Abstract
The first steps of wood degradation by fungi lead to the release of toxic compounds known as extractives. To better understand how lignolytic fungi cope with the toxicity of these molecules, a transcriptomic analysis of Phanerochaete chrysosporium genes was performed in the presence of oak acetonic extracts. It reveals that in complement to the extracellular machinery of degradation, intracellular antioxidant and detoxification systems contribute to the lignolytic capabilities of fungi, presumably by preventing cellular damages and maintaining fungal health. Focusing on these systems, a glutathione transferase (P. chrysosporium GTT2.1 [PcGTT2.1]) has been selected for functional characterization. This enzyme, not characterized so far in basidiomycetes, has been classified first as a GTT2 compared to the Saccharomyces cerevisiae isoform. However, a deeper analysis shows that the GTT2.1 isoform has evolved functionally to reduce lipid peroxidation by recognizing high-molecular-weight peroxides as substrates. Moreover, the GTT2.1 gene has been lost in some non-wood-decay fungi. This example suggests that the intracellular detoxification system evolved concomitantly with the extracellular ligninolytic machinery in relation to the capacity of fungi to degrade wood.
INTRODUCTION
Lignolytic basidiomycetes initiate wood decay by producing extracellular reactive oxygen species (ROS) that depolymerize lignocellulose (1, 2). Accordingly, induction of lignin peroxidases (LiPs) is associated with ROS production, oxidative damage to macromolecules, and induction of antioxidant enzymes, such as catalase, superoxide dismutase (SOD), glutathione (GSH) reductase (GR), and glutathione peroxidase (Gpx) (3). In wood-grown Postia placenta, the quantity of ROS produced by a laccase has been estimated to be large enough to contribute to incipient decay (2). Moreover, some highly reactive wood compounds (known as extractives) are released primarily during wood degradation processes. Some studies have reported the toxicity and antifungal activity of these molecules (4–7). This suggests that during wood degradation, fungal cells are under oxidative stress.
Thus, the lignolytic activity of fungi is closely related to their capacity to resist oxidants and toxic molecules, such as wood extractives or fungicides. Thus, fungi have developed an efficient detoxification system. It is composed of enzymes encoded by multigenic families, which are expanded in these genomes and exhibit versatile activities, allowing them to accept a broad range of compounds such as substrates (8, 9). This system is composed mainly of oxidases as cytochrome P450 monooxygenases (CytP450), conjugating enzymes, and transporters. CytP450-encoding genes are highly represented in saprophytic fungal genomes (10–12). Functional analysis and gene upregulation data suggested that the enriched P450 families of model basidiomycetes have a common physiological function, i.e., degradation of plant defense chemicals and plant material-derived compounds, especially lignin-derived compounds (13). Moreover, the catalytic versatility of these enzymes could be involved in fungal colonization of plant material. CytP450 copy numbers in the genomes of wood degraders is correlated with the glutathione transferase (GST) copy numbers (11). GST genes belong to the second step of detoxification and participate in cell response to oxidative stress. They are less duplicated than CytP450s, but saprotrophic fungi, such as the wood decayer Phanerochaete chrysosporium or the litter decomposer Coprinus cinereus, still exhibit a high number of GST-encoding genes compared to symbiotic fungi or biotrophic pathogens (11). P. chrysosporium exhibits 27 GST isoforms, which cluster into 7 main classes: GSTO, GHR, Ure2p, GSTFuA, GTT1, GTT2, and Phi (11). The cysteine-containing GSTs (GSTO and GHR) are quite well conserved among organisms and have been studied in humans, insects, plants, and fungi (11). The others are more specific to some organisms and have been studied less. In particular, GSTFuA, which has been described recently, is a fungus-specific class with atypical features (14). Some GSTFuA members exhibit a ligandin property with wood-related molecules. GTT1 and GTT2 are described exclusively in Saccharomyces cerevisiae, having an antioxidant role (15). While S. cerevisiae GTT2 (ScGTT2) displays only classical GST activity, ScGTT1 exhibits both classical GST activity and peroxidase activity with hydroperoxides (16). The crystal structure of ScGTT2 has been solved (17). A water molecule acts as the deprotonator of the glutathione sulfur atom instead of the classic catalytic residues, i.e., tyrosine, serine, and cysteine. To date, no other fungal homologue has been characterized. In P. chrysosporium, 2 GTT2-related (Joint Genome Institute [JGI] protein identifiers [ProtID] 6683 and 6766) and no GTT1 genes have been identified in the genome by sequence homology with the yeast isoforms (18). However, their roles are unknown.
The aim of this study was to better understand how P. chrysosporium copes with the putative toxicity of oak acetonic extracts. Using a transcriptomic approach, we show that oak acetonic extracts create oxidative stress in P. chrysosporium highlighted by induction of genes of the antioxidant and detoxification systems. In particular, the analysis has revealed the induction of the gene coding for P. chrysosporium GTT2.1 (PcGTT2.1), a GTT2-related glutathione transferase, which is highly active against peroxides and seems to have evolved concomitantly with the extracellular degradation system.
MATERIALS AND METHODS
Oak acetonic extract preparation and identification.
Oak heartwood samples were ground to a fine powder, passed through a 115-mesh sieve, and dried at 60°C until minimal weight was obtained. Extraction then was performed overnight with high-performance liquid chromatography (HPLC)-grade acetone using a Soxhlet apparatus. The solvent was removed from the crude extracts by evaporation to dryness under vacuum. The powder was resuspended in dimethyl sulfoxide (DMSO). Liquid chromatography-mass spectrometry (LC-MS) analyses of samples were carried out using a Shimadzu (Noisiel, France) LC-20A ultra-HPLC (UHPLC) system equipped with an autosampler and interfaced to a PDA UV detector SPD-20A, followed by an LC-MS 8030 triple-quadruple mass spectrometer. The separation was performed on a Luna C18 analytical column (inner diameter, 150 mm by 3 mm; Phenomenex, Le Pecq, France) using a linear gradient from 8 to 68% of methanol in water (containing 0.1% formic acid) at a flow rate of 0.4 ml/min. The injection volume was 2 μl. UV-visible spectra were recorded between 190 and 800 nm. Positive and negative ion electrospray mass spectrometric analyses were carried out at a unit resolution between 100 and 2,000 m/z at a scan speed of 15,000 U/s. The heat block and desolvation line temperatures were 400°C and 250°C, respectively. Nitrogen was used as a drying (15 liters/min) and nebulizing (3 liters/min) gas. The ion spray voltage was ±4,500 V. Data were acquired and analyzed using LabSolutions software, version 5.42SP4, from Shimadzu. Identification was achieved by comparison of experimental retention times and UV-MS spectra to bibliographic data and standard compounds.
Culture conditions.
Phanerochaete chrysosporium homocaryon RP78 was maintained in malt agar medium. Fungal plugs were used to inoculate liquid minimal medium containing 5 mM sodium acetate, pH 4.5, 1% glucose, 1 mM ammonium tartrate, 1% (vol/vol) base medium (20 g liter−1 KH2PO4, 5 g liter−1 MgSO4, 1 g liter−1 CaCl2), 7% (vol/vol) trace medium [1.5 g liter−1 nitrilotriacetate, 3 g liter−1 MgSO4, 1 g liter−1 NaCl, 0.1 g liter−1 FeSO4 · 7H2O, 0.1 g liter−1 CoCl2, 0.1 g liter−1 ZnSO4 · 7H2O, 0.1 g liter−1 CuSO4, 10 mg liter−1 AlK(SO4) · 12H2O, 10 mg liter−1 H3BO3, 10 mg liter−1 NaMoO4 · 2H2O], and 225 μM MnCl2 (19). To study the effect of oak acetonic extracts on P. chrysosporium gene expression, static culturing was performed at 37°C for 3 days. The medium was then replaced by fresh liquid minimal medium (30 ml) as described above, with or without extracts obtained from 200 mg of oak wood. The treatment was done at 37°C for 24 h. The mycelium then was rinsed with distilled water and frozen in liquid nitrogen for further RNA extraction.
Arrays.
The P. chrysosporium custom exon expression array (12×135K array) was manufactured by Roche NimbleGen Systems Ltd. (Madison, WI, USA). From a data set of 9,998 unique P. chrysosporium gene predictions, each array featured 6 unique probes per gene, all in duplicate.
Total RNA was extracted and purified from triplicate cultures using the RNeasy plant minikit (Qiagen) according to the manufacturer's instructions. The RNA was treated twice with DNase I (during purification as recommended in the manufacturer's protocol and directly added onto purified RNA) and cleaned with a Qiagen RNA cleanup kit. An additional purification step was performed to remove residual phenolic compounds due to oak treatment by precipitating RNA with 2 M LiCl. RNA was converted to double-stranded cDNA using the Smarter PCR cDNA synthesis kit (Clontech) according to the manufacturer's protocol and purified using the QIAquick PCR purification kit (Qiagen). Single-dye labeling of samples, hybridization procedures, data acquisition, background correction, and normalization were performed at the NimbleGen facilities (NimbleGen Systems, Reykjavik, Iceland) by following their standard protocol. Microarray probe intensities were quantile normalized across all chips. Average expression levels were calculated for each gene from the independent probes on the array and were used for further analysis. Natural log-transformed data were calculated and subjected to the Cyber-T statistical framework (http://cybert.ics.uci.edu/) (20) using the Standard t test unpaired two-conditions data module. Benjamini and Hochberg multiple-hypothesis testing corrections with false discovery rates (FDR) were used.
Transcripts with a significant P value (<0.05) were considered to be differentially expressed.
Cloning of PcGTT2.1 and heterologous expression and purification of the recombinant protein.
