Abstract
The development of ligands that can promote selective insertion of a metal into primary or secondary C(sp3)–H bonds is a central challenge in the field of C–H functionalization. Here, we report a rare example of catalyst-controlled primary and secondary C(sp3)–H arylation using two different ligands. Successive application of these ligands enables the sequential hetero-diarylation of an alanine derivative with two different aryl iodides affording a wide range of β-Ar-β-Ar'-α-amino acids with excellent levels of diastereoselectivity (d.r. > 20:1). Both configurations of the β-chiral center can be accessed by choosing the order in which the aryl iodides are installed, thus demonstrating the potential to construct tertiary chiral centers from a simple methyl group. The realization of this reactivity by electronic and steric modulation of the ligands may provide fundamental guidance for the future design of more effective and selective catalysts for C(sp3)–H activation.
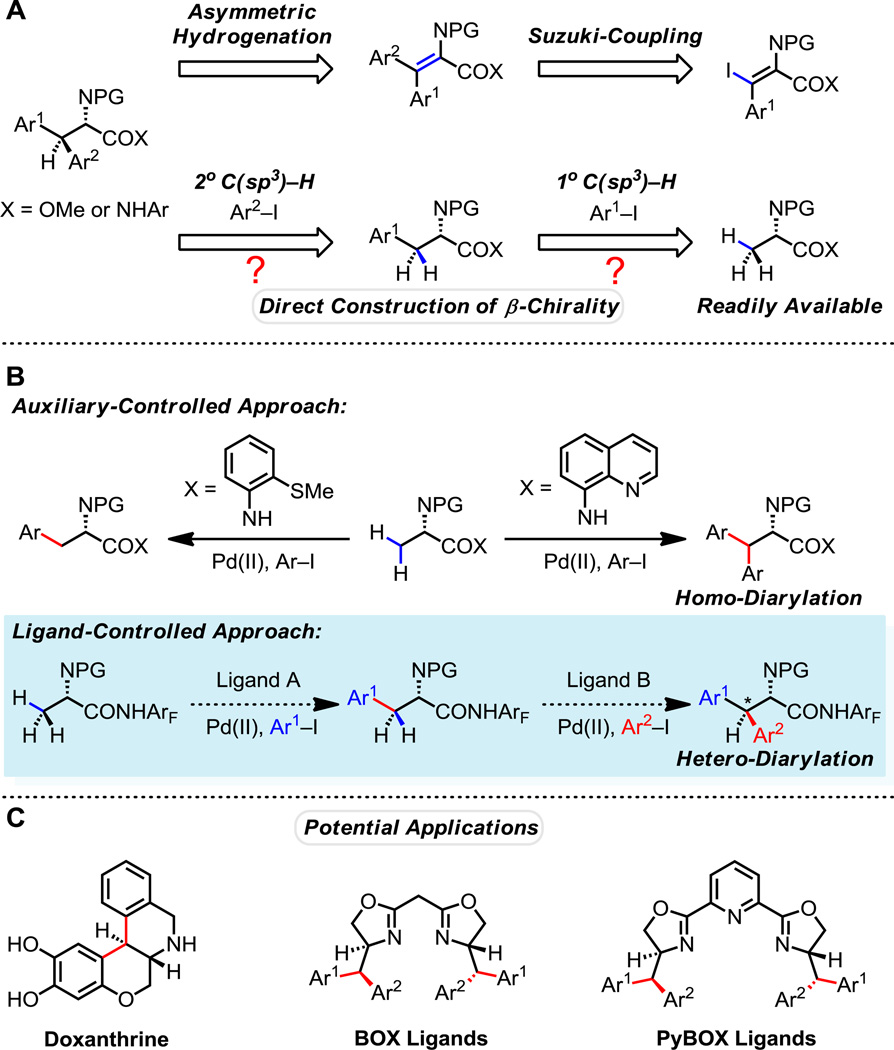
Unnatural amino acids are important building blocks in modern drug discovery, especially in peptide synthesis, enabling the introduction of diverse structural motifs and functional groups (1). The incorporation of unnatural amino acids can facilitate the study of protein-protein interactions and engender proteins with novel properties and functions (2). In addition, chiral amino acids are widely used as enantiomerically pure synthons for the synthesis of chiral ligands (3,4) and natural products (5). Although asymmetric hydrogenation is often the state-of-art method for the preparation of chiral α-amino acids (6), it remains practically challenging to access a structurally diverse collection of β-Ar-β-Ar'-α-amino acids in this way due to the difficulties associated with synthesis of the requisite enamide starting materials with defined alkene stereochemistry (Fig. 1A) (7).
