicity | |||
| Bothrops asper Asp49 myotoxins | 2.7 | 7, myotoxicity | Present study |
| 4, cytotoxicity |
Notes.
Determined by indirect hemolysis.
Analyzed qualitatively, and described as “minimal myotoxicity”.
On the other hand, in the case of elapid PLA2s (group I), a recent study on notexin and site-directed mutants has shown that the catalytically-inactive Asp49/Lys mutant retains a cytotoxic activity as high as 35–50% of that corresponding to the wild-type protein (Simonato et al., 2014), indicating that in addition to its catalytic-dependent toxic mechanism, it also exerts toxicity by a yet uncharacterized catalytic-independent action. Thus, elapid PLA2s may present important differences with viperid PLA2s regarding structure–function relationships and toxic mechanisms.
Altogether, current evidence on the mode of action of the two divergent subtypes of group II PLA2 myotoxins of viperids supports the notion that the basic Asp49 enzymes acquired this toxic activity by changes that directed their catalytic action towards the membrane phospholipids of muscle, whereas the Lys49 proteins, which lost catalytic activity along their evolutionary history due to critical substitutions (Petan, Križaj & Pungerčar, 2007), acquired changes that enabled them to directly alter membrane permeability via their specialized C-terminal region (Lomonte & Gutiérrez, 2011; Fernández et al., 2013).
Supplemental Information
Phospholipase A2 activity, colorimetrically quantified in a microplate reader–Cytotoxic activity, quantified by a UV kinetic assay–Myotoxic activity, quantified by a UV kinetic assay
Acknowledgments
The valuable contribution of Dr Julián Fernández in critical reading of the manuscript, and of Erika Camacho, Juan Manuel Díaz-Ureña, and Wan-Chih Tsai in various aspects of this work, is gratefully acknowledged. This study was performed in fulfillment of the M.Sc. degree in Biomedical Sciences of D Mora-Obando at Universidad de Costa Rica.
Funding Statement
Financial support was received from International Centre for Genetic Engineering and Biotechnology (ICGEB, Italy; CRP/COS13-01), and Vicerrectoría de Investigación (UCR; 741-B4-100). Sistema de Estudios de Posgrado, Universidad de Costa Rica (SEP-UCR) provided partial financial aid to D Mora-Obando during this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
Bruno Lomonte is an Academic Editor for PeerJ. The authors declare there are no competing interests.
Author Contributions
Diana Mora-Obando conceived and designed the experiments, performed the experiments, analyzed the data, wrote the paper, prepared figures and/or tables.
Cecilia Díaz analyzed the data, reviewed drafts of the paper.
Yamileth Angulo performed the experiments, analyzed the data, reviewed drafts of the paper.
José María Gutiérrez conceived and designed the experiments, analyzed the data, reviewed drafts of the paper.
Bruno Lomonte conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, reviewed drafts of the paper.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
In vivo assays performed in mice followed protocols approved by the Institutional Committee for the Use and Care of Animals (CICUA; 132-13), University of Costa Rica.
