Abstract
Objective
Interleukin-8 (IL8) receptors IL8RA and IL8RB (ILRA/B) on neutrophil membranes bind to IL8 with high affinity and play a critical role in neutrophil recruitment to sites of injury and/or inflammation. This study tested the hypothesis that administration of rat pulmonary arterial endothelial cells (ECs) overexpressing IL8RA/B can accelerate the adhesion of ECs to the injured lung and inhibit monocrotaline (MCT)-induced pulmonary inflammation, arterial thickening and hypertension, and right ventricular (RV) hypertrophy.
Approach and Results
The treatment groups included 10-wk-old ovariectomized Sprague-Dawley rats that received s.c. injection of phosphate-buffered-saline (Vehicle); a single injection of MCT (MCT alone, 60 mg/kg, s.c.); MCT followed by i.v. transfusion of ECs transduced with the empty adenoviral vector (Null-EC); and MCT followed by i.v. transfusion of ECs overexpressing IL8RA/B (IL8RA/B-EC, 1.5×106 cells/rat). Two days or 4 wks after MCT treatment, eNOS, iNOS, CINC-2β (IL8 equivalent in rat) and MCP-1 expression; neutrophil and macrophage infiltration into pulmonary arterioles, and arteriolar and alveolar morphology were measured by histological and immunohistochemical techniques. Pro-inflammatory cytokine/chemokine protein levels were measured by Multiplexed rat specific magnetic beads based sandwich immunoassay in total lung homogenates. Transfusion of IL8RA/B-ECs significantly reduced MCT-induced neutrophil infiltration and pro-inflammatory mediator (IL-8, MCP-1, iNOS, CINC and MIP-2) expression in lungs and pulmonary arterioles and alveoli, pulmonary artery pressure, and pulmonary arteriole and RV hypertrophy and remodeling.
Conclusion
These provocative findings suggest that targeted delivery of ECs overexpressing IL8RA/B is effective in repairing the injured pulmonary vasculature.
Keywords: Monocrotaline induced pulmonary vascular remodeling and hypertension, Targeted delivery of endothelial cells, Interleukin-8 receptors, Cell therapy
Introduction
The neutrophil chemoattractant interleukin-8 (IL8) (also named cytokine-induced neutrophil chemoattractant [CINC]-2β in rat) is expressed in the setting of acute vascular injury. Neutrophils migrate to injured tissue, triggering an initial pro-inflammatory response and facilitating the influx of other types of inflammatory cells e.g. monocytes/macrophages. Selective IL8 receptors (IL8RA and IL8RB; also named CXCR1 and CXCR2) on the cell membrane of neutrophils bind to IL8 or N-acetylated proline-glycine–proline (acPGP, a specific collagen-derived tripeptide fragment expressed in injured tissue and 3-D structurally similar to IL8) (1, 2) and serve as homing devices to orient neutrophils to target injured tissue.
We have recently developed an innovative strategy to improve the efficiency of cell-based therapy by intravenously (i.v.) transfusing rat aortic endothelial cells (RAECs) overexpressing IL8RA and IL8RB (IL8RA/B) into rats with balloon injury of the carotid artery (3) or myocardial infarction caused by ligation of the left anterior descending (LAD) coronary artery (4). We demonstrated that acute i.v. transfusion of RAECs equipped with IL8RA/B results in targeting and adherence of the transformed RAECs to injured carotid artery (3) or left ventricle (LV) (4), decreasing infiltration of activated neutrophils, inhibiting pro-inflammatory responses and reducing neointima formation in balloon injured carotid artery (3) or enhancing neovascularization in LAD ligation injured LV (4).
Using the rat model of monocrotaline (MCT)-induced lung injury, the current study tested the hypothesis that acute administration of pulmonary arterial endothelial cells (ECs) (5) equipped with IL8RA/B alleviates MCT-induced pulmonary vascular hypertrophy/remodeling and right ventricular (RV) hypertrophy by decreasing neutrophil infiltration and pro-inflammatory mediator production, as well as enhancing eNOS expression in ECs. Rat ECs transduced with adenoviral vectors carrying neutrophil IL8RA/B were transfused into the femoral vein of rats 24 hrs after MCT treatment. We demonstrated that, compared to the control groups, administration of ECs overexpressing IL8RA/B reduced MCT-induced neutrophil infiltration and pro-inflammatory mediator expression in pulmonary arterioles and alveoli, as well as pulmonary arteriolar and RV hypertrophy and remodeling in rats following experimentally induced pulmonary vascular injury.
Methods
A Supplement provides complete details of the methods in this study.
Results
Transfusion of IL8RA/B-ECs improved the growth and survival of MCT-treated rats
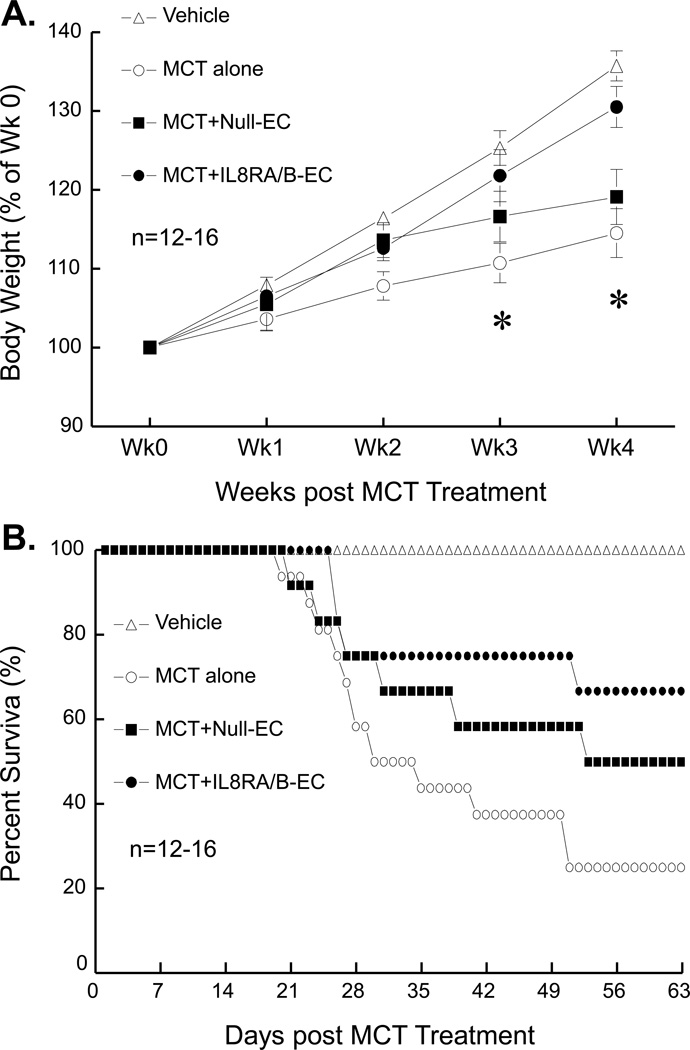
I.v. transfusion of ECs overexpressing IL8RA/B [IL8RA/B-EC, 1.5×106 cells per rat in 500 µl saline, 1 day after the MCT injection (60 mg/kg, s.c.)] resulted in greater weight gain than in MCT alone-treated and MCT+Null-EC (ECs transduced with control empty adenovirus) treated rats at 3–4 wks after MCT treatment (Figure 1A). Rats that received MCT alone began to die within 3–4 wks of treatment; survival was 25% of MCT alone rats, 50% of MCT+Null-EC rats, and 66.7% of MCT+IL8RA/B rats at 9 wks after MCT treatment (Figure 1B). All dead rats exhibited significant RV hypertrophy. The ratio of RV/(LV+septum [S]) (Fulton index) weight of MCT alone rats at death was 0.476±0.031 (n=12) in MCT alone rats, which was significantly greater (p<0.05) than that of Vehicle control rats 0.248±0.010 (n=12).
Figure 1.
