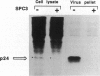
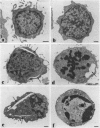
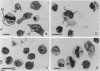
Abstract
The third variable region (V3 loop) of gp120, the HIV-1 surface envelope glycoprotein, plays a key role in HIV-1 infection and pathogenesis. Recently, we reported that a synthetic multibranched peptide (SPC3) containing eight V3-loop consensus motifs (GPGRAF) inhibited HIV-1 infection in both CD4+ and CD4- susceptible cells. In the present study, we investigated the mechanisms of action of SPC3 in these cell types--i.e., CD4+ lymphocytes and CD4- epithelial cells expressing galactosylceramide (GalCer), an alternative receptor for HIV-1 gp120. We found that SPC3 was a potent inhibitor of HIV-1 infection in CD4+ lymphocytes when added 1 h after initial exposure of the cells to HIV-1, whereas it had no inhibitory effect when present only before and/or during the incubation with HIV-1. These data suggested that SPC3 did not inhibit the binding of HIV-1 to CD4+ lymphocytes but interfered with a post-binding step necessary for virus entry. In agreement with this hypothesis, SPC3 treatment after HIV-1 exposure dramatically reduced the number of infected cells without altering gp120-CD4 interaction or viral gene expression. In contrast, SPC3 blocked HIV-1 entry into CD4-/GalCer+ human colon epithelial cells when present in competition with HIV-1 but had no effect when added after infection. Accordingly, SPC3 was found to inhibit the binding of gp120 to the GalCer receptor. Thus, the data suggest that SPC3 affects HIV-1 infection by two distinct mechanisms: (i) prevention of GalCer-mediated HIV-1 attachment to the surface of CD4-/GalCer+ cells and (ii) post-binding inhibition of HIV-1 entry into CD4+ lymphocytes.
Full text
PDF

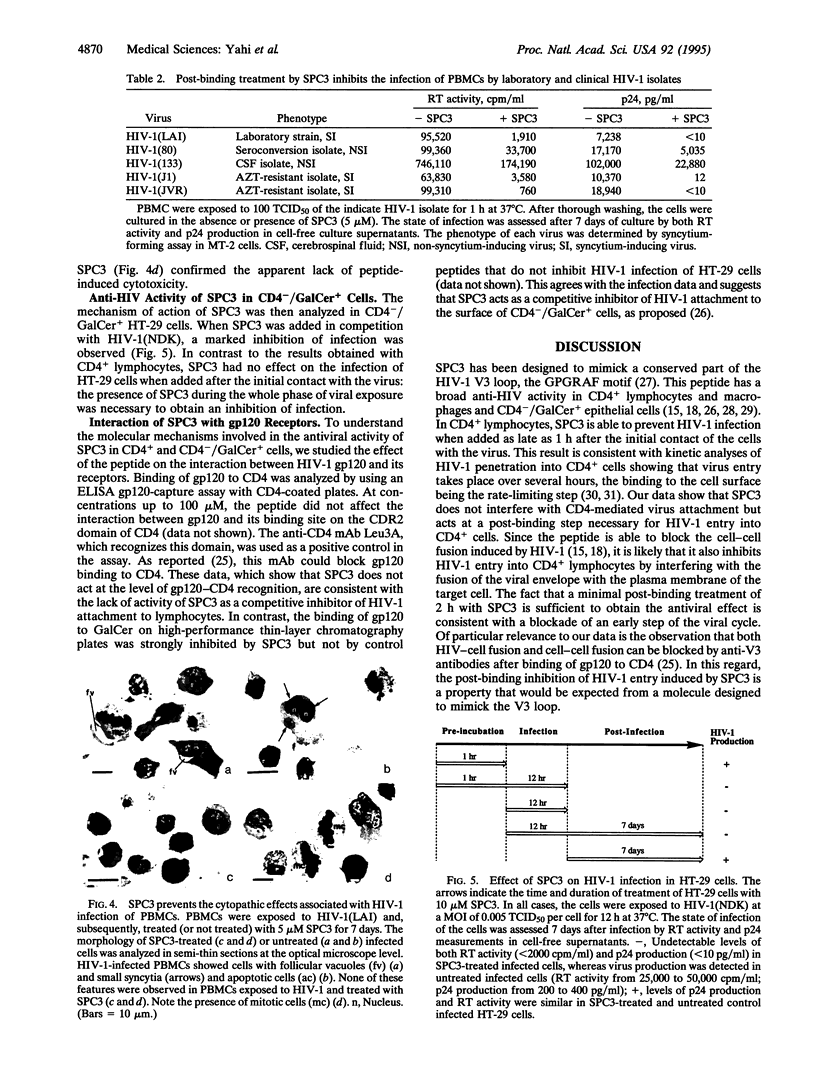

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P. Human immunodeficiency virus vaccines. Adv Virus Res. 1993;42:103–148. doi: 10.1016/s0065-3527(08)60084-6. [DOI] [PubMed] [Google Scholar]
- Cook D. G., Fantini J., Spitalnik S. L., Gonzalez-Scarano F. Binding of human immunodeficiency virus type I (HIV-1) gp120 to galactosylceramide (GalCer): relationship to the V3 loop. Virology. 1994 Jun;201(2):206–214. doi: 10.1006/viro.1994.1287. [DOI] [PubMed] [Google Scholar]
- De Rossi A., Pasti M., Mammano F., Panozzo M., Dettin M., Di Bello C., Chieco-Bianchi L. Synthetic peptides from the principal neutralizing domain of human immunodeficiency virus type 1 (HIV-1) enhance HIV-1 infection through a CD4-dependent mechanism. Virology. 1991 Sep;184(1):187–196. doi: 10.1016/0042-6822(91)90835-y. [DOI] [PubMed] [Google Scholar]
- Fantini J., Yahi N., Mabrouk K., Van Rietschoten J., Rochat H., Sabatier J. M. Multi-branched peptides based on the HIV-1 V3 loop consensus motif inhibit HIV-1 and HIV-2 infection in CD4+ and CD4- cells. C R Acad Sci III. 1993 Nov;316(11):1381–1387. [PubMed] [Google Scholar]
- Faure E., Yahi N., Zider A., Cavard C., Champion S., Fantini J. Physical contact with lymphocytes is required for reactivation of dormant HIV-1 in colonic epithelial cells: involvement of the HIV-1 LTR. Virus Res. 1994 Oct;34(1):1–13. doi: 10.1016/0168-1702(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Goudsmit J., Boucher C. A., Meloen R. H., Epstein L. G., Smit L., van der Hoek L., Bakker M. Human antibody response to a strain-specific HIV-1 gp120 epitope associated with cell fusion inhibition. AIDS. 1988 Jun;2(3):157–164. [PubMed] [Google Scholar]
