Abstract
Psychological stress caused by everyday life style contributes to health disparities experience by individuals. It affects many biomarkers, but cortisol “a steroid hormone” is known as a potential biomarker for psychological stress detection. Abnormal levels of cortisol, is indicative of conditions such as Cushing’s syndrome Addison’s disease, adrenal insufficiencies and more recently post-traumatic stress disorder (PTSD). Chromatographic techniques, which are traditionally used to detect cortisol, are a complex system requiring multistep extraction/purification. This limits its application for point-of-care (POC) detection of cortisol. However, electrochemical immunosensing of cortisol is a recent advancement towards POC application. This review highlights simple, low-cost, and label-free electrochemical immunosensing platforms which have been developed recently for sensitive and selective detection of cortisol in biofluids. Electrochemical detection is utilized for the detection of cortisol using Anti-Cortisol antibodies (Anti-Cab) covalently immobilized on nanostructures such as self-assembled monolayer (SAM), polymer composite, etc. for POC integration of sensors. The observed information can be used as prototype to understand behavioral changes in humans in case to case such as farmers, fire fighters, etc. Keeping the future directions and challenges in mind the focus of the BioMEMS and Microsystems Research Group at Florida International University is on development of POC devices for immunosensing, integration of these devices with microfluidics, cross validation with existing technologies, and analysis of real sample.
Keywords: Psychological Stress, Cortisol, Electrochemical Immunosensing, Point-of-Care Application
Introduction
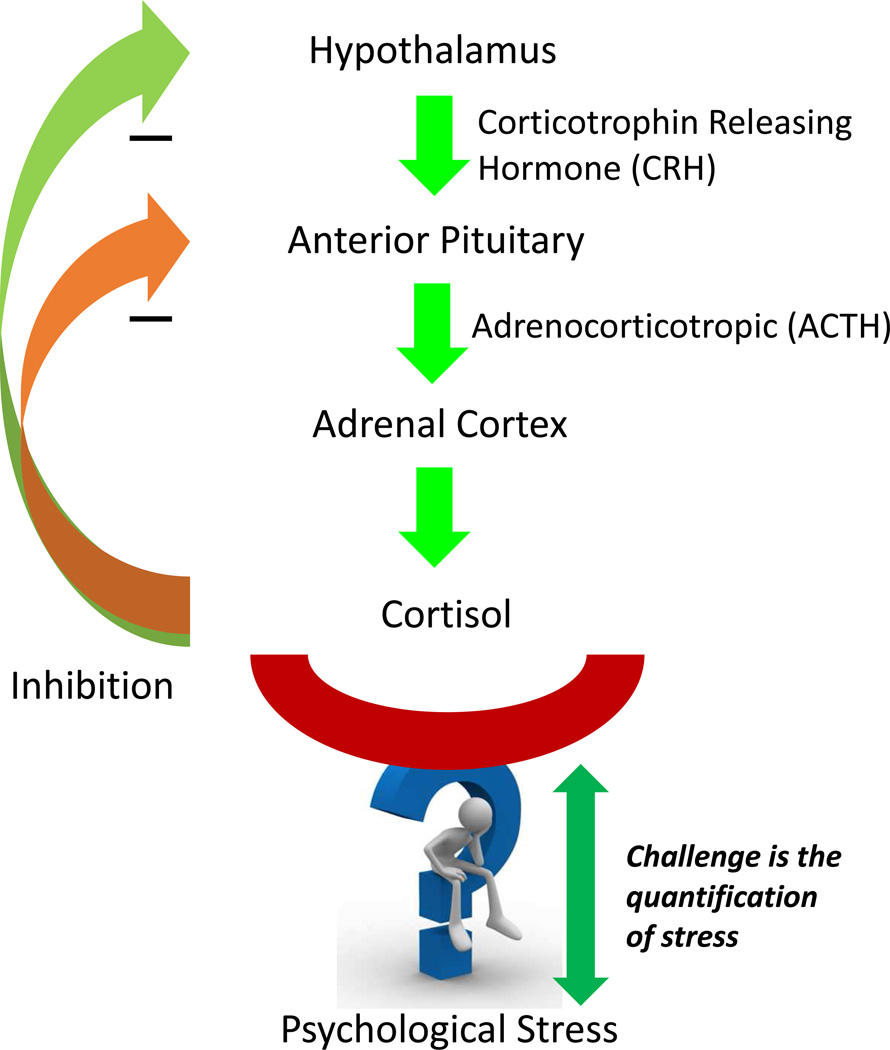
A life sustaining adrenal hormone, cortisol is essential to maintenance of homeostasis. It is secreted by the hypothalamic-pituitary-adrenal system and it is produced as part of body’s stress response (Fig. 1). It is called the “stress hormone” which influences and regulates various physiological processes such as blood pressure, glucose levels, immune responses, heart contractions, central nervous system activation, and carbohydrate metabolism [1]. It is known that cortisol levels vary throughout the day in a circadian rhythm with highest levels during daybreak and reaching its lowest level by night [2,3]. Thus understanding of abnormal levels of cortisol is important [4–7,1]. Abnormalities in cortisol are indicator of chronic conditions such as Cushing’s disease [8] due to excess cortisol levels, Addison’s disease [9] due to decreased cortisol levels, and adrenal insufficiencies [10–13]. Changes in behavioral and environmental conditions trigger cortisol secretion and hence its measurement at point-of-care (POC) has become vital to understand behavioral patterns in humans. Laboratory based techniques such as chromatography [14–16], radioimmunoassay (RIA) [17], electro-chemiluminescence immunoassay (ECLIA) [18–22], enzyme-linked immunosorbent assay (ELISA) [23–26], surface plasmon resonance (SPR) [27–30], and quartz crystal microbalance (QCM) [31] which make up the state-of-the-art in cortisol detection are complex systems requiring multistep extraction/purification of samples. The turn-around time from sampling to results for these systems is typically from days to a few weeks. In the recent years, electrochemical immunosensing has emerged as the promising technology for simple, cost-effective, and label free POC detection of cortisol in bio-fluids. Interstitial Fluid (ISF) [7], Blood [11], Urine [32], Sweat [33], and Saliva [34] are the sources of cortisol. Urine and saliva are relevant bio-fluids for detection of cortisol because only free cortisol is found in urine and 90% of free cortisol is in saliva. Harvesting these samples is completely non-invasive.