The open reading frame sequence encoding P. chrysosporium GTT2.1 (PcGTT2.1; JGI ProtID 6766) was amplified from a P. chrysosporium cDNA library using PcGTT2.1 forward and reverse primers (5′ CCCCCATGGCTCACCTCCCCAACTTTCTC 3′ and 5′ CCCCGGATCCTTAGTGGAGGGTCTCGGC 3′) and cloned into the NcoI and BamHI restriction sites (underlined in the primers) of pET-3d (Novagen). The amplified sequences encoded a protein in which an alanine has been added to improve protein production. For protein production, the Escherichia coli BL21(DE3) strain, containing the pSBET plasmid, was cotransformed with PcGTT2.1-pET-3d plasmid (21). Cultures were progressively amplified up to 2 liters in LB medium supplemented with ampicillin and kanamycin at 37°C. Protein expression was induced at exponential phase by adding 100 μM isopropyl β-d-thiogalactopyranoside for 4 h at 37°C. The cultures then were centrifuged for 15 min at 4,400 × g. The pellets were resuspended in 30 ml of TE-NaCl (30 mM Tris-HCl, pH 8.0, 1 mM EDTA, 200 mM NaCl) buffer. Cell lysis was performed by sonication (3 times for 1 min each with intervals of 1 min), and the soluble and insoluble fractions were separated by centrifugation for 30 min at 27,000 × g. The soluble part then was fractionated with ammonium sulfate in two steps, and the protein fraction precipitating between 40 and 80% of the saturation contained the recombinant protein, as estimated by 15% SDS-PAGE. The protein was purified by size-exclusion chromatography after loading the resolubilized fraction on an ACA44 (5- by 75-cm) column equilibrated in TE-NaCl buffer. The fractions containing the protein were pooled, dialyzed by ultrafiltration to remove NaCl, and loaded onto a DEAE-cellulose column (Sigma) in TE (30 mM Tris-HCl [pH 8.0], 1 mM EDTA) buffer. The proteins were eluted using a 0 to 0.4 M NaCl gradient. Finally, the fractions of interest were pooled, dialyzed, concentrated by ultrafiltration under nitrogen pressure (YM10 membrane; Amicon), and stored in TE buffer at −20°C. Purity was checked by SDS-PAGE. Protein concentration was determined spectrophotometrically using a molar extinction coefficient at 280 nm of 60,390 M−1 cm−1. A total of around 60 mg of pure PcGTT2.1 was obtained (final yield, 25 mg per liter of culture).
Activity measurements.
The activity measurements of PcGTT2.1 in thiol transferase activity with hydroxyethyldisulfide (HED) assay or for reduction of dehydroascorbate (DHA) were performed at 25°C as described by Couturier and coworkers (22). The classical GSH transferase activity was assessed with phenethyl isothiocyanate (ITC) prepared in 2% (vol/vol) acetonitrile, 1-chloro-2,4-dinitrobenzene (CDNB) prepared in DMSO, and 4-hydroxynonenal (HNE) in ethanol as substrates (14, 23). The reactions were monitored at 274 nm for ITC, 340 nm for CDNB, and 224 nm for HNE following the increase in absorbance arising from the formation of the S-glutathionylated adduct. The reactions with CDNB were performed in 100 mM phosphate buffer, pH 7.5, in the presence of glutathione (2 mM), while the reaction with ITC was performed at pH 6.5 with an identical GSH concentration. The conjugation of HNE with GSH was monitored in 50 mM phosphate buffer, pH 6.5, at 30°C (23). The apparent Kcat value for HNE was determined in the presence of 2 mM GSH using an HNE range from 0 to 0.5 mM. Glutathione peroxidase activities were monitored as described previously (14): peroxide (hydrogen peroxide [H2O2], tert-butyl hydroperoxide [tBOOH], and cumene hydroperoxide [CuOOH]) in 30 mM Tris-HCl, pH 8.0, was incubated in the presence of GSH, 200 μM NADPH, and 0.5 IU glutathione reductase (GR). The activity was analyzed by monitoring the decrease in absorbance arising from NADPH oxidation in this coupled enzyme assay system showing the formation of oxidized glutathione (GSSG). The Km value for GSH was determined using a GSH concentration ranging from 0 to 2 mM in the presence of 100 μM CuOOH. The apparent Kcat value for tBOOH was determined in the presence of 1 mM GSH using a tBOOH concentration ranging from 0 to 2 mM. The apparent Kcat value for CuOOH was determined in the presence of 1 mM GSH using a CuOOH range from 0 to 2 mM. The reactions were started by addition of the purified enzyme and monitored with a Cary 50 UV-visible spectrophotometer (Varian). The catalytic parameters were calculated using GraphPad software.
Yeast complementation.
Saccharomyces cerevisiae strains W303-1A (MATa ura3-1 ade2-1 leu2-3,112 trp1-1 his3-11,15 can1-1) and MML1022 (W303-1A Δgtt1::natMX4 Δgtt2::kanMX4) were employed. The MML1022 strain was constructed by standard genetic methods as described previously (24). MML1022 was transformed with either pCM190 or PcGTT2.1-pCM190 vector. The overexpression of PcGTT2.1 in the yeast strains was checked by Western blotting with rabbit polyclonal anti-PcGTT2.1 (1:1,000) using protein extracts (20 μg) from yeast cultures exponentially growing in SC supplemented with the required amino acids.
For sensitivity experiments, the growth of S. cerevisiae cells in liquid SD medium (25) under parallel separate treatments at 30°C was automatically recorded (by optical density at 600 nm) at 1-h intervals for 24 h, using individual 0.5-ml cultures in shaken microtiter plates sealed with oxygen-permeable plastic sheets, in a PowerWave XS (Biotek) apparatus at controlled temperature. Identical cell numbers (2 × 105) were inoculated initially in each parallel culture. Cells were treated with 0.2 mM tBOOH or 0.4 mM H2O2.
Lipid peroxidation measurement.
Lipid peroxidation has been measured in S. cerevisiae mutant MML1022 and PcGTT2.1-complemented mutant strains in the presence of tBOOH. Cells (2 × 107) were grown in liquid SD medium for 2 h and then treated with 0.2 mM tBOOH for 4 h. The Bioxytech HAE-586 kit (OxisResearch) was used to measure 4-hydroxyalkenal (HAE), which is an indicator of lipid peroxidation. The protocol used is the one described by the manufacturer.
Microarray data accession number.
The complete expression data set is available as a series (accession number GSE54542) at the Gene Expression Omnibus (GEO) at the National Center for Biotechnology Information (NCBI).
RESULTS AND DISCUSSION
Transcriptomic analysis. (i) Overview of gene expression.
The effect of oak acetonic extracts on P. chrysosporium gene expression was assessed by transcriptome analysis. The complete transcriptomic data are given in Table S1 in the supplemental material. LC-MS analysis of these extracts revealed that they mainly contain caffeic acid, castalagin/vescalagin, and ellagic acid, which are tannin-derived molecules (see Table S2). Besides their antioxidant properties, these molecules have toxic and antimicrobial activities (26, 27). Indeed, tannins have the ability to bind strongly to proteins, often causing precipitation and inactivation of enzymes (28).
The most upregulated genes (more than 10-fold) by incubation with oak acetonic extracts are reported in Table 1. These genes encode proteins involved in nutrition, nucleic acid modification, gene regulation, signaling, and stress responses. A limited number of genes coding for proteins involved in degradation of recalcitrant compounds, respiration, and folate and methionine metabolism also were found among the most upregulated genes. The induction of genes involved in amino acid (lysine-ketoglutarate reductase, taurine dioxygenase, arginase, and fumarylacetoacetate hydralase) and protein (protease) catabolism could be a way to recycle both intracellular carbon and nitrogen. In accordance with this, the gene coding for glutamine synthetase also is induced. In addition, some genes involved in mitochondrial respiration are induced, suggesting modification of the bioenergetic state of the fungus.
TABLE 1.
