Abstract
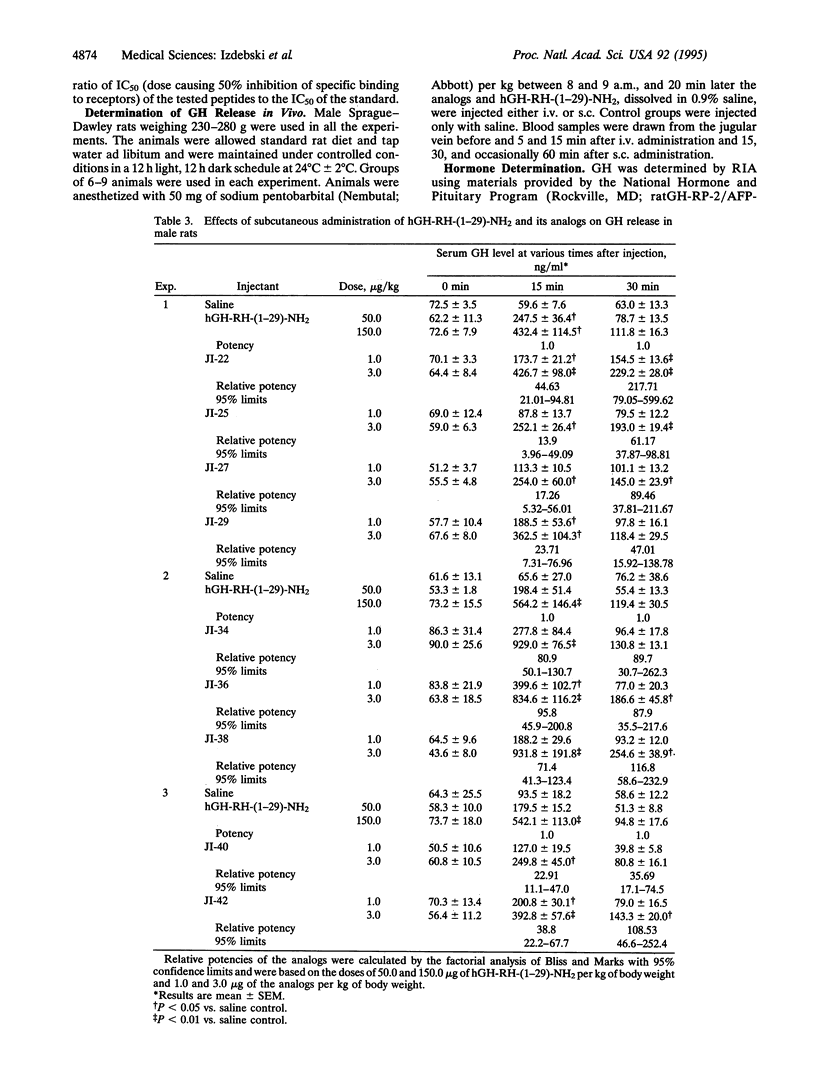
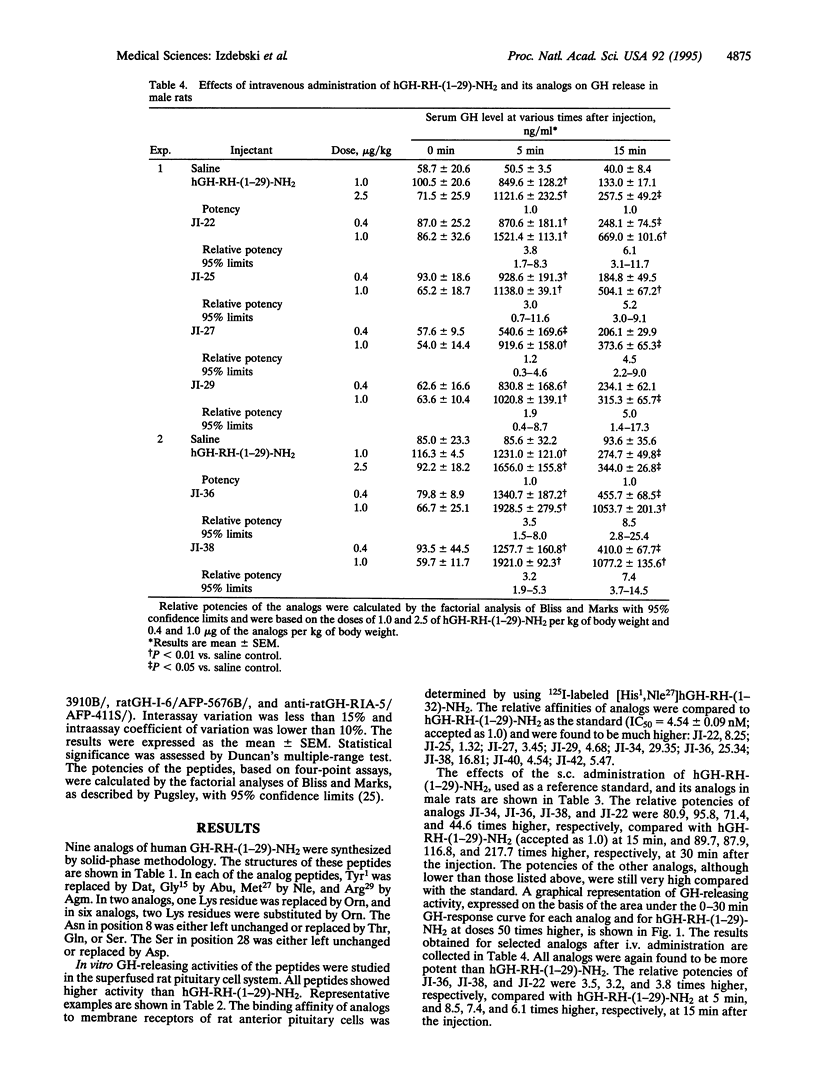
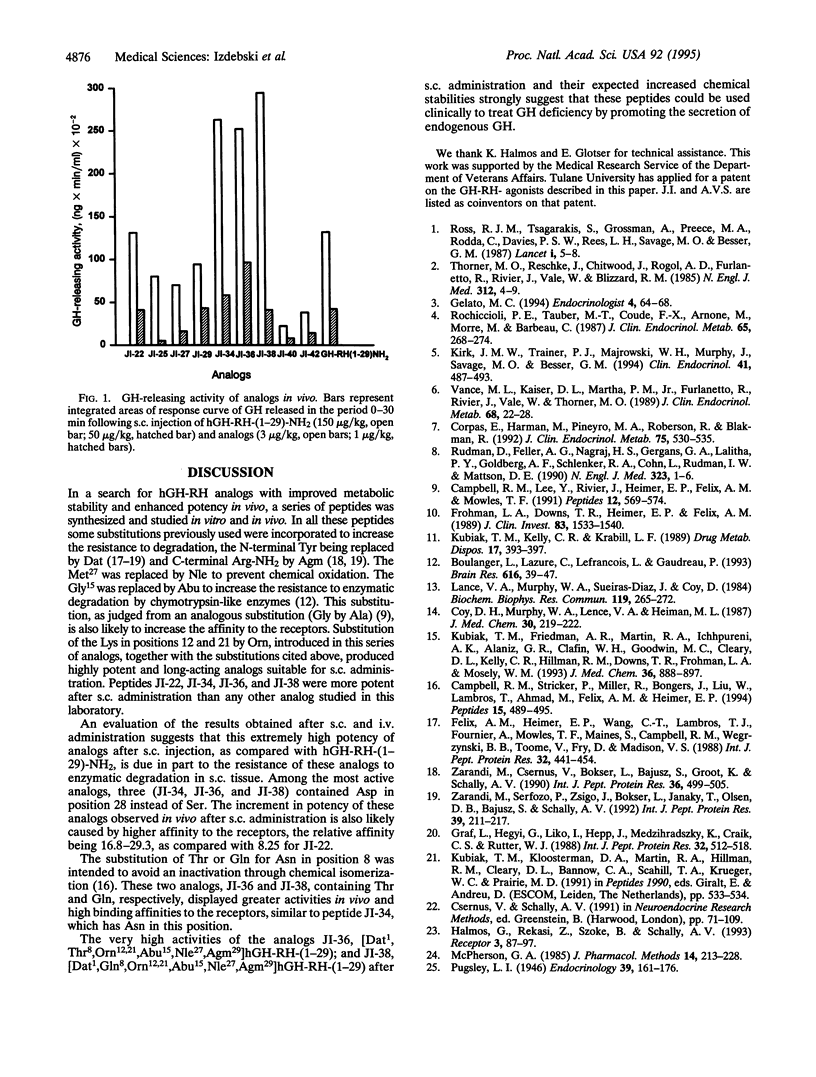
Analogs of the 29 amino acid sequence of human growth hormone-releasing hormone (hGH-RH) with agmatine (Agm) in position 29, desaminotyrosine (Dat) in position 1, norleucine (Nle) in position 27, and L-alpha-aminobutyric acid (Abu) in position 15 have been synthesized, and their biological activity was evaluated. Some peptides contained one or two residues of ornithine (Orn) instead of Lys in positions 12 and 21 and additional replacements in positions 8 and 28. All analogs were found to be more potent than hGH-RH-(1-29)-NH2 in the superfused rat pituitary cell system. In tests in vivo in rats after subcutaneous administration, the analogs JI-22, [Dat1, Orn12,21, Abu15, Nle27, Agm29]hGH-RH-(1-29); JI-34, [Dat1, Orn12,21,Abu15,Nle27, Asp28, Agm29]hGH-RH-(1-29); JI-36, [Dat1, Thr8, Orn12,21, Abu15,Nle27,Asp28,Agm29]hGH-RH-(1-29); and JI-38, [Dat1,Gln8, Orn12,21,Abu15,Nle27,Asp28,Agm29]hGH-RH-(1 -29) displayed a potency 44.6,80.9,95.8, and 71.4 times greater, respectively, than that of hGH-RH-(1-29)-NH2 at 15 min and 217.1, 89.7, 87.9, and 116.8 times greater at 30 min. After intravenous administration, JI-22, JI-36, and JI-38 were 3.2-3.8 times more potent than hGH-RH-(1-29)-NH2 at 5 min and 6.1-8.5 times more active at 15 min. All analogs were found to have higher binding affinities for GH-RH receptors on rat pituitary cells than hGH-RH-(1-29)-NH2. Because of high activity and greater stability, these analogs could be considered for therapy of patients with growth hormone deficiency.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boulanger L., Lazure C., Lefrançois L., Gaudreau P. Proteolytic degradation of rat growth hormone-releasing factor(1-29) amide in rat pituitary and hypothalamus. Brain Res. 1993 Jul 9;616(1-2):39–47. doi: 10.1016/0006-8993(93)90189-t. [DOI] [PubMed] [Google Scholar]
