Abstract
Post-transcriptional maturation of plant mitochondrial transcripts requires several steps. Among these, the generation of mature 5′ ends is still one of the most enigmatic processes. Toward a characterization of proteins involved in 5′ processing of mitochondrial transcripts in Arabidopsis (Arabidopsis thaliana), we now analyzed 5′ maturation of nad2 transcripts. Based on natural genetic variation affecting 5′ ends of nad2 transcripts in ecotype Can-0 and complementation studies we now identified RNA processing factor 7, which takes part in the generation of the 5′ terminus of the mature nad2 mRNA. RPF7 is a relatively short regular P-class pentatricopeptide repeat protein comprising seven canonical P repeats and a single short S repeat. The corresponding allele in Can-0 encodes a truncated version of this protein lacking two C-terminal repeats, which are essential for the function of RPF7. Furthermore we established transgenic plants expressing artifical microRNAs targeting the mitochondrial polynucleotide phosphorylase (PNPase), which results in substantial reduction of the PNPase mRNA levels and strong knockdown of this gene. Detailed quantitative studies of 5′ and 3′ extended nad2 precursor RNAs in these knockdown plants as well as in the rpf7–1 knockout mutant suggest that 5′ processing contributes to the stability of mitochondrial transcripts in plants.
Keywords: 5′ end processing, arabidopsis thaliana, RNA, RNA processing factor, mitochondria, pentatricopeptide repeat proteins, polynucleotide phosphorylase
Introduction
Since their discovery several years ago the function and/or biological role of a considerable number of pentatricopeptide repeat (PPR) proteins have been described. These proteins, characterized by degenerative, predominantly 35 amino acid long motifs, are involved in virtually all processes dealing with RNA. But clearly outstanding is their dominating role in post-transcriptional processes in plant mitochondria and chloroplasts.1-5
In these organelles, PPR proteins specify cytidines to be converted to uridines by RNA editing.6 PPR proteins involved in this process group into subclass PLS, according to their composition of canonical (P), short (S) and long (L) repeats.7 PPR proteins are also involved in intron splicing as well as in 5′ and 3′ end formation of organellar transcripts. The proteins participating in these processes belong to subclass P, composed predominantly of classical P motifs. These proteins also lack E or DYW domains, which were present at C-termini of most PLS-class proteins.3,7,8
PPR proteins are sequence-specific RNA binding proteins.9 The recognition of RNA occurs in a modular way by a specific interaction of the PPR motifs with distinct nucleotide identities. The combinatorial code by which distinct amino acid identities within a repeat interact with certain nucleotide identities has recently been elucidated and lately substantiated by the crystal structure analysis of maize PPR10 together with an 18 nucleotide RNA oligonucleotide representing the recognition site of the PSAJ transcript. According to the initial numbering system amino acids at positions 3, 6 and 1’ (position 1 of the following PPR) are involved in the nucleotide identity-specific interaction.8,10-12 Despite this substantial progress it is still impossible to predict the interaction of PPR proteins with distinct RNA targets.
Proteins containing large arrays of PPR motifs seem to have enzymatic functions only in extra domains as for instance the potential deaminase and/or endonucleolytic activity of the DYW domain of PLS subclass proteins or the putative endonucleolytic activity of the SMR domain found in a few P-class proteins.13-18 But it is assumed that the majority of P-class PPR proteins cooperates with other proteins, which by the nature of their enzymatic activity seem to determine the way in which a given RNA is processed. A general concerted function of P-class PPR proteins and exoribonucleases has been suggested for the post-transcriptional 5′ and 3′ end formation of plastid transcripts. For instance PPR10 binds to specific sites in different RNAs thereby blocking progression of 5′ or 3′ exoribonucleases. Thus the sequence-specific binding to the RNA determines the ends of mature transcripts. This mode of processing and stabilization has been indicated as a general mechanism for chloroplast transcript processing and stability.19,20
Studies in Arabidopsis (Arabidopsis thaliana) showed that a similar mechanism seems to exist in mitochondria. Two enzymes were found to be involved in 3′ to 5′ exonucleolytic trimming of the mature 3′ ends.21,22 The mitochondrial polynucleotide phosphorylase (PNPase) is capable to degrade large parts of the precursor transcript downstream of the mature 3′ terminus, whereas the fine-tuning of the 3′ termini is done by RNase R1 homolog (RNR1), which removes a few nucleotides left over by PNPase. Analogous to the plastid-type transcript processing, the P-class PPR protein MTSF1 binds to the 3′ terminal part of nad4 transcript and it is highly likely that this interaction impedes progression of the 3′ exonucleolytic activities and thus determines the mature 3′ end of this mRNA.23
In contrast to mitochondrial 3′ processing, the post-transcriptional maturation of 5′ ends apparently follows a completely different mode. These ends seem to be generated by an as yet unknown endonucleolytic activity. But similar to the above described exonucleolytic maturation processes, the 5′ processing also involves P-class PPR proteins here called RNA Processing Factors (RPFs). In Arabidopsis, several of these proteins have been identified.24 These include proteins that are similar to restorer of fertility (RF) from other plant species.25-27 RF proteins can rescue cytoplasmic male sterility (CMS), a maternal inherited deficiency to produce or release fertile pollen.28-31 Recently CMS in Arabidopsis has been reported, but a nuclear gene restoring male fertility in this system has not been identified.32 Thus it remains unclear, whether RF-like RPFs indeed function as fertility restorer in this model plant species.
Despite intensive research in recent years, many questions regarding 5′ processing of plant mitochondrial transcripts remained open. Still the molecular function of the individual RPFs is unknown as are the identities of other proteins required for this process. Finally, also the biological importance of post-transcriptional 5′ maturation is still enigmatic since defects in most mitochondrial RPFs did not cause any detrimental effects in the plants. Only the loss of RPF5, a canonical P-class PPR protein involved in processing of nad6 and atp9 mRNAs as well as 26S rRNA interferes with the seed germination.33
To obtain more information about 5′ processing in plant mitochondria, we now used a rare nad2 mRNA polymorphism in Arabidopsis accession Can-0 to identify the gene encoding RPF7. This protein, required for the efficient processing at the -122 5′ terminus of the nad2 mRNA, is a short P-class member, composed of seven canonical P and a single short S motif. A non-sense codon in the penultimate P-class repeat in the RPF7 allele in Can-0 causes a complete defect of the gene demonstrating that the C-terminal repeats are essential for the function of this protein in 5′ processing. A comparative quantitative RT-PCR analysis of nad2 transcripts in PNPase knockdown and rpf7–1 knockout plants suggested that both 5′ and 3′ end processing influences nad2 transcript levels.