Fig. 1.
(A) Two routes for synthesizing chiral β-Ar-β-Ar'-α-amino acids. (B) Two different approaches towards selective primary and secondary C(sp3)–H arylation. (C) Applications of β- Ar-β-Ar'-α-amino acid in drug discovery and asymmetric catalysis.
Over the past decade substantial progress has been achieved in the activation of the inert β-C(sp3)–H bonds of aliphatic carboxylic acids derivatives using chiral oxazolines (8), the 8-aminoquinoline auxiliary (9), and a variety of weakly coordinating amide directing groups (10, 11). In particular, the synthesis of unnatural amino acids via the direct β-functionalization of α-amino acids has been an area of extensive research since a seminal report by Corey (12, 13). We envision that a sequential hetero-diarylation of alanine with two different aryl iodides could potentially provide an efficient route for the preparation of β-Ar-β-Ar'-α-amino acids containing a β-chiral center (Fig. 1A). While the more strongly coordinating 8-aminoquinoline auxiliary developed by Daugulis is a remarkably powerful directing group for the β-arylation of alanine, this auxiliary provides predominantly β,β-homo-diarylated products, which prevents the installation of two different aryl groups in a step-wise manner. Mono-arylation of alanine can be achieved by switching to the use of the bi-dentate 2-thiomethylaniline directing group (Fig. 1B) (14, 15). However, for the purpose of preparing β-Ar-β-Ar'-α-amino acids it would be preferable to achieve this type of selectivity through catalyst control, rather than substrate control. Specifically, we envisioned that we could discover two different ligands, one that would selectively promote primary β-C(sp3)–H arylation, and another that would promote secondary β-C(sp3)–H arylation, using a common substrate. Fundamentally, the lack of appropriate ligands that are capable of cooperating with a weakly binding substrate on the metal center to promote C(sp3)–H bond cleavage and confer selectivity for primary or secondary β-C(sp3)–H bonds has largely hampered further development of C(sp3)–H activation reactions (Fig. 1B) (16–23). The successful achievement of this objective would have a significant and broad impact on the field of C–H functionalization.
Herein we report the discovery of a pyridine-based ligand that promotes the selective mono-arylation of primary C(sp3)–H bonds and a second, quinoline-based ligand that allows the subsequent arylation of secondary C(sp3)–H bonds. The stepwise use of these two ligands enables the introduction of two distinct aryl groups to produce a variety of β-Ar-β-Ar'-α-amino acids with excellent levels of diastereoselectivity by generating tertiary methine stereogenic centers from a simple methyl group (Fig. 1A). As such, both configurations at the new β-stereogenic center can be constructed by simply choosing the order of aryl group installation. These unnatural amino acid products are broadly useful as chiral building blocks for the synthesis of drug molecules such as doxanthrine (24), and a variety of new BOX and pyBOX chiral ligands, which are derived from α-amino alcohols (Fig. 1C) (3).
Our first challenge in the development of a versatile method for the preparation of stereo-defined β-Ar-β-Ar'-α-amino acids from alanine (Fig. 1B) was the design of a ligand that promotes selective mono-arylation of primary C(sp3)–H bonds. Our group has recently focused on the development of simple and weakly coordinating auxiliaries, such as N-methoxyamides and perfluorinated arylamides, to direct a wide range of C(sp3)–H activation reactions (10, 11). However, to date we have found that the use of these weakly coordinating auxiliaries is incompatible with the functionalization of the C(sp3)–H bonds of α-amino acids (10, 11). We recently demonstrated that the use of an alkoxypyridine ligand can match the weak coordination of the amide auxiliary (CONHArF) and facilitate secondary C(sp3)–H activation (albeit with only simple aliphatic amides) (25) indicating that pyridine-based ligands are capable of lowering the transition state energy of C(sp3)–H activation. This finding prompted us to test a diverse array of mono-dentate pyridine-based ligands for their ability to selectively promote primary C(sp3)–H activation, thereby allowing for highly mono-selective arylation of alanine-derived amide 1.
