Abstract
The study is a randomized phase II trial investigating graft-versus-host disease prophylaxis after non-myeloablative (90 mg/m2 fludarabine and 2 Gy total body irradiation) human leukocyte antigen matched unrelated donor transplantation. Patients were randomized as follows: arm 1 – tacrolimus 180 days and mycophenolate mofetil 95 days (n=69); arm 2 – tacrolimus 150 days and mycophenolate mofetil 180 days (n=71); arm 3 – tacrolimus 150 days, mycophenolate mofetil 180 days and sirolimus 80 days (n=68). All patients had sustained engraftment. Grade II-IV acute graft-versus-host disease rates in the 3 arms were 64%, 48% and 47% at Day 150, respectively (arm 3 vs. arm 1 (hazard ratio 0.62; P=0.04). Owing to the decreased incidence of acute graft-versus-host disease, systemic steroid use was lower at Day 150 in arm 3 (32% vs. 55% in arm 1 and 49% in arm 2; overall P=0.009 by hazard ratio analysis). The Day 150 incidence of cytomegalovirus reactivation was lower in arm 3 (arm 1, 54%; arm 2, 47%; arm 3, 22%; overall P=0.002 by hazard ratio analysis). Non-relapse mortality was comparable in the three arms at two years (arm 1, 26%; arm 2, 23%; arm 3, 18%). Toxicity rates and other outcome measures were similar between the three arms. The addition of sirolimus to tacrolimus and mycophenolate mofetil is safe and associated with lower incidence of acute graft-versus-host disease and cytomegalovirus reactivation. (clinicaltrials.gov identifier: 00105001).
Introduction
In recent years, it has become increasingly evident that after non-myeloablative hematopoietic cell transplantation (HCT), development of chronic graft-versus-host-disease (GvHD) is associated with the graft-versus-tumor effects (GvT) and lower relapse incidence, while acute GvHD only increases the risk of treatment-related mortality.1–4 The most frequently used post-grafting immunosuppressive regimen after non-myeloablative conditioning with fludarabine (FLU) and 2 Gy total body irradiation (TBI) is based on a calcineurin inhibitor, cyclosporine (CSP) or tacrolimus, and mycophenolate mofetil (MMF). The approach was translated from the canine model,5,6 and its efficacy and safety in human unrelated HLA-matched donor transplantation has subsequently been established in a series of phase I/II trials.7–9 However, with a grade II-IV acute GvHD rate of 53% at Day 100 after HLA-matched unrelated donor transplantation, improvement is still warranted.9
This phase II randomized clinical trial is part of an ongoing effort to optimize control of acute GvHD without reducing the GvT effect after unrelated donor transplantation. Patients were randomized between three different post-transplantation immunosuppressive regimens (referred to as arms). In arm 1, tacrolimus was administered for 180 days and MMF for 95 days. In arms 2 and 3, tacrolimus and MMF were administered for 150 and 180 days, respectively, with the addition of 80 days of sirolimus in arm 3. The immunosuppressive regimen used in arm 1 was similar to the GvHD prophylaxis used in our previous non-myeloablative unrelated donor transplantation trials, and thus designated as the reference arm. The primary objective was to determine which of the regimens in the experimental arms 2 and 3 could reduce grade II–IV acute GvHD to 40% or under, compared to the reference arm. The calcineurin inhibitor, tacrolimus, was chosen over CSP due to observations from previous randomized phase III studies in patients given high-dose conditioning where the incidence of acute GvHD was lower when tacrolimus was combined with methotrexate instead of CSP, although survival rates in both arms of those trials were comparable.10–12 The mTOR inhibitor, sirolimus, which may have a synergistic effect with tacrolimus,13,14 was chosen as a third immunosuppressive drug based on positive experiences from phase I and II HCT trials.15,16
Methods
Study design
The study was a randomized phase II trial including 11 transplant centers (Online Supplementary Table S1). The Fred Hutchinson Cancer Research Center (FHCRC) acted as coordinating center. The study was approved by institutional review boards at the FHCRC and at collaborating centers. All patients signed consent forms approved by the local institutional review boards. The study was registered at clinicaltrials.gov (identifier: 00105001). The manuscript was prepared in accordance with the CONSORT 2010 statement (Online Supplementary Figure S1).17 The primary objective was to determine whether either of the two experimental immunosuppressive regimens could reduce grades II–IV acute GvHD to 40% or under. Secondary objectives were to reduce the Day 200 non-relapse mortality (NRM) to 15% or under, and to lower corticosteroid use compared to the reference arm.