- Campbell R. M., Lee Y., Rivier J., Heimer E. P., Felix A. M., Mowles T. F. GRF analogs and fragments: correlation between receptor binding, activity and structure. Peptides. 1991 May-Jun;12(3):569–574. doi: 10.1016/0196-9781(91)90103-v. [DOI] [PubMed] [Google Scholar]
- Campbell R. M., Stricker P., Miller R., Bongers J., Liu W., Lambros T., Ahmad M., Felix A. M., Heimer E. P. Enhanced stability and potency of novel growth hormone-releasing factor (GRF) analogues derived from rodent and human GRF sequences. Peptides. 1994;15(3):489–495. doi: 10.1016/0196-9781(94)90211-9. [DOI] [PubMed] [Google Scholar]
- Corpas E., Harman S. M., Piñeyro M. A., Roberson R., Blackman M. R. Growth hormone (GH)-releasing hormone-(1-29) twice daily reverses the decreased GH and insulin-like growth factor-I levels in old men. J Clin Endocrinol Metab. 1992 Aug;75(2):530–535. doi: 10.1210/jcem.75.2.1379256. [DOI] [PubMed] [Google Scholar]
- Coy D. H., Murphy W. A., Lance V. A., Heiman M. L. Differential effects of N-terminal modifications on the biological potencies of growth hormone releasing factor analogues with varying chain lengths. J Med Chem. 1987 Jan;30(1):219–222. doi: 10.1021/jm00384a039. [DOI] [PubMed] [Google Scholar]
- Felix A. M., Heimer E. P., Wang C. T., Lambros T. J., Fournier A., Mowles T. F., Maines S., Campbell R. M., Wegrzynski B. B., Toome V. Synthesis, biological activity and conformational analysis of cyclic GRF analogs. Int J Pept Protein Res. 1988 Dec;32(6):441–454. doi: 10.1111/j.1399-3011.1988.tb01375.x. [DOI] [PubMed] [Google Scholar]
- Frohman L. A., Downs T. R., Heimer E. P., Felix A. M. Dipeptidylpeptidase IV and trypsin-like enzymatic degradation of human growth hormone-releasing hormone in plasma. J Clin Invest. 1989 May;83(5):1533–1540. doi: 10.1172/JCI114049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gráf L., Hegyi G., Likó I., Hepp J., Medzihradszky K., Craik C. S., Rutter W. J. Structural and functional integrity of specificity and catalytic sites of trypsin. Int J Pept Protein Res. 1988 Dec;32(6):512–518. doi: 10.1111/j.1399-3011.1988.tb01382.x. [DOI] [PubMed] [Google Scholar]