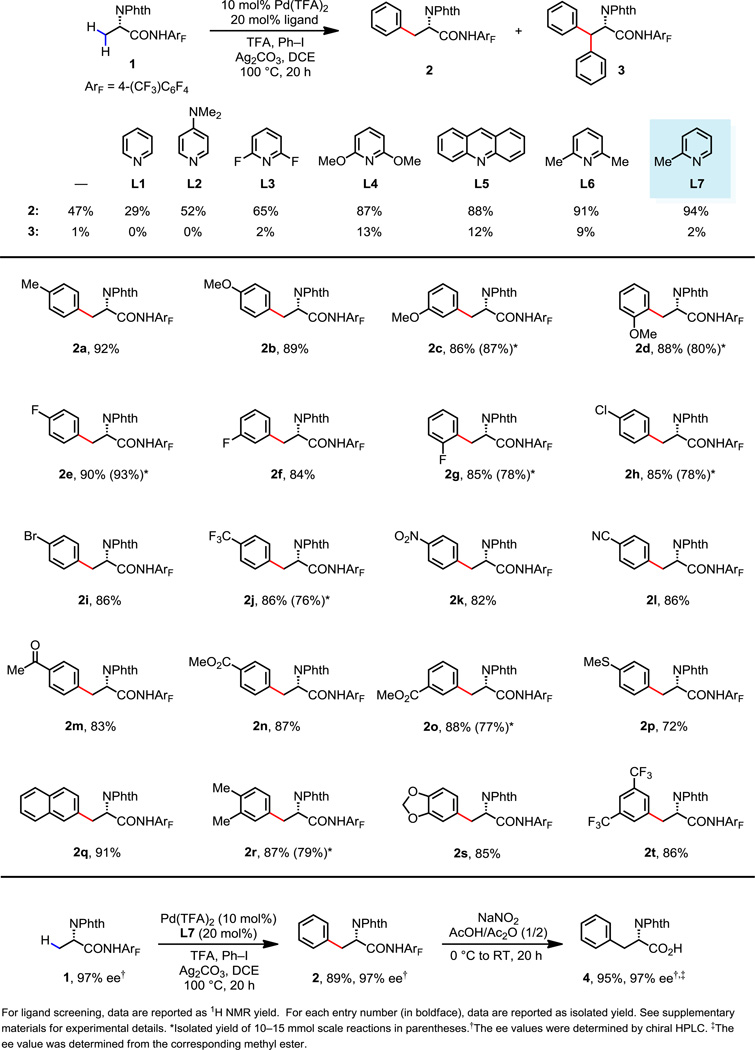
In order to obtain preliminary information regarding the reactivity of the CONHArF amide auxiliary with amino acid substrates, we initiated our experimental efforts by studying C(sp3)–H arylation of 1 under a variety of different reaction conditions in the absence of an ancillary ligand. Through extensive screening, we found that the use of 20 mol% trifluoroacetic acid (TFA) prevented substrate decomposition, which had been observed with 1 under previously developed basic conditions (25). Under the best conditions from this initial screen, mono-arylated product 2 could be isolated in 47% yield, along with full recovery of the remaining starting material (Fig. 2). Additional attempts to fine-tune various reaction parameters, however failed to improve the conversion. Based on these results, a library of pyridine ligands were tested for their efficiency of promoting mono-arylation in the presence of TFA. Pyridine and 4-dimethylaminopyridine (L1 and L2) are ineffective ligands for promoting mono-arylation. In these cases, we suspect that two ligand molecules may coordinate to Pd(II) in solution, depriving the weakly coordinating substrate of an open coordination site. In contrast, we found that 2,6-dimethoxypyridine, acridine, 2,6-lutidine and 2-picoline (L4–L7) are highly effective ligands for C(sp3)–H arylation. While the use of more electron-rich ligands (L4–L6) leads to substantial quantities of the undesired diarylated product 3, 2-picoline (L7) seems to possess an optimal balance of steric and electronic properties to provide 2 in high yield with an excellent level of selectivity for mono-arylation (NMR yield of 94%). The mono-arylation reaction also proceeded in the presence of 5 mol% of Pd(TFA)2 and 10 mol% of L7 to give the desired product 2 in 79% yield. In addition to this reactivity data, the intimate involvement of the ligand in the C(sp3)–H cleavage step is implicated by the results of an intramolecular kinetic isotope effect experiment (KIE); a significantly larger KIE is seen when L7 is used in this reaction (without ligand, kH/D = 6.0; with L7, kH/D = 8.1; see supplementary materials).