Most upregulated genes (>10-fold) in the oak extract treatment compared to the control conditiona
| ProtID v2.0 and grouping | ProtID v2.2 | Fold upregulation in oak vs control | Cyber-T |
Annotation | |
|---|---|---|---|---|---|
| ppde,p | BH | ||||
| Degradation and nutrition | |||||
| 40125 | 2919778 | 37.16 | 0.9996 | 4.95E−04 | Aspartyl protease |
| 9276 | 3043396 | 30.15 | 0.9984 | 1.96E−03 | Lysine-ketoglutarate reductase/saccharopine dehydrogenase |
| 130933 | 3043390 | 29.62 | 0.9981 | 2.49E−03 | Lysine-ketoglutarate reductase/saccharopine dehydrogenase |
| 34295 | 2889548 | 28.87 | 0.9779 | 3.30E−02 | Chloroperoxidase |
| 122114 | 3028920 | 24.75 | 0.9688 | 4.54E−02 | Predicted transporter (major facilitator superfamily) |
| 10661 | 2957918 | 21.67 | 0.9997 | 3.19E−04 | Taurine catabolism dioxygenase, TauD/TfdA |
| 4568 | 3033687 | 18.41 | 0.9905 | 1.41E−02 | Arginase family protein |
| 134181 | 3006107 | 16.28 | 0.9975 | 3.32E−03 | Voltage-gated shaker-like K+ channel, subunit beta/KCNAB/aryl-alcohol dehydrogenase |
| 41330 | 2922705 | 15.64 | 0.9912 | 1.30E−02 | Aromatic compound dioxygenase |
| 5517 | 2911054 | 13.77 | 0.9942 | 8.39E−03 | EXPN, distantly related to plant expansins |
| 5864 | 2828877 | 13.73 | 0.9898 | 1.52E−02 | Glutamine synthetase |
| 137220 | 3015932 | 12.51 | 0.9886 | 1.71E−02 | Predicted transporter (major facilitator superfamily) |
| 9336 | 3021560 | 12.51 | 0.9946 | 7.73E−03 | Predicted transporter (major facilitator superfamily) |
| 132735 | 3001100 | 11.69 | 0.9702 | 4.38E−02 | Predicted fumarylacetoacetate hydralase |
| 37522 | 2972234 | 11.40 | 0.9978 | 2.87E−03 | Glycoside hydrolase family 20 protein |
| 137453 | 2966954 | 11.36 | 0.9999 | 8.73E−05 | Glycosyltransferase family 2 protein |
| 121193 | 121193 | 11.10 | 0.9882 | 1.76E−02 | Lytic polysaccharide monooxygenase (formerly GH61)/carbohydrate-binding module family 1 protein |
| 135277 | 2990792 | 10.79 | 0.9671 | 4.75E−02 | Amino acid transporters |
| 4296 | 2953754 | 10.62 | 0.9840 | 2.41E−02 | Predicted transporter (major facilitator superfamily) |
| 137216 | 3024803 | 10.42 | 0.9826 | 2.61E−02 | Glycoside hydrolase family 7/carbohydrate-binding module family 1 protein |
| Folate/methionine pathway | |||||
| 5290 | 2722858 | 11.86 | 0.9990 | 1.27E−03 | Folylpolyglutamate synthase |
| 123437 | 3034009 | 10.39 | 0.9991 | 1.10E−03 | C1-tetrahydrofolate synthase |
| 125238 | 3032558 | 10.15 | 0.9996 | 4.44E−04 | Methylthioadenosine phosphorylase (MTAP) |
| Respiration | |||||
| 127904 | 2978093 | 17.30 | 0.9999 | 7.96E−05 | Protein involved in ubiquinone biosynthesis |
| 44349 | 2937022 | 16.81 | 0.9953 | 6.77E−03 | NADH:ubiquinone oxidoreductase, NDUFS6, 13-kDa subunit |
| 34038 | 2989470 | 13.99 | 0.9801 | 2.98E−02 | Cytochrome c oxidase, subunit Va/COX6 |
| 6157 | 2957600 | 11.85 | 0.9986 | 1.71E−03 | NADH-dehydrogenase (ubiquinone) |
| DNA/RNA modification, gene regulation | |||||
| 5211 | 2983373 | 23.13 | 0.9999 | 1.18E−04 | Nucleolar GTPase/ATPase p130 |
| 5606 | 5606 | 21.47 | 0.9875 | 1.86E−02 | Splicing coactivator SRm160/300, subunit SRm300 |
| 8396 | 1637283 | 20.24 | 0.9977 | 3.01E−03 | Splicing coactivator SRm160/300, subunit SRm300 |
| 6418 | 1283299 | 18.79 | 0.9938 | 9.06E−03 | CCCH-type Zn-finger protein |
| 2677 | 2677 | 18.20 | 0.9991 | 1.06E−03 | Nucleolar GTPase/ATPase p130 |
| 130038 | 2976481 | 17.05 | 1.0000 | 2.38E−07 | Inositol polyphosphate 5-phosphatase and related proteins |
| 7242 | 2915258 | 13.78 | 0.9885 | 1.72E−02 | Transcription factor of the Forkhead/HNF3 family |
| 129920 | 2899842 | 12.44 | 0.9908 | 1.37E−02 | Transcription factor TAFII-31 |
| 8180 | 2916937 | 10.95 | 0.9907 | 1.38E−02 | Nuclear pore complex, rNup107 component (ScNup84) |
| 5016 | 2668412 | 10.80 | 0.9753 | 3.70E−02 | RNA polymerase II, large subunit |
| 25553 | 2919747 | 10.64 | 0.9999 | 1.30E−04 | Cullins |
| 44155 | 2874226 | 10.42 | 0.9940 | 8.73E−03 | Putative translation initiation inhibitor UK114/IBM1 |
| 139682 | 2898028 | 10.39 | 0.9976 | 3.24E−03 | ATP-dependent RNA helicase |
| 39851 | 2987670 | 10.31 | 0.9886 | 1.71E−02 | Translational repressor MPT5/PUF4 and related RNA-binding proteins (Puf superfamily) |
| 6343 | 2912954 | 10.24 | 0.9985 | 1.84E−03 | Endoplasmic reticulum-to-Golgi-structure transport protein/RAD50-interacting protein 1 |
| 2869 | 2967072 | 10.20 | 0.9992 | 9.43E−04 | Uncharacterized conserved protein/Zn-finger protein |
| 32494 | 1905331 | 10.15 | 0.9989 | 1.40E−03 | Nuclear localization sequence-binding protein |
| Signaling and stress | |||||
| 2416 | 2901030 | 106.57 | 0.9993 | 8.71E−04 | Unknown/SSP putative |
| 41334 | 2904208 | 22.16 | 0.9992 | 9.71E−04 | Flavonol reductase/cinnamoyl-CoA reductase |
| 5667 | 2984197 | 20.42 | 0.9979 | 2.75E−03 | Tyrosine kinase specific for activated (GTP-bound) p21cdc42Hs |
| 3280 | 3003339 | 18.81 | 0.9945 | 7.97E−03 | Thaumatin |
| 5747 | 3005309 | 17.85 | 0.9939 | 8.85E−03 | Dual-specificity phosphatase |
| 133796 | 2981209 | 15.18 | 0.9919 | 1.19E−02 | Serine/threonine protein phosphatase |
| 38902 | 2989411 | 14.09 | 0.9955 | 6.51E−03 | Extracellular protein SEL-1 and related proteins |
| 1088 | 2965634 | 13.41 | 0.9902 | 1.46E−02 | RTA1-like protein |
| 122315 | 122315 | 11.99 | 0.9989 | 1.42E−03 | Peptide methionine sulfoxide reductase |
| 43951 | 3032803 | 11.63 | 0.9985 | 1.95E−03 | MAPEG |
| 43149 | 2914358 | 11.28 | 0.9959 | 5.95E−03 | GCN5-related N-acetyltransferase |
| 136604 | 2907053 | 10.32 | 0.9913 | 1.29E−02 | Thioredoxin/protein disulfide isomerase |
ProtID from P. chrysosporium v2.0 and v2.2 JGI databases are reported. Annotations are those retrieved from the v2.2 database. ppde,p, posterior probability of differential expression; BH, Benjamini and Hochberg. MAPEG, membrane-associated protein involved in eicosanoid and glutathione metabolism.
Interestingly, the most upregulated gene encodes a protein (ProtID 2416) with an unknown function but with the characteristics of small secreted proteins (SSPs). Indeed, it is predicted to be secreted, the sequence is less than 300 amino acids, and it contains many cysteines. Such proteins have been studied recently in Phlebia brevispora and Heterobasidion annosum (29). The authors focused their analysis on hydrophobins. A considerable expansion of the hydrophobin-encoding genes exists in basidiomycetes, probably in relation to their ecological preferences. The gene identified in our analysis does not belong to the hydrophobin family, since it does not exhibit the conserved eight-cysteine motif and has not been identified as a hydrophobin in genomic analysis (30). SSPs are of great interest in pathology and symbiosis research, since they could act as signaling molecules in fungi and are able to regulate gene expression of the plant host (31, 32). A putative thaumatin-encoding gene also is induced. Although thaumatin genes are well studied in plants, they have been discovered only recently in fungi (33). In saprophytic fungi, the role of these proteins still is unknown, but a signaling function is plausible.
Stress-related genes could be noticed, such as the ones coding for a peptide methionine sulfoxide reductase or an acetyltransferase. The oxidation of methionine into methionine sulfoxide (MetSO) is a reversible process, the reduction being catalyzed by methionine sulfoxide reductase. These oxidation/reduction steps could restore the biological activity of proteins by repairing oxidative damage (34). Few GCN5-related N-acetyltransferases have been characterized in fungi. A study shows that MPR1, belonging to the GCN5-related N-acetyltransferase, is involved in stress responses in S. cerevisiae. Indeed, it has been shown to regulate ROS caused by a toxic proline catabolism intermediate (35).
An accumulation of flavonol reductase/cinnamoyl-coenzyme A (CoA) reductase transcripts also has been highlighted in the presence of oak acetonic extract. The corresponding enzyme is an oxidoreductase acting on cinnamaldehyde, an organic compound naturally occurring in the bark of the cinnamon tree. This enzyme participates in the lignin biosynthesis pathway (36). Moreover, a 9-fold-elevated level of cinnamoyl-CoA reductase protein in P. chrysosporium under Cu stress suggests a role of this enzyme in stress response (37). In addition to the genes reported in Table 1, 73 genes exhibiting no homology in the databases are upregulated more than 10-fold.
(ii) Extracellular degradation of lignocellulose and aromatic compounds.
The induction of genes coding for enzymes involved in the extracellular degradation of aromatic compounds suggests putative extracellular oxidation of the tannin-derived molecules. Indeed, genes coding for a chloroperoxidase and a benzoquinone reductase and five genes coding for some class II peroxidases (lignin and manganese peroxidases) are induced by oak extracts from 2.25- to 28.87-fold (Table 2). Consistent with the induction of these genes, other genes coding for enzymes that generate H2O2 (the copper radical oxidase cro1, pyranose oxidase, and oxalate oxidase) also are upregulated. However, while glyoxal oxidase has been suggested to be the predominant source of extracellular H2O2, no corresponding gene was upregulated under our conditions. Inversely, some other oxidases are downregulated by oak extracts (GMC oxidoreductase, copper radical oxidases cro4 and cro6, and oxalate oxidase). These results can be analyzed in light of a previous transcriptomic analysis of P. chrysosporium grown on oak wood (38). The cDNAs highly expressed in that study on wood material also are globally upregulated in our study. In particular, LipD (ProtID 6811) and MnP1 (ProtID 140708) have been identified in both studies, suggesting a putative signaling/regulatory role of phenolics for gene expression.
TABLE 2.
