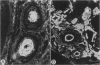
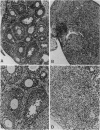
Abstract
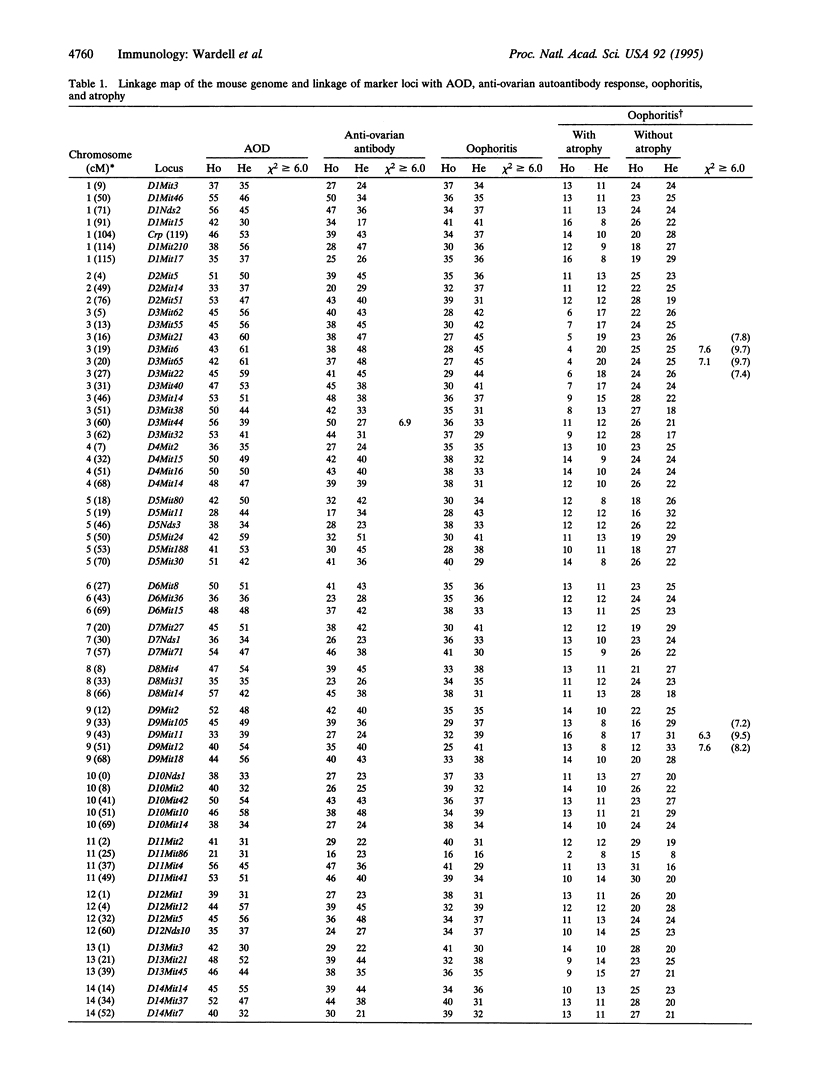
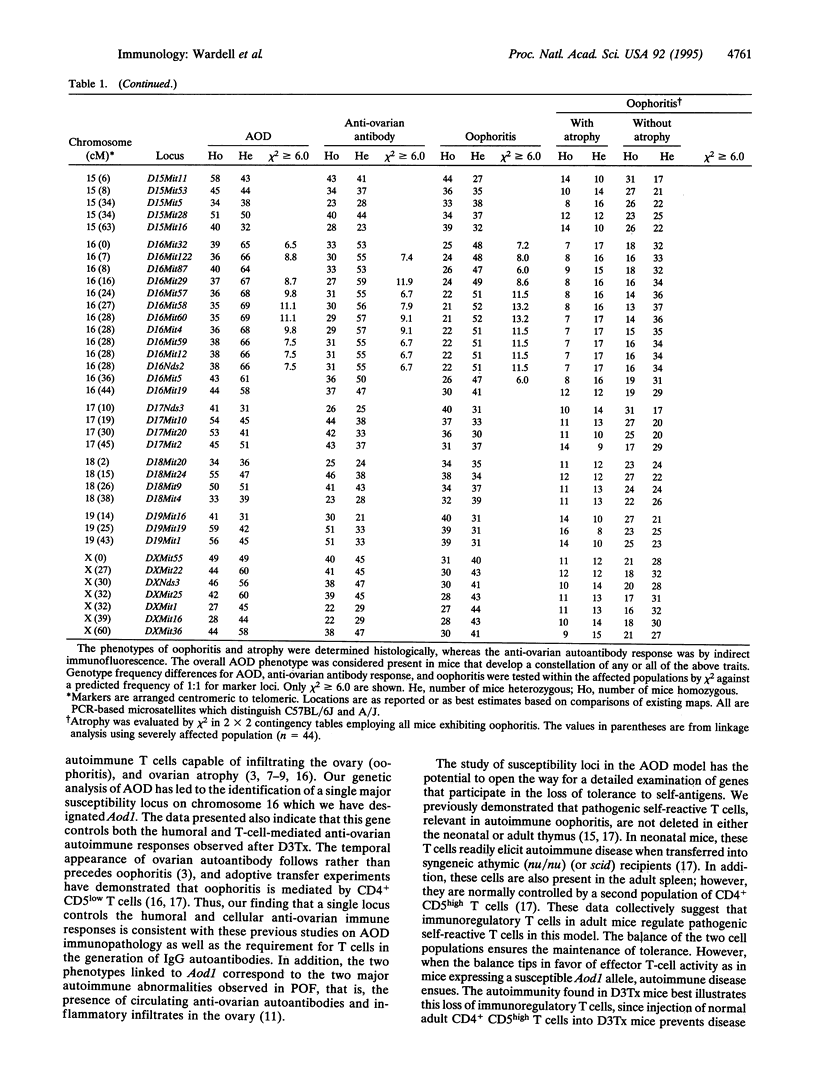
Mice thymectomized at three days of age (D3Tx) develop during adulthood a variety of organ-specific autoimmune diseases, including autoimmune ovarian dysgenesis (AOD). The phenotypic spectrum of AOD is characterized by the development of anti-ovarian autoantibodies, oophoritis, and atrophy. The D3Tx model of AOD is unique in that disease induction depends exclusively on perturbation of the normal developing immune system, is T-cell-mediated, and is strain specific. For example, D3Tx A/J mice are highly susceptible to AOD, whereas C57BL/6J mice are resistant. After D3Tx, self ovarian antigens, expressed at physiological levels, trigger an autoimmune response capable of eliciting disease. The D3Tx model provides, therefore, the opportunity to focus on the mechanisms of self-tolerance that are relevant to disease pathogenesis. Previous studies indicate that the principal mechanisms involved in AOD susceptibility are genetically controlled and govern developmental processes associated with the induction and maintenance of peripheral tolerance. We report here the mapping of the Aod1 locus to mouse chromosome 16 within a region encoding several loci of immunologic relevance, including scid, Igl1, VpreB, Igll, Igl1r, Mtv6 (Mls-3), Ly-7, Ifnar, and Ifgt.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderuccio F., Toh B. H., Tan S. S., Gleeson P. A., van Driel I. R. An autoimmune disease with multiple molecular targets abrogated by the transgenic expression of a single autoantigen in the thymus. J Exp Med. 1993 Aug 1;178(2):419–426. doi: 10.1084/jem.178.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich W. F., Miller J. C., Steen R. G., Merchant M., Damron D., Nahf R., Gross A., Joyce D. C., Wessel M., Dredge R. D. A genetic map of the mouse with 4,006 simple sequence length polymorphisms. Nat Genet. 1994 Jun;7(2 Spec No):220–245. doi: 10.1038/ng0694supp-220. [DOI] [PubMed] [Google Scholar]
- Dietrich W., Katz H., Lincoln S. E., Shin H. S., Friedman J., Dracopoli N. C., Lander E. S. A genetic map of the mouse suitable for typing intraspecific crosses. Genetics. 1992 Jun;131(2):423–447. doi: 10.1093/genetics/131.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake C. G., Babcock S. K., Palmer E., Kotzin B. L. Genetic analysis of the NZB contribution to lupus-like autoimmune disease in (NZB x NZW)F1 mice. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):4062–4066. doi: 10.1073/pnas.91.9.4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowell D., Mason D. Evidence that the T cell repertoire of normal rats contains cells with the potential to cause diabetes. Characterization of the CD4+ T cell subset that inhibits this autoimmune potential. J Exp Med. 1993 Mar 1;177(3):627–636. doi: 10.1084/jem.177.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy D. L., Bacher J., Hodgen G. D. Thymic regulation of primate fetal ovarian-adrenal differentiation. Biol Reprod. 1985 Jun;32(5):1127–1133. doi: 10.1095/biolreprod32.5.1127. [DOI] [PubMed] [Google Scholar]