Fig. 2.
Synthesis of phenylalanine derivatives via ligand-promoted primary C(sp3)–H arylation.
The potential applicability of this ligand-controlled mono-arylation protocol in the preparation of diverse chiral β-Ar-β-Ar'-α-amino acids is demonstrated by the selective arylation of 1 with a broad range of aryl coupling partners. As shown in Figure 2, phenylalanine derivatives with electron-rich or electron poor-groups in the ortho-, meta-, or para-positions can be synthesized in high yields. This reaction is tolerant of halide substituents and a wide range of polar functional groups. Arylation with 4-methylthiophenyl iodide also proceeds to give the arylated product (2p) in a synthetically useful yield, indicating that the pyridine ligand is able to out-compete the methylthio group for coordination at Pd(II). The reaction of 1 with 2-iodonaphthalene to give 2q is particularly useful, as the resulting product could be used to synthesize bioactive peptides that block cell cycle progression in HeLa cells (26). Arylation with di-substituted aryl iodides is also efficient, giving 2r, 2s, and 2t in > 85% yields. Deprotection of 2s would afford L-DOPA, which is widely used in the treatment of Parkinson’s disease (27).
When conducted at 100 °C, these reactions are typically completed within 20 hours with no racemization of the stereogenic center. Subsequent removal of the auxiliary can be accomplished under mild conditions without loss of enantiomeric purity (Fig. 2) and the resultant compounds are readily converted to the corresponding Fmoc-protected unnatural amino acids following literature procedures (see supplementary materials). The auxiliary 2,3,5,6-tetrafluoro-4-(trifluoromethyl)aniline is readily prepared from octafluorotoluene ($0.47/g) on 100 gram scale or purchased directly from Aldrich. Due to these practical advantages, we have prepared a variety of mono-arylated alanines on 10 mmol scale to facilitate peptide drug discovery in collaboration with BMS (2c–2e, 2g, 2h, 2j, 2o, 2r).
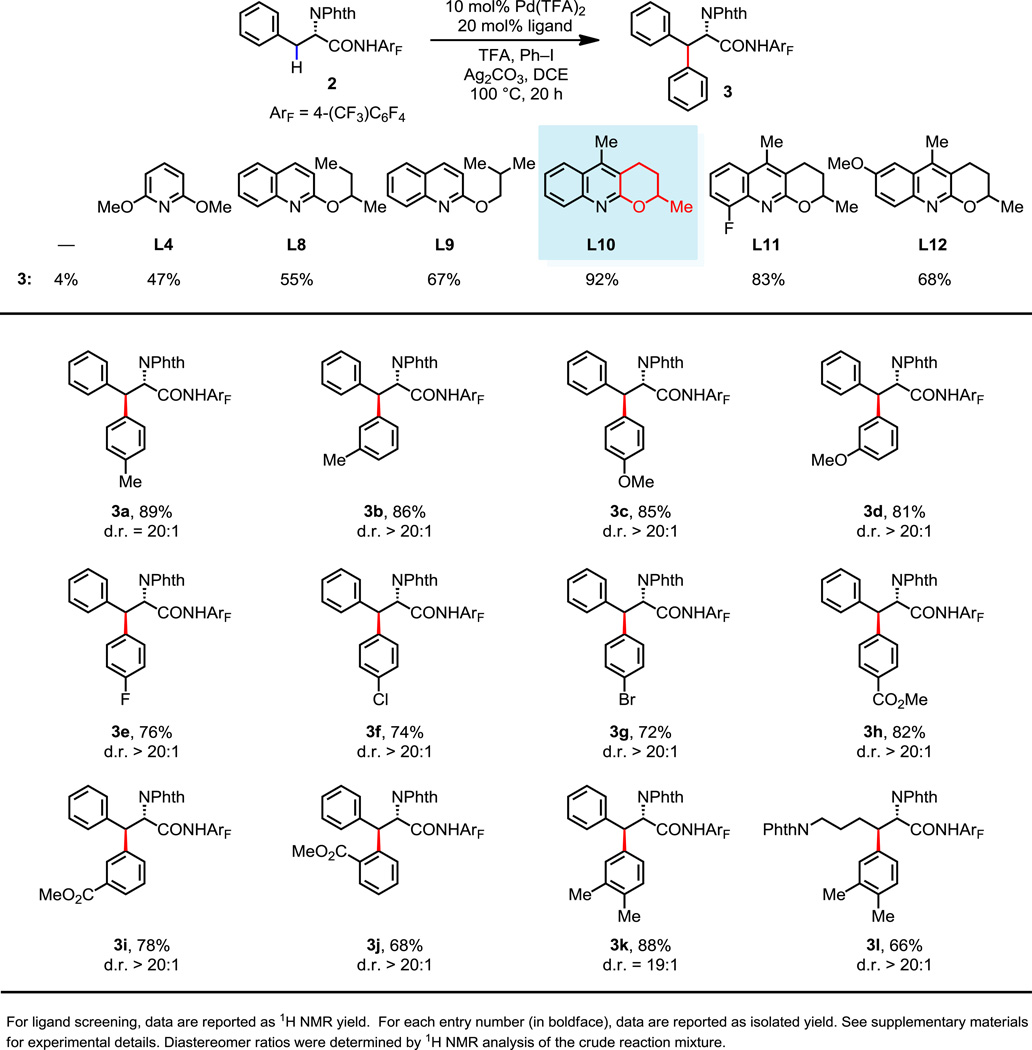