Results
In Can-0 nad2 precursor RNAs accumulate to high levels
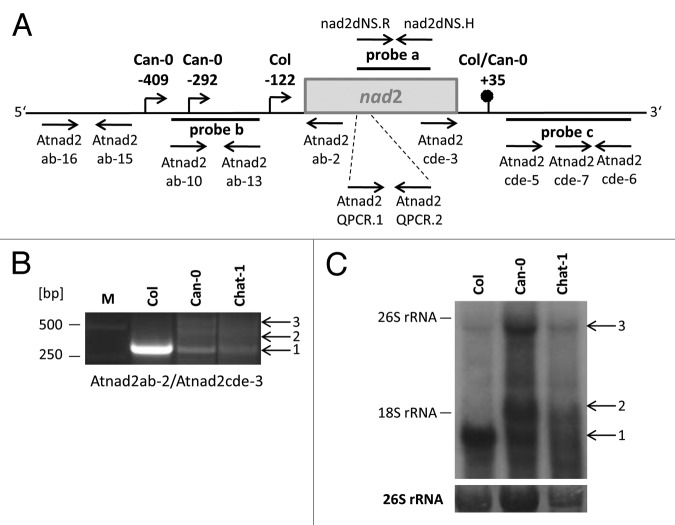
In a previous study of mitochondrial transcripts in different Arabidopsis (Arabidopsis thaliana) accessions, we detected an atypical nad2 RNA pattern in Can-0 (Canary Islands, Spain).34 In contrast to Col (Columbia, USA) and all other accessions tested, RT-PCR of circularized RNA (CR-RT-PCR) indicated a reduction of the mature nad2 transcript species with a 5′ end at position -122 in this accession. At the same time two additional CR-RT-PCR products identified 5′ termini at positions -292 and -409 that were not seen in Col and the other ecotypes (Fig. 1A and B), whereas the 3′ extremities of the nad2 transcripts were found to be identical in Can-0 and the other accessions.34 This result was confirmed by primer extension as well as northern blot analyses, which demonstrated the reduced levels of the mature nad2 mRNA with a length of 1,650 nucleotides (nt) to be accompanied by a comparatively strong accumulation of larger precursor of approximately 1,950 and 3,000 nt (Fig. 1C).34 Furthermore, the nad2 transcript analysis in reciprocal Can-0 x Col and Col x Can-0 F1 and F2 hybrids indicated the biparental inheritance of the abnormal nad2 transcript pattern suggesting this mRNA phenotype to be linked to a nuclear encoded gene, which we designated RNA Processing Factor 7 (RPF7).24,34
Figure 1. In Arabidopsis accessions Can-0 and Chat-1 nad2 precursor RNAs accumulate to higher levels. (A) Schema of the nad2 reading frame (gray box). 5′ (bent arrows) and 3′ ends (pinhead) are indicated, their positions are given relative to the translation start codon (5′ end; NATG, n = -1) or to the translation stop codon (3′ end; TAAN, n = +1). Locations of the northern blot hybridization probes are indicated by black lines. Primers used for nad2 transcript analysis are represented by horizontal arrows. (B) CR-RT-PCR analysis of nad2 RNAs with primer pair Atnad2ab-2/Atnad2cde-3. Accessions are given above the image. Sizes of DNA marker fragments are indicated in the left margin. PCR products are marked on the right hand side (product 1, 340 bp; product 2, 510 bp; product 3, 620 bp). This figure has been assembled from different images. (C) northern blot hybridization with probe a representing parts of the nad2 reading frame. Positions of the cytoplasmic 26S and 18S rRNAs are marked. Stained 26S rRNA was used as loading control (bottom part). Prominent transcripts are marked on the right hand side (transcript 1, ~1,650 nucleotides (nt); transcript 2, ~1,950 nt; transcript 3, ~3,000 nt).
At2g28050 encodes RPF7 required for post-transcriptional nad2 mRNA maturation
To identify the RPF7 gene, we established a Can-0 x Col and Col x Can-0 F2 mapping population of 286 individuals. CR-RT-PCR analysis of the F2 plants identified the Can-0-type nad2 transcripts in 64 plants, while all others exhibit the Col-type pattern (Table 1). A ratio of 1:3.5 (Can-0:Col) demonstrated that the Can-0 nad2 mRNA phenotype is recessive. These numbers also strongly suggested that only a single gene is responsible for the atypical nad2 transcript pattern in Can-0. To map the RPF7 gene, genomic markers (insertion/deletion (indel)) previously established to differentiate between the Ler and Col genotypes,35 were tested for their ability to discriminate between the Can-0 and Col genotypes and were subsequently used to map the RPF7 gene (Table S1). This linkage analysis identified the RPF7 locus on the lower arm of chromosome 2 (Fig. S1A). The use of additional markers mapped the RPF7 locus to a 0.8 Mbp region between positions 11.6 and 12.4 Mbp (Fig. S1B). This chromosome section contains 182 genes including At2g27800 and At2g28050 encoding PPR proteins, which were considered to be the best candidates for RPF7.
Table 1. CR-RT-PCR analysis on nad2 of Can-0 x Col and Col x Can-0 F2 hybrid plants.
| F2 Can-0 x Col | F2 Col x Can-0 | total | |
|---|---|---|---|
|
number of plants with - nad2 Col phenotype - nad2 Can-0 phenotype |
78 30 |
144 34 |
222 64 |
|
percentage - nad2 Col phenotype - nad2 Can-0 phenotype |
72% 28% |
81% 19% |
78% 22% |
| ratio Can-0:Col | 1: 2.6 | 1: 4.3 | 1: 3.5 |
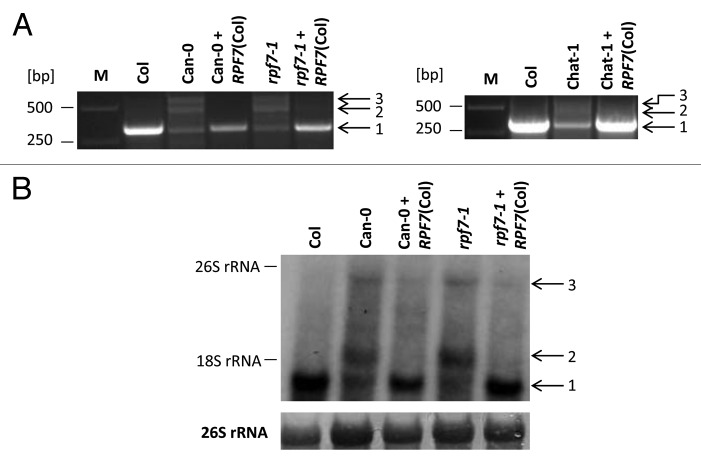
To finally identify RPF7, the Col alleles of each of the two candidate genes including 5′ and 3′ flanking regions were cloned into plant transformation vector pMDC123 and stably introduced into accession Can-0. Several individual lines were selected, tested for the presence of the transgene and investigated by CR-RT-PCR. The examination of three Can-0 plants containing At2g27800 did not reveal any changes in the nad2 mRNA-derived product pattern, excluding this gene to encode RPF7 (Fig. S2). In contrast, CR-RT-PCR and northern blot analyses consistently showed that the introduction of At2g28050 restored nad2 mRNA maturation in Can-0 plants (Fig. 2A, left panel and Figure 2B). To further confirm this result, we examined the mutant SALK_058434 containing a T-DNA insertion in the At2g28050 gene. The localization of the T-DNA within the N-terminal part of the At2g28050 reading frame (at position 499 relative to the translation start codon) provokes a knockout of this gene (Fig. S3). CR-RT-PCR as well as northern blot hybridizations revealed identical product and transcript patterns in this mutant and in Can-0, further corroborating, that At2g28050 encodes RPF7. In addition, the introduction of the Col allele of the At2g28050 gene into this mutant rescued nad2 transcript formation, identical to what was seen upon the integration of this gene into Can-0 (Fig. 2A, left panel and Figure 2B).