Patients were randomized between three immunosuppressive treatment regimens and stratified according to transplant center (FHCRC vs. other), number of prior chemotherapy regimens (<3 vs. ≥3) and age (<55 vs. ≥55 years).
Patients with advanced hematologic malignancies (Table 1) treatable by non-myeloablative conditioned allogeneic HCT were eligible for the study. Donors were unrelated, high-resolution typed for HLA-A, -B, -C, -DRB1 and -DQB1, allele level matched (10/10) and no more than a single allele disparity for either HLA-A, -B, or -C was allowed.18
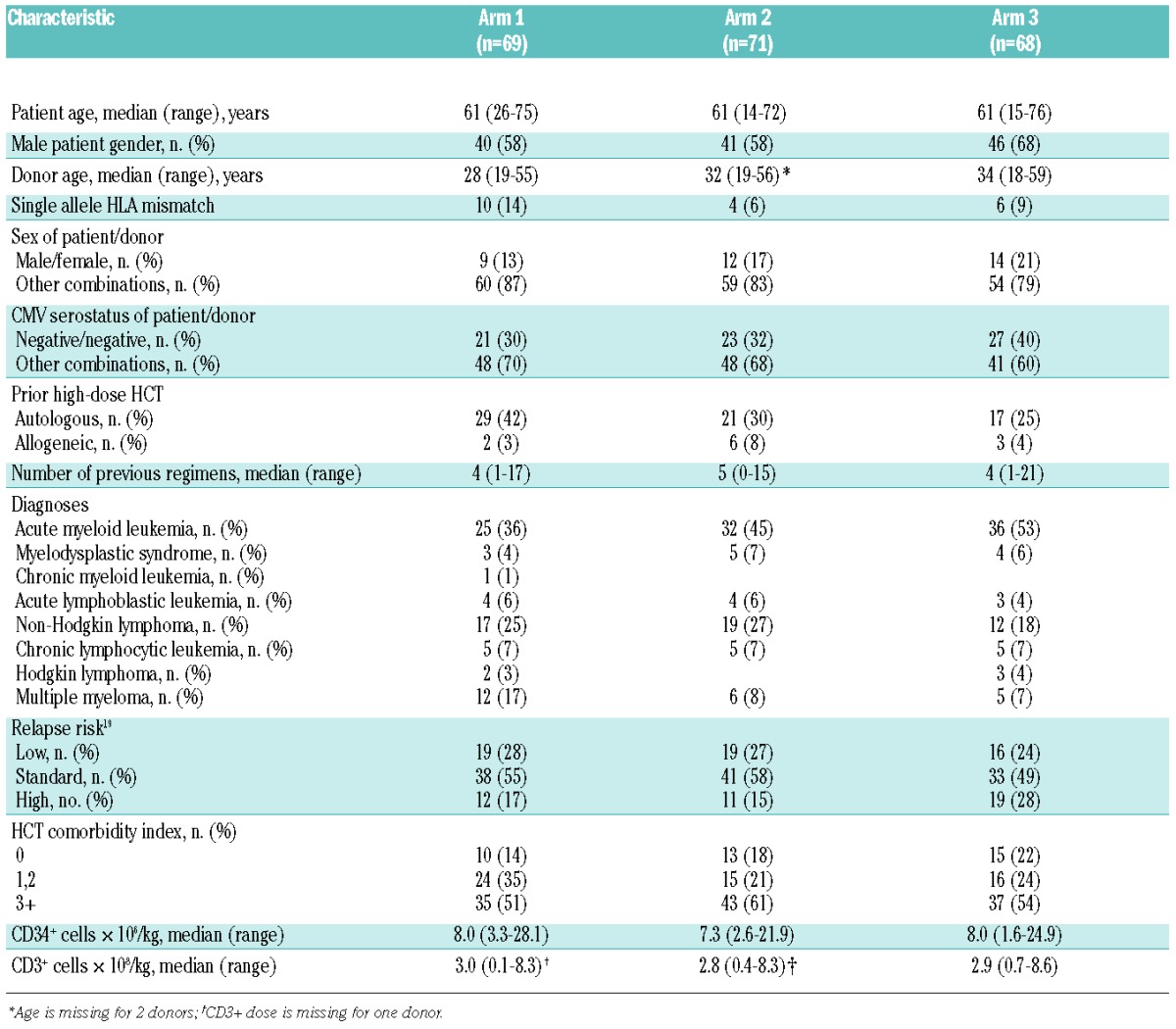
Table 1.