The development of this highly selective ligand-promoted mono-arylation protocol set the stage for identifying a second ligand that can promote the subsequent arylation of the secondary β-C(sp3)–H bonds. As previously mentioned, our recently disclosed procedure for the ligand-promoted arylation of secondary C(sp3)–H bonds (25) is not compatible with amino acid substrates. However, the formation of minor amounts of the diarylated product 3 from amide 1 with ligand L4 (13% yield, Fig 2) suggested that these new conditions could be effective for secondary β-C(sp3)–H bond arylation with an appropriate ligand. We were pleased to find that the arylation of phenylalanine-derived amide 2, in the presence of ligand L4, affords the desired product 3 in 47% yield. The modest success of this ligand and our previously reported 2-alkoxylquinoline ligand L8 (25) led us to explore a variety of electron-rich 2-alkoxylpyridine and 2-alkoxylquinoline ligands for this secondary C(sp3)–H bond arylation.
The significant improvement in reaction efficiency with ligands L8 and L9 suggested that the 2-substituted quinoline motif possesses steric properties that favored mono- rather than bis-coordination to Pd(II), which is a prerequisite for effective substrate binding. Cognizant of the correlation between electron donating ligands and an increased efficiency of secondary C(sp3)–H bond cleavage, we decided to examine the impact of further increasing the electron-donating ability of the alkoxyl group by synthesizing the tricyclic ligand L10, in which the conformation of the lone pairs on the oxygen atom is rigidified to favor π-conjugation with the pyridine ring. This rational ligand design led to a dramatic improvement in reaction efficiency, affording product 3 in 92% yield. Further electronic modification of L10 to weaken or strengthen the coordinating ability of the quinoline led to a decrease in product yield.
Under these optimized reaction conditions amide 2 can be arylated with a broad range of electron-rich and electron-poor aryl iodides in high yield (Fig. 3). ortho-Substituted aryl iodides are also compatible with this reaction despite the known steric hindrance associated with the arylation of secondary C(sp3)–H bonds (3j). Ligand L10 also promotes the arylation of the secondary C(sp3)–H bonds of the corresponding lysine-derived amide to afford 3l in 66% yield, thus demonstrating the utility of this ligand for the syntheses of β-alkyl-β-aryl-α-amino acids. Importantly, these arylation reactions all proceeded with high levels of diastereoselectivity (d.r. > 20:1), which may be attributed to the steric repulsion between the α-phthalimido group and the β-substituent in the transition state of the directed cyclopalladation.