A) Body weight and B) survival rate of monocrotaline (MCT) and IL8RA/B-EC or Null-EC treated rats. Vehicle control rats were age-matched ovariectomized Sprague-Dawley rats that did not receive MCT injection or EC transfusion. MCT alone rats received injection of MCT (60 mg/kg, s.c.) on day 0. MCT+Null-EC rats received MCT injection (60 mg/kg, s.c.) followed by i.v. transfusion of pulmonary arterial endothelial cells (ECs) transduced with control empty adenovirus (1.5×106 cells per rat, 24 hrs after the MCT injection). MCT+IL8RA/B-EC rats received MCT injection (60 mg/kg, s.c.) followed by i.v. transfusion of ECs overexpressing IL8RA/B (1.5×106 cells per rat, 1 day after the MCT injection). Results are means±SEM. In panel A), two-way ANOVA was used to analyze the interaction between time and body weight changes (p<0.05) among 4 groups, and one-way ANOVA was used to test the differences in body weight at the same time point. When the overall F test result of ANOVA was significant, a multiple-comparison Tukey post-hoc test was applied to test the difference between groups. * Indicates significant differences (p<0.05) among groups by ANOVA.
Transfusion of IL8RA/B-ECs improved pulmonary artery blood flow in MCTtreated rats
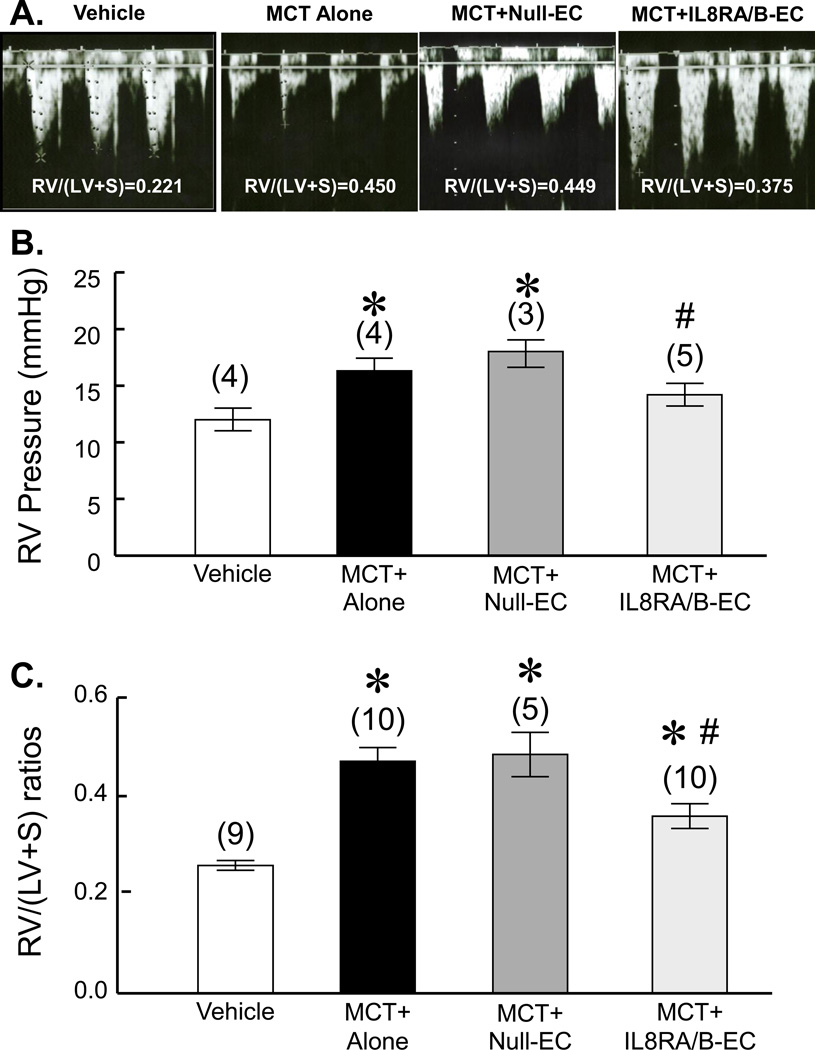
Doppler imaging echocardiographic analysis was used to visualize pulmonary artery outflow. We used the change in the shape of the pulmonary artery waveform as an index of pulmonary arterial hypertension at 4 wks after MCT treatment according to the report of Jones et.al (6). As shown in Figure 2A, the development of a pulmonary artery midsysystolic notch in MCT-treated and MCT+IL8RA/B-EC-treated rats was significant at 4 wks after the MCT treatment. Transfusion of IL8RA/B-ECs partially corrected the MCT-induced midsystolic pulmonary artery notching. RV weight (measured after rats were sacrificed) related to the change in waveform of pulmonary artery flow.
Figure 2.
A) Representative pulmonary outflow wave forms show MCT-induced midsystolic pulmonary artery notching. Doppler of pulmonary outflow was recorded in the parasternal view at the level of the aortic valve. 5 rats were measured in each group 4 wks after MCT treatment. Vehicle control rats did not receive MCT injection or EC transfusion; MCT alone rats received MCT injection (60 mg/kg, s.c.) without EC transfusion; MCT+Null-EC rats received MCT injection followed by i.v. transfusion of ECs (1.5×106 cells per rat, 24 hrs after the MCT injection) transduced with control empty adenovirus; and MCT+IL8RA/B-EC rats received MCT injection followed by i.v. transfusion of ECs overexpressing IL8RA/B (1.5×106 cells per rat, 24 hrs after the MCT injection). Note a) the midsystolic pulmonary artery notching was associated with right ventricular (RV) hypertrophy [RV/(LV+S) weight ratio], an index of pulmonary arterial hypertension; and b) transfusion of ECs overexpressing IL8RA/B (IL8RA/B-EC) prevented the MCT-induced change in the shape of the PA wave form. (B) Transfusion of ILRA/B-EC attenuated MCT-induced right ventricular (RV) hypertension, and C) RV hypertrophy at 4 wks post MCT injection. Mean RV pressure was measured directly by inserting a 23 gauge needle (connected to a transducer) into RV after the chest was opened using a Grass Model 7 polygraph. Note that the RV pressure measured using the open chest method was lower than that obtained in the same model using a closed chest method (30). RV weights were normalized by the weights of left ventricle and septum (LV+S) (Fulton index). Results are means±SEM, (n)=number of rats. * p<0.05 vs. respective Vehicle control group; # p<0.05 vs. respective MCT alone group. One way ANOVA was used to analyze the data. When the overall F test result of ANOVA was significant, a multiple-comparison Tukey post-hoc test was applied.
Transfusion of IL8RA/B-ECs inhibited MCT-induced RV hypertrophy and hypertension at 4 wks after MCT treatment
MCT alone- and MCT-Null-EC-treated rats developed increased mean RV pressure (an index of chronic pulmonary arterial hypertension, assessed by inserting a 23 gauge needle directly into the RV in anesthetized open-chest rats) (Figure 2B). The chronic pulmonary arterial hypertension in MCT alone and MCT-Null-EC-treated rats was confirmed by the presence of significant RV hypertrophy [RV/(LV+S) ratios] (Figure 2C). Transfusion of ECs that overexpress IL8RA/B significantly inhibited MCT-induced RV hypertension and hypertrophy, while transfusion of Null-ECs had no effect.