- Harada S., Koyanagi Y., Yamamoto N. Infection of HTLV-III/LAV in HTLV-I-carrying cells MT-2 and MT-4 and application in a plaque assay. Science. 1985 Aug 9;229(4713):563–566. doi: 10.1126/science.2992081. [DOI] [PubMed] [Google Scholar]
- Harouse J. M., Bhat S., Spitalnik S. L., Laughlin M., Stefano K., Silberberg D. H., Gonzalez-Scarano F. Inhibition of entry of HIV-1 in neural cell lines by antibodies against galactosyl ceramide. Science. 1991 Jul 19;253(5017):320–323. doi: 10.1126/science.1857969. [DOI] [PubMed] [Google Scholar]
- Harouse J. M., Kunsch C., Hartle H. T., Laughlin M. A., Hoxie J. A., Wigdahl B., Gonzalez-Scarano F. CD4-independent infection of human neural cells by human immunodeficiency virus type 1. J Virol. 1989 Jun;63(6):2527–2533. doi: 10.1128/jvi.63.6.2527-2533.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S. S., Boyle T. J., Lyerly H. K., Cullen B. R. Identification of the envelope V3 loop as the primary determinant of cell tropism in HIV-1. Science. 1991 Jul 5;253(5015):71–74. doi: 10.1126/science.1905842. [DOI] [PubMed] [Google Scholar]
- LaRosa G. J., Davide J. P., Weinhold K., Waterbury J. A., Profy A. T., Lewis J. A., Langlois A. J., Dreesman G. R., Boswell R. N., Shadduck P. Conserved sequence and structural elements in the HIV-1 principal neutralizing determinant. Science. 1990 Aug 24;249(4971):932–935. doi: 10.1126/science.2392685. [DOI] [PubMed] [Google Scholar]
- Merrifield B. Solid phase synthesis. Science. 1986 Apr 18;232(4748):341–347. doi: 10.1126/science.3961484. [DOI] [PubMed] [Google Scholar]
- Nardelli B., Lu Y. A., Shiu D. R., Delpierre-Defoort C., Profy A. T., Tam J. P. A chemically defined synthetic vaccine model for HIV-1. J Immunol. 1992 Feb 1;148(3):914–920. [PubMed] [Google Scholar]
- Nehete P. N., Arlinghaus R. B., Sastry K. J. Inhibition of human immunodeficiency virus type 1 infection and syncytium formation in human cells by V3 loop synthetic peptides from gp120. J Virol. 1993 Nov;67(11):6841–6846. doi: 10.1128/jvi.67.11.6841-6846.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orloff G. M., Orloff S. L., Kennedy M. S., Maddon P. J., McDougal J. S. Penetration of CD4 T cells by HIV-1. The CD4 receptor does not internalize with HIV, and CD4-related signal transduction events are not required for entry. J Immunol. 1991 Apr 15;146(8):2578–2587. [PubMed] [Google Scholar]
- Palker T. J., Clark M. E., Langlois A. J., Matthews T. J., Weinhold K. J., Randall R. R., Bolognesi D. P., Haynes B. F. Type-specific neutralization of the human immunodeficiency virus with antibodies to env-encoded synthetic peptides. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1932–1936. doi: 10.1073/pnas.85.6.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieber E. P., Federle C., Reiter C., Krauss S., Gürtler L., Eberle J., Deinhardt F., Riethmüller G. The monoclonal CD4 antibody M-T413 inhibits cellular infection with human immunodeficiency virus after viral attachment to the cell membrane: an approach to postexposure prophylaxis. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10792–10796. doi: 10.1073/pnas.89.22.10792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusche J. R., Javaherian K., McDanal C., Petro J., Lynn D. L., Grimaila R., Langlois A., Gallo R. C., Arthur L. O., Fischinger P. J. Antibodies that inhibit fusion of human immunodeficiency virus-infected cells bind a 24-amino acid sequence of the viral envelope, gp120. Proc Natl Acad Sci U S A. 1988 May;85(9):3198–3202. doi: 10.1073/pnas.85.9.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier J. M., Zerrouk H., Darbon H., Mabrouk K., Benslimane A., Rochat H., Martin-Eauclaire M. F., Van Rietschoten J. P05, a new leiurotoxin I-like scorpion toxin: synthesis and structure-activity relationships of the alpha-amidated analog, a ligand of Ca(2+)-activated K+ channels with increased affinity. Biochemistry. 1993 Mar 23;32(11):2763–2770. doi: 10.1021/bi00062a005. [DOI] [PubMed] [Google Scholar]
- Shioda T., Levy J. A., Cheng-Mayer C. Macrophage and T cell-line tropisms of HIV-1 are determined by specific regions of the envelope gp120 gene. Nature. 1991 Jan 10;349(6305):167–169. doi: 10.1038/349167a0. [DOI] [PubMed] [Google Scholar]
- Spire B., Sire J., Zachar V., Rey F., Barré-Sinoussi F., Galibert F., Hampe A., Chermann J. C. Nucleotide sequence of HIV1-NDK: a highly cytopathic strain of the human immunodeficiency virus. Gene. 1989 Sep 30;81(2):275–284. doi: 10.1016/0378-1119(89)90188-1. [DOI] [PubMed] [Google Scholar]
- Tam J. P. Synthetic peptide vaccine design: synthesis and properties of a high-density multiple antigenic peptide system. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5409–5413. doi: 10.1073/pnas.85.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateno M., Gonzalez-Scarano F., Levy J. A. Human immunodeficiency virus can infect CD4-negative human fibroblastoid cells. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4287–4290. doi: 10.1073/pnas.86.11.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahi N., Baghdiguian S., Moreau H., Fantini J. Galactosyl ceramide (or a closely related molecule) is the receptor for human immunodeficiency virus type 1 on human colon epithelial HT29 cells. J Virol. 1992 Aug;66(8):4848–4854. doi: 10.1128/jvi.66.8.4848-4854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahi N., Fantini J., Mabrouk K., Tamalet C., de Micco P., van Rietschoten J., Rochat H., Sabatier J. M. Multibranched V3 peptides inhibit human immunodeficiency virus infection in human lymphocytes and macrophages. J Virol. 1994 Sep;68(9):5714–5720. doi: 10.1128/jvi.68.9.5714-5720.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahi N., Sabatier J. M., Baghdiguian S., Gonzalez-Scarano F., Fantini J. Synthetic multimeric peptides derived from the principal neutralization domain (V3 loop) of human immunodeficiency virus type 1 (HIV-1) gp120 bind to galactosylceramide and block HIV-1 infection in a human CD4-negative mucosal epithelial cell line. J Virol. 1995 Jan;69(1):320–325. doi: 10.1128/jvi.69.1.320-325.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahi N., Sabatier J. M., Nickel P., Mabrouk K., Gonzalez-Scarano F., Fantini J. Suramin inhibits binding of the V3 region of HIV-1 envelope glycoprotein gp120 to galactosylceramide, the receptor for HIV-1 gp120 on human colon epithelial cells. J Biol Chem. 1994 Sep 30;269(39):24349–24353. [PubMed] [Google Scholar]