References
- Andrião-Escarso et al. (2000).Andrião-Escarso SH, Soares AM, Rodrigues VM, Angulo Y, Díaz C, Lomonte B, Gutiérrez JM, Giglio JR. Myotoxic phospholipases A2 in Bothrops snake venoms: effect of chemical modifications on the enzymatic and pharmacological properties of bothropstoxins from Bothrops jararacussu. Biochimie. 2000;82:755–763. doi: 10.1016/S0300-9084(00)01150-0. [DOI] [PubMed] [Google Scholar]
- Angulo & Lomonte (2009).Angulo Y, Lomonte B. Biochemistry and toxicology of toxins purified from the venom of the snake Bothrops asper. Toxicon. 2009;54:949–957. doi: 10.1016/j.toxicon.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Arni & Ward (1996).Arni RK, Ward RJ. Phospholipase A2 — a structural review. Toxicon. 1996;34:827–841. doi: 10.1016/0041-0101(96)00036-0. [DOI] [PubMed] [Google Scholar]
- Bultrón, Thelestam & Gutiérrez (1993).Bultrón E, Thelestam M, Gutiérrez JM. Effects on cultured mammalian cells of myotoxin III, a phospholipase A2 isolated from Bothrops asper (terciopelo) venom. Biochimica et Biophysica Acta (BBA)—Molecular Cell Research. 1993;1179:253–259. doi: 10.1016/0167-4889(93)90080-9. [DOI] [PubMed] [Google Scholar]
- Chioato et al. (2002).Chioato L, de Oliveira AHC, Ruller R, Sá JM, Ward RJ. Distinct sites for myotoxic and membrane-damaging activities in the C-terminal region of a Lys49-phospholipase A2. Biochemical Jounal. 2002;366:971–976. doi: 10.1042/BJ20020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chioato & Ward (2003).Chioato L, Ward RJ. Mapping structural determinants of biological activities in snake venom phospholipases A2 by sequence analysis and site directed mutagenesis. Toxicon. 2003;42:869–883. doi: 10.1016/j.toxicon.2003.11.027. [DOI] [PubMed] [Google Scholar]
- Cintra-Francischinelli et al. (2010).Cintra-Francischinelli M, Pizzo P, Angulo Y, Gutiérrez JM, Montecucco C, Lomonte B. The C-terminal region of a Lys49 myotoxin mediates Ca2+ influx in C2C12 myotubes. Toxicon. 2010;55:590–596. doi: 10.1016/j.toxicon.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Cintra-Francischinelli et al. (2009).Cintra-Francischinelli M, Pizzo P, Rodrigues-Simioni L, Ponce-Soto L, Rossetto O, Lomonte B, Gutiérrez JM, Pozzan T, Montecucco C. Calcium imaging of muscle cells treated with snake myotoxins reveals toxin synergism and presence of receptors. Cellular and Molecular Life Sciences. 2009;66:1718–1728. doi: 10.1007/s00018-009-9053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Oreiro & Gutiérrez (1997).Díaz-Oreiro C, Gutiérrez JM. Chemical modification of histidine and lysine residues of myotoxic phospholipases A2 isolated from Bothrops asper and Bothrops godmani snake venoms: effects on enzymatic and pharmacological properties. Toxicon. 1997;35:241–252. doi: 10.1016/S0041-0101(96)00128-6. [DOI] [PubMed] [Google Scholar]
- Díaz et al. (1991).Díaz C, Gutiérrez JM, Lomonte B, Gené JA. The effect of myotoxins isolated from Bothrops snake venoms on multilamellar liposomes: relationship to phospholipase A2, anticoagulant and myotoxic activities. Biochimica et Biophysica Acta. 1991;1070:455–460. doi: 10.1016/0005-2736(91)90086-N. [DOI] [PubMed] [Google Scholar]
- Díaz et al. (1993).Díaz C, Gutiérrez JM, Lomonte B, Núñez J. p-Bromophenacyl bromide modification of Bothrops asper myotoxin II, a lysine-49 phospholipase A2, affects its pharmacological activities. Toxicon. 1993;31:1202–1206. doi: 10.1016/0041-0101(93)90136-7. [DOI] [PubMed] [Google Scholar]