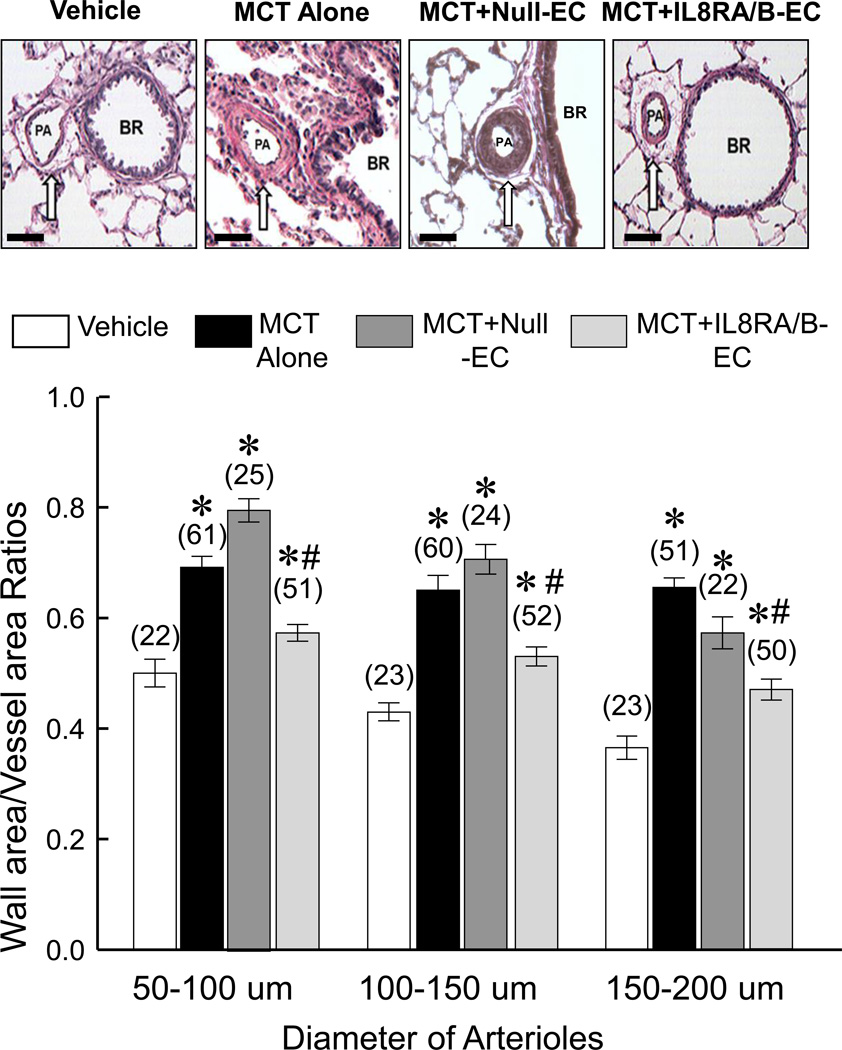
Transfusion of IL8RA/B-ECs inhibited MCT-induced pulmonary arteriolar hypertrophy and remodeling at 4 wks after MCT treatment
MCT treatment markedly increased the medial area and decreased adventitial tissue in 50–200 µm pulmonary arterioles 4 wks after the MCT treatment (Figure 3). Transfusion with IL8RA/B-ECs prevented the development of medial hypertrophy and preserved the adventitial architecture of these vessels. In contrast, transfusion with Null-ECs had no effect on MCT-induced pulmonary vascular hypertrophy and remodeling.
Figure 3.
Transfusion of IL8RA/B-ECs attenuated MCT-induced pulmonary arterial hypertrophy and remodeling at 4 wks post MCT injection. (Top) Representative H/E stained micrographs of pulmonary arterioles (PA) adjacent to the bronchi (BR) in lungs of rats that received Vehicle, MCT alone, MCT+Null-EC, or MCT+IL8RA/B-EC. MCT induced medial hypertrophy and disappearance of adventitial tissue in pulmonary arterioles. (Bottom) Bar graphs show the quantitation of vascular hypertrophy of small (50–100 µm), medium (100–150 µm) and large (150–200 µm) pulmonary arterioles. Bar scale = 100 µm. Ratios of medial area (area between the internal and external elastic lamellae) to total vessel area (area within the external elastic lamella) were calculated of lung sections from 5–6 rats in each group. (n)= number of vessels. Results are means±SEM. * p<0.05 vs. respective Vehicle control groups. # p<0.05 vs. respective MCT alone and MCT+Null-EC groups. One way ANOVA was used to analyze the data, followed by a multiple-comparison Tukey post-hoc test.
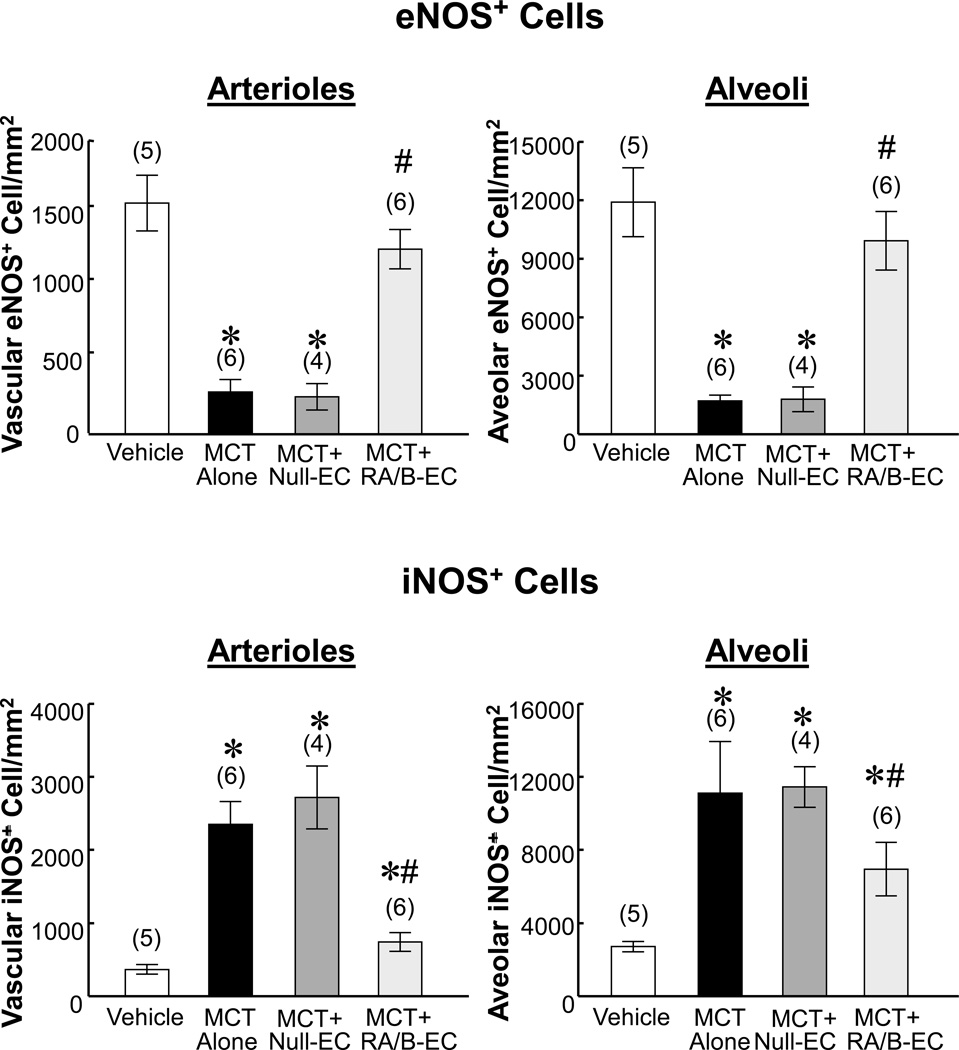
Transfusion of IL8RA/B-ECs prevented MCT-induced decreases in endothelial nitric oxide synthase (eNOS) and increases in inducible NOS (iNOS) in pulmonary arterioles and alveoli at 2 days after MCT treatment (and one day after IL8RA/B-EC treatment)
Immunohistochemical staining demonstrated large decreases in number of eNOS positive cells in pulmonary arterioles and alveoli of MCT-treated and MCT+Null- EC-treated rats (Figure 4 top and Supplemental Figures II–III). Transfusion with IL8RA/B-ECs attenuated this effect on eNOS expression in pulmonary arterioles and alveoli of rats treated with MCT. In contrast, MCT treatment resulted in large increases in number of iNOS positive cells in pulmonary arterioles and alveoli (Figure 4 bottom and Supplemental Figures IV–V). Transfusion with IL8RA/B-ECs, but not Null-ECs, significantly inhibited the MCT-induced increase in iNOS expression in both arterioles and alveoli in injured lungs. The number of iNOS positive cells in the injured lung was negatively correlated with the number of eNOS positive cells (R=−0.887, p<0.01 for arterioles;, and R=−0.552, p<0.05 for alveoli).