Figure 2. The Col allele of At2g28050 (RPF7) restores the efficient 5′ maturation of the nad2 mRNA in Can-0, Chat-1 and in the rpf7–1 mutant (SALK_058434). (A) CR-RT-PCR analysis of nad2 transcripts in Can-0, the rpf7–1 mutant (left panel) and Chat-1 (right panel) and in the same plant lines transformed with the Col allele of RFF7 (+RPF7 (Col)). (B) northern blot hybridization performed with a probe complementary to the sequence of the open reading frame (probe a, indicated in Figure 1). Labeling is identical to Figure 1.
Collectively these results unambiguously show that RPF7 required for maturation of the nad2 transcript is encoded by At2g28050. Accordingly we designated this gene RPF7 and the corresponding mutant rpf7–1 (SALK_058434).
To investigate the potential involvement of RPF7 in additional 5′ processing events all other major mitochondrial transcripts were analyzed by CR-RT-PCR. These experiments did not reveal any difference in the pattern between Col and rpf7–1 suggesting that RPF7 is not involved in additional 5′ maturation reactions affecting known mitochondrial transcripts in Arabidopsis (Fig. S4).
The last PPR motifs are essential for the function of RPF7 in nad2 processing
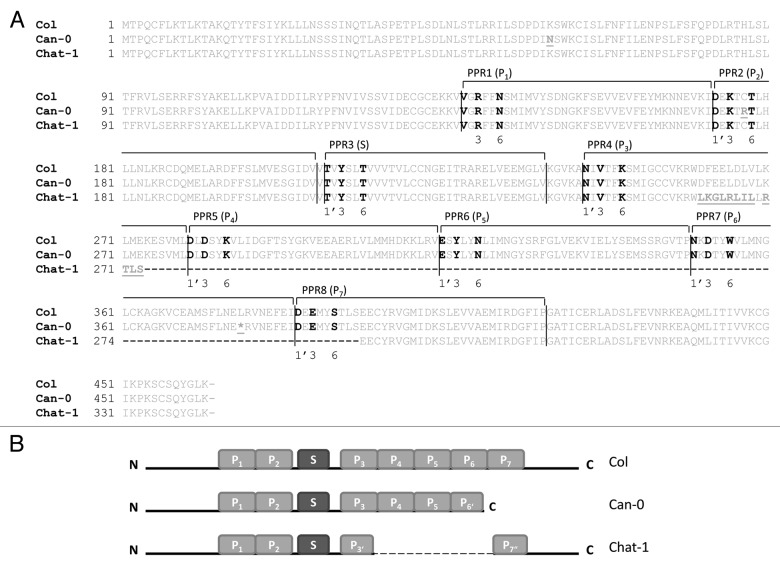
As revealed by the RNA gel blot analysis, the nad2 transcript pattern is identical in Can-0 and the rpf7–1 mutant (Fig. 2B). Since the T-DNA insertion in rpf7–1 is located in sequences coding for PPR motif 1, provoking a complete knockout of the gene (Fig. S3), we analyzed the RPF7 allele in Can-0 to identify the reason for the gene defect. A semi-quantitative RT-PCR (qRT-PCR) analysis revealed that there is no substantial difference between RPF7 transcript accumulation in Col and Can-0 (Fig. S5). The inspection of this gene in the recently published Can-0 sequence identified a thymidine (Col) to adenosine (Can-0) exchange converting a Leu codon into a premature translation stop codon at the end of PPR motif 7 (Fig. 3, P6).36 In addition, a few other differences were observed in comparison to the Col DNA sequence,37 but only two of them cause changes in the amino acid sequences (Fig. 3A, position 60: Lys (Col) ↔ Asn (Can-0) and position 177: Cys (Col) ↔ Arg (Can-0)). To verify the observed discrepancies, we amplified this gene and its 190 and 540 bp 5′ and 3′ flanking regions from Can-0 and sequenced the complete PCR product. This analysis revealed a complete agreement of our sequence with Can-0 reference sequence corroborating the observed nucleotide and amino acid differences. Together with the observation that Can-0 exhibits the same nad2 transcript phenotype as the rpf7–1 knockout mutant these results suggested the amino acid differences and/or the C-terminal truncation to completely abolish the function of the RPF7 allele in Can-0. To collect further information about this protein in Arabidopsis, we inspected RPF7 amino acid sequences from several other accessions (http://signal.salk.edu/atg1001/index.php). This compilation revealed that the RPF7 from Chat-1 (Chateaudun, France) contains several amino acid substitutions in the C-terminal PPR motif (http://1001genomes.org/data/Salk/releases/current/TAIR10/strains/Chat-1). To check the influence of these deviating amino acids on the function of this protein, we used CR-RT-PCR to analyze nad2 transcripts in this accession. This amplification reaction generated a product pattern identical to those found in Can-0 and rpf7–1 suggesting that the RPF7 allele in Chat-1 is not functional, which is confirmed by the rescue of the CR-RT-PCR wild-type pattern upon the introduction of the RPF7 allele from Col into Chat-1 (Fig. 1B Figure 2A, right panel). This result was confirmed by a northern blot hybridization which demonstrated an excessive accumulation of 5′ extended nad2 precursor RNAs consistent with the RNA pattern seen in Can-0 and rpf7–1 (Fig. 1C and Figure 2B). To verify the unusual RPF7 amino acid sequence in Chat-1, we amplified this gene from this ecotype. Sequencing of the resulting product did not confirm the reported amino acid exchanges instead revealed a large deletion within the RFP7 gene in Chat-1. This allele encodes only truncated repeats P3 and P7 and completely lacks PPR motifs P4 to P6 demonstrating that this RPF7 allele is defective (Fig. 3). Considering the nad2 transcript pattern in rpf7–1 and Chat-1, which both harbor RPF7 knockout alleles, and the nad2 RNA pattern in Can-0, we concluded that the latter accession also encodes a non-functional RPF7 gene, here caused by the presence of a premature translation stop codon within PPR motif P6. In summary these results strongly suggest that PPR motifs P6 and P7 are critical for RPF7 function in nad2 transcript maturation.