Fig. 3.
Ligand-promoted secondary C(sp3)–H arylation of phenylalanine and lysine derivatives.
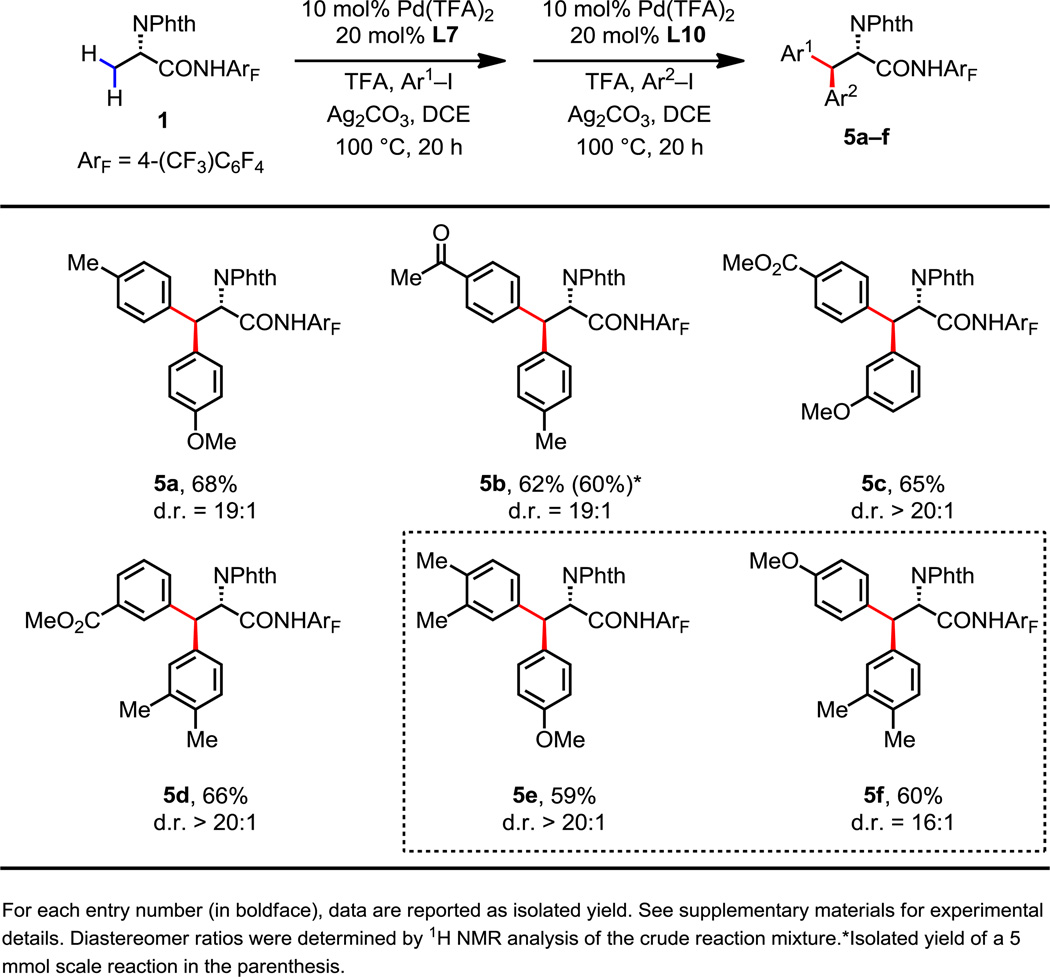
Importantly, ligands L7 and L10, which enable the arylation of primary and secondary β-C(sp3)–H bonds, respectively, can be employed for the sequential one-pot incorporation of two distinct aryl groups onto the β-carbon of alanine-derivative 1 (Fig. 4). Thus, following the completion of the mono-arylation of 1 with an aryl iodide using L7, we can add L10 and a second aryl iodide to provide a β-Ar-β-Ar'-α-amino acid. This one-pot procedure is successfully applied to both electron-rich and electron-deficient aryl iodides to prepare a variety of amino acids (5a–5d) in good yields with excellent levels of diastereoselectivity. Notably, by simply switching the order of the addition of the two different aryl iodides, the inverse configuration at the β-stereogenic center can be obtained (5e and 5f). When conducted on a 5 mmol scale the desired product 5b was isolated in 60% yield. Currently, this ligand controlled C(sp3)–H arylation protocol is being applied to the gram-scale synthesis of bioactive peptides in collaboration with BMS.