Regulation of genes coding for the lignin degradation systema
| ProtID |
Fold upregulation in oak vs control | Cyber-T |
Annotation | ||
|---|---|---|---|---|---|
| v2.0 | v2.2 | ppde,p | BH | ||
| 34295 | 2889548 | 28.87 | 0.9779 | 3.30E−02 | Chloroperoxidase |
| 10307 | 2979875 | 7.16 | 1.0000 | 3.49E−05 | Benzoquinone reductase BQR1 |
| 878 | 2896529 | 6.33 | 0.9727 | 4.08E−02 | Class II peroxidase MnP |
| 6811 | 1386770 | 4.26 | 0.9755 | 3.67E−02 | Class II peroxidase LiPD |
| 140708 | 8191 | 3.97 | 0.9661 | 4.87E−02 | Class II peroxidase MnP1 |
| 6250 | 2911114 | 3.58 | 0.9834 | 2.49E−02 | Class II peroxidase |
| 124009 | 2416765 | 3.41 | 0.9829 | 2.57E−02 | Copper radical oxidase cro1 |
| 131217 | 3003453 | 2.66 | 0.9854 | 2.19E−02 | Oxalate oxidase/decarboxylase |
| 137275 | 2977507 | 2.53 | 0.9661 | 4.87E−02 | Pyranose oxidase pox |
| 123914 | 123914 | 2.25 | 0.9909 | 1.36E−02 | Class II peroxidase |
| 129887 | 3008599 | 0.47 | 0.9814 | 2.80E−02 | Benzoquinone reductase BQR3 |
| 6199 | 6199 | 0.20 | 0.9988 | 1.53E−03 | GMC oxidoreductase |
| 1932 | 3023991 | 0.16 | 0.9978 | 2.93E−03 | Iron-binding glycoprotein |
| 37905 | 2894758 | 0.12 | 0.9892 | 1.61E−02 | Copper radical oxidase cro6 |
| 8882 | 1717398 | 0.10 | 0.9953 | 6.74E−03 | Copper radical oxidase cro4 |
| 136169 | 2908112 | 0.06 | 0.9937 | 9.09E−03 | Oxalate oxidase/decarboxylase |
Genes coding for carbohydrate active enzymes (CAZymes) also have been found differentially expressed under our conditions (Table 3). Again, some of the genes induced by oak extracts are highly expressed in wood (38). This is the case for some cellobiohydrolases (ProtID 127029, 129072, and 133052), endoglucanases (ProtID 129325, 41563, and 31049), β-glucosidase (ProtID 8072), endoxylanase (ProtID 133788, 138715, and 138345), and acetyl xylan esterase (ProtID 130517) (Table 3; also see Table S1 in the supplemental material) (38). Inversely, we show a downregulation leading to very low expression of genes coding for alcohol oxidase (ProtID 126879), cellobiohydrolase (ProtID 137372), endoglucanase (ProtID 6458 and 8466), and endomannanase (ProtID 140501), while from 72 to 377 tags have been reported for these genes in the culture with oak. Since glucose was used as a carbon source in the present study, one can expect that in a natural biotope (glucose poor), more CAZY could be expressed. Indeed, it has been shown that cellulolytic and xylanolytic genes are regulated by transcriptional factors XYR1 and CRE1 in Trichoderma reesei depending on the carbon source, with glucose acting as a repressor (39).
TABLE 3.
Regulation of genes coding for the CAZymesa
| ProtID |
Fold upregulation in oak vs control | Cyber-T |
Annotation | ||
|---|---|---|---|---|---|
| v2.0 | v2.2 | ppde,p | BH | ||
| 37522 | 2972234 | 11.40 | 0.9978 | 2.87E−03 | Glycoside hydrolase family 20 |
| 121193 | 2980158 | 11.10 | 0.9882 | 1.76E−02 | Lytic polysaccharide monooxygenase (formerly GH61) |
| 137216 | 3024803 | 10.42 | 0.9826 | 2.61E−02 | Glycoside hydrolase family 7 |
| 40899 | 2978123 | 9.44 | 0.9905 | 1.41E−02 | Glycoside hydrolase family 18 |
| 129072 | 2976248 | 8.97 | 0.9711 | 4.27E−02 | Glycoside hydrolase family 7 |
| 42616 | 42616 | 7.56 | 0.9833 | 2.50E−02 | Lytic polysaccharide monooxygenase (formerly GH61) |
| 39389 | 2914306 | 5.14 | 1.0000 | 8.72E−06 | Glycoside hydrolase family 16 |
| 4967 | 2982894 | 4.34 | 0.9791 | 3.12E−02 | Lytic polysaccharide monooxygenase (formerly GH61) |
| 1106 | 2965653 | 4.28 | 0.9654 | 4.95E−02 | Six-hairpin glycosidase-like |
| 9257 | 2919526 | 4.21 | 0.9758 | 3.63E−02 | Glycoside hydrolase family 3 |
| 130517 | 2918304 | 4.09 | 0.9889 | 1.66E−02 | Carbohydrate-binding module family 1/carbohydrate esterase family 15 protein |
| 1924 | 2899497 | 3.27 | 0.9817 | 2.74E−02 | Glycoside hydrolase family 85 |
| 138813 | 2909460 | 2.77 | 0.9980 | 2.64E−03 | Glycoside hydrolase family 15/carbohydrate-binding module family 20 |
| 132605 | 2974311 | 2.75 | 0.9864 | 2.04E−02 | Glycoside hydrolase family 63 |
| 121774 | 2989703 | 2.38 | 0.9821 | 2.69E−02 | Glycoside hydrolase family 5 |
| 4590 | 2907097 | 2.12 | 0.9914 | 1.27E−02 | Six-hairpin glycosidase-like |
| 10320 | 3003776 | 0.04 | 0.9977 | 3.04E−03 | Lytic polysaccharide monooxygenase (formerly GH61) |
| 333 | 2896433 | 0.03 | 0.9999 | 8.05E−05 | Glycoside hydrolase family 43 |
| 134001 | 3010808 | 0.03 | 1.0000 | 4.03E−06 | Carbohydrate-binding module family 1/glycoside hydrolase family 27 |
| 140501 | 140501 | 0.03 | 1.0000 | 2.80E−05 | Carbohydrate-binding module family 1/glycoside hydrolase family 5 |
| 4550 | 2579514 | 0.03 | 1.0000 | 4.85E−07 | Glycoside hydrolase family 47 |
| 125033 | 3005667 | 0.02 | 0.9986 | 1.71E−03 | Glycoside hydrolase family 27 |
| 128442 | 3003144 | 0.01 | 1.0000 | 1.83E−05 | Glycoside hydrolase family 3 |
Prot ID from P. chrysosporium v2.0 and v2.2 JGI databases are reported. Annotations are those retrieved from v2.2.
(iii) Intracellular detoxification systems.
Genes coding for proteins involved in the antioxidant response, such as methionine sulfoxide reductase and disulfide isomerase, are induced by oak acetonic extracts. Moreover, the induction of two glutathione reductase (GR1 and GR3) genes suggests an intracellular accumulation of oxidized glutathione, evidence for oxidative stress. However, another GR (GR2), which is predicted to be directed to mitochondria, is downregulated. Similarly, a smaller amount of Mn-dependent superoxide dismutase transcripts has been detected under the oak extract conditions. This result is in accordance with oxidative stress at the mitochondrial level and with the induction of LiP expression by the intracellular ROS (3).
In accordance, YAP1, which is the central regulator of oxidative gene expression, is 5-fold induced. Moreover, the formation of peroxides inside the cell is supported by the upregulation of genes coding for a peroxiredoxin, PrxII.2, and a catalase. Focusing on the intracellular degradative pathways of toxic compounds, we highlighted the induction of 12 genes coding for CytP450 known as phase I detoxification enzymes and 5 and 3 genes coding for the phase II-conjugating glycosyl transferases (GT) and glutathione transferases, respectively (Table 4).
TABLE 4.
Regulation of genes coding for the detoxification systema
| ProtID v2.0 and grouping | ProtID v2.2 | Fold upregulation in oak vs control | Cyber-T |
Annotation | |
|---|---|---|---|---|---|
| ppde,p | BH | ||||
| Antioxidant system | |||||
| 122315 | 122315 | 11.99 | 0.9989 | 1.42E−03 | Methionine sulfoxide reductase MsrA |
| 136604 | 2907053 | 10.32 | 0.9913 | 1.29E−02 | Disulfide isomerase |
| 135167 | 135167 | 8.54 | 0.9974 | 3.58E−03 | Glutathione reductase GR3 |
| 7851 | 2915724 | 5.32 | 0.9817 | 2.75E−02 | YAP1 |
| 125657 | 125657 | 4.08 | 0.9976 | 3.18E−03 | Peroxiredoxin PrxII.2 |
| 128306 | 3025652 | 3.97 | 0.9902 | 1.46E−02 | Catalase Cat1 |
| 876 | 2974699 | 2.29 | 0.9936 | 9.31E−03 | Glutathione reductase GR1 |
| 127288 | 3002838 | 0.40 | 0.9863 | 2.07E−02 | Catalase Cat3 |
| 127266 | 2013640 | 0.19 | 0.9915 | 1.26E−02 | Catalase Cat5 |
| 10525 | 3027920 | 0.11 | 0.9999 | 7.06E−05 | Glutathione reductase GR2 |
| 138910 | 2982879 | 0.01 | 1.0000 | 1.30E−08 | Superoxide dismutase MnSOD2 |
| Cytochrome P450 monooxygenases | |||||
| 7086 | 3030400 | 8.21 | 0.9962 | 5.37E−03 | CYP502B1 |
| 131322 | 2980309 | 7.24 | 0.9699 | 4.41E−02 | CYP5144F1 |
| 131921 | 3029016 | 6.86 | 0.9742 | 3.87E−02 | CYP5144C7 |
| 9146 | 2990306 | 5.81 | 0.9942 | 8.35E−03 | |
| 138612 | 2909718 | 5.80 | 0.9994 | 7.43E−04 | CYP5035A2 |
| 137485 | 3081758 | 5.49 | 0.9994 | 7.11E−04 | CYP63C2 |
| 38849 | 2974374 | 4.21 | 0.9958 | 6.09E−03 | CYP5146A2 |
| 137321 | 2977643 | 3.99 | 0.9768 | 3.47E−02 | CYP63C1 |
| 37971 | 2965345 | 2.64 | 0.9689 | 4.53E−02 | CYP5144A11 |
| 139146 | 3035514 | 2.46 | 0.9954 | 6.63E−03 | CYP5144C6 |
| 9478 | 2919941 | 2.32 | 0.9987 | 1.66E−03 | CYP5140A1 |
| 132579 | 2897041 | 2.14 | 0.9901 | 1.48E−02 | CYP5146B1 |
| 3368 | 2903228 | 0.04 | 0.9726 | 4.09E−02 | CYP5141A2 |
| 1664 | 2976048 | 0.04 | 1.0000 | 2.56E−05 | CYP5143A1 |
| 3661 | 3003571 | 0.04 | 0.9985 | 1.83E−03 | CYP5147A2 |
| 38024 | 2895038 | 0.03 | 1.0000 | 1.45E−06 | CYP5144A2 |
| 6327 | 3005638 | 0.03 | 1.0000 | 6.35E−06 | CYP5145A3 |
| 798 | 2896486 | 0.02 | 1.0000 | 6.48E−06 | CYP5144D5-CYP5144J1 |
| 8707 | 2989550 | 0.02 | 1.0000 | 9.28E−07 | CYP512B2 |
| 8912 | 2963503 | 0.02 | 1.0000 | 3.03E−05 | CYP5035B2 |
| 5055 | 2908523 | 0.01 | 1.0000 | 6.71E−06 | CYP5144A12 |
| 38012 | 3090395 | 0.01 | 0.9999 | 5.22E−05 | CYP5037A1 |
| 138753 | 2692082 | 0.01 | 1.0000 | 9.51E−07 | CYP5144B1 |
| 761 | 3022655 | 0.01 | 1.0000 | 3.07E−06 | CYP5144C2 |
| 5054 | 5054 | 0.01 | 0.9999 | 1.39E−04 | CYP5144A13 |
| Glycosyl transferases | |||||
| 137453 | 2966954 | 11.36 | 0.9999 | 8.73E−05 | GT family 2 |
| 3697 | 2904243 | 8.84 | 0.9814 | 2.79E−02 | GT family 8 |
| 7550 | 1511835 | 8.07 | 0.9899 | 1.51E−02 | GT family 8 |
| 2561 | 2901646 | 6.14 | 0.9769 | 3.46E−02 | GT family 8 |
| 40855 | 2989061 | 5.08 | 0.9817 | 2.75E−02 | GT family 3 |
| 1287 | 2898363 | 0.20 | 0.9999 | 1.33E−04 | GT family 8 |
| 132866 | 3008474 | 0.06 | 0.9683 | 4.61E−02 | GT family 4 |
| 125979 | 125979 | 0.02 | 1.0000 | 2.99E−05 | GT family 8 |
| Glutathione transferases | |||||
| 43951 | 3032803 | 11.63 | 0.9985 | 1.95E−03 | MAPEG |
| 6766 | 3006031 | 8.51 | 0.9989 | 1.30E−03 | GTT2.1 |
| 5122 | 2908587 | 5.07 | 0.9992 | 9.88E−04 | GST FuA3 |
| 2268 | 2977134 | 0.05 | 0.9999 | 1.20E−04 | Ure2p8 |
| 5118 | 2685965 | 0.33 | 0.9716 | 4.22E−02 | GST FuA1 |
| 5119 | 2687275 | 0.12 | 0.9950 | 7.20E−03 | GST FuA2 |
The CytP450ome has been quite extensively investigated in P. chrysosporium, especially thanks to a functional library in yeast (8, 9). These enzymes are highly versatile and inducible. The isoforms found induced in our experiment have not been functionally characterized yet, except for ProtID 7086 and 138612, which modify carbazole, dibenzothiophene, and ethoxycoumarin for the first one and naproxen for the second (9). Members from CYP5144 and CYP63 exhibit catalytic activities toward environmentally persistent and toxic high-molecular-weight polycyclic aromatic compounds, such as phenanthrene, pyrene or benzo(a)pyrene, alkylphenols, and alkane (40, 41). Evidence of the involvement of ProtID 131921 in the degradation of the endocrine disruptor chemical nonylphenol also has been demonstrated (42). Transcriptomic experiments showed that ProtID 139146 and 132579 gene expression is induced after anthracen and anthrone treatments (43). All of these studies demonstrate the potential of this enzyme family as biocatalysts to handle environmental mixed pollution. It is also important to note that many CytP450 genes are downregulated in our experiment.