Figure 3. Sequences and structures of RPF7 proteins from different Arabidopsis accessions. (A) Amino acid sequences are given in the one letter code. Divergent amino acids are given in bold and underlined letters. A premature translation stop codon is represented by an asterisk (*). The deletion in Chat-1 is indicated by a dashed line. Crucial positions (3, 6 and 1’) are given in black. The pentatricopeptide repeats are given according to a previous study above the sequence.7 (B) Cartoons demonstrating the structures of the distinct RPF7 proteins from the different accessions (indicated on the right hand side). Light gray boxes represent canonical P motifs (P1–7) whereas an S repeat is given as dark gray box. Incomplete motifs are marked with one (C-terminal truncated: P3‘,6‘) or two primes (N-terminal truncated: P7“).
5′ unprocessed nad2 precursor transcripts over-accumulate in plants with severely reduced levels of Polynucleotide Phosphorylase (PNPase)
Although 5′ maturation of mitochondrial transcripts is frequently observed, virtually nothing is known about the relevance of this process in higher plants. Previous studies of RPF5 required for 5′ maturation of 26S rRNA as well as nad6 and atp9 mRNAs, revealed that the knockout of this gene leads to an accumulation of nad6 precursor RNAs while both mature and 5′ un-processed 26S rRNA are substantially reduced.33 This observation indicated that the 5′ extended 26S rRNA precursors are less stable than the 5′ mature RNA species suggesting that 5′ processing is a prerequisite for adequate accumulation of the mature 26S rRNA species. In plant mitochondria, PNPase, a 3′ to 5′ exoribonuclease, is involved in the generation of mature 3′ termini of transcripts such as atp9 mRNA but is also required for the removal of RNAs from the total mitochondrial transcript pool.21,22
To check whether there is any connection between 5′ processing and the degradation of non-processed RNAs by PNPase, we established a transgenic Arabidopsis line expressing an artifical microRNA (amiRNA) targeting the corresponding mRNA (amiR-PNP-3, Fig. S6A). AmiR-PNP-3 was directed to the PNPase mRNA segment coding for the K homology (KH) RNA binding and recognition domain and substantially reduced the target RNA to about 12% of wild-type level (Fig. S6B). These plants were characterized by a severely retarded growth and development as well as by undulated leaves (Fig. S7). Since previously established PNPase co-suppression lines were found to be impaired in atp9 mRNA 3′ processing,21,22 we analyzed this transcript in the amiR-PNP-3 plants. Both CR-RT-PCR and RT-PCR indicated excessive amounts of 3′ extended atp9 transcripts in PNPase knockdown plants (Fig. S8), which is consistent with previous reports.21,22 These results demonstrated that the knockdown of PNPase in the amiR-PNP-3 plants provokes severely impaired 3′ processing of atp9 mRNAs.
To check the influence of the PNPase knockdown on nad2 RNAs, we performed northern blot analyses of amiR-PNP-3, Col wild-type, Can-0 wild-type, rpf7–1 plants, as well as Can-0 wild-type and rpf7–1 lines complemented with the RPF7 allele from Col (Fig. 4). A probe binding to the nad2 reading frame (Fig. 1, probe a) detected the mature nad2 mRNA in Col as well as in Can-0 and rpf7–1 containing the intact RPF7 allele from Col. Low levels of mature transcript species were also seen in Can-0 and rpf7–1 (Fig. 4A, transcript 1). A transcript of this size (~1,650 nt) is also seen in the amiR-PNP-3 plant, where also larger precursor molecules were present, of which the 3,000 nt large molecules seem to be identical with those seen in Can-0 and rpf7–1 (Fig. 4A, transcript 3). To the contrary, the approximately 1,950 nt large transcript was not detected in the amiR-PNP-3 plant but was seen both in Can-0 and rpf7–1 (Fig. 4A, transcript 2). In addition, RNA species smaller than the mature transcript were detected in all samples, but were particularly strong in the amiR-PNP-3 sample. A hybridization with a probe binding to regions upstream of the -122 5′ end (Fig. 1, probe b) revealed only very weak signals corresponding to larger precursor molecules in samples from plants containing an intact RPF7 allele from Col (Fig. 4B, Col, Can-0 + RPF7 (Col), rpf7–1 + RPF7 (Col)) whereas stronger signals were seen in samples derived from plants with defective RPF7 genes (Fig. 4B, Can-0 and rpf7–1). Strongest signals were observed in the RNA sample from the amiR-PNP-3 plant (Fig. 4B, amiR-PNP-3). These signals indicate a comparatively strong accumulation of 5′ un-processed nad2 transcripts, most of them being larger precursor molecules identical to those detected with probe a. However, probe b detected also RNA smaller than the mature nad2 mRNA (Fig. 4B, transcripts 4 and 5).
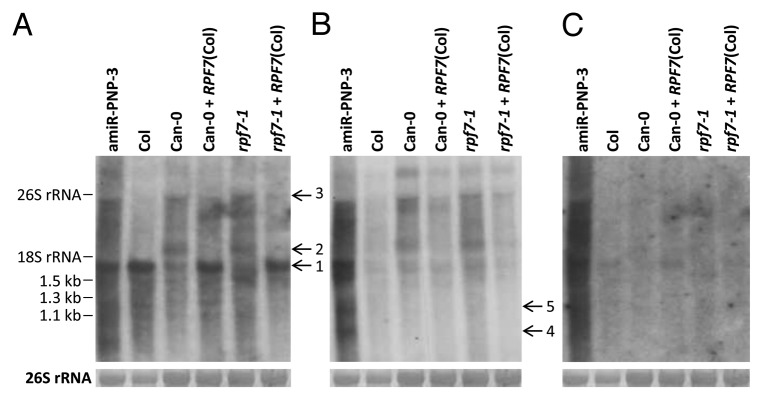
Figure 4. Accumulation of large nad2 transcripts in amiR-PNP-3 plants. Northern blot analyses of PNPase knockdown, Col wild-type, Can-0 wild-type, rpf7–1 plants, as well as Can-0 wild-type and rpf7–1 lines complemented with the RPF7 allele from Col (+RPF7 (Col)). Probes used in these analyses are indicated in Figure 1 and represent the nad2 reading frame (A), sequences upstream of the mature 5′ end (-122) (B) and the region downstream of the mature 3′ terminus (C). All hybridizations were done with the same membrane. Labeling corresponds to Figure 1. Loading of the RNA gels was checked by a methylene blue staining of cytoplasmic rRNAs on the membrane (26S rRNA).
When the same RNA samples were hybridized with probe c corresponding to sequences downstream of the mature 3′ end at position +35 (Fig. 1), signals were only seen in the PNPase knockdown plant (Fig. 4C). Thus 3′ extended precursor molecules accumulate only in amiR-PNP-3 plants, whereas no such RNAs were detectable in the other plants independent of the presence or absence of a functional RPF7 allele.