- dos Santos et al. (2009).dos Santos JI, Fernandes CAH, Magro AJ, Fontes MRM. The intriguing phospholipases A2 homologues: relevant structural features on myotoxicity and catalytic inactivity. Protein and Peptide Letters. 2009;16:887–893. doi: 10.2174/092986609788923310. [DOI] [PubMed] [Google Scholar]
- Faure & Bon (1988).Faure G, Bon C. Crotoxin, a phospholipase A2 neurotoxin from the South American rattlesnake Crotalus durissus terrificus: purification of several isoforms and comparison of their molecular structure and of their biological activities. Biochemistry. 1988;27:730–738. doi: 10.1021/bi00402a036. [DOI] [PubMed] [Google Scholar]
- Fernandes et al. (2013).Fernandes CAH, Comparetti EJ, Borges RJ, Huancahuire-Vega S, Ponce-Soto LA, Marangoni S, Soares AM, Fontes MRM. Structural bases for a complete myotoxic mechanism: crystal structures of two non-catalytic phospholipases A2-like from Bothrops brazili venom. Biochimica et Biophysica Acta. 2013;1834:2772–2781. doi: 10.1016/j.bbapap.2013.10.009. [DOI] [PubMed] [Google Scholar]
- Fernandes et al. (2010).Fernandes CAH, Marchi-Salvador DP, Salvador GM, Silva MCO, Costa TR, Soares AM, Fontes MRM. Comparison between apo and complexed structures of bothropstoxin-I reveals the role of Lys122 and Ca2+-binding loop region for the catalytically inactive Lys49-PLA2s. Journal of Structural Biology. 2010;171:31–43. doi: 10.1016/j.jsb.2010.03.019. [DOI] [PubMed] [Google Scholar]
- Fernández et al. (2013).Fernández J, Caccin P, Koster G, Lomonte B, Gutiérrez JM, Montecucco C, Postle AD. Muscle phospholipid hydrolysis by Bothrops asper Asp49 and Lys49 phospholipase A2 myotoxins—distinct mechanisms of action. FEBS Journal. 2013;280:3878–3886. doi: 10.1111/febs.12386. [DOI] [PubMed] [Google Scholar]
- Francis et al. (1991).Francis B, Gutiérrez JM, Lomonte B, Kaiser II. Myotoxin II from Bothrops asper (Terciopelo) venom is a lysine-49 phospholipase A2. Archives of Biochemistry and Biophysics. 1991;284:352–359. doi: 10.1016/0003-9861(91)90307-5. [DOI] [PubMed] [Google Scholar]
- Fry & Wüster (2004).Fry BG, Wüster W. Assembling an arsenal: origin and evolution of the snake venom proteome inferred from phylogenetic analysis of toxin sequences. Molecular Biology and Evolution. 2004;21:870–883. doi: 10.1093/molbev/msh091. [DOI] [PubMed] [Google Scholar]
- Fuly et al. (2000).Fuly AL, Calil-Elias S, Zingali RB, Guimarães JA, Melo PA. Myotoxic activity of an acidic phospholipase A2 isolated from Lachesis muta (Bushmaster) snake venom. Toxicon. 2000;38:961–972. doi: 10.1016/S0041-0101(99)00208-1. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnakone et al. (1984).Gopalakrishnakone P, Dempster DW, Hawgood BJ, Elder HY. Cellular and mitochondrial changes induced in the structure of murine skeletal muscle by crotoxin, a neurotoxic phospholipase A2 complex. Toxicon. 1984;22:85–98. doi: 10.1016/0041-0101(84)90141-7. [DOI] [PubMed] [Google Scholar]
- Gutiérrez & Lomonte (2013).Gutiérrez JM, Lomonte B. Phospholipases A2: unveiling the secrets of a functionally versatile group of snake venom toxins. Toxicon. 2013;62:27–39. doi: 10.1016/j.toxicon.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Gutiérrez & Ownby (2003).Gutiérrez JM, Ownby CL. Skeletal muscle degeneration induced by venom phospholipases A2: insights into the mechanisms of local and systemic myotoxicity. Toxicon. 2003;42:915–931. doi: 10.1016/j.toxicon.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Gutiérrez, Ownby & Odell (1984).Gutiérrez JM, Ownby CL, Odell GV. Isolation of a myotoxin from Bothrops asper venom: partial characterization and action on skeletal muscle. Toxicon. 1984;22:115–128. doi: 10.1016/0041-0101(84)90144-2. [DOI] [PubMed] [Google Scholar]