Figure 1.
Cortisol is secretion is regulated by HPA axis as a part of body’s stress response. As a negative feedback due to stress, CRH is secreted from the hypothalamus which stimulates release of ACTH from anterior pituitary. This acts on the adrenal glands to release cortisol.
Electrochemical Sensing of Cortisol
In the past few years there have been many reports of electrochemical immunosensing of cortisol using electrochemical impedance spectroscopy (EIS) [35] and cyclic voltammetry (CV) [36]. Self-assembled monolayer (SAM) [37], Au nanowires [38], Au-PANI nanocomposite [39], AgOAg-PANI nanocomposite [40], and Graphene [41] based electrochemical cortisol immunosensors have been developed. These immunosensors have been integrated with microfluidic systems for POC sensing [36,42]. This section gives an inclusive overview of the various electrochemical detection platforms integrated for quantification of cortisol. POC cortisol sensing is also discussed in this section.
Electrochemical Immunosensing of Cortisol
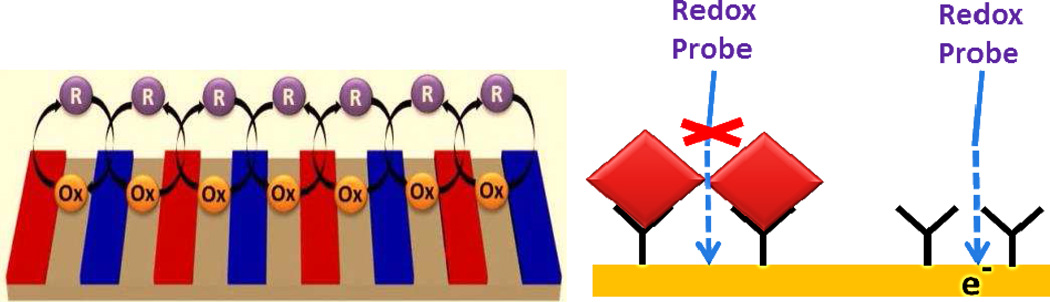
Electrochemical immunosensing has emerged as a promising label-free detection technology alternative to optical detection. It is based on the principle of measuring the changes in the electrical properties of a conductive substrate due to the adsorption of an analyte on the surface functionalized with antibodies sensitive to the analyte. Cyclic voltammetry (CV) and Electrochemical Impedance Spectroscopy (EIS) are two common methods of electrochemical analysis. The electrical change is attributed to the change in the concentration of the electro active redox species at the electrode (Fig. 2). Due to the mature processing techniques of the microelectronics industry, fabrication of microelectrodes is possible which provides high sensitivity and very low detection limits. The inexpensive batch fabrication and simplicity of the electronic circuit for electrochemical detection has brought up electrochemical immunosensing up to speed with other immunosensing techniques [43] Multi-parametric-analysis electrochemical immunosensor has shown great potential for detection of desired biomarkers at POC due to high sensitivity and ease of fabrication using nanotechnology [44].
Figure 2.
Schematic of strategies used for electrochemical sensing
Cortisol Sensing Platforms: A Recent Update
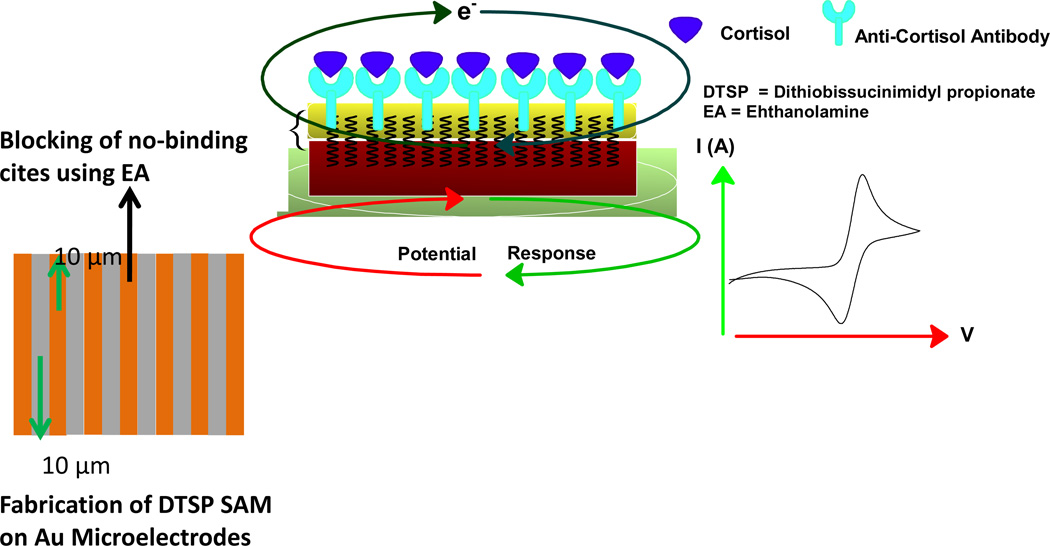
Dithiobis(succinimidyl propionate) (DTSP) SAM modified interdigitated microelectrodes (IDμEs) were used for the immobilization of cortisol specific monoclonal antibody (C-Mab) to detect cortisol using EIS [37]. The electrodes were also modified with Ethanol Amine (EA) to block the unreacted succinimidyl group on DTSP SAM and to remove unbound antibodies from the electrode surface. The immunosensor exhibited the sensitivity of 7.9 kΩ (pg mL−1)−1 and it accurately detected cortisol in the range of 0.36 pg mL−1 to 0.36 ng mL−1 in saliva. This work was further proceeded using the same immunosensing strategy for cortisol detection in interstitial fluid (ISF) in vitro and had sensitivity of 6.4 kΩ (pg mL−1)−1[45]. EA/C-Mab/DTSP/Au (Fig. 3) based biosensor can accurately detect cortisol in the range of 0.36 pg mL−1 to 0.36 ng mL−1 with the detection limit of 0.36 pg mL−1 in ISF. ISF was extracted through micropores created on the stratum corneum layer of the skin by means of vacuum pressure for detection of cortisol via EA/C-Mab/DTSP/Au based immunosensor [7]. The performance of this EIS based immunosensor was validated using ELISA. This confirmed the feasibility of using impedance based biosensor as a disposable cortisol detector, capable of working with complex bodily fluids (e.g., saliva and ISF) at point-of-care. The researchers also proposed that cortisol monitoring of continuously harvested ISF over 24 hours even when the subject is asleep can project commercial viability for in vivo real-time cortisol sensor device. The results from their research suggested that a circadian rhythm could be established between the subject’s ISF and the saliva samples collected over 24 hour time-periods.