Fig. 4.
Synthesis of β-aryl, β-Ar'-α-amino acids via sequential C(sp3)–H arylation.
In summary, we have discovered two ligands that can promote selective activation of either primary or secondary C(sp3)–H bonds. The use of these two ligands enables the sequential hetero-diarylation of alanine-derived amides in one pot with high levels of diastereoselectivity, thus providing a versatile method for preparing β-Ar-β-Ar'-α-amino acids. The significant influence of the steric and electronic properties of ligands on the reactivity of a palladium catalyst in the activation of primary and secondary C(sp3)–H bonds provides invaluable guidance for the further development of more effective and selective catalysts for C(sp3)–H activation.
Supplementary Material
Acknowledgements
We gratefully acknowledge The Scripps Research Institute and the NIH (NIGMS, 2R01GM084019) for their financial support.
Footnotes
Supplementary Materials:
Materials and Methods
Tables S1 to S3
NMR Spectra
References (28–33)
References and Notes
- 1.Brik A. Chemistry and biology of peptides, proteins and beyond. Bioorg. Med. Chem. 2013;21:3398–3399. doi: 10.1016/j.bmc.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 2.Liu CC, Schultz PG. Adding new chemistries to the genetic code. Annu. Rev. Biochem. 2010;79:413–444. doi: 10.1146/annurev.biochem.052308.105824. [DOI] [PubMed] [Google Scholar]
- 3.Pfaltz A. Chiral semicorrins and related nitrogen heterocycles as ligands in asymmetric catalysis. Acc. Chem. Res. 1993;26:339–345. [Google Scholar]
- 4.Han S, Miller SJ. Asymmetric catalysis at a distance: catalytic, site-selective phosphorylation of teicoplanin. J. Am. Chem. Soc. 2013;135:12414–12421. doi: 10.1021/ja406067v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sardina FJ, Rapoport H. Enantiospecific synthesis of heterocycles from α-amino acids. Chem. Rev. 1996;96:1825–1872. doi: 10.1021/cr9300348. [DOI] [PubMed] [Google Scholar]
- 6.Tang W, Zhang X. New chiral phosphorus ligands for enantioselective hydrogenation. Chem. Rev. 2003;103:3029–3069. doi: 10.1021/cr020049i. [DOI] [PubMed] [Google Scholar]
- 7.Ferreira PMT, Monteiro LS, Pereira G. Synthesis of substituted oxazoles from N-acyl-β-hydroxyamino acid derivatives. Eur. J. Org. Chem. 2008:4676–4683. [Google Scholar]
- 8.Giri R, Chen X, Yu J-Q. Palladium-catalyzed asymmetric iodination of unactivated C–H bonds under mild conditions. Angew. Chem. Int. Ed. 2005;44:2112–2115. doi: 10.1002/anie.200462884. [DOI] [PubMed] [Google Scholar]
- 9.Zaitsev VG, Shabashov D, Daugulis O. Highly regioselective arylation of sp3 C–H bonds catalyzed by palladium acetate. J. Am. Chem. Soc. 2005;127:13154–13155. doi: 10.1021/ja054549f. [DOI] [PubMed] [Google Scholar]
- 10.Wang D-H, Wasa M, Giri R, Yu J-Q. Pd(II)-catalyzed cross-coupling of sp3 C–H bonds with sp2 and sp3 boronic acids using air as the oxidant. J. Am. Chem. Soc. 2008;130:7190–7191. doi: 10.1021/ja801355s. [DOI] [PubMed] [Google Scholar]
- 11.Wasa M, Engle KM, Yu J-Q. Pd(0)/PR3-catalyzed intermolecular arylation of sp3 C–H bonds. J. Am. Chem. Soc. 2009;131:9886–9887. doi: 10.1021/ja903573p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reddy BVS, Reddy LR, Corey EJ. Novel acetoxylation and C–C coupling reactions at unactivated positions in α-amino acid derivatives. Org. Lett. 2006;8:3391–3394. doi: 10.1021/ol061389j. [DOI] [PubMed] [Google Scholar]