Pre-transplant demographics.
The protocol was opened in January 2005 and closed in August 2009 after accruing 208 patients. The database was analyzed as of August 2013 and median follow up was 4.9 (range 0.5–8.4) years.
Treatment
Patients were conditioned with FLU (30 mg/m2/day) on Days -4, -3, and -2 before receiving 2 Gy TBI at a rate of 0.06–0.07 Gy/min from a linear accelerator on the day of HCT (Day 0). Donor peripheral blood stem cells (PBSC) were collected as previously described.8 For post-grafting immunosuppression, patients were randomized between three regimens; henceforward in this report these will be referred to as arms 1–3. In arm 1, 15 mg/kg of MMF was given p.o. t.i.d. from Day 0 until Day 30, then b.i.d until Day 40 and in the absence of GvHD tapered off by Day 96. Tacrolimus 0.06 mg/kg was administered orally b.i.d. from Day -3 to 100 and in the absence of GvHD tapered off by Day 180. In arm 2, the same doses of MMF and tacrolimus were used, but MMF was b.i.d. from Day 30 to Day 150 and tapered over one month, while tacrolimus was continued to Day 100 and tapered over 50 days. In arm 3, the MMF and tacrolimus dosing schedules were the same as for arm 2, but with the addition of sirolimus started at Day -3 at 2 mg p.o. q.d. and adjusted to attain trough levels of 3–12 ng/mL. Sirolimus was stopped at Day 80 without a taper. In arms 1 and 2, tacrolimus trough levels were targeted between 10–15 ng/mL for the first 28 days and thereafter between 7.5 and 15 ng/mL. In arm 3, tacrolimus trough levels were targeted between 5 and 10 ng/mL during sirolimus administration.
Eligibility criteria, patient evaluations and statistical analyses are described in the Online Supplementary Appendix.
Results
Patients
Sixty-nine patients were randomized into arm 1, 71 into arm 2, and 68 into arm 3. Patients’ demographics are summarized in Table 1. Patients received G-CSF mobilized PBSC from 10/10 HLA matched (90%) or 1 allele mismatched (at HLA-A, -B, or -C) unrelated donors (10%). Underlying diseases were: acute myeloid leukemia (AML) (n=93); myelodysplastic syndrome (MDS) (n=12); chronic myeloid leukemia (CML) (n=1); acute lymphoblastic leukemia (ALL) (n=11); non-Hodgkin lymphoma (NHL) (n=48); chronic lymphocytic leukemia (CLL) (n=15); Hodgkin lymphoma (HL) (n=5) and multiple myeloma (MM) (n=23), with slightly more patients with AML compared to NHL in arm 3. Relapse risk scores19 and HCT-comorbidity index (HCT-CI)20 were evenly distributed among arms. Thirty-seven percent of the patients had at least one prior high-dose HCT (32% autologous and 5% allogeneic). Seventy-eight percent had the high-dose HCT as part of a planned tandem transplant. All 23 patients with MM had high-dose autologous HCT prior to entering the trial.
Two patients each in arms 1 and 2, and 3 patients in arm 3, all with CD20+ B-cell lymphomas, were concurrently enrolled on a study protocol (clinicaltrials.gov identifier:00867529) studying the effects of peri-transplant rituximab (Days -3, 10, 24 and 38). Due to the even distribution of patients treated with peri-transplant rituximab, they were included in subsequent analyses, as any impact of rituximab on GvHD would affect the study arms equally.