Figure 4.
Effects of IL8RA/B-EC or Null-EC transfusion on MCT-induced decrease in eNOS expression and increase in iNOS expression in pulmonary arterioles and alveoli at 4 wks after MCT treatment. Positive immunostained eNOS or iNOS cells on arterioles or alveoli in each 400x microscopic field were counted, and 10 microscopic fields on each lung section were randomly selected for counting. (n)= number of rats. Results are means±SEM. * p<0.05 vs. respective Vehicle control groups. # p<0.05 vs. respective MCT alone and MCT+Null-EC groups. One way ANOVA was used to analyze the data, followed by a multiple-comparison Tukey post-hoc test. Representative micrographs are shown in Supplemental Figures II–V.
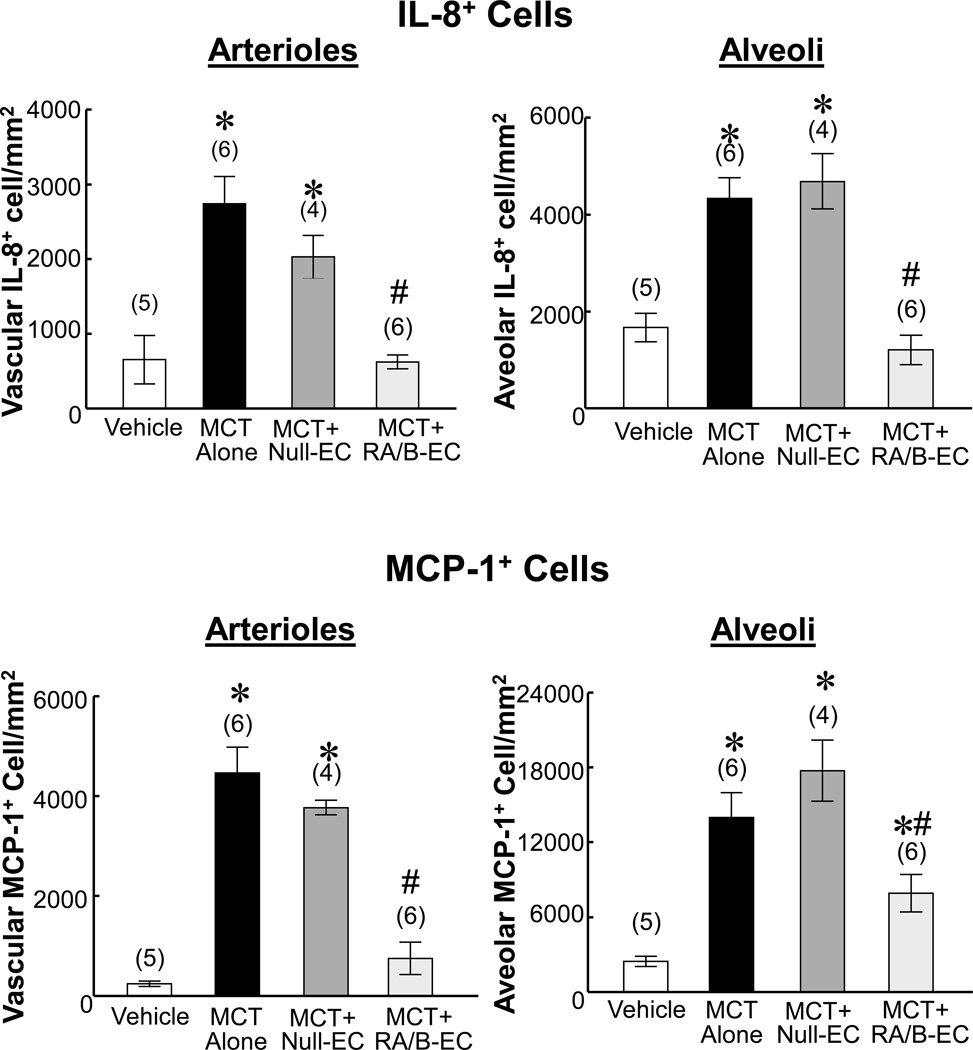
Transfusion of IL8RA/B-ECs inhibited MCT-induced increases in IL8 (cytokine-induced neutrophil chemoattractant [CINC]-2β in rat) and monocyte chemoattractant protein (MCP)-1 positive cells in injured lungs at 2 days after MCT treatment
Immunohistochemical staining demonstrated large increases in number of IL8 and MCP-1 positive cells in arterioles and alveoli in lungs of MCT-treated and MCT+Null-EC-treated rats (Figure 5 and Supplement Figures VI–IX). Transfusion with IL8RA/B-ECs resulted in major reductions in number of IL8 and MCP-1 cells in these vessels compared to MCT-treated and MCT+Null-EC-treated rats. The number of IL8 positive cells in the injured lung was positively correlated with the number of MCP-1 positive cells (R=0.823, p<0.01 for arterioles; and R=0.614, p<0.05 for alveoli).
Figure 5.
Effects of IL8RA/B-EC or Null-EC transfusion on MCT-induced increase in IL-8 and MCP-1 expression on pulmonary arterioles and alveoli at 4 wks after MCT treatment. Positive immunostained IL-8 (CINC-2β) or MCP-1 cells in arterioles or alveoli in each 400x microscopic field were counted, and 10 microscopic fields in each lung section were randomly selected for counting. (n)= number of rats. Results are means±SEM. * p<0.05 vs. respective Vehicle control groups. # p<0.05 vs. respective MCT alone and MCT+Null-EC groups. One way ANOVA was used to analyze the data, followed by a multiple-comparison Tukey post-hoc test. CINC-2β-cytokine induced neutrophil chemoattractant-2-beta (equivalent to human IL-8); MCP-1 - monocyte chemotactic protein-1. Representative micrographs are shown in Supplement Figures VI–IX.
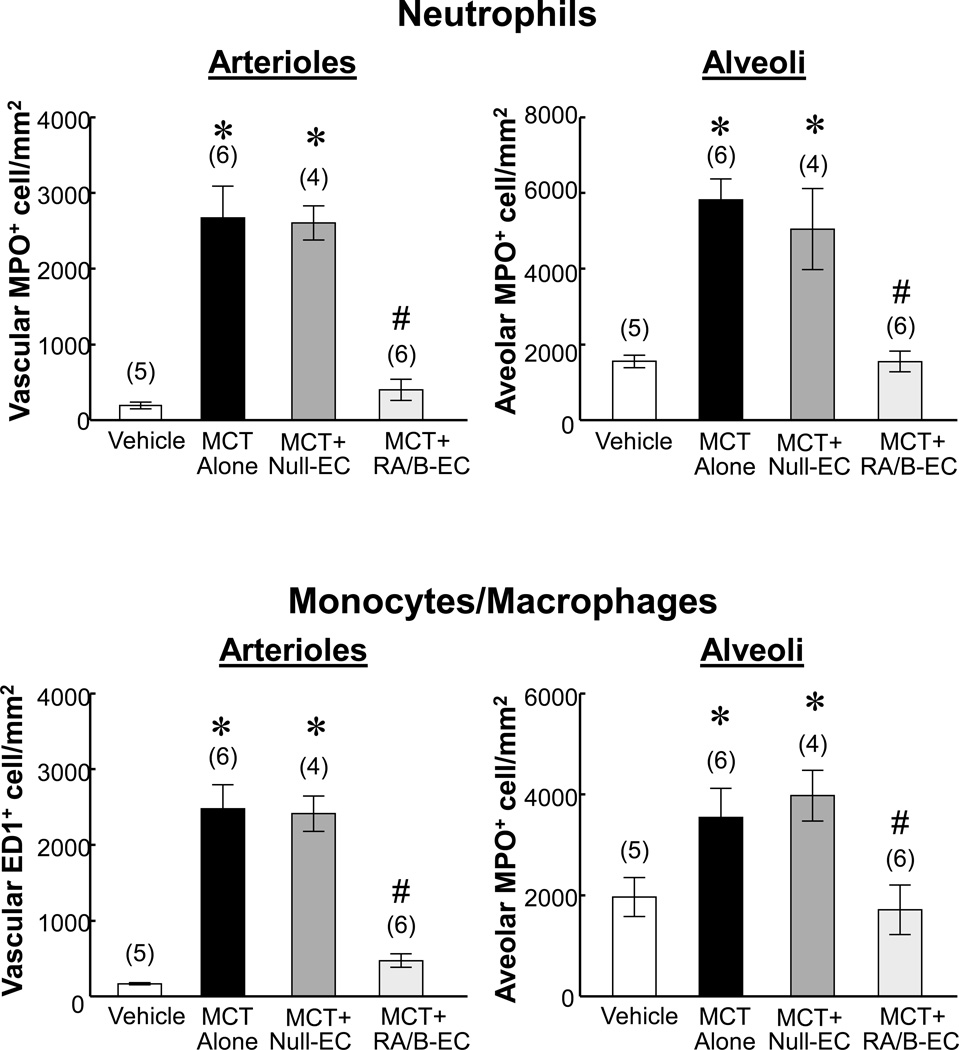
Transfusion of IL8RA/B-ECs inhibited neutrophil and monocyte/macrophage infiltration in injured lungs at 2 days after MCT treatment
Immunohistochemical staining demonstrated large numbers of neutrophils (myeloperoxidase [MPO]-positive) and monocytes/macrophages (ED1-positive) in arterioles and alveoli in lungs of MCT-treated and MCT+Null-EC-treated rats. Numbers of neutrophils and monocytes/macrophages were greatly reduced following IL8RA/B-EC treatment (Figure 6 and Supplement Figures X–XIII). The numbers of neutrophil and monocyte/macrophage were positively correlated with numbers of IL8 and MCP-1 (Figure 5) positive cells in pulmonary arterioles (R=0.655, p<0.05 for neutrophil/IL8; R=0.916, p<0.01 for monocyte/macrophage) and alveoli (R=0.741, p<0.05 for neutrophil/IL8; R=0.260. p>0.05 for monocyte/macrophage), respectively.