Figure 3.
Schematic of EA/C-Mab/DTSP/Au Immunosensor
In the recent years, explorations of nanomaterials and nanoparticle-polymer composites have shown great promises to enhance the sensitivity and selectivity of cortisol detection. A competitive immunoassay fabricated using anti-cortisol antibody immobilized onto protein A-modified magnetic particles and cortisol antigen labeled with alkaline phosphatase (AP), under optimized parameters, exhibited a linear range between 5.0 pg mL−1 to 150 ng mL−1 and a limit of detection was 3.5 pg mL−1 in DPV investigations [46]. The fabricated sensor was highly sensitive, selective with respect to other corticosteroid compounds closely related to cortisol and demonstrated by analyzing certified human serum containing cortisol.
Gold nanoparticles (NPs) have been used to increase the active surface area of the Au NP/ G-DTBP Protein/ Au electrode based electrochemical cortisol immunosensor [47]. The Protein G –DTBP scaffold aided by providing an orientation controlled immobilization. The immunosensor showed sensitivity of 1.6 µA (pg mL−1)−1 with a detection limit of 16 pg mL−1 and demonstrated excellent performance in case of laboratory prepared samples and real-life blood samples. In another study, electrophoretically fabricated polyaniline (PANI)-Au nanocomposite was utilized to immobilize C-Mab covalently via EDC-NHS chemistry to fabricate a mediator and label free electrochemical immunosensor for cortisol estimation using CV technique [39]. The detection range of this nanocomposite based cortisol immunosensor was in the range of 0.36 pg mL−1 to 36 ng mL−1 with a detection limit of 0.36 pg mL−1 and sensitivity of 4.5 µA (g mL−1)−1. It was found to be selective against BSA and α-hydroxy progesterone.
Another viable method for a quick and easy-to-use sensor was realized for immobilization of anti-cortisol antibody via NHS-WSC-hydrochloride SAM chemistry on platinum electrode [48]. In this immunosensor change in current was observed as a result of the competitive reaction between sample cortisol and glucose oxidase-labeled conjugate and the current was inversely proportional to the cortisol concentration in the sample. The sensor exhibited a faster response time of 10 minute when compared to other sensors which require a 30 minute incubation time. However, the cortisol sensor had a higher detection limit of 1 ngmL−1. The detected current was 200 nA at 200mg dL−1 glucose concentration which the authors propose is sufficient for a rapid, easy, and noninvasive analysis of cortisol in saliva samples.
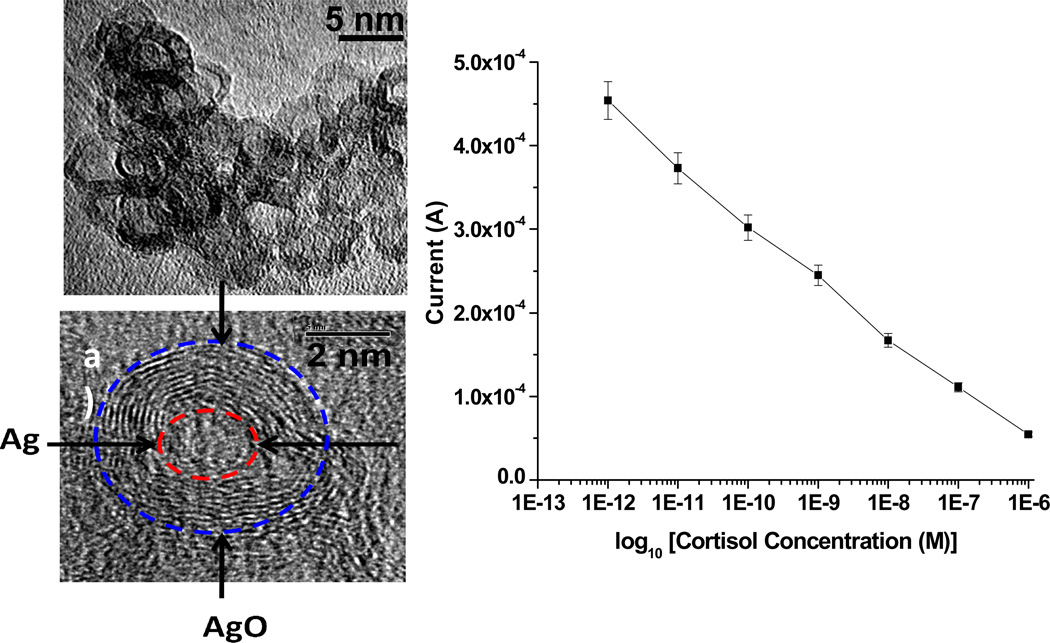
Electrophoretically deposited PANI-core-shell AgAgO NP (~5nm) nanocomposite films have been explored for Anti-cortisol (Anti-Cab) antibody immobilization via EDC/NHS chemistry for mediator/label free electrochemical detection of cortisol [40]. Authors proposed that nanocomposite exhibited a high magnitude of current response, which resulted in high electron transport at the electrode-electrolyte interface without a mediator (Fig. 4). This immunosensor exhibited a wide linear detection range of 0.36 pg mL−1 to 0.36 µg mL−1, a detection limit of 0.64 pg/mL, and high sensitivity of 183 µA (g mL−1)−1 with a regression coefficient of 0.998.