The second detoxification phase consists of conjugating reactions performed by many transferases and, in particular, GTs and GSTs. GSTs are at the interface between compound elimination and oxidative stress rescue. In this study, we identified 3 GST genes, which are upregulated by oak acetonic extracts (MAPEG, GSTFuA3, and GTT2.1). The first gene encodes a membrane-associated protein involved in eicosanoid and glutathione metabolism (MAPEG). This superfamily includes structurally related membrane proteins with diverse functions of widespread origin. The eukaryotic MAPEG members can be subdivided into six families: MGST1, MGST2, and MGST3, leukotriene C-4 synthase (LTC4), 5-lipoxygenase activating protein (FLAP), and prostaglandin E synthase. Protein overexpression and enzyme activity analysis demonstrated that all proteins catalyzed the conjugation of CDNB with reduced glutathione. Thus, glutathione transferase activity can be regarded as a common denominator for a majority of MAPEG members throughout the kingdoms of life, whereas glutathione peroxidase activity occurs in representatives from the MGST1, MGST2, MGST3, and PGES subfamilies (44). Based on sequence homology, it appears that the Phanerochaete isoform belongs to MGST3, but it has not been functionally characterized yet. GSTFuA3 belongs to a newly identified GST class (18, 45). Four members of this class (GSTFuA1 to GSTFuA4) exhibit a ligandin property toward wood compounds, such as coniferaldehyde, syringaldehyde, vanillin, chloronitrobenzoic acid, hydroxyacetophenone, and catechins, suggesting a role in sequestration and transport of the toxic molecules inside the cell (14, 45). GSTFuA3, which is the only member of this family that is able to reduce peroxides, is induced in our study, while GSTFuA1 and GSTFuA2 are downregulated. GTT2.1 was first defined by its sequence homology to GTT2 from S. cerevisiae (18). ScGTT2 exhibits GST activity with CDNB and seems to be crucial in the response to peroxides (46–48). However, no homologue has been characterized yet in other species. In P. chrysosporium, 2 genes have been identified: PcGTT2.1 (ProtID 6766) and PcGTT2.2 (ProtID 6683). While PcGTT2.1 is induced in our study (8.5-fold), PcGTT2.2 shows an expression ratio of only 1.8 in the oak extract treatment compared to the control (see Table S1 in the supplemental material).
Functional characterization of PcGTT2.1. (i) Enzymatic activities.
The activity pattern of recombinant PcGTT2.1 has been determined using various substrates (Table 5). No thiol transferase and reductase activities with hydroxyethyl disulfide (HED) and dehydroascorbate (DHA) or GSH-transferase activity could be detected with the classical substrates CDNB and ITC. A weak GSH transferase activity has been measured with HNE, one of the major end products of lipid peroxidation. Moreover, PcGTT2.1 exhibited peroxidase activity with tBOOH and CuOOH but not with H2O2. With organic peroxides, the reaction catalyzed by PcGTT2.1 likely involves first a transfer of GSH onto the peroxide, which is rapidly removed by a second glutathione molecule to form GSSG and alcohol. This scheme was supported by the formation of GSSG that we have detected with NADPH-dependent GR method-based activity and mass spectrometry (data not shown). With peroxides as a substrate, the specific activity of PcGTT2.1 is substantially higher than that of fungal glutathione peroxidases. As an example, GPX2 from S. cerevisiae exhibits similar affinity but almost 7-fold-less activity than PcGTT2.1 toward tBOOH (49). Other fungal GSTs have been found to be able to reduce peroxides; however, they do so with less efficiency than PcGTT2.1 (14, 50) (Table 5). Moreover, PcGTT2.1 has a very strong affinity for CuOOH, suggesting high specificity for high-molecular-weight peroxides.
TABLE 5.
Kinetic parameters of PcGTT2.1 in H2O2, tBOOH, CuOOH, and HNE activity assays compared to those of other fungal peroxidase-like enzymesa
| Enzyme | Substrate |
Reference or source | |||||||
|---|---|---|---|---|---|---|---|---|---|
| H2O2 |
tBOOH |
CuOOH |
HNE |
||||||
| Km (mM) | Kcat (s−1) | Km (mM) | Kcat (s−1) | Km (mM) | Kcat (s−1) | Km (mM) | Kcat (s−1) | ||
| PcGTT2.1 | NA | NA | 0.55 ± 0.24 | 725.00 ± 119.90 | 0.011 ± 0.002 | 500.00 ± 13.52 | 0.15 ± 0.03 | 0.78 ± 0.07 | This study |
| ScGpx2 | 0.17 | 0.99 | 0.31 | 109.00 | ND | ND | ND | ND | 49 |
| ScUre2p | 4.30 | 0.36 | 7.90 | 0.07 | 7.80 | 0.37 | ND | ND | 50 |
| PcGSTFuA3 | NA | NA | NA | NA | 2.04 | 1.27 | ND | ND | 14 |
Experimental details are given in Materials and Methods. NA, not active; ND, not determined; Sc, S. cerevisiae; Pc, P. chrysosporium.
In humans, GST can modulate the intracellular concentrations of HNE by affecting its generation during lipid peroxidation by reducing hydroperoxides and also by converting it into a glutathione conjugate (51). Since PcGTT2.1 reacts with HNE, albeit rather slowly, it seems that it could be involved in both reactions. Thus, besides reducing oxidative stress, it could have a key role by modulating HNE content, whose amount regulates stress signaling events and apoptosis.
(ii) Yeast complementation.
PcGTT2.1 shares only 16.3% and 17.6% identity with ScGTT1 and ScGTT2, respectively, as defined by global sequence alignment using Lalign (http://www.ch.embnet.org/software/LALIGN_form.html). Nevertheless, the overexpression of PcGTT2.1 in a yeast strain deficient in both GTT1 and GTT2 genes rescued the growth of S. cerevisiae in the presence of H2O2 and tBOOH (Fig. 1A and B). While ScGTT2 displays only classical GSH transferase activity, ScGTT1 exhibits both classical GSH transferase activity and peroxidase activity with hydroperoxides (16). Moreover, it has been shown that exposure of GTT1/GTT2 double mutant strains to peroxides caused oxidative stress and increased lipid peroxidation (48). Lipid peroxides are unstable and decompose to form a complex series of malondialdehyde and HAE. HAE can be used as an indicator of lipid peroxidation (52). Under our conditions, less free 4-hydroxyalkenals were detected in the complemented strain compared to the mutant (Fig. 1C). This observation strengthens the proposal for a role of PcGTT2.1 in reducing lipid peroxidation in vivo.
FIG 1.