Rejections, chimerism and peripheral blood cell changes
All patients had sustained engraftment. Near-complete granulocyte donor chimerism was achieved promptly, with no significant differences between arms (Figure 1A). Median donor T-cell chimerism was lower in arm 3 on Days +28 (arm 1, 94%; arm 2, 84%; arm 3, 74%; P<0.001) and +84 (arm 1, 95%; arm 2, 95%; arm 3, 82%; P=0.003) (Figure 1B). Median Day +28 donor NK cell chimerism (n=83), which was only analyzed in FHCRC patients, was also lower in arm 3 and correlated with donor T-cell chimerism (Pearson’s correlation coefficient r=0.47; P<0.0001).
Figure 1.
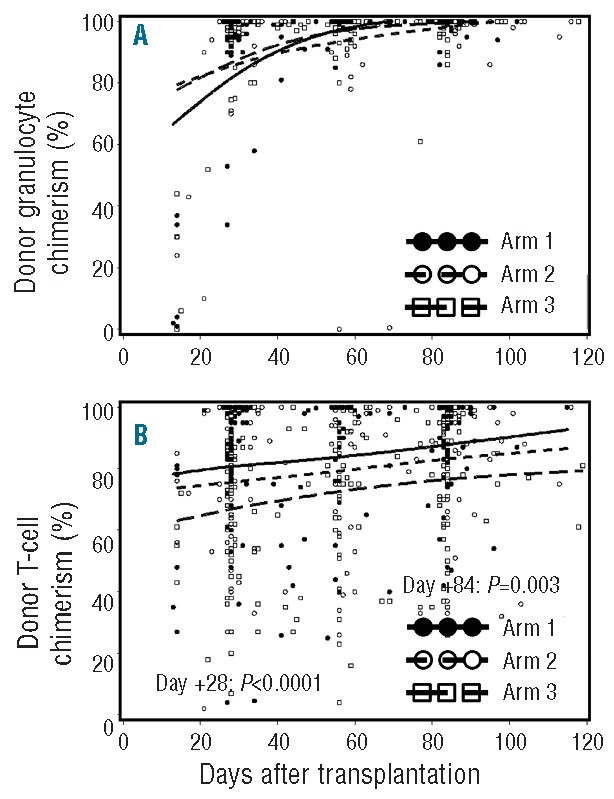
Donor granulocyte and T-cell chimerism. Percent donor granulocyte (A) and T-cell chimerism (B) in arm 1 (n=69), arm 2 (n=71) and arm 3 (n=68). Horizontal lines represent medians, and dots represent the individual data points. P values are two-tailed.
Compared to arm 1, the median ANC nadirs in arms 2 and 3 were significantly lower [arm 1, 0.32 (range 0–1.42) cells/μL; arm 2, 0.25 (range 0–2.38) cells/μL; arm 3, 0.15 (range 0–1.68) cells/μL; P<0.0001], while the median period with ANC below 500 cells/μL was longer in arm 3 [arm 1, 3 (range 0–22) days; arm 2, 3 (range 0–37) days; arm 3, 9 (range 0–34); P<0.0001]. The number of patients who required G-CSF treatment for prolonged neutropenia (persistence or development of granulocyte counts below 500 cells/μL past Day +21 after HCT) was similar in all arms (arm 1, 17%; arm 2, 18%; arm 3, 18%; P=0.98).
Donor lymphocyte infusion
Donor lymphocyte infusion (DLI) was not offered on this protocol, and patients with low chimerism or disease progression were eligible for ongoing DLI protocols or treatment plans.
A total of 14 (7%) patients received DLI. In arm 1, 5 patients received DLI, 2 for low chimerism and 3 for relapse. Three patients received DLI in arm 2, 2 of whom for low chimerism and one for progressive disease. In arm 3, 6 patients were treated with DLI, of whom 2 for low chimerism and 4 for relapse.