Figure 4.
a & b) TEM image of AgAgO NP; c) Calibration curve obtained from the electrochemical response studies of BSA/Anti-Cab/AgAgO-PANI/Au immunoelectrode as a function of cortisol concentration (1 pM to 1 µM) [40].
Reduced graphene oxide (rGO) modified gold interdigitated array electrode (Au-IDA) based immunosensor for detection of cortisol in saliva has also been reported [41]. rGO modified electrodes are desirable due to the fact that it is the most economical way of obtaining graphene like structures and graphene is recognized for its unique conductivity. The electrochemical activity of the electrode was studied on p-aminophenol (pAP) as it is a good probe for electrochemical immunoassay and the redox cycle was confirmed between the anode and cathode. It was found that denser rGO provide better reactivity for pAP. The electrode was also used to estimate cortisol level in human saliva. The CV response of this electrochemical immunoassay demonstrated a useful cortisol detection range of 1–8 ng mL−1 with a detection limit of 1 ng mL−1. However other sensing parameter such as sensitivity was not reported.
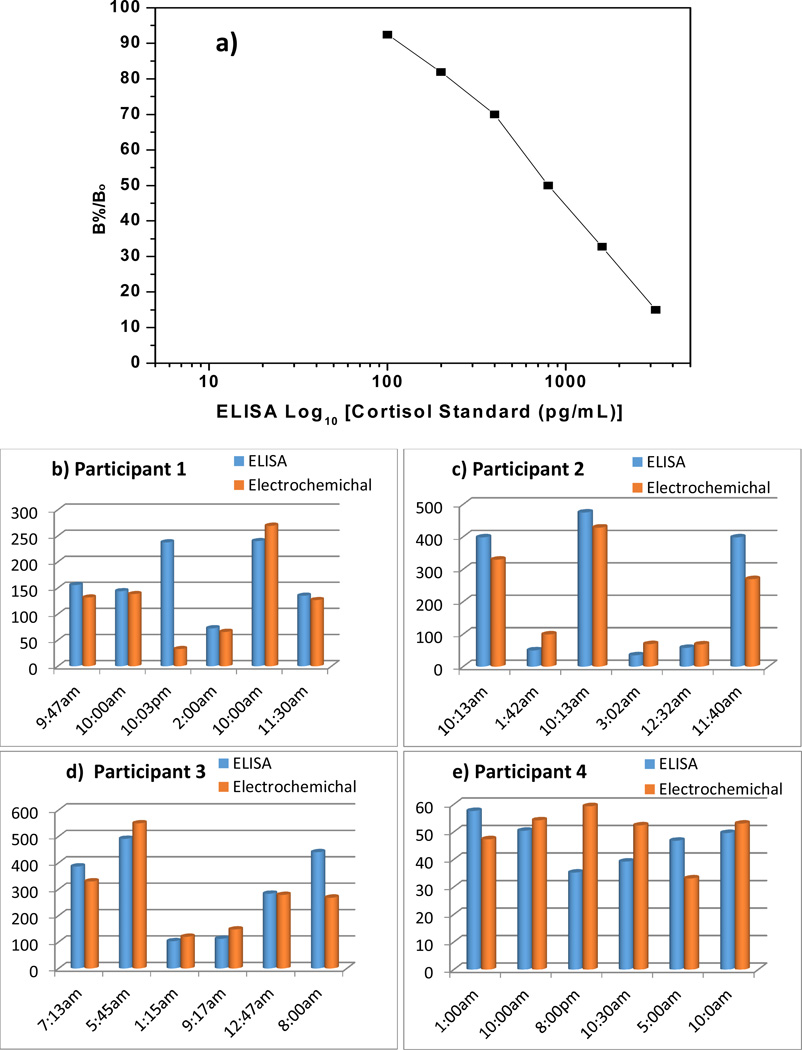
Very recently the BioMEMS and Microsystems research group at Florida International University reported EA/Anti-Cab/DTSP(SAM)/Au IDEs based electrochemical immunosensors for cortisol detection in saliva collected at different time intervals from two participants (Fig. 5 b & c) [49]. The sensor exhibited a detection range of 10 pg mL−1 to 100 ng mL−1 with a detection limit of 10 pg mL−1 and sensitivity of 6µA (pg mL−1)−1. The obtained results correlated well with results from the standard ELISA technique (Fig. 5a) and such a validation was performed for the first time for cortisol estimation in saliva. The aim of this work is to establish a cortisol sensing protocol using saliva samples collected from human subjects along with validation of the results with the standard ELISA technique. In order to develop that protocol this study has continued and data from two more participants were obtained which has been included in this review (Fig. d & e). This developed protocol will be used to understand the behavioral changes of farm workers on exposure to pesticide.
Figure 5.
a) A calibration curve obtained for determination of the cortisol concentrations in saliva samples using ELISA. Comparison of results obtained from ELISA and Electrochemical immunosensor for participant 1 (b), participant 2 (c), participant 3 (d), and participant 4 (e).