The differential accumulation of nad2 RNAs was also addressed by Real-Time qRT-PCR. A PCR amplifying sequences in the 5′ terminal half of the nad2 mRNA revealed a decrease of nad2 transcript species both in rpf7–1 (x 0.8) and amiR-PNP-3 (x 0.5) plants (Fig. 5A). In contrast, an amplification reaction covering the 5′ region revealed that in both rpf7–1 (x 1.6) and amiR-PNP-3 (x 1.7) plants 5′ extended precursors accumulate to levels approximately 2-fold higher than in wild type (Fig. 5B), which was consistent with the results of the northern experiments (Fig. 4B). On the contrary, an analogous investigation of nad2 3′ extended precursor RNAs detected an increase only in the PNPase knockdown line (x 21.9), whereas the level of such RNAs were found to be reduced to about 70% of wild-type levels in the rpf7–1 mutant (Fig. 5C). Likewise, this result was consistent with the northern blot hybridization albeit the reduced RNA levels in rpf7–1 could not be seen in this experiment (Figs. 4C and 5C).
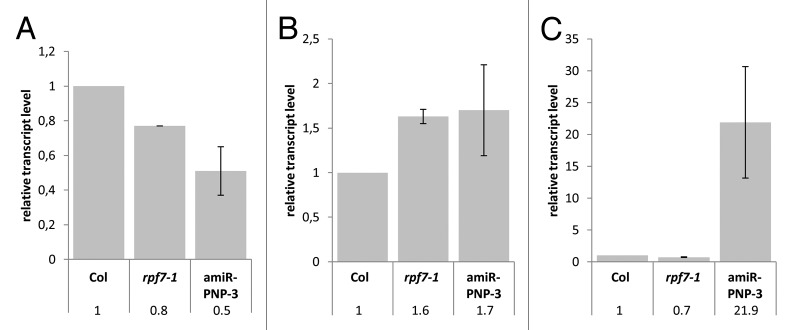
Figure 5. Quantitative analysis of nad2 transcripts in distinct plant lines. Real-Time qRT-PCR of nad2 RNAs covering the reading frame (A), 5′ region (B) and 3′ region (C). The nad2 transcript levels are given relative to the level in Col wild type, which was arbitrarily set to 1.
In summary, these results demonstrate that 5′ un-processed nad2 precursor transcripts accumulate in plants with defective RPF7 alleles. Likewise 5′ extended precursors accumulate in PNPase knockdown plants demonstrating that this enzyme eliminates such RNAs from the steady-state RNA pool in mitochondria. Consistent with previous observations, a strong over-accumulation was seen for 3′ extended precursors upon the knockdown of PNPase confirming the importance of this enzyme in 3′ processing. Interestingly, a slight reduction of 3′ un-processed nad2 transcripts was found in the rpf7–1 mutant, suggesting that impaired 5′ processing might additionally destabilize RNA molecules.
Discussion
At2g28050 encodes RPF7 essential for efficient 5′ end formation of nad2 transcripts
In Arabidopsis a number of RPFs have been described. These factors, exclusively P-class PPR proteins comprising 14 PPR motifs or more, are required for efficient 5′ processing of the major transcript species transcribed from one, two or three different mitochondrial genes,25-27,33 but as found recently by the analysis of the Restorer of Fertility-like PPR protein 9 (RFL9), are also involved in 5′ processing of rare RNA molecules in particular ecotypes.38 The relatively high number of PPR motifs present in these proteins allowed to predict the putative binding sites on the RNA targets, which is exclusively found upstream of the 5′ end of the mature transcript.24 Now we identified the gene At2g28050 encoding RPF7. Several lines of evidence support that this protein is required for efficient post-transcriptional generation of the -122 5′ end of the major nad2 mRNA. First, CR-RT-PCR, northern and Real-Time qRT-PCR analyses demonstrated that 5′ extended nad2 precursor RNAs over-accumulate in Can-0, Chat-1 and rpf7–1, all of them lines with defective RPF7 alleles (Figs. 1, 2, 4 and 5). Second, unlike precursor molecules, mature nad2 mRNA levels are severely reduced in theses plant lines, as found by CR-RT-PCR and northern blot experiments (Figs. 1, 2 and 4). Third, the introduction of the functional RPF7 allele from Col into Can-0, Chat-1 and rpf7–1 restored nad2 processing identical to what is seen in Col wild-type plants (Fig. 2). Collectively these data provide clear evidence for the important role of RPF7 for 5′ maturation of the major nad2 mRNA. A comprehensive CR-RT-PCR screening further suggests that RPF7 is exclusively involved in nad2 mRNA processing and that it plays no substantial role in 5′ or 3′ end maturation of any other known mitochondrial transcript (Fig. S4). This might be also true for plastid-located transcripts. A previous study found that the putative targeting peptide of RPF7 fused to the green fluorescent protein directs this chimeric polypeptide to mitochondria, strongly suggesting that the genuine RPF7 is exclusively imported into these organelles in vivo.4 However, experiments with the full-length RPF7:GFP fusions were not successful, so that additional subcellular localizations are unlikely but cannot be ultimately excluded.
Is another PPR protein required for nad2 processing?
In addition to RPF5, RPF7 is the second regular P-class PPR protein involved in 5′ processing that does not belong to the clade of RF-like proteins. But it is the first protein of this type that is identified on the basis of natural genetic variation. In contrast to previously described mitochondrial RNA polymorphisms, variation of nad2 transcript sizes is a relatively rare event among ecotypes. Originally identified in a single ecotype (Can-0),34 this polymorphism was now found also in Chat-1 and data from the 1001 genome project suggest that a deletion similar to the one found in Chat-1 might also inactivate RPF7 in the ecotype Utrecht. These data demonstrate, that the screening of 5′ and 3′ ends of mitochondrial transcripts in many more ecotypes will identify further processing factors involved in mitochondrial 5′ processing and might eventually also reveal other non-PPR proteins involved in 5′ processing.