- Halmos G., Rekasi Z., Szoke B., Schally A. V. Use of radioreceptor assay and cell superfusion system for in vitro screening of analogs of growth hormone-releasing hormone. Receptor. 1993 Summer;3(2):87–97. [PubMed] [Google Scholar]
- Kirk J. M., Trainer P. J., Majrowski W. H., Murphy J., Savage M. O., Besser G. M. Treatment with GHRH(1-29)NH2 in children with idiopathic short stature induces a sustained increase in growth velocity. Clin Endocrinol (Oxf) 1994 Oct;41(4):487–493. doi: 10.1111/j.1365-2265.1994.tb02580.x. [DOI] [PubMed] [Google Scholar]
- Kubiak T. M., Friedman A. R., Martin R. A., Ichhpurani A. K., Alaniz G. R., Claflin W. H., Goodwin M. C., Cleary D. L., Kelly C. R., Hillman R. M. Position 2 and position 2/Ala15-substituted analogs of bovine growth hormone-releasing factor (bGRF) with enhanced metabolic stability and improved in vivo bioactivity. J Med Chem. 1993 Apr 2;36(7):888–897. doi: 10.1021/jm00059a014. [DOI] [PubMed] [Google Scholar]
- Kubiak T. M., Kelly C. R., Krabill L. F. In vitro metabolic degradation of a bovine growth hormone-releasing factor analog Leu27-bGRF(1-29)NH2 in bovine and porcine plasma. Correlation with plasma dipeptidylpeptidase activity. Drug Metab Dispos. 1989 Jul-Aug;17(4):393–397. [PubMed] [Google Scholar]
- Lance V. A., Murphy W. A., Sueiras-Diaz J., Coy D. H. Super-active analogs of growth hormone-releasing factor (1-29)-amide. Biochem Biophys Res Commun. 1984 Feb 29;119(1):265–272. doi: 10.1016/0006-291x(84)91647-4. [DOI] [PubMed] [Google Scholar]
- McPherson G. A. Analysis of radioligand binding experiments. A collection of computer programs for the IBM PC. J Pharmacol Methods. 1985 Nov;14(3):213–228. doi: 10.1016/0160-5402(85)90034-8. [DOI] [PubMed] [Google Scholar]
- Rochiccioli P. E., Tauber M. T., Coude F. X., Arnone M., Morre M., Uboldi F., Barbeau C. Results of 1-year growth hormone (GH)-releasing hormone-(1-44) treatment on growth, somatomedin-C, and 24-hour GH secretion in six children with partial GH deficiency. J Clin Endocrinol Metab. 1987 Aug;65(2):268–274. doi: 10.1210/jcem-65-2-268. [DOI] [PubMed] [Google Scholar]