Figure 6.
Effects of IL8RA/RB-EC or Null-EC transfusion on MCT-induced neutrophil and monocyte/macrophage infiltration at 4 wks after MCT treatment. Positive immunostained neutrophils and monocytes/macrophages in arterioles or alveoli in each 400x microscopic field were counted, and 10 microscopic fields on each lung section were randomly selected for counting. (n)= number of rats. Results are means±SEM. * p<0.05 vs. respective Vehicle control groups. # p<0.05 vs. respective MCT alone and MCT+Null-EC groups. One way ANOVA was used to analyze the data, followed by a multiple-comparison Tukey post-hoc test. Representative microscopic micrographs are shown in Supplement Figures X–XIII.
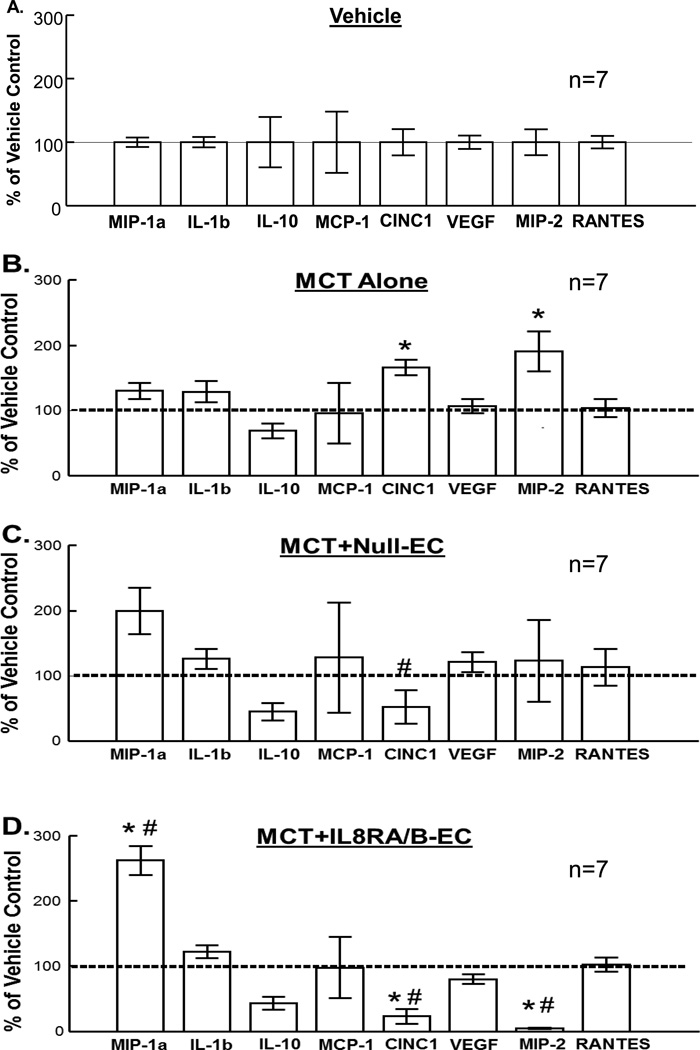
Transfusion of IL8RA/B-ECs prevented MCT-induced increases in cytokine-induced neutrophil chemoattractant-1 (CINC1, rat homologue to human IL-8) and macrophage inflammatory protein-2 (MIP-2) expression in lungs at 2 days after MCT treatment (and one day after IL8RA/B-EC treatment)
In separate groups of rats, Multiplexed rat specific magnetic beads-based sandwich immunoassay demonstrated significant increases in protein levels of pro-inflammatory mediators CINC1 and MIP-2 in whole lung homogenates of rats treated with MCT (Figure 7B). In contrast, transfusion with IL8RA/B-ECs significantly decreased CINC1 and MIP-2 and increased macrophage inflammatory protein-1 alpha (MIP-1a) protein expression in lungs of MCT+IL8RA/B-EC rats compared to those of Vehicle control or MCT-treated rats (Figure 7D). MCT-Null-EC-treatment was also associated with decreased CINC1 and increased MIP-1a levels (Figures 7C&D). Neither MCT treatment nor EC transfusion altered levels of the other cytokines/chemokines measured [e.g., interleukin-1 beta (IL-1b), interleukin-10 (IL-10), monocyte chemotactic protein-1 (MCP-1), vascular endothelial growth factor (VEGF), and regulated on activation, normal T cell expressed and secreted (RANTES)(chemokine ligand 5,CCL5). The concentrations of cytokine/chemokine in lungs of Vehicle control rats (in pg/µg, means±SEM, n=7) were: MIP-1a (0.143±0.011); IL-1b (1.059±0.087); IL-10 (0.143±0.011); MCP-1 (0.189±0.091); CINC1 (0.211±0.044); VEGF (4.951±0.515); MIP-2 (0.123±0.025); and RANTES (4.589±0.049). Levels of tumor necrosis factor alpha (TNF-α) and IL-6 were too low to be detected by the Multiplex analysis.
Figure 7.
Effects of (A) Vehicle, (B) MCT treatment, and/or (C) Null-EC, (D) IL8RA/B-EC transfusion on cytokine/chemokine protein expression in lungs of MCT-treated rats at 2 days after MCT treatment (1 day after transfusion of ECs). Cytokine/chemokine levels were measured in whole lung homogenates using commercially available Multiplexed rat specific magnetic beads-based sandwich immunoassay kits with the Luminex xMAP analyzer (Milliplex Rat Cytokine/Chemokine Panel, Millipore). Cytokine/chemokine level was normalized by total protein concentration in each sample and then standardized (in %) by (A) mean values of respective Vehicle control rats (horizontal line in each panel). Results are means±SEM. (n)= number of rats in each group. * p<0.05 vs. respective Vehicle control groups. # p<0.05 vs. respective MCT alone groups. One way ANOVA was used to analyze the data, followed by a multiple-comparison Tukey post-hoc test. MIP-1a - Macrophage Inflammatory Protein-1 alpha (CCL3); IL-1b – Interleukin-1 beta; IL-10 – Interleukin-10 ; MCP-1 - Monocyte Chemotactic Protein-1; CINC1 - Cytokine Induced Neutrophil Chemoattractant-1 (rat homologue to human IL-8); VEGF – Vascular Endothelial Growth Factor; MIP-2 – Macrophage Inflammatory Protein-2 (a CXC chemokine that stimulates neutrophil mobilization) ; RANTES – Regulated on Activation, Normal T cell Expressed and Secreted, also called chemokine ligand 5 (CCL5).