Graft-versus-host disease
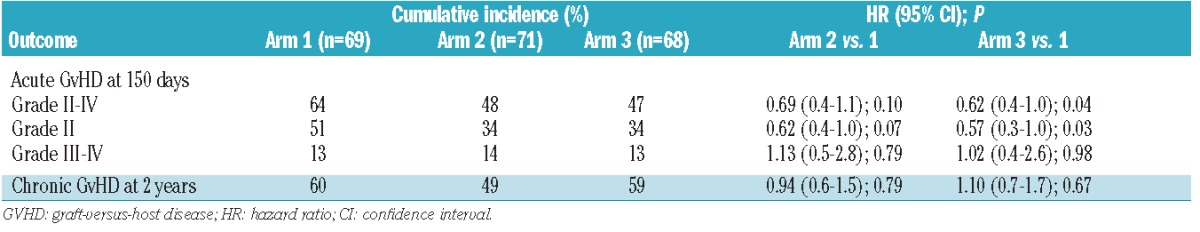
The cumulative incidences of Day 150 grade II-IV acute GvHD in arms 2 and 3 were lower compared to arm 1 (arm 1: 64%; arm 2: 48%; arm 3: 47%), although the overall difference was not statistically significant (P=0.09 by hazard ratio (HR) analysis) (Figure 2A). The incidence of grade III-IV acute GvHD was similar in all arms (arm 1, 13%; arm 2, 14%; arm 3, 13%; overall P=0.96). However, a comparison of arms 2 and 3 to the reference arm (arm 1) (Table 2) suggests that the incidence of grade II acute GvHD was lower in these arms (P=0.10 for arm 2 vs. arm 1 and P=0.04 arm 3 vs. arm 1). Among patients with grades II–IV acute GvHD, the proportion with skin involvement was 61%, 74% and 19% (P<0.0001), and the proportion with gut involvement was 80%, 74% and 100% (P=0.009) in arms 1, 2 and 3, respectively (Online Supplementary Table S2). No patients experienced acute GvHD of the liver. Acute GvHD was confirmed by biopsy in 72%, 71% and 88% of patients in arms 1, 2, and 3, respectively.
Figure 2.
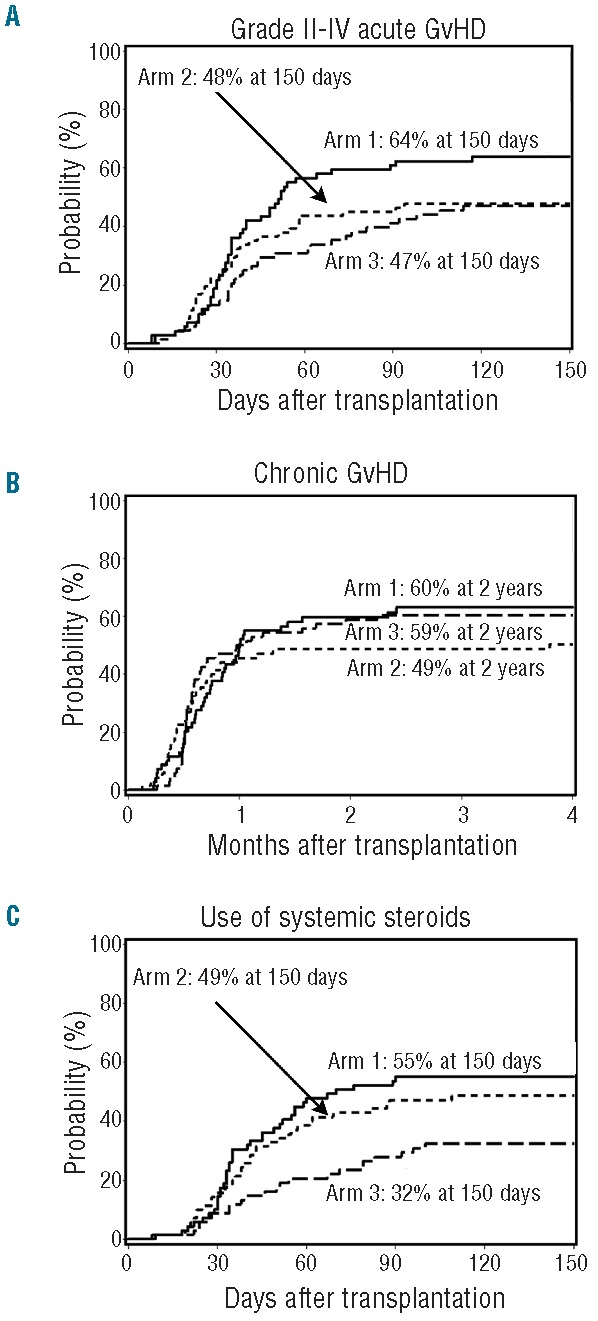
Graft-versus-host disease and use of systemic steroids. Cumulative incidence of grade II to IV acute (A) and chronic (B) GvHD among patients in arm 1 (n=69), arm 2 (n=71) and arm 3 (n=68). (C) Cumulative incidence of use of systemic steroids in arm 1 (n=69), arm 2 (n=71) and arm 3 (n=68).