A comparison of the analytical performance of electrochemical based cortisol immunosensors is provided in Table 1:
| Substrate | Immobilizing Matrix |
Detection Limit |
Sensitivity | Sample | Technique | Ref |
|---|---|---|---|---|---|---|
| Au IDμEs | DTSP SAM | 0.36 pg mL−1 | 3.2 kΩ (pg mL−1)−1 | Saliva/ ISF | EIS | [35] |
| Au IDμEs | DTSP SAM | 0.36 pg mL−1 | 7.9 kΩ (pg mL−1)−1 | Saliva | EIS | [37] |
| PANI protected Au Nanoparticles/ Au IDμEs | EDC/NHS | 0.36 pg mL−1 | 4.5 µA (g mL−1)−1 | Cortisol in PBS solution | CV, DPV | [39] |
| Coreshell Ag@AgO-PANI/ Au IDμEs | EDC/NHS | 0.64 pgmL−1 | 183 µA (g mL−1)−1 | Cortisol in PBS | CV | [40] |
| Reduced Graphene (rGo)/Au IDA | - | 1 ng mL−1 | - | Saliva | CV | [41] |
| Au IDμEs | DTSP SAM | 0.36 pg mL−1 | 6.4 kΩ (pg mL−1)−1 | ISF | EIS | [45] |
| Screen Printed Carbon Electrode | Potein A-modified magnetic particle | 3.5 pg mL−1 | - | Serum | DPV | [46] |
| Au Nanoparticle/Au IDμEs | DTBP-Protein G Scaffold | 16 pg mL−1 | 1.6 µA (pg mL−1)−1 | Blood | Square wave voltammetry | [47] |
| Pt Electrode | NHS-WSC SAM | 1 ng mL−1 | 200 nA (200mg dL−1)−1 | Saliva | Current by GOD-cortisol reaction | [48] |
| Au IDμEs | DTSP SAM | 10 pg mL−1 | 6 µA (pg mL−1)−1 | Saliva | CV | [49] |
Cortisol Sensing at Point-of-care
Due to constraints of portability, cost, analysis time or requirement of highly skilled personnel for system operation, microfluidic systems have enabled development of POC chemical and biological assays. Advantages of a microfluidic system include small sample volumes, precise control of fluidic routines, repeatable sensing protocols, controlled environment for bio-molecule reaction, reduced form factor and application at POC. The error associated with human handling is also eliminated due to the high degree of automation that exists in microfluidic systems, thereby reducing the percentage of false results. These features have drawn focus in integrating biosensors into microfluidic environment with a goal to create POC sensors.
To analyze salivary cortisol at POC, a portable, rapid and hand held biosensor based on disposable immune-chromatographic test strip has been proposed [50]. An immunechromatographic test-strip (5 × 1.5 × 50 mm3) consisting of glucose oxidase (GOD)-cortisol conjugate was synthesized for molecular recognition of cortisol. The fabricated cortisol biosensor based on calorimetric protocol detects cortisol concentrations between 1 to 10 ng mL−1 within 25 min. Another SAM based cortisol immunosensor has been proposed by the same research group integrated with a fluid control mechanism which has both a vertical flow and a lateral flow [42]. A competitive reaction between the sample cortisol and GOD-labeled conjugate was found to be inversely related to the concentration of cortisol and can be detected electrochemically. This sensor exhibited a salivary cortisol detection range from 0.1–10 ng mL−1 with regression coefficient of 0.98. This immunosensor was able to detect cortisol within 35 min and could be reused. The results correlated well with ELISA. These immunosensors exhibited capability for an on-site and easy-to-use biosensor for detection of salivary cortisol.
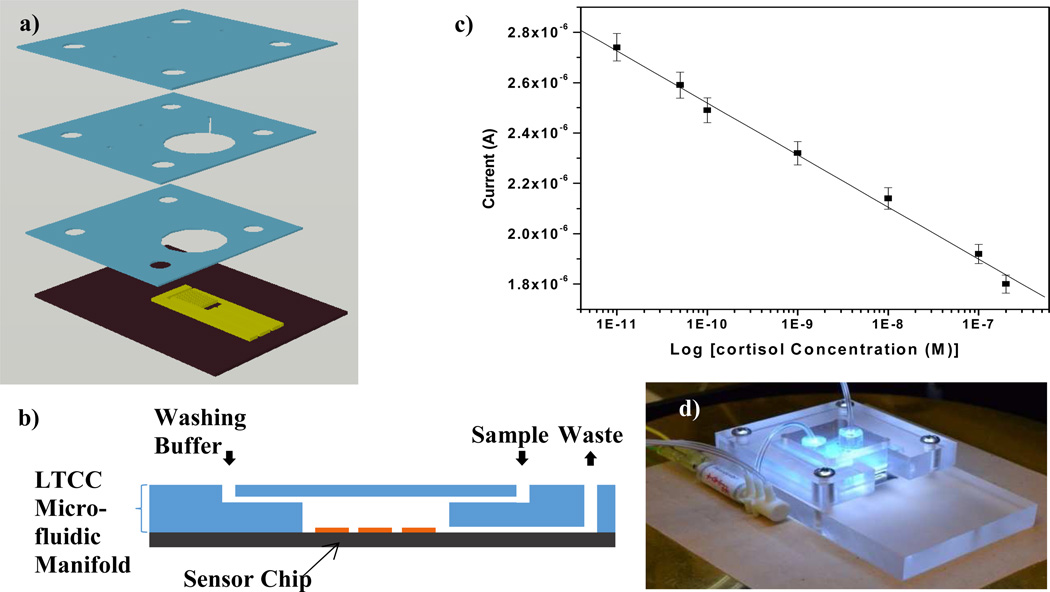
A fully automated low temperature co-fired ceramic (LTCC) based microfluidic system integrated with an electrochemical immunosensor (Fig. 6) has been developed at BioMEMS and Research Laboratory [36]. Authors designed and optimized 3D microfluidic architecture (150 µm width) and successfully integrated with SAM modified IDμEs based electrochemical immunosensor for detection of cortisol. The integrated sensor exhibited a linear range of 36 pg mL−1 to 36 ng mL−1 at a sensitivity of 0.575 µA M in an automated and controlled microfluidic environment. This LTCC based electrochemical cortisol detection device is a step toward realizing development for non-invasive, point of care measurement of human cortisol in desired bio-fluids.
Figure 6.
a) Exploded view of the microfluidic system; b) close up of the LTCC microfluidic channel; c) cortisol detection using the immunosensor; and d) Fully automated microfluidic system [36]
The above mentioned electrochemical immunosensor integrated with MEMS technology could be interfaced to a wireless health monitoring system that could transfer sensor data over existing wide-area networks such as the internet and a cellular phone network to enable real-time remote monitoring of subjects. The area of miniaturized electronics integrated with electrochemical biosensors has great significance for on-site diagnostics. The junction of laboratory bio-sensing protocols and miniaturized electronics can be explored for mass production with great prospects of commercialization.