Discussion
The current study has shown for the first time that i.v. transfusion of pulmonary arterial ECs overexpressing IL8RA/B homing devices significantly enhances eNOS expression and inhibits expression of iNOS and other pro-inflammatory mediators, as well as and infiltration of neutrophils and monocytes/macrophages in lungs of rats treated with MCT. These anti-inflammatory effects of targeting ECs to the injured lung effectively prevented pulmonary vascular hypertrophy and remodeling, and loss of RV function following MCT treatment. These results support our hypothesis that ECs overexpressing IL8RA/B mimic the behavior of neutrophils that target IL8 and acPGP expressed in acutely injured lungs, thus inhibiting pro-inflammatory responses, accelerating endothelial repair and decreasing vascular hypertrophy and remodeling in injured lungs, thus preserving RV function after acute injury by MCT. Targeting delivery of ECs equipped with the homing device (IL8 receptors) to the site of pulmonary injury, as demonstrated in this study, provides a potential novel strategy for the treatment of pulmonary vascular diseases.
Pulmonary arterial hypertension is a complex disorder that is characterized by remodeling of pulmonary arteries and increased pulmonary vascular resistance, resulting in progressive elevation in pulmonary arterial pressure and subsequent RV failure and death (7,8). Progressive structural remodeling of small pulmonary arteries and arterioles is associated with excessive proliferation of pulmonary arterial smooth muscle cells (PASMCs).
A single s.c. MCT injection has been shown to induce pulmonary hypertension over a period of 3–4 wks in the rat. The active hepatic metabolite of MCT (MCT pyrrole) selectively damages the pulmonary vascular endothelium and stimulates the proliferation of PASMCs, resulting in pulmonary arterial remodeling and hypertrophy, pulmonary arterial hypertension and RV dysfunction (9). Enhancing regeneration of endothelium (re-endothelialization) in injured vessels is thus a potential therapeutic target to rescue tissue from critical vascular injury. After severe endothelial injury occurs in MCT treated animals, protection of pulmonary arterial structure and function can decrease pulmonary arterial hypertension, thus preventing RV dysfunction and reducing mortality. Therapeutic endothelial repair has thus become a promising new method of treatment for patients with pulmonary arterial diseases.
Cell- and gene-based therapies for pulmonary arterial diseases have proliferated over the past years, but with limited success (10,11,12). Cell-based therapies have been shown to alleviate pulmonary hypertension and RV dysfunction, but the majority of the studies are currently at the preclinical level in animal models (11,13,14,15,16). An important limitation of cell therapy is that, since the introduced cells lack selective homing device(s) to target injured tissues, they must be injected locally or directly into the affected tissue to increase therapeutic efficiency. Such invasive procedures are costly and difficult to adapt for widespread clinical use. Other major hurdles for successful cell therapies include 1) cell type selection (e.g. stem cells or differentiated cells), 2) cell delivery mode (e.g. peripherally or directly into tissue), 3) timing of cell delivery (e.g. acute or delayed treatment), 4) rejection of transplanted cells (e.g. autologous or heterologous transplantation), 5) age of cells, and most importantly, 6) targeting delivery of cells to damaged organs to maximize therapeutic effects. Several strategies including growth factor delivery (15,17), gene therapy (14,18,19) and stem cell therapy (16,17) have been developed to promote vascular repair in pulmonary vascular diseases. Among them, cell-based therapy has emerged as the most promising therapeutic tool for treatment of pulmonary hypertension.
Both non-selective bone marrow-derived stem cells (MSCs) and selective endothelial progenitor cells (EPCs) have been used in rodent models of hypoxia- or MCT-induced pulmonary arterial remodeling and hypertension (11,14–17). Intra-tracheal administration of MSCs has been shown to attenuate neonatal hyperoxia-induced lung injury (16). Transplantation of MSCs overexpressing hepatocyte growth factor, alone or in combination with granulocyte colony-stimulating factor, attenuates the development of MCT-induced pulmonary arterial hypertension in rats (15). Localized jugular vein delivery of prostacyclin-producing EPCs attenuates MCT-induced RV systolic pressure increase, RV hypertrophy, and pulmonary vessel wall thickening. These effects have led to functional recovery of pulmonary arteries (17).
A critical limitation for the therapeutic application of exogenous MSCs or EPCs is the lack of guidance for the cells to reach target tissue to elicit maximal therapeutic efficiency. Since the introduced cells lack a selective homing device to target the injured tissues, they must be introduced directly into the affected tissue (e.g. injured lung tissue or vessels), procedures that are invasive and may be hazardous. In the current study, the injured pulmonary arterioles are the therapeutic target for treatment of the MCT-induced injury. We have generated RAECs overexpressing IL8RA/B that can mimic the behavior of neutrophils in binding to IL8 that is produced by injured tissues (3,4). We have previously demonstrated that adenoviral transduction did not change the expression of EC makers (vWF and CD31) in ECs transduced with Ad-ILRA/RB-GFP (green fluorescence protein) vector. The percentage of ECs expressing the GFP marker peaked (~90%) at 3 days after transduction and gradually decreased during cell proliferation (50% at passage 1 and < 10% at passage 2), Similarly, the intensity of GFP staining diminished over time with cell proliferation (4). The in vitro proliferative activity of adenoviral transduced IL8RA/RB-ECs was the same as un-transduced control ECs and the migratory activity of IL8RA/RB-EC to IL8 (in a dose-dependent manner, assessed by Boyden chamber technique) was significantly greater compared to un-transduced cells (3). We also showed that IL8RA/B-ECs can compete with neutrophils to attach to activated endothelial monolayers in vitro and that i.v. transfusion of these transduced ECs into rats with balloon injured carotid arteries or LAD ligation injured LV resulted in targeting and adhesion of the transduced RAECs to injured arterial or cardiac tissues (3,4). Transfusion of RAECs overexpressing IL8RA/B resulted in decreased neutrophil infiltration and inflammatory mediator expression, accelerated re-endothelializtion, neo-vascularization, and reduced neointima formation in injured arteries (3) or LAD ligation induced myocardial infarction (4). The goal of the current study was to develop novel approaches and strategies to safely and attenuate the contribution of neutrophils to the pulmonary vascular injury response without impairing resistance to infection. This approach targets delivery of ECs equipped with homing device (IL8RA/B) to injured pulmonary vessels, thus efficiently inhibiting neutrophil and monocyte/macrophage infiltration and pro-inflammatory responses. Targeting delivery of ECs to sites of pulmonary vascular injury provides a novel therapeutic strategy for pulmonary disease. Findings from the current (using a pulmonary vascular injury model) and our previous studies [using a vascular injury model (3), and a cardiac injury model (4)] not only provide a novel strategy for therapeutic interventions for cardiopulmonary vascular diseases, but also fundamentally advance the field of cell-based therapy.
Acute inflammatory responses to MCT injection promote vascular EC apoptosis, pulmonary arterial hypertrophy/fibrosis/ remodeling, and impairing pulmonary arterial and RV function (20,21). Neutrophils migrate to injured tissue in response to the IL8 and acPGP (a tripeptide fragment of collagen that is 3-D structurally similar to IL8) that are expressed and released in large amounts by injured tissues (1,2). Interactions between IL8 and its cognate receptors on neutrophil membranes play a key role in host defense and disease responses following recruitment of activated neutrophils to sites of inflammation (22). IL8 binds to selective IL8RA/B on the neutrophil surface (1,23), and activated IL8RA/B induce expression of chemotactic mediators that trigger local inflammation. Neutrophils are the main leukocyte subset that interacts with the damaged endothelium, triggers the initial pro-inflammatory response, and facilitates the influx of other classes of inflammatory cells e.g. monocytes/macrophages and T cells in the setting of acute vascular injury (24,25). Using this background knowledge, we have developed an innovative strategy to equip ECs with a neutrophil homing device in order to target their delivery to injured tissues.