Table 2.
Cumulative incidence of graft-versus-host disease.
The 2-year cumulative incidence of chronic GvHD was similar in all study arms (arm 1, 60%; arm 2, 49%; arm 3, 59%; overall P=0.80 by HR analysis) (Table 2) (Figure 2B). The cumulative 150-day incidence of systemic steroid use was 55% in arm 1, 49% in arm 2, and 32% in arm 3 (overall P=0.009 by HR analysis). The incidences of Day 150 steroid use were comparable between arms 1 and 2 [HR: 0.86 (95%CI: 0.5–1.4); P=0.51], and significantly lower in arm 3 when compared to arm 1 [HR: 0.47 (95%CI: 0.3–0.8); P=0.004] (Figure 2C).
Regimen-related toxicities, infections and non-relapse mortality
When toxicities unrelated to the blood and bone marrow were analyzed, 32% of all patients experienced 1 or more grade 3–4 toxicities with no difference between arms (arm 1, 35%; arm 2, 27%; and arm 3, 34%; P=0.54) (Online Supplementary Table S3). Importantly, no patients experienced veno-occlusive disease of the liver, thrombocytic microangiopathy, hypercholesterolemia or hypertriglyceridemia. One patient in arm 1 developed post-transplant lymphoproliferative disease three months after transplantation and was treated successfully with 4 doses of rituximab. No difference in the incidence of bacterial infections (arm 1, 62%; arm 2, 45%; arm 3, 59%; overall P=0.30 by HR analysis) or fungal infections (arm 1, 20%; arm 2, 16%; arm 3, 19%; overall P=0.95 by HR analysis) between arms was observed. However, Day 150 incidence of viral infections was significantly lower in arm 3 (arm 1, 62%; arm 2, 62%; arm 3, 37%; overall P=0.03 by HR analysis), which was mainly due to a reduction in risk of cytomegalovirus (CMV) reactivation in arm 3 (arm 1, 54%; arm 2, 47%; arm 3, 22%; overall P=0.002 by HR analysis) (Table 3 and Figure 3). Of the 95 patients who experienced one or more CMV reactivations, only 17 (arm 1, n=9; arm 2, n=5; arm 3, n=3) had overt CMV infections, primarily of the gut. Only 2 patients, one in each of arms 2 and 3, experienced Epstein Barr virus reactivation. There was no difference in the incidence of other viral infections between arms (arm 1, 17%; arm 2, 31%; arm 3, 22%; overall P=0.08 by HR analysis) (Table 3).
Table 3.
Cox regression analyses of incidence of cytomegalovirus (CMV) reactivation and non-CMV infection.
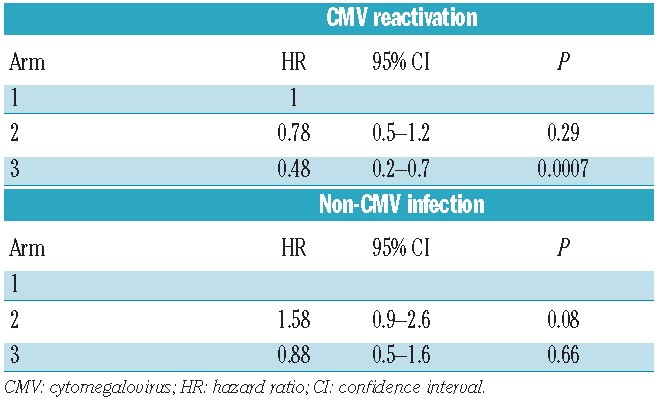
Figure 3.