Conclusion and Future Prospects
This mini review provides an update on low-cost and label free electrochemical detection of cortisol using anti-cortisol antibody covalently immobilized on nanomaterial enabled immunosensors. These reported electrochemical immunosensors have the potential to be integrated with wearable POC devices for continuous monitoring of cortisol levels. This review also highlights the microfluidic devices which have been integrated with these immunosensors to provide rapid information on cortisol levels. These devices form the current state-of-the-art in POC devices for cortisol sensing. These devices coupled with continuous bio-fluid harvesting systems can allow a continuous readout of cortisol levels for real-time analysis of patients subjected to stress induced by surrounds and situations. The obtained rhythmic information on cortisol levels can be used to understand behavioral patterns of individuals in field.
There have been inherent limitations of the above mentioned cortisol immunosensors that have been designed in field and on-site cortisol detection. Low shelf-life of immunosensor at room temperature and denaturation of antibody due to the environmental factors (temperature fluctuations, humidity, change in pH, exposure to light etc.) limits their application in the field for diurnal cortisol measurement. The encapsulation of antibody to retain its bio-integrity [51] and single domain antibodies (sdAb) [52,53] which shows potential as inherently immune to changes in temperature can be the possible solution to overcome the issue to antibody stability. These strategies are not yet in practice. However, currently at BioMEMS and Microsystems laboratory the aim is to analyze the affinity and thermal stability of sdAb for electrochemical detection of cortisol to overcome the problem of temperature dependent denaturation of the antibodies. The ability of these small antigen-binding molecules to refold after heating to achieve their original structure [52] makes them attractive candidates for POC wearable devices. Thus, there is a great scope to conduct research in the area of developing miniaturized electrochemical devices to monitor cortisol cycle for personalized health care.
Further work at BioMEMS and Microsystems laboratory at Florida International University will be focused on fabrication of nanowire and nanoparticle based mediator free and label free immunosensing of biomarker applications. It is well regarded that nanowires will replace other competing nanostructures such as nanotubes in sensing platforms, due to their high aspect ratio, tailorable properties, and surface modification [54]. Efforts are also continuous towards exploring nanocomposites for cortisol sensing [39,40]. It is observed that understanding of their properties and development morphologies is crucial, if these nanostructures contribute significantly or enhance current sensing parameters such as sensitivity and selectivity. Future sensors will require refined development techniques which can be modified for greater sensitivity and stability.
Acknowledgements
This work was supported by the National Science Foundation (NSF) ASSIST Nanosystems ERC (EEC-1160483) and National Institute of Health (NIH) 1RO1-DA027049. The author Rajesh Kumar acknowledges the University Grant Commission (UGC), India for the award of Raman Fellowship (ID 1001, 2013-14).
References
- 1.Kaushik A, Vasudev A, Arya SK, Pasha SK, Bhansali S. Recent advances in cortisol sensing technologies for point-of-care application. Biosensors and Bioelectronics. 2014;53(0):499–512. doi: 10.1016/j.bios.2013.09.060. [DOI] [PubMed] [Google Scholar]
- 2.Corbalan-Tutau D, Madrid JA, Nicolas F, Garaulet M. Daily profile in two circadian markers "melatonin and cortisol" and associations with metabolic syndrome components. Physiol Behav. 2014;123:231–235. doi: 10.1016/j.physbeh.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Nicolson NA. Handbook of Physiological Research Methods in Health Psychology. SAGE Publications, Inc. SAGE Publications, Inc; Thousand Oaks, CA.: Measurement of Cortisol. [Google Scholar]
- 4.Delahanty DL, Raimonde AJ, Spoonster E. Initial posttraumatic urinary cortisol levels predict subsequent PTSD symptoms in motor vehicle accident victims. Biol Psychiatry. 2000;48(9):940–947. doi: 10.1016/s0006-3223(00)00896-9. [DOI] [PubMed] [Google Scholar]
- 5.Yehuda R, Halligan SL, Grossman R. Childhood trauma and risk for PTSD: relationship to intergenerational effects of trauma, parental PTSD, and cortisol excretion. Dev Psychopathol. 2001;13(3):733–753. doi: 10.1017/s0954579401003170. [DOI] [PubMed] [Google Scholar]
- 6.Yehuda R, Halligan SL, Bierer LM. Cortisol levels in adult offspring of Holocaust survivors: relation to PTSD symptom severity in the parent and child. Psychoneuroendocrinology. 2002;27(1–2):171–180. doi: 10.1016/s0306-4530(01)00043-9. [DOI] [PubMed] [Google Scholar]
- 7.Venugopal M, Arya SK, Chornokur G, Bhansali S. A realtime and continuous assessment of cortisol in ISF using electrochemical impedance spectroscopy. Sensors and Actuators A: Physical. 2011;172(1):154–160. doi: 10.1016/j.sna.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McEwen BS. Cortisol, Cushing’s Syndrome, and a Shrinking Brain—New Evidence for Reversibility. The Journal of Clinical Endocrinology & Metabolism. 2002;87(5):1947–1948. doi: 10.1210/jcem.87.5.9999. [DOI] [PubMed] [Google Scholar]
- 9.Edwards OM, Courtenay-Evans RJ, Galley JM, Hunter J, Tait AD. Changes in cortisol metabolism following rifampicin therapy. Lancet. 1974;2(7880):548–551. [PubMed] [Google Scholar]