- Kojima A., Prehn R. T. Genetic susceptibility to post-thymectomy autoimmune diseases in mice. Immunogenetics. 1981;14(1-2):15–27. doi: 10.1007/BF00344296. [DOI] [PubMed] [Google Scholar]
- Kojima A., Sakakura T., Tanaka Y., Nishizuka Y. Sterility in neonatally thymectomized female mice: its nature and prevention by the injection of spleen cells. Biol Reprod. 1973 Apr;8(3):358–361. doi: 10.1093/biolreprod/8.3.358. [DOI] [PubMed] [Google Scholar]
- Kojima A., Taguchi O., Nishizuka Y. Experimental production of possible autoimmune castritis followed by macrocytic anemia in athymic nude mice. Lab Invest. 1980 Apr;42(4):387–395. [PubMed] [Google Scholar]
- Kojima A., Tanaka-Kojima Y., Sakakura T., Nishizuka Y. Spontaneous development of autoimmune thyroiditis in neonatally thymectomized mice. Lab Invest. 1976 Jun;34(6):550–557. [PubMed] [Google Scholar]
- LaBarbera A. R., Miller M. M., Ober C., Rebar R. W. Autoimmune etiology in premature ovarian failure. Am J Reprod Immunol Microbiol. 1988 Mar;16(3):115–122. doi: 10.1111/j.1600-0897.1988.tb00180.x. [DOI] [PubMed] [Google Scholar]
- Michael S. D., Taguchi O., Nishizuka Y. Effect of neonatal thymectomy on ovarian development and plasma LH, FSH, GH and PRL in the mouse. Biol Reprod. 1980 Mar;22(2):343–350. doi: 10.1093/biolreprod/22.2.343. [DOI] [PubMed] [Google Scholar]
- Miyake T., Taguchi O., Ikeda H., Sato Y., Takeuchi S., Nishizuka Y. Acute oocyte loss in experimental autoimmune oophoritis as a possible model of premature ovarian failure. Am J Obstet Gynecol. 1988 Jan;158(1):186–192. doi: 10.1016/0002-9378(88)90808-3. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y., Sakakura T., Taguchi O. Mechanism of ovarian tumorigenesis in mice after neonatal thymectomy. Natl Cancer Inst Monogr. 1979 May;(51):89–96. [PubMed] [Google Scholar]
- Nishizuka Y., Sakakura T. Thymus and reproduction: sex-linked dysgenesia of the gonad after neonatal thymectomy in mice. Science. 1969 Nov 7;166(3906):753–755. doi: 10.1126/science.166.3906.753. [DOI] [PubMed] [Google Scholar]
- Reeves R. H., Irving N. G., Miller R. D. Encyclopedia of the mouse genome III. October 1993. Mouse chromosome 16. Mamm Genome. 1993;4(Spec No):S223–S229. doi: 10.1007/BF00360842. [DOI] [PubMed] [Google Scholar]