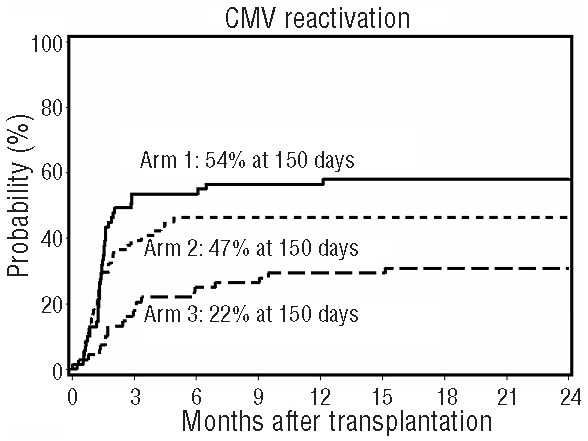
Viral infections. Cumulative incidence of cytomegalovirus reactivation in arm 1 (n=69), arm 2 (n=71) and arm 3 (n=68).
A total of 56 patients died of NRM. The cumulative incidences of NRM were similar in all arms at 200 days (arm 1, 4%; arm 2, 9%; arm 3, 3%) and at two years (arm 1, 26%; arm 2, 23%; arm 3, 18%; overall P=0.55 by HR analysis) (Figure 4A). GvHD alone accounted for 5 deaths in arm 1, 2 deaths in arm 2, and 3 deaths in arm 3. The most common cause of NRM mortality was GvHD with infection (arm 1, n=10; arm 2, n=10; arm 3, n=8). Infection alone accounted for 3 deaths in arm 1, 5 in arm 2, and 3 in arm 3. One patient each in arms 2 and 3 died of other causes, both unrelated to the transplant (pancreatic cancer and a motor vehicle accident). In arm 1, 3 patients died of cerebral events, while 2 died of causes unrelated to the transplant (metastatic gastric cancer and accidental drowning).
Figure 4.
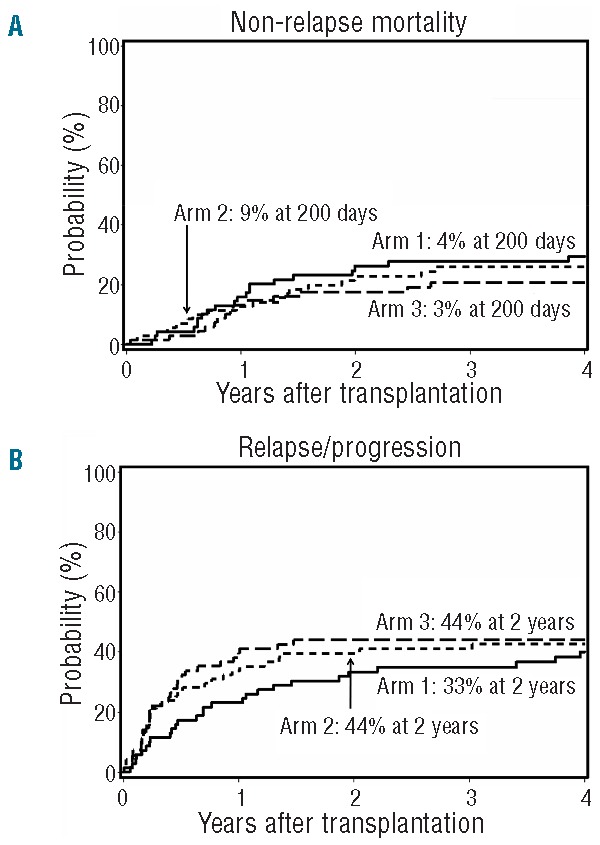
Non-relapse mortality and relapse or progression. (A) Cumulative incidence of non-relapse mortality among patients in arm 1 (n=69), arm 2 (n=71) and arm 3 (n=68). (B) Cumulative incidence of relapse or progression among patients in arm 1 (n=69), arm 2 (n=71) and arm 3 (n=68).