- Gutiérrez et al. (1995).Gutiérrez JM, Romero M, Díaz C, Borkow G, Ovadia M. Isolation and characterization of a metalloproteinase with weak hemorrhagic activity from the venom of the snake Bothrops asper (terciopelo) Toxicon. 1995;33:19–29. doi: 10.1016/0041-0101(94)00138-X. [DOI] [PubMed] [Google Scholar]
- Harris & Cullen (1990).Harris JB, Cullen MJ. Muscle necrosis caused by snake venoms and toxins. Electron Microscopy Reviews. 1990;3:183–211. doi: 10.1016/0892-0354(90)90001-9. [DOI] [PubMed] [Google Scholar]
- Holzer & Mackessy (1996).Holzer M, Mackessy SP. An aqueous endpoint assay of snake venom phospholipase A2. Toxicon. 1996;34:149–1155. doi: 10.1016/0041-0101(96)00057-8. [DOI] [PubMed] [Google Scholar]
- Kaiser et al. (1990).Kaiser II, Gutierrez JM, Plummer D, Aird SD, Odell GV. The amino acid sequence of a myotoxic phospholipase from the venom of Bothrops asper. Archives of Biochemistry and Biophysics. 1990;278:319–325. doi: 10.1016/0003-9861(90)90266-2. [DOI] [PubMed] [Google Scholar]
- Kini (1997).Kini RM. Venom phospholipase A2 enzymes. structure, function, and mechanisms. Chichester: John Wiley & Sons; 1997. p. 511. [Google Scholar]
- Lomonte, Angulo & Calderón (2003).Lomonte B, Angulo Y, Calderón L. An overview of Lysine-49 phospholipase A2 myotoxins from crotalid snake venoms and their structural determinants of myotoxic action. Toxicon. 2003;42:885–901. doi: 10.1016/j.toxicon.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Lomonte et al. (1999).Lomonte B, Angulo Y, Rufini S, Cho W, Giglio JR, Ohno M, Daniele JJ, Geoghegan P, Gutiérrez JM. Comparative study of the cytolytic activity of myotoxic phospholipases A2 on mouse endothelial (tEnd) and skeletal muscle (C2C12) cells in vitro. Toxicon. 1999;37:145–158. doi: 10.1016/S0041-0101(98)00171-8. [DOI] [PubMed] [Google Scholar]
- Lomonte, Angulo & Santamaría (2003).Lomonte B, Angulo Y, Santamaría C. Comparative study of synthetic peptides corresponding to region 115–129 in Lys49 myotoxic phospholipases A2 from snake venoms. Toxicon. 2003;42:307–312. doi: 10.1016/S0041-0101(03)00149-1. [DOI] [PubMed] [Google Scholar]
- Lomonte et al. (2009).Lomonte B, Angulo Y, Sasa M, Gutiérrez JM. The phospholipase A2 homologues of snake venoms: biological activities and their possible adaptive roles. Protein and Peptide Letters. 2009;16:860–876. doi: 10.2174/092986609788923356. [DOI] [PubMed] [Google Scholar]
- Lomonte & Carmona (1992).Lomonte B, Carmona E. Individual expression patterns of myotoxin isoforms in the venom of the snake Bothrops asper. Comparative Biochemistry and Physiology. 1992;102B:325–329. doi: 10.1016/0305-0491(92)90129-f. [DOI] [PubMed] [Google Scholar]
- Lomonte & Gutiérrez (1989).Lomonte B, Gutiérrez JM. A new muscle damaging toxin, myotoxin II, from the venom of the snake Bothrops asper (terciopelo) Toxicon. 1989;27:725–733. doi: 10.1016/0041-0101(89)90039-1. [DOI] [PubMed] [Google Scholar]
- Lomonte & Gutiérrez (2011).Lomonte B, Gutiérrez JM. Phospholipases A2 from Viperidae snake venoms: how do they induce skeletal muscle damage? Acta Chimica Slovenica. 2011;58:647–659. [PubMed] [Google Scholar]