- Smith H., Chen I. M., Kubo R., Tung K. S. Neonatal thymectomy results in a repertoire enriched in T cells deleted in adult thymus. Science. 1989 Aug 18;245(4919):749–752. doi: 10.1126/science.2788921. [DOI] [PubMed] [Google Scholar]
- Smith H., Lou Y. H., Lacy P., Tung K. S. Tolerance mechanism in experimental ovarian and gastric autoimmune diseases. J Immunol. 1992 Sep 15;149(6):2212–2218. [PubMed] [Google Scholar]
- Smith H., Sakamoto Y., Kasai K., Tung K. S. Effector and regulatory cells in autoimmune oophoritis elicited by neonatal thymectomy. J Immunol. 1991 Nov 1;147(9):2928–2933. [PubMed] [Google Scholar]
- Sudweeks J. D., Todd J. A., Blankenhorn E. P., Wardell B. B., Woodward S. R., Meeker N. D., Estes S. S., Teuscher C. Locus controlling Bordetella pertussis-induced histamine sensitization (Bphs), an autoimmune disease-susceptibility gene, maps distal to T-cell receptor beta-chain gene on mouse chromosome 6. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3700–3704. doi: 10.1073/pnas.90.8.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi O., Kojima A., Nishizuka Y. Experimental autoimmune prostatitis after neonatal thymectomy in the mouse. Clin Exp Immunol. 1985 Apr;60(1):123–129. [PMC free article] [PubMed] [Google Scholar]
- Taguchi O., Nishizuka Y. Experimental autoimmune orchitis after neonatal thymectomy in the mouse. Clin Exp Immunol. 1981 Nov;46(2):425–434. [PMC free article] [PubMed] [Google Scholar]
- Taguchi O., Nishizuka Y., Sakakura T., Kojima A. Autoimmune oophoritis in thymectomized mice: detection of circulating antibodies against oocytes. Clin Exp Immunol. 1980 Jun;40(3):540–553. [PMC free article] [PubMed] [Google Scholar]
- Todd J. A., Aitman T. J., Cornall R. J., Ghosh S., Hall J. R., Hearne C. M., Knight A. M., Love J. M., McAleer M. A., Prins J. B. Genetic analysis of autoimmune type 1 diabetes mellitus in mice. Nature. 1991 Jun 13;351(6327):542–547. doi: 10.1038/351542a0. [DOI] [PubMed] [Google Scholar]
- Tung K. S., Smith S., Matzner P., Kasai K., Oliver J., Feuchter F., Anderson R. E. Murine autoimmune oophoritis, epididymoorchitis, and gastritis induced by day 3 thymectomy. Autoantibodies. Am J Pathol. 1987 Feb;126(2):303–314. [PMC free article] [PubMed] [Google Scholar]
- Tung K. S., Smith S., Teuscher C., Cook C., Anderson R. E. Murine autoimmune oophoritis, epididymoorchitis, and gastritis induced by day 3 thymectomy. Immunopathology. Am J Pathol. 1987 Feb;126(2):293–302. [PMC free article] [PubMed] [Google Scholar]
- Watson M. L., Rao J. K., Gilkeson G. S., Ruiz P., Eicher E. M., Pisetsky D. S., Matsuzawa A., Rochelle J. M., Seldin M. F. Genetic analysis of MRL-lpr mice: relationship of the Fas apoptosis gene to disease manifestations and renal disease-modifying loci. J Exp Med. 1992 Dec 1;176(6):1645–1656. doi: 10.1084/jem.176.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]