We chose eNOS and iNOS as target genes to assess endothelial function in MCT-treated lungs. Endothelial NOS (eNOS) has been used as an index of vascular EC function (26). In healthy endothelium, nitric oxide (NO) formed via eNOS plays a crucial role in the regulation of vascular blood flow through vasodilatation and decreased vascular resistance. Further, in gene therapy studies, eNOS has been shown to promote re-endothelialization and inhibits intimal hyperplasia in injured blood vessels (27). In contrast, an increase in iNOS expression in the injured vasculature promotes inflammation and vascular smooth muscle cell (VSMC) migration and proliferation in the setting of atherosclerosis and angioplasty-induced restenosis. A common feature of many forms of vascular dysfunction is the contribution of reactive oxygen species (ROS) and reactive nitrogen species (RNS) to vascular injury. Primary sources of ROS and RNS in VSMCs are the cytokine-regulated iNOS and NADPH oxidase. Nox-derived ROS modulates NO bioavailability by altering the expression of iNOS. Reaction of NO produced by iNOS with superoxide yields peroxynitrite, which contributes to the pathogenesis of vascular dysfunction and hypertension (28). Therefore, the goal to repair the vascular injury is to restore eNOS expression and decrease iNOS expression. In the current study, our IL8RA/B-ECs treatment in MCT-treated rats has successfully reached this goal.
We also demonstrated that, compared with MCT alone and MCT+Null-EC rats, transfusion with ECs overexpressing IL8RA/B one day after MCT treatment decreased expression of the pro-inflammatory chemoattractants IL8 and MCP-1 expression, as well as neutrophil and monocyte/macrophage infiltration into pulmonary arterioles and alveoli (as shown by immunohistochemical analysis in Figures 5 and 6), and reduced pulmonary arteriole hypertrophy in association with preserved RV function in rats treated with MCT. IL8RA/B-EC transfusion reversed the MCT treatment induced increases in CINC1 and MIP-2 protein levels in lungs, confirming the results of the immunohistochemical analysis. Similar to CINC-2β, CINC1 is a rat homologue of human IL-8, and MIP-2 is a member of the family of CXC chemokines and a neutrophil chemoattractant that plays a role in inflammatory, immune, and wound healing responses in lung. MIP2 and CINCs constitute a chemokine gradient that orchestrates influx of neutrophils into lung, leading to accumulation of neutrophils during acute lung injury (29). Our data indicate that transfusion of ECs overexpressing IL8-RA/B has protective effects after acute pulmonary vascular injury.
In summary, the current study clearly provides proof of principle that targeted delivery of ECs is effective in repairing injured tissue. Results in Figure 7 demonstrated significant increases in protein levels of a number of cytokines and chemokines in lung homogenates at 2 days after MCT treatment, clear evidence of lung injury. Expression of these pro-inflammatory molecules is attenuated by treatment with IL8RA/B-EC (and to a lesser extent with null-EC), indicating reversal of the injury response. We suggest that the targeted delivery of ECs to sites of pulmonary injury provides a novel therapeutic strategy for pulmonary vascular disease. This novel strategy offers advantages over existing methods that include: 1) Elimination of need for local injection of cells into injured organs due to the homing device. Many existing cell therapies require direct injection of cells into injured organs, which are costly and carry substantial risk for the recipient; 2) Selective inhibition of inflammatory responses at the site of injury with healthy ECs to compete with pro-inflammatory neutrophils and monocytes/macrophages; 3) Preservation of resident cell survival by a combination of inhibiting inflammation and promoting re-endothelization and/or re-vascularization by transplanted ECs. We hypothesize that targeted EC therapy will also have beneficial effects in forms of chronic vascular injury involved neutrophil and monocyte/macrophage infiltration and inflammation. Future studies are needed to test this hypothesis.
Supplementary Material
SIGNIFICANCE.
Cell therapies for pulmonary arterial diseases have proliferated over the past years, but with limited success. A critical limitation is the lack of guidance for the cells to reach target tissue to elicit maximal therapeutic efficiency, thus they must be introduced directly into the affected tissue. Such invasive procedures are costly and difficult to adapt for widespread clinical use. We have developed an innovative strategy to overcome this hurdle by intravenously transfusing rat endothelial cells overexpressing the neutrophil interleukin-8 receptors (IL8R) into rat with pulmonary vascular injury and hypertension induced by monocrotaline. We demonstrated that, equipped with the IL8R homing device, transfused endothelial cells mimic the behavior of neutrophils to bind to IL8 produced in injured lungs, inhibit pro-inflammatory responses, decrease vascular hypertrophy and remodeling in injured lungs, thus preserve right ventricular function following pulmonary vascular injury induced by monocrotaline.
Acknowledgements
This work was supported, in part, by UAB CCVC William W. Featheringill Innovative Award (Chen YF); National Heart, Lung, and Blood Institute grants: RO1HL116727 (Chen YF), RO1HL087980 (Oparil S); by American Heart Association Grant AHA SDG0930098N (Hage FG); and by Veterans Affairs Biomedical Laboratory Research & Development Service Merit Award OMB 4040-0001 (Hage FG).
Non standard Abbreviations and Acronyms
- CCL5
chemokine ligand 5
- CINC
cytokine-induced neutrophil chemoattractant
- ECs
endothelial cells
- EPCs
endothelial progenitor cells
- IL
Interleukin
- IL8RA/B
Interleukin-8 receptors A and B
- LAD
left anterior descending
- MCP-1
monocyte chemoattractant protein
- MCT
monocrotaline
- MIP
macrophage inflammatory protein
- MPO
myeloperoxidase
- MSCs
marrow-derived stem cells
- NO
nitric oxide
- eNOS
endothelial nitric oxide synthase
- iNOS
inducible nitric oxide synthase
- PASMCs
pulmonary arterial smooth muscle cells
- acPGP
N-acetylated proline-glycine–proline
- RAECs
rat aortic endothelial cells
- RANTES
regulated on activation, normal T cell expressed and secreted
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- RV
right ventricular
- TNF-α
tumor necrosis factor alpha
- VEGF
vascular endothelial growth factor
Footnotes
Disclosures
None
References
- 1.Stillie R, Farooq SM, Gordon JR, Stadnyk AW. The functional significance behind expressing two IL-8 receptor types on PMN. J Leukoc Biol. 2009;86:529–543. doi: 10.1189/jlb.0208125. [DOI] [PubMed] [Google Scholar]
- 2.Snelgrove RJ. Targeting of a common receptor shared by CXCL8 and N-Ac-PGP as a therapeutic strategy to alleviate chronic neutrophilic lung diseases. Eur J Pharmacol. 2011;667:1–5. doi: 10.1016/j.ejphar.2011.05.073. [DOI] [PubMed] [Google Scholar]