- Lomonte & Kahan (1988).Lomonte B, Kahan L. Production and partial characterization of monoclonal antibodies to Bothrops asper (terciopelo) myotoxin. Toxicon. 1988;26:675–689. doi: 10.1016/0041-0101(88)90249-8. [DOI] [PubMed] [Google Scholar]
- Lomonte et al. (1994).Lomonte B, Moreno E, Tarkowski A, Hanson LÅ, Maccarana M. Neutralizing interaction between heparins and myotoxin II, a Lys-49 phospholipase A2 from Bothrops asper snake venom. Identification of a heparin-binding and cytolytic toxin region by the use of synthetic peptides and molecular modeling. Journal of Biological Chemistry. 1994;269:29867–29873. [PubMed] [Google Scholar]
- Lomonte & Rangel (2012).Lomonte B, Rangel J. Snake venom Lys49 myotoxins: from phospholipases A2 to non-enzymatic membrane disruptors. Toxicon. 2012;60:520–530. doi: 10.1016/j.toxicon.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Magro et al. (2005).Magro AJ, Takeda AAS, Soares AM, Fontes MRM. Structure of BthA-I complex with p-bromophenacyl bromide: possible correlations with lack of pharmacological activity. Acta Crystallographica. 2005;D61:1670–1677. doi: 10.1107/S0907444905029598. [DOI] [PubMed] [Google Scholar]
- Maraganore et al. (1984).Maraganore JM, Merutka G, Cho W, Welches W, Kézdy FJ, Heinrikson RL. A new class of phospholipases A2 with lysine in place of aspartate 49. The Journal of Biological Chemistry. 1984;259:13839–13843. [PubMed] [Google Scholar]
- Marchi-Salvador et al. (2009).Marchi-Salvador DP, Fernandes CAH, Silveira LB, Soares AM, Fontes MRM. Crystal structure of a phospholipase A2 homolog complexed with p-bromophenacyl bromide reveals important structural changes associated with the inhibition of myotoxic activity. Biochimica et Biophysica Acta. 2009;1794:1583–1590. doi: 10.1016/j.bbapap.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Montecucco, Gutiérrez & Lomonte (2008).Montecucco C, Gutiérrez JM, Lomonte B. Cellular pathology induced by snake venom phospholipase A2 myotoxins and neurotoxins: common aspects of their mechanisms of action. Cellular and Molecular Life Sciences. 2008;65:2897–2912. doi: 10.1007/s00018-008-8113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora-Obando et al. (2014).Mora-Obando D, Guerrero-Vargas J, Prieto-Sánchez R, Beltrán J, Rucavado A, Sasa M, Gutiérrez JM, Ayerbe S, Lomonte B. Proteomic and functional profiling of the venom of Bothrops ayerbei from Cauca, Colombia, reveals striking interspecific variation with Bothrops asper venom. Journal of Proteomics. 2014;96:159–172. doi: 10.1016/j.jprot.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Núñez, Angulo & Lomonte (2001).Núñez CE, Angulo Y, Lomonte B. Identification of the myotoxic site of the Lys49 phospholipase A2 from Agkistrodon piscivorus piscivorus snake venom: synthetic C-terminal peptides from Lys49, but not from Asp49 myotoxins, exert membrane-damaging activities. Toxicon. 2001;39:1587–1594. doi: 10.1016/S0041-0101(01)00141-6. [DOI] [PubMed] [Google Scholar]
- Petan, Križaj & Pungerčar (2007).Petan T, Križaj I, Pungerčar J. Restoration of enzymatic activity in a Ser-49 phospholipase A2 homologue decreases its Ca2+-independent membrane-damaging activity and increases its toxicity. Biochemistry. 2007;46:12795–12809. doi: 10.1021/bi701304e. [DOI] [PubMed] [Google Scholar]
- Renetseder et al. (1988).Renetseder R, Dijkstra BW, Huizinga K, Kalk KH, Drenth J. Crystal structure of bovine pancreatic phospholipase A2 covalently inhibited by p-bromo-phenacyl-bromide. Journal of Molecular Biology. 1988;200:181–199. doi: 10.1016/0022-2836(88)90342-7. [DOI] [PubMed] [Google Scholar]