- 10.Gatti R, Antonelli G, Prearo M, Spinella P, Cappellin E, De Palo EF. Cortisol assays and diagnostic laboratory procedures in human biological fluids. Clinical Biochemistry. 2009;42(12):1205–1217. doi: 10.1016/j.clinbiochem.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Levine A, Zagoory-Sharon O, Feldman R, Lewis JG, Weller A. Measuring cortisol in human psychobiological studies. Physiol Behav. 2007;90(1):43–53. doi: 10.1016/j.physbeh.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 12.de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6(6):463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 13.Djuric Z, Bird CE, Furumoto-Dawson A, Rauscher GH, Ruffin MTt, Stowe RP, Tucker KL, Masi CM. Biomarkers of Psychological Stress in Health Disparities Research. Open Biomark J. 2008;1:7–19. doi: 10.2174/1875318300801010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klopfenstein BJ, Purnell JQ, Brandon DD, Isabelle LM, DeBarber AE. Determination of cortisol production rates with contemporary liquid chromatography–mass spectrometry to measure cortisol-d3 dilution after infusion of deuterated tracer. Clinical Biochemistry. 2011;44(5–6):430–434. doi: 10.1016/j.clinbiochem.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L-Q, Kang X-J, Sun J, Deng J-J, Gu Z-Z, Lu Z-H. Application of nanofiber-packed SPE for determination of salivary-free cortisol using fluorescence precolumn derivatization and HPLC detection. Journal of Separation Science. 2010;33(15):2369–2375. doi: 10.1002/jssc.201000071. [DOI] [PubMed] [Google Scholar]
- 16.Gao W, Xie Q, Jin J, Qiao T, Wang H, Chen L, Deng H, Lu Z. HPLC-FLU detection of cortisol distribution in human hair. Clinical Biochemistry. 2010;43(7–8):677–682. doi: 10.1016/j.clinbiochem.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Appel D, Schmid RD, Dragan CA, Bureik M, Urlacher VB. A fluorimetric assay for cortisol. Anal Bioanal Chem. 2005;383(2):182–186. doi: 10.1007/s00216-005-0022-9. [DOI] [PubMed] [Google Scholar]
- 18.van Aken MO, Romijn JA, Miltenburg JA, Lentjes EGWM. Automated Measurement of Salivary Cortisol. Clinical Chemistry. 2003;49(8):1408–1409. doi: 10.1373/49.8.1408. [DOI] [PubMed] [Google Scholar]
- 19.Carrozza C, Corsello SM, Paragliola RM, Ingraudo F, Palumbo S, Locantore P, Sferrazza A, Pontecorvi A, Zuppi C. Clinical accuracy of midnight salivary cortisol measured by automated electrochemiluminescence immunoassay method in Cushing's syndrome. Annals of Clinical Biochemistry. 2010;47(3):228–232. doi: 10.1258/acb.2010.010020. [DOI] [PubMed] [Google Scholar]
- 20.Lippi G, De Vita F, Salvagno GL, Gelati M, Montagnana M, Guidi GC. Measurement of morning saliva cortisol in athletes. Clinical Biochemistry. 2009;42(9):904–906. doi: 10.1016/j.clinbiochem.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 21.Yaneva M, Kirilov G, Zacharieva S. Midnight salivary cortisol, measured by highly sensitive electrochemiluminescence immunoassay, for the diagnosis of Cushing’s syndrome. Central European Journal of Medicine. 2009;4(1):59–64. [Google Scholar]
- 22.Shi H, Xu X, Ding Y, Liu S, Li L, Kang W. Determination of cortisol in human blood sera by a new Ag(III) complex–luminol chemiluminescent system. Analytical Biochemistry. 2009;387(2):178–183. doi: 10.1016/j.ab.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Lewis JG, Elder PA. An enzyme-linked immunosorbent assay (ELISA) for plasma cortisol. Journal of Steroid Biochemistry. 1985;22(5):673–676. doi: 10.1016/0022-4731(85)90222-5. [DOI] [PubMed] [Google Scholar]
- 24.Manenschijn L, Koper JW, Lamberts SW, van Rossum EF. Evaluation of a method to measure long term cortisol levels. Steroids. 2011;76(10–11):1032–1036. doi: 10.1016/j.steroids.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Shimada M, Takahashi K, Ohkawa T, Segawa M, Higurashi M. Determination of Salivary Cortisol by ELISA and Its Application to the Assessment of the Circadian Rhythm in Children. Hormone Research in Paediatrics. 1995;44(5):213–217. doi: 10.1159/000184628. [DOI] [PubMed] [Google Scholar]
- 26.Small BC, Davis KB. Validation of a Time-Resolved Fluoroimmunoassay for Measuring Plasma Cortisol in Channel Catfish Ictalurus punctatus. Journal of the World Aquaculture Society. 2002;33(2):184–187. [Google Scholar]
- 27.Mitchell JS, Lowe TE, Ingram JR. Rapid ultrasensitive measurement of salivary cortisol using nano-linker chemistry coupled with surface plasmon resonance detection. Analyst. 2009;134(2):380–386. doi: 10.1039/b817083p. [DOI] [PubMed] [Google Scholar]
- 28.Shankaran DR, Gobi KV, Miura N. Recent advancements in surface plasmon resonance immunosensors for detection of small molecules of biomedical, food and environmental interest. Sensors and Actuators B: Chemical. 2007;121(1):158–177. [Google Scholar]
- 29.Stevens RC, Soelberg SD, Near S, Furlong CE. Detection of cortisol in saliva with a flow-filtered, portable surface plasmon resonance biosensor system. Anal Chem. 2008;80(17):6747–6751. doi: 10.1021/ac800892h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee H-J, Lee J-H, Moon H-S, Jang I-S, Choi J-S, Yook J-G, Jung H-I. A planar split-ring resonator-based microwave biosensor for label-free detection of biomolecules. Sensors and Actuators B: Chemical. 2012;169(0):26–31. [Google Scholar]
- 31.Atashbar MZ, Bejcek B, Vijh A, Singamaneni S. QCM biosensor with ultra thin polymer film. Sensors and Actuators B: Chemical. 2005;107(2):945–951. [Google Scholar]
- 32.Brossaud J, Ducint D, Gatta B, Molimard M, Tabarin A, Corcuff J. Urinary cortisol metabolites in corticotroph and adrenal tumours. Endocrine Abstracts. 2012;29:P55. [Google Scholar]