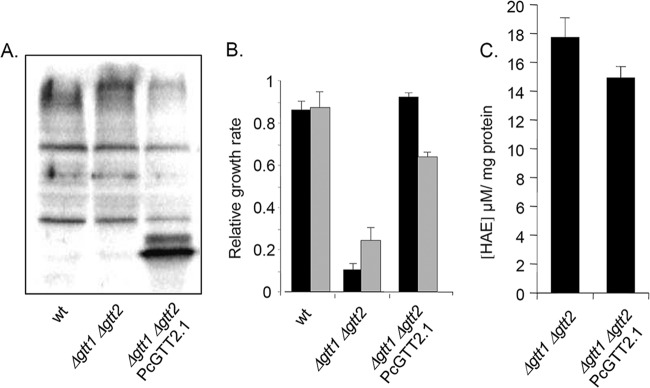
Functional analysis of PcGTT2.1. (A) Western blot confirming PcGTT2.1 overexpression in the yeast Δgtt1 Δgtt2 mutant. (B) Sensitivity of PcGTT2.1-complemented yeast strains to tert-butyl hydroperoxide (black bars) and hydrogen peroxide (gray bars). The growth of S. cerevisiae wild-type (wt; W303-1A) and Δgtt1 Δgtt2 mutant (MML1022) cells was automatically recorded as described in Materials and Methods. Exponential growth rates of treated cultures with the indicated agent concentrations, as well as control untreated cultures, were calculated, and the treated versus untreated ratios are represented. Bars correspond to the means from three independent experiments (plus standard deviations). (C) Measurement of 4-hydroxyalkenals (HAE) in Δgtt1 Δgtt2 mutants and in Δgtt1 Δgtt2 mutants complemented with PcGTT2.1. Bars correspond to the means from four independent experiments (plus standard deviations).
(iii) Evolution of GTT2 genes.
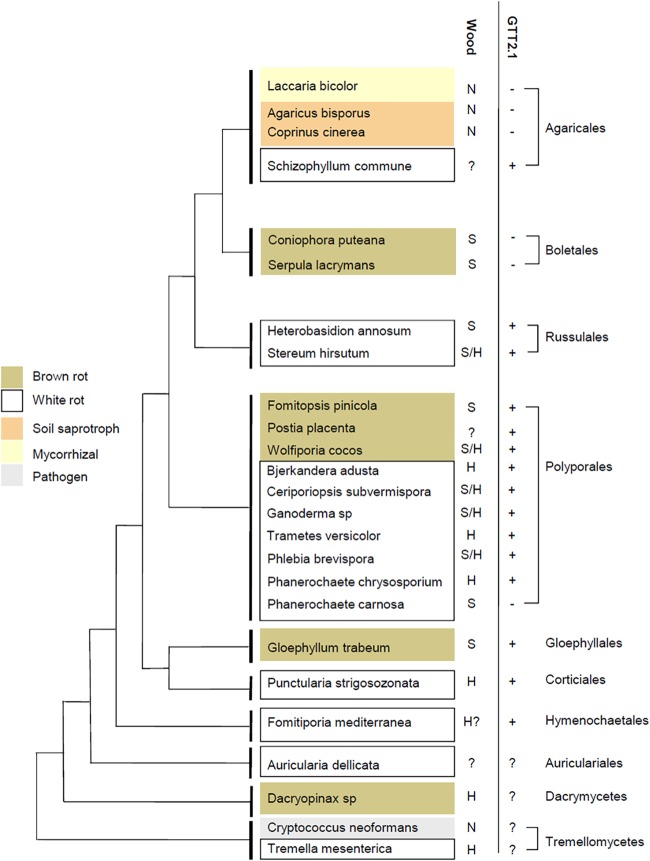
PcGTT2.1 orthologues have been searched in the available basidiomycete genomes from the MycoCosm database of the Joint Genome Institute (53). A phylogenetic analysis allowed us to identify 2 groups that we named GTT2.1 and GTT2.2 (see Fig. S1 in the supplemental material). In P. chrysosporium, both genes locate on the same scaffold; however, more than 200 kb separate them, suggesting that the duplication event is not very recent. While GTT2.2 orthologues have been identified in almost all other considered fungi except Agaricus bisporus and Serpula lacrymans, GTT2.1 gene losses could have occurred in the lineage leading to the Boletales and within lineages in the Agaricales, leading to the non-wood-decay species Agaricus bisporus, Coprinopsis cinerea, and Laccaria bicolor (Fig. 2). The presence of GTT2.1 was investigated in 15 mycorrhizal species within the Boletales and Agaricales lineages, and only one orthologue was detected in Suillus species (data not shown). An interesting point is that Phanerochaete carnosa, which is a white rot fungus, does not exhibit any GTT2.1 homologue. While taxonomically close, P. carnosa and P. chrysosporium differ in that P. carnosa has been isolated almost exclusively from softwood, while P. chrysosporium was isolated mainly from hardwood. Globally, P. carnosa and P. chrysosporium similarly reduce the total phenolic content of various sapwood samples. P. carnosa transforms a higher fraction of phenolics in most of the heartwood samples, including those from softwood species, while P. chrysosporium transformed a broader range of heartwood phenolics in maple, a hardwood species (12). The accumulation of PcGTT2.1 transcripts in the presence of phenolics from oak heartwood suggests that PcGTT2.1 has a role in this process.
FIG 2.
Presence of GTT2.1 gene in various basidiomycetes in relation to their taxonomic distribution and nutritional modes. Wood preference is reported. S, softwood; H, hardwood; N, nondegrader. This schematic tree has been constructed based on the chronogram made by Floudas et al. (58) and the phylogenetic tree shown in Fig. S1 in the supplemental material.
Looking at wood specificities of the various ligninolytic fungi, it is noticeable that all fungi growing on hardwood exhibit a GTT2.1 isoform. Softwood and hardwood fibers differ in the structure and composition of their hemicellulosic polymers and lignin (54). Indeed, softwood hemicelluloses are constituted mainly of galactoglucomannans and arabinoglucuronoxylans, while hardwood hemicelluloses contain mainly glucuronoxylans and glucomannans. Similarly, softwood lignins are mainly comprised of guaiacyl units, while hardwood lignins contain guaiacyl and syringyl units. The chemical composition of extractives depends directly on the wood species, and important variability exists among the ones mentioned above, leading to important variations in their content, chemical composition, and biological properties (55). A recent study showed that transgenic poplar lines enriched in syringyl lignin are more resistant to fungal degradation, suggesting a toxic effect of these subunits (56).
Given the detoxification activity of PcGTT2.1, our observation suggests a link between the maintenance of GTT2.1 in wood-degrader genomes and the selective pressure exerted by the toxic molecules released during wood degradation. It is an interesting point, since it suggests that the adaptation of fungi to their habitat occurs not only through their extracellular machinery of wood degradation (57, 58) but also through their ability to survive in toxic environments. A comparative genomic analysis did not reveal any differences in gene content for the classical antioxidant systems in P. chrysosporium, C. cinereus, and L. bicolor genomes (59). However, large variations have been observed in the detoxification system, including CytP450 and GSTs (11). These multigenic families obviously are good markers of adaptation.
Conclusions.
Genomic, transcriptomic, and proteomic data have considerably enriched our knowledge concerning the regulation and evolution of wood degradation systems (12, 58, 60–62). Our transcriptomic analysis is original in that it focuses on the effect of oak-derived molecules, especially tannins, on P. chrysosporium gene expression. It reveals that in complement to the extracellular machinery of degradation, intracellular antioxidant and detoxification systems contribute to the lignolytic capabilities of fungi by preventing cellular damage and maintaining fungal health. In particular, the functional characterization of PcGTT2.1 suggests that the protein has evolved to specifically reduce lipid peroxidation during wood degradation. We believe that this is an interesting example of the neofunctionalization of a protein driven by selective pressure.
Supplementary Material
ACKNOWLEDGMENTS
The UMR IAM is supported by a grant overseen by the French National Research Agency (ANR) as part of the Investissements d'Avenir program (ANR-11-LABX-0002-01, Laboratory of Excellence ARBRE). This work also was supported by the French National Research Agency (project number ANR-09-BLAN-0012).
We have no conflicts of interest to declare.
Footnotes
Published ahead of print 8 August 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02103-14.