Unlike previously characterized RPFs, RPF7 is a rather small protein (calculated molecular mass 52.9 kDa) comprising only seven canonical P repeats and a single S repeat. Thus the binding site of this protein is clearly smaller than 12 nucleotides or more, which are usually bound by a PPR protein.8 Therefore it is highly likely that this protein exhibits a rather relaxed specificity in its binding to the target RNA. In line with this, it is not feasible to predict the binding site of RPF7 on the nad2 transcript, as it was done for other RPFs.24 Nevertheless our analysis revealed that the C-terminal repeats are essential for function, strongly suggesting that they are involved in the sequence-specific RNA-protein interaction. A defect in this gene results in a specific reduction of nad2 5′ processing efficiency (Figs. 1, 2 and 4), whereas all other major mitochondrial transcripts are not affected (Fig. S4). This is somewhat similar to THA8, a small protein with only four PPR motifs. This polypeptide also exhibits a very relaxed specificity predominantly binding to G-A-rich sequences with only specifically contacting a G residue, however, splicing of only two distinct introns is affected by the knockout of this protein.39,40
We assume that an additional PPR protein is required to efficiently define the cleavage site. This assumption is supported by the relatively high levels of mature nad2 mRNA in RPF7-defective plant lines, where this transcript was still detectable by northern blot hybridization (Fig. 1) and where levels of this transcript are above the usually observed residual amounts. A similar situation has been observed for the function of RPF5 in 5′ processing of atp9 mRNAs. Here the knockout of RPF5 leads to a moderate increase of atp9 precursor RNAs, while the level of the mature transcript apparently remains unaffected. Thus RPF5 influences only 5′ processing efficiency and it seems clear that another processing factor is required for 5′ processing of atp9 transcripts.33
5′ un-processed transcripts are substrates for PNPase
Although 5′ processing is required for mRNA maturation of many mitochondrial genes the function and biological relevance of this process is still unclear.24 The knockout of most RPFs did not have any detrimental effects on plant fitness and only the inactivation of RPF5 seems to have a negative influence on seed germination.24 The absence of this factor, which is also required for 26S rRNA processing, leads to a severe decrease of total 26S rRNAs, most of them being 5′ un-processed. This observation indicated that 5′ processing influences the stability of the 26S rRNA. Thus we established plants, in which the expression of PNPase is strongly decreased by the function of an amiRNA, which reduced the PNPase mRNA to about 12% of wild-type level (Fig. S6B). Consistent with previous results obtained by PNPase co-suppression lines, 3′ processing of atp9 RNAs is impaired demonstrating the reduced function of PNPase in the knockdown plants (Fig. S8).21 In addition, both the co-suppression and amiR-PNP-3 lines showed a severely retarded growth and development as well as undulated or curled leaves (Fig. S7). But in contrast to the co-suppression plants, the amiR-PNP-3 plants flowered and set seeds, which facilitates the cultivation and investigation of these lines.
As expected, 3′ extended nad2 precursor RNAs over-accumulate in the PNPase knockdown plants (Fig. 5C, x 21.9). But also 5′ extended nad2 precursor transcripts were found to be increased in these plants, albeit to a lesser extent (Fig. 5B, x 1.7). This increase is in the same range as in the rpf7–1 line (Fig. 5B, x 1.6). Interestingly, 3′ extended nad2 transcripts are even found to be reduced in the rpf7–1 mutant in comparison to wild type (Fig. 5C, x 0.7). This observation suggests that severely reduced 5′ processing of nad2 transcripts interferes with the stability of these RNA similar to what have been observed for 26S rRNA.33 These observations raise the possibility that 5′ processing somehow interferes with transcript stability, but still it remains unclear whether the reduced stability of 5′ un-processed transcripts is a general phenomenon in plant mitochondria. Furthermore, the amplification reaction directed to the nad2 reading frame revealed a total decrease of nad2 transcripts both in rpf7–1 and PNPase knockdown plants (Fig. 5A, x 0.8 and 0.5). In case of rpf7–1, this is clearly consistent with northern analysis (Fig. 4A). And indeed the northern data also indicated the mature nad2 mRNA level to be reduced in PNPase knockdown plants, however, there seemed to be a lot of other nad2 transcripts to be detected, which might not be covered by the Real-Time qRT-PCR (Fig. 4A). Unfortunately attempts to establish a substantial knockdown PNPase in the rpf7–1 background have not yet been successful, which prevented to investigate the simultaneous influence of both 5′ and 3′ processing transcript levels.
To address this potential biological relevance of 5′ processing further studies of many more mitochondrial transcripts are required and the now established PNPase knockdown line will be a convenient tool for such a comprehensive study.
Material and Methods
Plant material
Arabidopsis accessions, T-DNA insertion lines or F1 hybrids are either obtained from Rhonda Meyer (IPK, Gatersleben) or The European Arabidopsis Stock Centre (NASC, http: http://arabidopsis.info). Arabidopsis plants were cultivated in a growth chamber as described.33 Crossings (Can-0 x Col) were done following an established protocol.41
RNA and DNA analyses
Total cellular RNA was purified from about 28 d-old Arabidopsis plants as reported previously.26,27 CR-RT-PCR experiments were done according to established protocols.34 northern blot analyses were performed with Biodyne A nylon membranes according to protocols given by the manufacturer (Pall Corporation, http://www.pall.com). Probes for hybridization were amplified with primer pairs nad2dNS.R/nad2dNS.H (nad2 orf = probe a), Atnad2ab-10/Atnad2ab-13 (nad2 upstream = probe b) and Atnad2cde-5/ Atnad2cde-6 (nad2 downstream probe c) and labeled applying the Rediprime II DNA Labeling System (GE Healthcare, http://www3.gehealthcare.de) and 50 µCi α32P-dCTP. The approximate locations and sizes of these probes are given in Figure 1A. Loading of the RNA gels was monitored by methylene blue staining of the membrane. Semi-qRT-PCR was done according to standard protocols.42 Real-Time qRT-PCR was performed as described previously.33 Primers used to determine PNPase transcript levels are AtmtPNPaseQPCR.1 and AtmtPNPaseQPCR.2. For the analysis of nad2 transcripts the following primer pairs were used: Atnad2QPCR.1/Atnad2QPCR.2 (reading frame), Atnad2ab-15/Atnad2ab-16 (5′ region) and Atnad2cde-6/Atnad2cde-7 (3′ region). QPCR analyses were done in four technical replicates (PNPase) from at least two independent biological replicates (nad2). Oligonucleotide sequences are listed in Table S2.
Total DNA was extracted from Arabidopsis plants following an earlier reported protocol.43 DNA sequencing was performed commercially (4baselab, http://www.4base-lab.de/index_4.html, and GATC-biotech, http://www.gatc-biotech.com/de/index.html). All other procedures for the investigation of nucleic acids were performed following standard protocols.42
Complementation experiments
The genes encoding RPF7 and At2g27800, respectively, were amplified from total DNA extracted from accession Col with primer pairs At2g28050_kompl.H2/At2g28050_kompl.R and At2g27800_kompl.H/At2g27800_kompl.R. The corresponding DNA products included 190 and 540 bp (RPF7) or 355 and 502 bp (At2g27800) 5′ and 3′ flanking sequences, respectively. The corresponding PCR product was cloned into pMDC123 via SgsI and PacI restriction sites.44 To exclude negative effects arising from potential amplification errors, a mixture of different clones of the complementation construct was stably introduced into different Arabidopsis lines via floral dip.45 Transformants were selected by their resistance to Basta and tested for the presence of the transgene using standard PCR.