- Scott et al. (1992).Scott DL, Achari A, Vidal JC, Sigler PB. Crystallographic and biochemical studies of the (inactive) Lys-49 phospholipase A2 from the venom of Agkistrodon piscivorus piscivorus. Journal of Biological Chemistry. 1992;267:22645–22657. [PubMed] [Google Scholar]
- Simonato et al. (2014).Simonato M, Morbiato L, Zorzi V, Caccin P, Fernández J, Massimino ML, de Laureto PP, Tonello F. Production in Escherichia coli, folding, purification and characterization of notexin with wild type sequence and with N-terminal and catalytic site mutations. Toxicon. 2014;88:11–20. doi: 10.1016/j.toxicon.2014.06.009. [DOI] [PubMed] [Google Scholar]
- Soares et al. (2001b).Soares AM, Andrião-Escarso SH, Bortoleto RK, Rodrigues-Simioni L, Arni RK, Ward RJ, Gutiérrez JM, Giglio JR. Dissociation of enzymatic and pharmacological properties of piratoxins-I and -III, two myotoxic phospholipases A2 from Bothrops pirajai snake venom. Archives of Biochemistry and Biophysics. 2001b;387:188–196. doi: 10.1006/abbi.2000.2244. [DOI] [PubMed] [Google Scholar]
- Soares, Fontes & Giglio (2004).Soares AM, Fontes MRM, Giglio JR. Phospholipase A2 myotoxins from Bothrops snake venoms: structure–function relationship. Current Organic Chemistry. 2004;8:1677–1690. doi: 10.2174/1385272043369610. [DOI] [Google Scholar]
- Soares & Giglio (2003).Soares AM, Giglio JR. Chemical modifications of phospholipases A2 from snake venoms: effects on catalytic and pharmacological properties. Toxicon. 2003;42:855–868. doi: 10.1016/j.toxicon.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Soares et al. (2000).Soares AM, Guerra-Sá R, Borja-Oliveira C, Rodrigues VM, Rodrigues-Simioni L, Rodrigues V, Fontes MRM, Lomonte B, Gutiérrez JM, Giglio JR. Structural and functional characterization of BnSP-7, a lysine-49 myotoxic phospholipase A2 homologue from Bothrops neuwiedi pauloensis venom. Archives of Biochemistry and Biophysics. 2000;378:201–209. doi: 10.1006/abbi.2000.1790. [DOI] [PubMed] [Google Scholar]
- Soares et al. (2001a).Soares AM, Mancin AC, Cecchini AL, Arantes EC, França C, Gutiérrez JM, Giglio JR. Effects of chemical modifications of crotoxin B, the phospholipase A2 subunit of crotoxin from Crotalus durissus terrificus snake venom, on its enzymatic and pharmacological activities. International Journal of Biochemistry and Cell Biology. 2001a;33:877–888. doi: 10.1016/S1357-2725(01)00065-6. [DOI] [PubMed] [Google Scholar]
- Valiente et al. (1992).Valiente C, Moreno E, Sittenfeld A, Lomonte B, Gutiérrez JM. An electrophoretic study on phospholipase A2 isoenzymes in the venoms of Central American crotaline snakes. Toxicon. 1992;30:815–823. doi: 10.1016/0041-0101(92)90379-J. [DOI] [PubMed] [Google Scholar]
- Volwerk, Pieterson & de Haas (1974).Volwerk JJ, Pieterson WA, de Haas GH. Histidine at the active site of phospholipase A2. Biochemistry. 1974;13:1446–1454. doi: 10.1021/bi00704a020. [DOI] [PubMed] [Google Scholar]
- Zhao et al. (1998).Zhao H, Tang L, Wang X, Zhou Y, Lin Z. Structure of a snake venom phospholipase A2 modified by p-bromo-phenacyl-bromide. Toxicon. 1998;36:875–886. doi: 10.1016/S0041-0101(97)00169-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phospholipase A2 activity, colorimetrically quantified in a microplate reader–Cytotoxic activity, quantified by a UV kinetic assay–Myotoxic activity, quantified by a UV kinetic assay