REFERENCES
- 1.Hunt CG, Houtman CJ, Jones DC, Kitin P, Korripally P, Hammel KE. 2013. Spatial mapping of extracellular oxidant production by a white rot basidiomycete on wood reveals details of ligninolytic mechanism. Environ. Microbiol. 15:956–966. 10.1111/1462-2920.12039 [DOI] [PubMed] [Google Scholar]
- 2.Wei DS, Houtman CJ, Kapich AN, Hunt CG, Cullen D, Hammel KE. 2010. Laccase and its role in production of extracellular reactive oxygen species during wood decay by the brown rot basidiomycete Postia placenta. Appl. Environ. Microbiol. 76:2091–2097. 10.1128/AEM.02929-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belinky PA, Flikshtein N, Lechenko S, Gepstein S, Dosoretz CG. 2003. Reactive oxygen species and induction of lignin peroxidase in Phanerochaete chrysosporium. Appl. Environ. Microbiol. 69:6500–6506. 10.1128/AEM.69.11.6500-6506.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feraydoni V, Hosseinihashemi SK. 2012. Effect of walnut heartwood extractives, acid copper chromate, and boric acid on white-rot decay resistance of treated beech sapwood. Bioresources 7:2393–2402 [Google Scholar]
- 5.Ramirez MGL, Ruiz HGO, Arzate FN, Gallegos MAC, Enriquez SG. 2012. Evaluation of fungi toxic activity of tannins and a tannin-copper complex from the mesocarp of Cocos nucifera Linn. Wood Fiber Sci. 44:357–364 [Google Scholar]
- 6.Wu CC, Wu CL, Huang SL, Chang HT. 2012. Antifungal activity of liriodenine from Michelia formosana heartwood against wood-rotting fungi. Wood Sci. Technol. 46:737–747. 10.1007/s00226-011-0428-9 [DOI] [Google Scholar]
- 7.Dorado J, Claassen FW, van Beek TA, Lenon G, Wijnberg JB, Sierra-Alvarez R. 2000. Elimination and detoxification of softwood extractives by white-rot fungi. J. Biotechnol. 80:231–240. 10.1016/S0168-1656(00)00264-9 [DOI] [PubMed] [Google Scholar]
- 8.Syed K, Yadav JS. 2012. P450 monooxygenases (P450ome) of the model white rot fungus Phanerochaete chrysosporium. Crit. Rev. Microbiol. 38:339–363. 10.3109/1040841X.2012.682050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirosue S, Tazaki M, Hiratsuka N, Yanai S, Kabumoto H, Shinkyo R, Arisawa A, Sakaki T, Tsunekawa H, Johdo O, Ichinose H, Wariishi H. 2011. Insight into functional diversity of cytochrome P450 in the white-rot basidiomycete Phanerochaete chrysosporium: involvement of versatile monooxygenase. Biochem. Biophys. Res. Commun. 407:118–123. 10.1016/j.bbrc.2011.02.121 [DOI] [PubMed] [Google Scholar]
- 10.Doddapaneni H, Chakraborty R, Yadav JS. 2005. Genome-wide structural and evolutionary analysis of the P450 monooxygenase genes (P450ome) in the white rot fungus Phanerochaete chrysosporium: evidence for gene duplications and extensive gene clustering. BMC Genomics 6:92. 10.1186/1471-2164-6-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morel M, Meux E, Mathieu Y, Thuillier A, Chibani K, Harvengt L, Jacquot JP, Gelhaye E. 2013. Xenomic networks variability and adaptation traits in wood decaying fungi. Microb. Biotechnol. 6:248–263. 10.1111/1751-7915.12015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki H, MacDonald J, Syed K, Salamov A, Hori C, Aerts A, Henrissat B, Wiebenga A, Vankuyk PA, Barry K, Lindquist E, LaButti K, Lapidus A, Lucas S, Coutinho P, Gong YC, Samejima M, Mahadevan R, Abou-Zaid M, de Vries RP, Igarashi K, Yadav JS, Grigoriev IV, Master ER. 2012. Comparative genomics of the white-rot fungi, Phanerochaete carnosa and P. chrysosporium, to elucidate the genetic basis of the distinct wood types they colonize. BMC Genomics 13:444. 10.1186/1471-2164-13-444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Syed K, Shale K, Pagadala NS, Tuszynski J. 2014. Systematic identification and evolutionary analysis of catalytically versatile cytochrome P450 monooxygenase families enriched in model basidiomycete fungi. PLoS One 9:e86683. 10.1371/journal.pone.0086683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathieu Y, Prosper P, Favier F, Harvengt L, Didierjean C, Jacquot JP, Morel-Rouhier M, Gelhaye E. 2013. Diversification of fungal specific class A glutathione transferases in saprotrophic fungi. PLoS One 8:e80298. 10.1371/journal.pone.0080298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collinson EJ, Grant CM. 2003. Role of yeast glutaredoxins as glutathione S-transferases. J. Biol. Chem. 278:22492–22497. 10.1074/jbc.M301387200 [DOI] [PubMed] [Google Scholar]
- 16.Garcera A, Barreto L, Piedrafita L, Tamarit J, Herrero E. 2006. Saccharomyces cerevisiae cells have three omega class glutathione S-transferases acting as 1-Cys thiol transferases. Biochem. J. 398:187–196. 10.1042/BJ20060034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma XX, Jiang YL, He YX, Bao R, Chen YX, Zhou CZ. 2009. Structures of yeast glutathione-S-transferase GTT2 reveal a new catalytic type of GST family. EMBO Rep. 10:1320–1326. 10.1038/embor.2009.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morel M, Ngadin AA, Droux M, Jacquot JP, Gelhaye E. 2009. The fungal glutathione S-transferase system. Evidence of new classes in the wood-degrading basidiomycete Phanerochaete chrysosporium. Cell. Mol. Life Sci. 66:3711–3725. 10.1007/s00018-009-0104-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tien M, Kirk TK. 1988. Lignin peroxidase of Phanerochaete chrysosporium. Methods Enzymol. 161:238–249. 10.1016/0076-6879(88)61025-1 [DOI] [Google Scholar]
- 20.Baldi P, Long AD. 2001. A Bayesian framework for the analysis of microarray expression data: regularized t-test and statistical inferences of gene changes. Bioinformatics 17:509–519. 10.1093/bioinformatics/17.6.509 [DOI] [PubMed] [Google Scholar]
- 21.Schenk PM, Baumann S, Mattes R, Steinbiss HH. 1995. Improved high-level expression system for eukaryotic genes in Escherichia coli using T7 RNA polymerase and rare ArgtRNAs. Biotechniques 19:196–200 [PubMed] [Google Scholar]
- 22.Couturier J, Koh CS, Zaffagnini M, Winger AM, Gualberto JM, Corbier C, Decottignies P, Jacquot JP, Lemaire SD, Didierjean C, Rouhier N. 2009. Structure-function relationship of the chloroplastic glutaredoxin S12 with an atypical WCSYS active site. J. Biol. Chem. 284:9299–9310. 10.1074/jbc.M807998200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alin P, Danielson UH, Mannervik B. 1985. 4-Hydroxyalk-2-enals are substrates for glutathione transferase. FEBS Lett. 179:267–270. 10.1016/0014-5793(85)80532-9 [DOI] [PubMed] [Google Scholar]
- 24.Barreto L, Garcera A, Jansson K, Sunnerhagen P, Herrero E. 2006. A peroxisomal glutathione transferase of Saccharomyces cerevisiae is functionally related to sulfur amino acid metabolism. Eukaryot. Cell 5:1748–1759. 10.1128/EC.00216-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sherman F. 2002. Getting started with yeast. Methods Enzymol. 350:3–41. 10.1016/S0076-6879(02)50954-X [DOI] [PubMed] [Google Scholar]
- 26.Stojkovic D, Petrovic J, Sokovic M, Glamoclija J, Kukic-Markovic J, Petrovic S. 2013. In situ antioxidant and antimicrobial activities of naturally occurring caffeic acid, p-coumaric acid and rutin, using food systems. J. Sci. Food Agric. 93:3205–3208. 10.1002/jsfa.6156 [DOI] [PubMed] [Google Scholar]
- 27.Field FA, Lettinga J. 1992. Toxicity of tannic compounds to microorganisms, p 673–692 In Hemingway RW, Laks PE. (ed), Plant polyphenols: synthesis, properties, significance. Basic life sciences, vol. 59 Plenum Press, New York, NY [Google Scholar]
- 28.Bennick A. 2002. Interaction of plant polyphenols with salivary proteins. Crit. Rev. Oral Biol. Med. 13:184–196. 10.1177/154411130201300208 [DOI] [PubMed] [Google Scholar]
- 29.Mgbeahuruike AC, Kovalchuk A, Chen H, Ubhayasekera W, Asiegbu FO. 2013. Evolutionary analysis of hydrophobin gene family in two wood-degrading basidiomycetes, Phlebia brevispora and Heterobasidion annosum s.l. BMC Evol. Biol. 13:240. 10.1186/1471-2148-13-240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mgbeahuruike AC, Kovalchuk A, Asiegbu FO. 2013. Comparative genomics and evolutionary analysis of hydrophobins from three species of wood-degrading fungi. Mycologia 105:1471–1478. 10.3852/13-077 [DOI] [PubMed] [Google Scholar]
- 31.Hacquard S, Joly DL, Lin YC, Tisserant E, Feau N, Delaruelle C, Legue V, Kohler A, Tanguay P, Petre B, Frey P, Van de Peer Y, Rouze P, Martin F, Hamelin RC, Duplessis S. 2012. A comprehensive analysis of genes encoding small secreted proteins identifies candidate effectors in Melampsora larici-populina (poplar leaf rust). Mol. Plant Microbe Interact. 25:279–293. 10.1094/MPMI-09-11-0238 [DOI] [PubMed] [Google Scholar]
- 32.Plett JM, Kemppainen M, Kale SD, Kohler A, Legue V, Brun A, Tyler BM, Pardo AG, Martin F. 2011. A secreted effector protein of Laccaria bicolor is required for symbiosis development. Curr. Biol. 21:1197–1203. 10.1016/j.cub.2011.05.033 [DOI] [PubMed] [Google Scholar]
- 33.Petre B, Major I, Rouhier N, Duplessis S. 2011. Genome-wide analysis of eukaryote thaumatin-like proteins (TLPs) with an emphasis on poplar. BMC Plant Biol. 11:33. 10.1186/1471-2229-11-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levine RL, Mosoni L, Berlett BS, Stadtman ER. 1996. Methionine residues as endogenous antioxidants in proteins. Proc. Natl. Acad. Sci. U. S. A. 93:15036–15040. 10.1073/pnas.93.26.15036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nomura M, Takagi H. 2004. Role of the yeast acetyltransferase MPR1 in oxidative stress: regulation of oxygen reactive species caused by a toxic proline catabolism intermediate. Proc. Natl. Acad. Sci. U. S. A. 101:12616–12621. 10.1073/pnas.0403349101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li L, Cheng X, Lu S, Nakatsubo T, Umezawa T, Chiang VL. 2005. Clarification of cinnamoyl co-enzyme A reductase catalysis in monolignol biosynthesis of aspen. Plant Cell Physiol. 46:1073–1082. 10.1093/pcp/pci120 [DOI] [PubMed] [Google Scholar]