Artificial microRNA constructs
Cloning of artificial micro RNAs (amiRNAs) targeting mitochondrial PNPase (At5g14580) constructs followed an approach applying overlap PCRs with vector pRS300 containing the miRNA319a backbone and the primers listed in Table S2.46 The PCR products representing the amiRNA gene were then cloned into pMDC123 vectors containing a CaMV 35S promoter and NOS terminator using the SmaI/EcoRV and SacI restriction sites. These constructs were checked by sequence analysis and introduced into Arabidopsis Col plants as mentioned in the previous section.
In silico studies
DNA sequence analyses were done using multiple Blast tools on the NCBI server.47 The different RPF7 alleles were inspected on http://1001genomes.org/accessions.html). Amino acid sequences were aligned using ClustalW.48
Supplementary Material
Acknowledgments
We thank Uli Tengler and Conny Guha for perfect technical support. We are also very grateful to Rhonda Meyer for the kind gift of Arabidopsis accessions as well as of Col x Can-0 F1 hybrids and to Joachim Forner for providing vector pRS300. This work was supported by the Deutsche Forschungsgemeinschaft (Bi-590/10–2).
References
- 1.Small ID, Peeters N. The PPR motif - a TPR-related motif prevalent in plant organellar proteins. Trends Biochem Sci. 2000;25:46–7. doi: 10.1016/S0968-0004(99)01520-0. [DOI] [PubMed] [Google Scholar]
- 2.Aubourg S, Boudet N, Kreis M, Lecharny A. In Arabidopsis thaliana, 1% of the genome codes for a novel protein family unique to plants. Plant Mol Biol. 2000;42:603–13. doi: 10.1023/A:1006352315928. [DOI] [PubMed] [Google Scholar]
- 3.Schmitz-Linneweber C, Small I. Pentatricopeptide repeat proteins: a socket set for organelle gene expression. Trends Plant Sci. 2008;13:663–70. doi: 10.1016/j.tplants.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Colcombet J, Lopez-Obando M, Heurtevin L, Bernard C, Martin K, Berthomé R, Lurin C. Systematic study of subcellular localization of Arabidopsis PPR proteins confirms a massive targeting to organelles. RNA Biol. 2013;10:1557–75. doi: 10.4161/rna.26128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakamura T, Yagi Y, Kobayashi K. Mechanistic insight into pentatricopeptide repeat proteins as sequence-specific RNA-binding proteins for organellar RNAs in plants. Plant Cell Physiol. 2012;53:1171–9. doi: 10.1093/pcp/pcs069. [DOI] [PubMed] [Google Scholar]
- 6.Yagi Y, Tachikawa M, Noguchi H, Satoh S, Obokata J, Nakamura T. Pentatricopeptide repeat proteins involved in plant organellar RNA editing. RNA Biol. 2013;10:1419–25. doi: 10.4161/rna.24908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lurin C, Andrés C, Aubourg S, Bellaoui M, Bitton F, Bruyère C, Caboche M, Debast C, Gualberto J, Hoffmann B, et al. Genome-wide analysis of Arabidopsis pentatricopeptide repeat proteins reveals their essential role in organelle biogenesis. Plant Cell. 2004;16:2089–103. doi: 10.1105/tpc.104.022236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barkan A, Small I. Pentatricopeptide repeat proteins in plants. Annu Rev Plant Biol. 2014;65:415–42. doi: 10.1146/annurev-arplant-050213-040159. [DOI] [PubMed] [Google Scholar]
- 9.Delannoy E, Stanley WA, Bond CS, Small ID. Pentatricopeptide repeat (PPR) proteins as sequence-specificity factors in post-transcriptional processes in organelles. Biochem Soc Trans. 2007;35:1643–7. doi: 10.1042/BST0351643. [DOI] [PubMed] [Google Scholar]
- 10.Barkan A, Rojas M, Fujii S, Yap A, Chong YS, Bond CS, Small I. A combinatorial amino acid code for RNA recognition by pentatricopeptide repeat proteins. PLoS Genet. 2012;8:e1002910. doi: 10.1371/journal.pgen.1002910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yagi Y, Hayashi S, Kobayashi K, Hirayama T, Nakamura T. Elucidation of the RNA recognition code for pentatricopeptide repeat proteins involved in organelle RNA editing in plants. PLoS One. 2013;8:e57286. doi: 10.1371/journal.pone.0057286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yin P, Li Q, Yan C, Liu Y, Liu J, Yu F, Wang Z, Long J, He J, Wang HW, et al. Structural basis for the modular recognition of single-stranded RNA by PPR proteins. Nature. 2013;504:168–71. doi: 10.1038/nature12651. [DOI] [PubMed] [Google Scholar]
- 13.Chateigner-Boutin AL, Small I. Plant RNA editing. RNA Biol. 2010;7:213–9. doi: 10.4161/rna.7.2.11343. [DOI] [PubMed] [Google Scholar]
- 14.Chateigner-Boutin AL, Small I. Organellar RNA editing. Wiley Interdiscip Rev RNA. 2011;2:493–506. doi: 10.1002/wrna.72. [DOI] [PubMed] [Google Scholar]
- 15.Fujii S, Small I. The evolution of RNA editing and pentatricopeptide repeat genes. New Phytol. 2011;191:37–47. doi: 10.1111/j.1469-8137.2011.03746.x. [DOI] [PubMed] [Google Scholar]
- 16.Liu S, Melonek J, Boykin LM, Small I, Howell KA. PPR-SMRs: ancient proteins with enigmatic functions. RNA Biol. 2013;10:1501–10. doi: 10.4161/rna.26172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okuda K, Chateigner-Boutin AL, Nakamura T, Delannoy E, Sugita M, Myouga F, Motohashi R, Shinozaki K, Small I, Shikanai T. Pentatricopeptide repeat proteins with the DYW motif have distinct molecular functions in RNA editing and RNA cleavage in Arabidopsis chloroplasts. Plant Cell. 2009;21:146–56. doi: 10.1105/tpc.108.064667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashimoto M, Endo T, Peltier G, Tasaka M, Shikanai T. A nucleus-encoded factor, CRR2, is essential for the expression of chloroplast ndhB in Arabidopsis. Plant J. 2003;36:541–9. doi: 10.1046/j.1365-313X.2003.01900.x. [DOI] [PubMed] [Google Scholar]
- 19.Pfalz J, Bayraktar OA, Prikryl J, Barkan A. Site-specific binding of a PPR protein defines and stabilizes 5′ and 3′ mRNA termini in chloroplasts. EMBO J. 2009;28:2042–52. doi: 10.1038/emboj.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prikryl J, Rojas M, Schuster G, Barkan A. Mechanism of RNA stabilization and translational activation by a pentatricopeptide repeat protein. Proc Natl Acad Sci U S A. 2011;108:415–20. doi: 10.1073/pnas.1012076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perrin R, Lange H, Grienenberger JM, Gagliardi D. AtmtPNPase is required for multiple aspects of the 18S rRNA metabolism in Arabidopsis thaliana mitochondria. Nucleic Acids Res. 2004;32:5174–82. doi: 10.1093/nar/gkh852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perrin R, Meyer EH, Zaepfel M, Kim YJ, Mache R, Grienenberger JM, Gualberto JM, Gagliardi D. Two exoribonucleases act sequentially to process mature 3′-ends of atp9 mRNAs in Arabidopsis mitochondria. J Biol Chem. 2004;279:25440–6. doi: 10.1074/jbc.M401182200. [DOI] [PubMed] [Google Scholar]