- 3.Xing D, Li P, Gong K, Yu H, Yang Z, Hage F, Oparil S, Chen YF. Endothelial cells overexpressing interleukin-8 (IL8) receptors reduce inflammatory and neointimal responses to arterial injury. Circulation. 2012;125:1533–1541. doi: 10.1161/CIRCULATIONAHA.111.078436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao X, Zhang W, Xing D, Li P, Fu J, Gong K, Hage FG, Oparil S, Chen YF. Endothelial cells overexpressing IL-8 receptor reduce cardiac remodeling and dysfunction following myocardial infarction. Am J Physiol Heart Circ Physiol. 2013;305:H590–H598. doi: 10.1152/ajpheart.00571.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King J, Hamil T, Creighton J, Wu S, Bhat P, McDonald F, Stevens T. Structural and functional characteristics of lung macro- and microvascular endothelial cell phenotypes. Microvasc Res. 2004;67:139–151. doi: 10.1016/j.mvr.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Jones JE, Mendes L, Rudd MA, Russo G, Loscalzo J, Zhang YY. Serial noninvasive assessment of progressive pulmonary hypertension in a rat model. Am J Physiol Heart Circ Physiol. 2002;283:H364–H371. doi: 10.1152/ajpheart.00979.2001. [DOI] [PubMed] [Google Scholar]
- 7.Mathew R. Pulmonary hypertension: current therapy and future prospects. Cardiovasc Hematol Agents Med Chem. 2011;9:165–182. doi: 10.2174/187152511797037501. [DOI] [PubMed] [Google Scholar]
- 8.Voelkel NF, Gomez-Arroyo J, Abbate A, Bogaard HJ, Nicolls MR. Pathobiology of pulmonary arterial hypertension and right ventricular failure. Eur Respir J. 2012;40:1555–1565. doi: 10.1183/09031936.00046612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson DW, Segall HJ, Pan LC, Lamé MW, Estep JE, Morin D. Mechanisms and pathology of monocrotaline pulmonary toxicity. Crit Rev Toxicol. 1992;22:307–325. doi: 10.3109/10408449209146311. [DOI] [PubMed] [Google Scholar]
- 10.Dewachter L, Dewachter C, Naeije R. New therapies for pulmonary arterial hypertension: an update on current bench to bedside translation. Expert Opin Investig Drugs. 2010;19:469–488. doi: 10.1517/13543781003727099. [DOI] [PubMed] [Google Scholar]
- 11.Weiss DJ. Current status of stem cells and regenerative medicine in lung biology and diseases. Stem Cells. 2014;32:16–25. doi: 10.1002/stem.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Y, O'Callaghan DS, Humbert M. An Update on Medical Therapy for Pulmonary Arterial Hypertension. Curr Hypertens Rep. 2013;15:614–622. doi: 10.1007/s11906-013-0394-8. [DOI] [PubMed] [Google Scholar]
- 13.Lee C, Mitsialis SA, Aslam M, Vitali SH, Vergadi E, Konstantinou G, Sdrimas K, Fernandez-Gonzalez A, Kourembanas S. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. 2012;126:2601–2611. doi: 10.1161/CIRCULATIONAHA.112.114173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farkas L, Kolb M. Vascular repair and regeneration as a therapeutic target for pulmonary arterial hypertension. Respiration. 2013;85:355–364. doi: 10.1159/000350177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo Y, Su L, Li Y, Guo N, Xie L, Zhang D, Zhang X, Li H, Zhang G, Wang Y, Liu C. The synergistic therapeutic effect of hepatocyte growth factor and granulocyte colony-stimulating factor on pulmonary hypertension in rats. Heart Vessels. 2013 Aug 10; doi: 10.1007/s00380-013-0395-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Ramachandran S, Suguihara C, Drummond S, Chatzistergos K, Klim J, Torres E, Huang J, Hehre D, Rodrigues CO, McNiece IK, Hare JM, Young KC. Bone Marrow-derived c-kit positive cells attenuate neonatal hyperoxia-induced lung Injury. Cell Transplant. 2013 May 22; doi: 10.3727/096368913X667736. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou L, Chen Z, Vanderslice P, So SP, Ruan KH, Willerson JT, Dixon RA. Endothelial-like progenitor cells engineered to produce prostacyclin rescue monocrotaline-induced pulmonary arterial hypertension and provide right ventricle benefits. Circulation. 2013;128:982–994. doi: 10.1161/CIRCULATIONAHA.113.003139. [DOI] [PubMed] [Google Scholar]
- 18.Caruso P, MacLean MR, Khanin R, McClure J, Soon E, Southgate M, MacDonald RA, Greig JA, Robertson KE, Masson R, Denby L, Dempsie Y, Long L, Morrell NW, Baker AH. Dynamic changes in lung microRNA profiles during the development of pulmonary hypertension due to chronic hypoxia and monocrotaline. Arterioscler Thromb Vasc Biol. 2010;30:716–723. doi: 10.1161/ATVBAHA.109.202028. [DOI] [PubMed] [Google Scholar]
- 19.Yuan K, Orcholski M, Tian X, Liao X, de Jesus Perez VA. MicroRNAs: promising therapeutic targets for the treatment of pulmonary arterial hypertension. Expert Opin Ther Targets. 2013;17:557–564. doi: 10.1517/14728222.2013.765863. [DOI] [PubMed] [Google Scholar]
- 20.Ghodsi F, Will JA. Changes in pulmonary structure and function induced by monocrotaline intoxication. Am J Physiol. 1981;240:H149–H155. doi: 10.1152/ajpheart.1981.240.2.H149. [DOI] [PubMed] [Google Scholar]
- 21.Gomez-Arroyo JG, Farkas L, Alhussaini AA, Farkas D, Kraskauskas D, Voelkel NF, Bogaard HJ. The monocrotaline model of pulmonary hypertension in perspective. Am J Physiol Lung Cell Mol Physiol. 2012;302:L363–L369. doi: 10.1152/ajplung.00212.2011. [DOI] [PubMed] [Google Scholar]
- 22.Skelton NJ, Quan C, Reilly D, Lowman H. Structure of a CXC chemokine-receptor fragment in complex with interleukin-8. Structure. 1999;7:157–168. doi: 10.1016/S0969-2126(99)80022-7. [DOI] [PubMed] [Google Scholar]
- 23.Fan X, Patera AC, Pong-Kennedy A, Deno G, Gonsiorek W, Manfra DJ, Vassileva G, Zeng M, Jackson C, Sullivan L, Sharif-Rodriguez W, Opdenakker G, Van Damme J, Hedrick JA, Lundell D, Lira SA, Hipkin RW. Murine CXCR1 is a functional receptor for GCP-2/CXCL6 and interleukin-8/CXCL8. J Biol Chem. 2007;82:11658–11666. doi: 10.1074/jbc.M607705200. [DOI] [PubMed] [Google Scholar]
- 24.Ionita MG, van den Borne P, Catanzariti LM, Moll FL, de Vries JP, Pasterkamp G, Vink A, de Kleijn DP. High neutrophil numbers in human carotid atherosclerotic plaques are associated with characteristics of rupture-prone lesions. Arterioscler Thromb Vasc Biol. 2010;30:1842–1848. doi: 10.1161/ATVBAHA.110.209296. [DOI] [PubMed] [Google Scholar]
- 25.Rotzius P, Thams S, Soehnlein O, Kenne E, Tseng CN, Björkström NK, Malmberg KJ, Lindbom L, Eriksson EE. Distinct infiltration of neutrophils in lesion shoulders in ApoE−/− mice. Am J Pathol. 2010;177:493–500. doi: 10.2353/ajpath.2010.090480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toda N, Toda H. Coronary hemodynamic regulation by nitric oxide in experimental animals: recent advances. Eur J Pharmacol. 2011;667:41–49. doi: 10.1016/j.ejphar.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 27.O'Connor DM, O'Brien T. Nitric oxide synthase gene therapy: progress and prospects. Expert Opin Biol Ther. 2009;9:867–878. doi: 10.1517/14712590903002047. [DOI] [PubMed] [Google Scholar]
- 28.Ginnan R, Guikema BJ, Halligan KE, Singer HA, Jourd'heuil D. Regulation of smooth muscle by inducible nitric oxide synthase and NADPH oxidase in vascular proliferative diseases. Free Radic Biol Med. 2008;44:1232–1245. doi: 10.1016/j.freeradbiomed.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo RF, Ward PA. Mediators and regulation of neutrophil accumulation in inflammatory responses in lung: insights from the IgG immune complex model. Free Radic Biol Med. 2002;33:303–310. doi: 10.1016/s0891-5849(02)00823-7. [DOI] [PubMed] [Google Scholar]
- 30.Jasińska-Stroschein M1, Owczarek J, Łuczak A, Orszulak-Michalak D. The beneficial impact of fasudil and sildenafil on monocrotaline-induced pulmonary hypertension in rats: a hemodynamic and biochemical study. Pharmacology. 2013;91:178–184. doi: 10.1159/000346921. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.