- 33.Russell E, Koren G, Rieder M, Van Uum SH. The detection of cortisol in human sweat: implications for measurement of cortisol in hair. Ther Drug Monit. 2014;36(1):30–34. doi: 10.1097/FTD.0b013e31829daa0a. [DOI] [PubMed] [Google Scholar]
- 34.VanBruggen MD, Hackney AC, McMurray RG, Ondrak KS. The relationship between serum and salivary cortisol levels in response to different intensities of exercise. Int J Sports Physiol Perform. 2011;6(3):396–407. doi: 10.1123/ijspp.6.3.396. [DOI] [PubMed] [Google Scholar]
- 35.Arya SK, Chornokur G, Venugopal M, Bhansali S. Antibody modified gold micro array electrode based electrochemical immunosensor for ultrasensitive detection of cortisol in saliva and ISF. Procedia Engineering. 2010;5(0):804–807. doi: 10.1016/j.bios.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vasudev A, Kaushik A, Tomizawa Y, Norena N, Bhansali S. An LTCC-based microfluidic system for label-free, electrochemical detection of cortisol. Sensors and Actuators B: Chemical. 2013;182(0):139–146. [Google Scholar]
- 37.Arya SK, Chornokur G, Venugopal M, Bhansali S. Antibody functionalized interdigitated micro-electrode (IDmicroE) based impedimetric cortisol biosensor. Analyst. 2010;135(8):1941–1946. doi: 10.1039/c0an00242a. [DOI] [PubMed] [Google Scholar]
- 38.Kumar A, Aravamudhan S, Gordic M, Bhansali S, Mohapatra SS. Ultrasensitive detection of cortisol with enzyme fragment complementation technology using functionalized nanowire. Biosensors and Bioelectronics. 2007;22(9–10):2138–2144. doi: 10.1016/j.bios.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 39.Arya SK, Dey A, Bhansali S. Polyaniline protected gold nanoparticles based mediator and label free electrochemical cortisol biosensor. Biosensors and Bioelectronics. 2011;28(1):166–173. doi: 10.1016/j.bios.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 40.Kaushik A, Vasudev A, Arya SK, Bhansali S. Mediator and label free estimation of stress biomarker using electrophoretically deposited Ag@AgO–polyaniline hybrid nanocomposite. Biosensors and Bioelectronics. 2013;50(0):35–41. doi: 10.1016/j.bios.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 41.Ueno Y, Furukawa K, Hayashi K, Takamura M, Hibino H, Tamechika E. Graphene-modified interdigitated array electrode: fabrication, characterization, and electrochemical immunoassay application. Anal Sci. 2013;29(1):55–60. doi: 10.2116/analsci.29.55. [DOI] [PubMed] [Google Scholar]
- 42.Yamaguchi M, Matsuda Y, Sasaki S, Sasaki M, Kadoma Y, Imai Y, Niwa D, Shetty V. Immunosensor with fluid control mechanism for salivary cortisol analysis. Biosensors and Bioelectronics. 2013;41(0):186–191. doi: 10.1016/j.bios.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ricci F, Adornetto G, Palleschi G. A review of experimental aspects of electrochemical immunosensors. Electrochimica Acta. 2012;84(0):74–83. [Google Scholar]
- 44.Wan Y, Su Y, Zhu X, Liu G, Fan C. Development of electrochemical immunosensors towards point of care diagnostics. Biosens Bioelectron. 2013;47:1–11. doi: 10.1016/j.bios.2013.02.045. [DOI] [PubMed] [Google Scholar]
- 45.Arya SK, Chornokur G, Venugopal M, Bhansali S. Dithiobis(succinimidyl propionate) modified gold microarray electrode based electrochemical immunosensor for ultrasensitive detection of cortisol. Biosens Bioelectron. 2010;25(10):2296–2301. doi: 10.1016/j.bios.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moreno-Guzman M, Eguilaz M, Campuzano S, Gonzalez-Cortes A, Yanez-Sedeno P, Pingarron JM. Disposable immunosensor for cortisol using functionalized magnetic particles. Analyst. 2010;135(8):1926–1933. doi: 10.1039/c0an00206b. [DOI] [PubMed] [Google Scholar]
- 47.Liu X, Zhao R, Mao W, Feng H, Wong DK. Detection of cortisol at a gold nanoparticle|Protein G-DTBP-scaffold modified electrochemical immunosensor. Analyst. 2011;136(24):5204–5210. doi: 10.1039/c1an15411g. [DOI] [PubMed] [Google Scholar]
- 48.Yamaguchi M, Matsuda Y, Yoshikawa S, Sasaki M, Imai Y, Niwa D, Shetty V. Rapid hormone immunosensor with fluid control mechanism. Solid-State Sensors, Actuators and Microsystems Conference (TRANSDUCERS) 2011 16th International; 5–9 June 2011; 2011. pp. 1164–1167. [Google Scholar]
- 49.Pasha SK, Kaushik A, Vasudev A, Snipes SA, Bhansali S. Electrochemical Immunosensing of Saliva Cortisol. Journal of The Electrochemical Society. 2014;161(2):B3077–B3082. [Google Scholar]
- 50.Yamaguchi M, Yoshikawa S, Tahara Y, Niwa D, Imai Y, Shetty V. Point-of-use measurement of salivary cortisol levels; Sensors, 2009 IEEE; 25–28 Oct. 2009; 2009. pp. 343–346. [Google Scholar]
- 51.Chantasirichot S, Ishihara K. Electrospun phospholipid polymer substrate for enhanced performance in immunoassay system. Biosens Bioelectron. 2012;38(1):209–214. doi: 10.1016/j.bios.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 52.Goldman ER, Anderson GP, Liu JL, Delehanty JB, Sherwood LJ, Osborn LE, Cummins LB, Hayhurst A. Facile generation of heat-stable antiviral and antitoxin single domain antibodies from a semisynthetic llama library. Anal Chem. 2006;78(24):8245–8255. doi: 10.1021/ac0610053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goldman ER, Anderson GP, Conway J, Sherwood LJ, Fech M, Vo B, Liu JL, Hayhurst A. Thermostable llama single domain antibodies for detection of botulinum A neurotoxin complex. Anal Chem. 2008;80(22):8583–8591. doi: 10.1021/ac8014774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bauer LA, Birenbaum NS, Meyer GJ. Biological applications of high aspect ratio nanoparticles. Journal of Materials Chemistry. 2004;14:517–526. [Google Scholar]