- 23.Haïli N, Arnal N, Quadrado M, Amiar S, Tcherkez G, Dahan J, Briozzo P, Colas des Francs-Small C, Vrielynck N, Mireau H. The pentatricopeptide repeat MTSF1 protein stabilizes the nad4 mRNA in Arabidopsis mitochondria. Nucleic Acids Res. 2013;41:6650–63. doi: 10.1093/nar/gkt337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Binder S, Stoll K, Stoll B. P-class pentatricopeptide repeat proteins are required for efficient 5′ end formation of plant mitochondrial transcripts. RNA Biol. 2013;10:1511–9. doi: 10.4161/rna.26129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hölzle A, Jonietz C, Törjek O, Altmann T, Binder S, Forner J. A RESTORER OF FERTILITY-like PPR gene is required for 5′-end processing of the nad4 mRNA in mitochondria of Arabidopsis thaliana. Plant J. 2011;65:737–44. doi: 10.1111/j.1365-313X.2010.04460.x. [DOI] [PubMed] [Google Scholar]
- 26.Jonietz C, Forner J, Hildebrandt T, Binder S. RNA PROCESSING FACTOR3 is crucial for the accumulation of mature ccmC transcripts in mitochondria of Arabidopsis accession Columbia. Plant Physiol. 2011;157:1430–9. doi: 10.1104/pp.111.181552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jonietz C, Forner J, Hölzle A, Thuss S, Binder S. RNA PROCESSING FACTOR2 is required for 5′ end processing of nad9 and cox3 mRNAs in mitochondria of Arabidopsis thaliana. Plant Cell. 2010;22:443–53. doi: 10.1105/tpc.109.066944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Budar F, Pelletier G. Male sterility in plants: occurrence, determinism, significance and use. C R Acad Sci III. 2001;324:543–50. doi: 10.1016/S0764-4469(01)01324-5. [DOI] [PubMed] [Google Scholar]
- 29.Chase CD. Cytoplasmic male sterility: a window to the world of plant mitochondrial-nuclear interactions. Trends Genet. 2007;23:81–90. doi: 10.1016/j.tig.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Hanson MR, Bentolila S. Interactions of mitochondrial and nuclear genes that affect male gametophyte development. Plant Cell. 2004;16(Suppl):S154–69. doi: 10.1105/tpc.015966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujii S, Toriyama K. Genome barriers between nuclei and mitochondria exemplified by cytoplasmic male sterility. Plant Cell Physiol. 2008;49:1484–94. doi: 10.1093/pcp/pcn102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gobron N, Waszczak C, Simon M, Hiard S, Boivin S, Charif D, Ducamp A, Wenes E, Budar F. A cryptic cytoplasmic male sterility unveils a possible gynodioecious past for Arabidopsis thaliana. PLoS One. 2013;8:e62450. doi: 10.1371/journal.pone.0062450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hauler A, Jonietz C, Stoll B, Stoll K, Braun H-P, Binder S. RNA Processing Factor 5 is required for efficient 5′ cleavage at a processing site conserved in RNAs of three different mitochondrial genes in Arabidopsis thaliana. Plant J. 2013;74:593–604. doi: 10.1111/tpj.12143. [DOI] [PubMed] [Google Scholar]
- 34.Stoll B, Stoll K, Steinhilber J, Jonietz C, Binder S. Mitochondrial transcript length polymorphisms are a widespread phenomenon in Arabidopsis thaliana. Plant Mol Biol. 2013;81:221–33. doi: 10.1007/s11103-012-9993-z. [DOI] [PubMed] [Google Scholar]
- 35.Jander G, Norris SR, Rounsley SD, Bush DF, Levin IM, Last RL. Arabidopsis map-based cloning in the post-genome era. Plant Physiol. 2002;129:440–50. doi: 10.1104/pp.003533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gan X, Stegle O, Behr J, Steffen JG, Drewe P, Hildebrand KL, Lyngsoe R, Schultheiss SJ, Osborne EJ, Sreedharan VT, et al. Multiple reference genomes and transcriptomes for Arabidopsis thaliana. Nature. 2011;477:419–23. doi: 10.1038/nature10414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arabidopsis Genome Initiative Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature. 2000;408:796–815. doi: 10.1038/35048692. [DOI] [PubMed] [Google Scholar]
- 38.Arnal N, Quadrado M, Simon M, Mireau H. A restorer-of-fertility like pentatricopeptide repeat gene directs ribonucleolytic processing within the coding sequence of rps3-rpl16 and orf240a mitochondrial transcripts in Arabidopsis thaliana. Plant J. 2014;78:134–45. doi: 10.1111/tpj.12463. [DOI] [PubMed] [Google Scholar]
- 39.Ke J, Chen RZ, Ban T, Zhou XE, Gu X, Tan MH, Chen C, Kang Y, Brunzelle JS, Zhu JK, et al. Structural basis for RNA recognition by a dimeric PPR-protein complex. Nat Struct Mol Biol. 2013;20:1377–82. doi: 10.1038/nsmb.2710. [DOI] [PubMed] [Google Scholar]
- 40.Khrouchtchova A, Monde RA, Barkan A. A short PPR protein required for the splicing of specific group II introns in angiosperm chloroplasts. RNA. 2012;18:1197–209. doi: 10.1261/rna.032623.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weigel D, Glazebrook J. Arabidopsis, a laboratory manual. Cold Spring Habor, New York: Cold Spring Harbor Laboratory Press, 2002. [Google Scholar]
- 42.Sambrook J, Russel DW. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 2001. [Google Scholar]
- 43.Edwards K, Johnstone C, Thompson C. A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res. 1991;19:1349. doi: 10.1093/nar/19.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Curtis MD, Grossniklaus U. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol. 2003;133:462–9. doi: 10.1104/pp.103.027979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–43. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 46.Ossowski S, Schwab R, Weigel D. Gene silencing in plants using artificial microRNAs and other small RNAs. Plant J. 2008;53:674–90. doi: 10.1111/j.1365-313X.2007.03328.x. [DOI] [PubMed] [Google Scholar]
- 47.McGinnis S, Madden TL. BLAST: at the core of a powerful and diverse set of sequence analysis tools. Nucleic Acids Res. 2004;32:W20-5. doi: 10.1093/nar/gkh435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–8. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.