- 37.Ozcan S, Yildirim V, Kaya L, Albrecht D, Becher D, Hecker M, Ozcengiz G. 2007. Phanerochaete chrysosporium soluble proteome as a prelude for the analysis of heavy metal stress response. Proteomics 7:1249–1260. 10.1002/pmic.200600526 [DOI] [PubMed] [Google Scholar]
- 38.Sato S, Feltus FA, Iyer P, Tien M. 2009. The first genome-level transcriptome of the wood-degrading fungus Phanerochaete chrysosporium grown on red oak. Curr. Genet. 55:273–286. 10.1007/s00294-009-0243-0 [DOI] [PubMed] [Google Scholar]
- 39.Castro LDS, Antoniêto AC, Pedersoli WR, Silva-Rocha R, Persinoti GF, Silva RN. 2014. Expression pattern of cellulolytic and xylanolytic genes regulated by transcriptional factors XYR1 and CRE1 are affected by carbon source in Trichoderma reesei. Gene Expr. Patterns 14:88–95. 10.1016/j.gep.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 40.Syed K, Doddapaneni H, Subramanian V, Lam YW, Yadav JS. 2010. Genome-to-function characterization of novel fungal P450 monooxygenases oxidizing polycyclic aromatic hydrocarbons (PAHs). Biochem. Biophys. Res. Commun. 399:492–497. 10.1016/j.bbrc.2010.07.094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Syed K, Porollo A, Lam YW, Grimmett PE, Yadav JS. 2013. Cyp63a2, a catalytically versatile fungal P450 monooxygenase capable of oxidizing higher-molecular-weight polycyclic aromatic hydrocarbons, alkylphenols, and alkanes. Appl. Environ. Microbiol. 79:2692–2702. 10.1128/AEM.03767-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Subramanian V, Yadav JS. 2009. Role of P450 monooxygenases in the degradation of the endocrine-disrupting chemical nonylphenol by the white rot fungus Phanerochaete chrysosporium. Appl. Environ. Microbiol. 75:5570–5580. 10.1128/AEM.02942-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chigu NL, Hirosue S, Nakamura C, Teramoto H, Ichinose H, Wariishi H. 2010. Cytochrome P450 monooxygenases involved in anthracene metabolism by the white-rot basidiomycete Phanerochaete chrysosporium. Appl. Microbiol. Biotechnol. 87:1907–1916. 10.1007/s00253-010-2616-1 [DOI] [PubMed] [Google Scholar]
- 44.Bresell A, Weinander R, Lundqvist G, Raza H, Shimoji M, Sun TH, Balk L, Wiklund R, Eriksson J, Jansson C, Persson B, Jakobsson PJ, Morgenstern R. 2005. Bioinformatic and enzymatic characterization of the MAPEG superfamily. FEBS J. 272:1688–1703. 10.1111/j.1742-4658.2005.04596.x [DOI] [PubMed] [Google Scholar]
- 45.Mathieu Y, Prosper P, Buee M, Dumarcay S, Favier F, Gelhaye E, Gerardin P, Harvengt L, Jacquot JP, Lamant T, Meux E, Mathiot S, Didierjean C, Morel M. 2012. Characterization of a Phanerochaete chrysosporium glutathione transferase reveals a novel structural and functional class with ligandin properties. J. Biol. Chem. 287:39001–39011. 10.1074/jbc.M112.402776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi JH, Lou W, Vancura A. 1998. A novel membrane-bound glutathione S-transferase functions in the stationary phase of the yeast Saccharomyces cerevisiae. J. Biol. Chem. 273:29915–29922. 10.1074/jbc.273.45.29915 [DOI] [PubMed] [Google Scholar]
- 47.Herrero E, Ros J, Tamarit J, Belli G. 2006. Glutaredoxins in fungi. Photosynth. Res. 89:127–140. 10.1007/s11120-006-9079-3 [DOI] [PubMed] [Google Scholar]
- 48.Mariani D, Mathias CJ, da Silva CG, Herdeiro RD, Pereira R, Panek AD, Eleutherio ECA, Pereira MD. 2008. Involvement of glutathione transferases, Gtt1 and Gtt2, with oxidative stress response generated by H2O2 during growth of Saccharomyces cerevisiae. Redox Rep. 13:246–254. 10.1179/135100008X309028 [DOI] [PubMed] [Google Scholar]
- 49.Tanaka T, Izawa S, Inoue Y. 2005. Gpx2, encoding a phospholipid hydroperoxide glutathione peroxidase homologue, codes for an atypical 2-Cys peroxiredoxin in Saccharomyces cerevisiae. J. Biol. Chem. 280:42078–42087. 10.1074/jbc.M508622200 [DOI] [PubMed] [Google Scholar]
- 50.Bai M, Zhou JM, Perrett S. 2004. The yeast prion protein Ure2 shows glutathione peroxidase activity in both native and fibrillar forms. J. Biol. Chem. 279:50025–50030. 10.1074/jbc.M406612200 [DOI] [PubMed] [Google Scholar]
- 51.Awasthi YC, Yang Y, Tiwari NK, Patrick B, Sharma A, Li J, Awasthi S. 2004. Regulation of 4-hydroxynonenal-mediated signaling by glutathione S-transferases. Free Radic. Biol. Med. 37:607–619. 10.1016/j.freeradbiomed.2004.05.033 [DOI] [PubMed] [Google Scholar]
- 52.Esterbauer H, Schaur RJ, Zollner H. 1991. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 11:81–128. 10.1016/0891-5849(91)90192-6 [DOI] [PubMed] [Google Scholar]
- 53.Grigoriev IV, Nordberg H, Shabalov I, Aerts A, Cantor M, Goodstein D, Kuo A, Minovitsky S, Nikitin R, Ohm RA, Otillar R, Poliakov A, Ratnere I, Riley R, Smirnova T, Rokhsar D, Dubchak I. 2012. The genome portal of the Department of Energy Joint Genome Institute. Nucleic Acids Res. 40:D26–D32. 10.1093/nar/gkr947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sjöström E. 1981. Wood polysaccharides, p 49–82 Wood chemistry: fundamentals and applications. Academic Press, New York, NY [Google Scholar]
- 55.Fengel D, Wegener G. 1984. Chemical composition and analysis of wood, p 183–267 In de Gruyter W. (ed), Wood chemistry, ultrastructures, reactions. Remagen, Berlin, Germany [Google Scholar]
- 56.Skyba O, Douglas CJ, Mansfield SD. 2013. Syringyl-rich lignin renders poplars more resistant to degradation by wood decay fungi. Appl. Environ. Microbiol. 79:2560–2571. 10.1128/AEM.03182-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eastwood DC, Floudas D, Binder M, Majcherczyk A, Schneider P, Aerts A, Asiegbu FO, Baker SE, Barry K, Bendiksby M, Blumentritt M, Coutinho PM, Cullen D, de Vries RP, Gathman A, Goodell B, Henrissat B, Ihrmark K, Kauserud H, Kohler A, LaButti K, Lapidus A, Lavin JL, Lee YH, Lindquist E, Lilly W, Lucas S, Morin E, Murat C, Oguiza JA, Park J, Pisabarro AG, Riley R, Rosling A, Salamov A, Schmidt O, Schmutz J, Skrede I, Stenlid J, Wiebenga A, Xie XF, Kues U, Hibbett DS, Hoffmeister D, Hogberg N, Martin F, Grigoriev IV, Watkinson SC. 2011. The plant cell wall-decomposing machinery underlies the functional diversity of forest fungi. Science 333:762–765. 10.1126/science.1205411 [DOI] [PubMed] [Google Scholar]
- 58.Floudas D, Binder M, Riley R, Barry K, Blanchette RA, Henrissat B, Martinez AT, Otillar R, Spatafora JW, Yadav JS, Aerts A, Benoit I, Boyd A, Carlson A, Copeland A, Coutinho PM, de Vries RP, Ferreira P, Findley K, Foster B, Gaskell J, Glotzer D, Gorecki P, Heitman J, Hesse C, Hori C, Igarashi K, Jurgens JA, Kallen N, Kersten P, Kohler A, Kues U, Kumar TKA, Kuo A, LaButti K, Larrondo LF, Lindquist E, Ling A, Lombard V, Lucas S, Lundell T, Martin R, McLaughlin DJ, Morgenstern I, Morin E, Murat C, Nagy LG, Nolan M, Ohm RA, Patyshakuliyeva A, Rokas A, Ruiz-Duenas FJ, Sabat G, Salamov A, Samejima M, Schmutz J, Slot JC, John FS, Stenlid J, Sun H, Sun S, Syed K, Tsang A, Wiebenga A, Young D, Pisabarro A, Eastwood DC, Martin F, Cullen D, Grigoriev IV, Hibbett DS. 2012. The paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science 336:1715–1719. 10.1126/science.1221748 [DOI] [PubMed] [Google Scholar]
- 59.Morel M, Kohler A, Martin F, Gelhaye E, Rouhier N. 2008. Comparison of the thiol-dependent antioxidant systems in the ectomycorrhizal Laccaria bicolor and the saprotrophic Phanerochaete chrysosporium. New Phytol. 180:391–407. 10.1111/j.1469-8137.2008.02498.x [DOI] [PubMed] [Google Scholar]
- 60.Fernandez-Fueyo E, Ruiz-Duenas FJ, Ferreira P, Floudas D, Hibbett DS, Canessa P, Larrondo LF, James TY, Seelenfreund D, Lobos S, Polanco R, Tello M, Honda Y, Watanabe T, San RJ, Kubicek CP, Schmoll M, Gaskell J, Hammel KE, St. John FJ, Vanden Wymelenberg A, Sabat G, BonDurant SS, Syed K, Yadav JS, Doddapaneni H, Subramanian V, Lavin JL, Oguiza JA, Perez G, Pisabarro AG, Ramirez L, Santoyo F, Master E, Coutinho PM, Henrissat B, Lombard V, Magnuson JK, Kues U, Hori C, Igarashi K, Samejima M, Held BW, Barry KW, LaButti KM, Lapidus A, Lindquist EA, Lucas SM, Riley R, Salamov AA, Hoffmeister D, Schwenk D, Hadar Y, Yarden O, de Vries RP, Wiebenga A, Stenlid J, Eastwood D, Grigoriev IV, Berka RM, Blanchette RA, Kersten P, Martinez AT, Vicuna R, Cullen D. 2012. Comparative genomics of Ceriporiopsis subvermispora and Phanerochaete chrysosporium provide insight into selective ligninolysis. Proc. Natl. Acad. Sci. U. S. A. 109:5458–5463. 10.1073/pnas.1119912109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kang YM, Prewitt ML, Diehl SV. 2009. Proteomics for biodeterioration of wood (Pinus taeda L.): challenging analysis by 2-D PAGE and MALDI-TOF/TOF/MS. Int. Biodeterior. Biodegrad. 63:1036–1044. 10.1016/j.ibiod.2009.07.008 [DOI] [Google Scholar]
- 62.MacDonald J, Suzuki H, Master ER. 2012. Expression and regulation of genes encoding lignocellulose-degrading activity in the genus Phanerochaete. Appl. Microbiol. Biotechnol. 94:339–351. 10.1007/s00253-012-3937-z [DOI] [PubMed] [Google Scholar]
- 63.Morel M, Ngadin AA, Jacquot JP, Gelhaye E. 2009. Reactive oxygen species in Phanerochaete chrysosporium. Relationship between extracellular oxidative and intracellular antioxidant systems. Adv. Bot. Res. 52:153–186. 10.1016/S0065-2296(10)52006-8 [DOI] [Google Scholar]
- 64.Vanden Wymelenberg A, Gaskell J, Mozuch M, Kersten P, Sabat G, Martinez D, Cullen D. 2009. Transcriptome and secretome analyses of Phanerochaete chrysosporium reveal complex patterns of gene expression. Appl. Environ. Microbiol. 75:4058–4068. 10.1128/AEM.00314-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.