- 13.Feng Y, Chen G. Total synthesis of Celogentin C by stereoselective C–H activation. Angew. Chem. Int. Ed. 2010;49:958–961. doi: 10.1002/anie.200905134. [DOI] [PubMed] [Google Scholar]
- 14.Shabashov D, Daugulis O. Auxiliary-assisted palladium-catalyzed arylation and alkylation of sp2 and sp3 carbon–hydrogen bonds. J. Am. Chem. Soc. 2010;132:3965–3972. doi: 10.1021/ja910900p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tran LD, Daugulis O. Nonnatural amino acid synthesis by using carbon–hydrogen bond functionalization methodology. Angew. Chem. Int. Ed. 2012;51:5188–5191. doi: 10.1002/anie.201200731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodríguez N, Romero-Revilla JA, Fernández-Ibáñez MÁ, Carretero JC. Palladium-catalyzed N-(2-pyridyl)sulfonyl-directed C(sp3)–H γ-arylation of amino acid derivatives. Chem. Sci. 2013;4:175–179. [Google Scholar]
- 17.Shang R, Ilies L, Matsumoto A, Nakamura E. β-Arylation of carboxamides via iron-catalyzed C(sp3)–H bond activation. J. Am. Chem. Soc. 2013;135:6030–6032. doi: 10.1021/ja402806f. [DOI] [PubMed] [Google Scholar]
- 18.Simmons EM, Hartwig JF. Catalytic functionalization of unactivated primary C–H bonds directed by an alcohol. Nature. 2012;483:70–73. doi: 10.1038/nature10785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasegawa N, Charra V, Inoue S, Fukumoto Y, Chatani N. Highly regioselective carbonylation of unactivated C(sp3)–H bonds by ruthenium carbonyl. J. Am. Chem. Soc. 2011;133:8070–8073. doi: 10.1021/ja2001709. [DOI] [PubMed] [Google Scholar]
- 20.Baudoin O. Transition metal-catalyzed arylation of unactivated C(sp3)–H bonds. Chem. Soc. Rev. 2011;40:4902–4911. doi: 10.1039/c1cs15058h. [DOI] [PubMed] [Google Scholar]
- 21.Lyons TW, Sanford MS. Palladium-catalyzed ligand-directed C–H functionalization reactions. Chem. Rev. 2010;110:1147–1169. doi: 10.1021/cr900184e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jazzar R, Hitce J, Renaudat A, Sofack-Kreutzer J, Baudoin O. Functionalization of organic molecules by transition-metal-catalyzed C(sp3)–H activation. Chem. Eur. J. 2010;16:2654–2672. doi: 10.1002/chem.200902374. [DOI] [PubMed] [Google Scholar]
- 23.Wasa M, Engle KM, Yu J-Q. Cross-coupling of C(sp3)–H bonds with organometallic reagents via Pd(II)/Pd(0) catalysis. Isr. J. Chem. 2010;50:605–616. doi: 10.1002/ijch.201000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cueva JP, et al. trans-2,3-Dihydroxy-6a,7,8,12b-tetrahydro-6H-chromeno[3,4-c]isoquinoline: synthesis, resolution, and preliminary pharmacological characterization of a new dopamine D1 receptor full agonist. J. Med. Chem. 2006;49:6848–6857. doi: 10.1021/jm0604979. [DOI] [PubMed] [Google Scholar]
- 25.Wasa M, et al. Ligand-enabled methylene C(sp3)–H bond activation with a Pd(II) catalyst. J. Am. Chem. Soc. 2012;134:18570–18572. doi: 10.1021/ja309325e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wildemann D, et al. Nanomolar inhibitors of the peptidyl prolyl cis/trans isomerase Pin1 from combinatorial peptide libraries. J. Med. Chem. 2006;49:2147–2150. doi: 10.1021/jm060036n. [DOI] [PubMed] [Google Scholar]
- 27.Cotzias GC, Van Woert MH, Schiffer LM. Aromatic amino acids and modification of parkinsonism. N. Engl. J. Med. 1967;276:374–379. doi: 10.1056/NEJM196702162760703. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.