- Ross R. J., Rodda C., Tsagarakis S., Davies P. S., Grossman A., Rees L. H., Preece M. A., Savage M. O., Besser G. M. Treatment of growth-hormone deficiency with growth-hormone-releasing hormone. Lancet. 1987 Jan 3;1(8523):5–8. doi: 10.1016/s0140-6736(87)90699-4. [DOI] [PubMed] [Google Scholar]
- Rudman D., Feller A. G., Nagraj H. S., Gergans G. A., Lalitha P. Y., Goldberg A. F., Schlenker R. A., Cohn L., Rudman I. W., Mattson D. E. Effects of human growth hormone in men over 60 years old. N Engl J Med. 1990 Jul 5;323(1):1–6. doi: 10.1056/NEJM199007053230101. [DOI] [PubMed] [Google Scholar]
- Thorner M. O., Reschke J., Chitwood J., Rogol A. D., Furlanetto R., Rivier J., Vale W., Blizzard R. M. Acceleration of growth in two children treated with human growth hormone-releasing factor. N Engl J Med. 1985 Jan 3;312(1):4–9. doi: 10.1056/NEJM198501033120102. [DOI] [PubMed] [Google Scholar]
- Vance M. L., Kaiser D. L., Martha P. M., Jr, Furlanetto R., Rivier J., Vale W., Thorner M. O. Lack of in vivo somatotroph desensitization or depletion after 14 days of continuous growth hormone (GH)-releasing hormone administration in normal men and a GH-deficient boy. J Clin Endocrinol Metab. 1989 Jan;68(1):22–28. doi: 10.1210/jcem-68-1-22. [DOI] [PubMed] [Google Scholar]
- Zarandi M., Csernus V., Bokser L., Bajusz S., Groot K., Schally A. V. Synthesis and in vitro and in vivo activity of analogs of growth hormone-releasing hormone (GH-RH) with C-terminal agmatine. Int J Pept Protein Res. 1990 Dec;36(6):499–505. doi: 10.1111/j.1399-3011.1990.tb00988.x. [DOI] [PubMed] [Google Scholar]
- Zarandi M., Serfozo P., Zsigo J., Bokser L., Janaky T., Olsen D. B., Bajusz S., Schally A. V. Potent agonists of growth hormone-releasing hormone. Part I. Int J Pept Protein Res. 1992 Mar;39(3):211–217. doi: 10.1111/j.1399-3011.1992.tb00791.x. [DOI] [PubMed] [Google Scholar]