Relapse and survival
There was no difference between the three arms in the overall cumulative incidence of relapse/progression (arm 1, 33%; arm 2, 40%; arm 3, 44%; overall P=0.65 by HR analysis) (Figure 4B) or relapse-related mortality (arm 1, 22%; arm 2, 32%; arm 3, 38%; overall P=0.32 by HR analysis) at two years post transplant. Analyzing lymphoid and myeloid diseases separately, no difference in relapse/progression was observed between the three arms at two years post transplant for lymphoid diseases (arm 1, 38%; arm 2, 33%; arm 3, 43%; overall P=0.89 by HR analysis). However, for myeloid diseases, a non-significant trend towards increased relapse/progression rates was observed in arms 2 and 3 (arm 1, 28%; arm 2, 46%; arm 3, 45%; overall P=0.14 by HR analysis). Overall, no difference was observed in progression-free survival (PFS) (P=0.96) or overall survival (OS) (P=0.93) among the three arms (Figure 5A and B, respectively).
Figure 5.
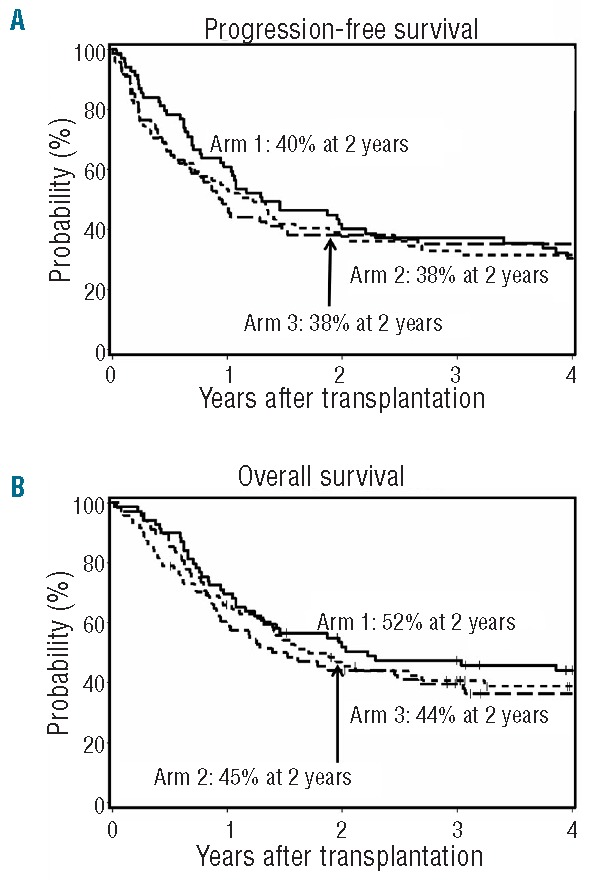
Progression-free and overall survival. Cumulative incidences of progression-free survival (A) and overall survival (B) among patients in arm 1 (n=69), arm 2 (n=71) and arm 3 (n=68).
Discussion
In the current study, we randomized 208 patients undergoing HLA-matched unrelated HCT conditioned with FLU and 2 Gy TBI to either our standard GvHD prophylactic regimen consisting of MMF and tacrolimus, or experimental regimens with different durations of tacrolimus and MMF administration with (arm 3) or without (arm 2) sirolimus. All patients in the three arms engrafted and no rejections were observed. No relevant clinical differences in outcome measures were observed between our standard regimen (arm 1) and arm 2, where tacrolimus was tapered for a shorter period and MMF administration prolonged.
Overall, the immunosuppressive regimens used in the three arms were well tolerated, as outlined in Online Supplementary Table S2. Across all arms, neither unacceptable cytopenias nor mucositis were observed and Day 200 NRM was low (3–9%). Unlike a recent retrospective study in unrelated myeloablative HCT, where a grade III–IV acute GvHD rate of 36.5% was observed after GvHD prophylaxis with tacrolimus and MMF,21 the grade III–IV acute GvHD rates in the current study ranged from 13–14% at Day 150.
In arm 3, where sirolimus was added to the prophylactic regimen, slower development of T-cell donor chimerism was observed. Although no difference was observed in grade II–IV acute GvHD when all arms were compared together, a significantly lower incidence of grade II–IV acute GvHD was observed when the sirolimus regimen (arm 3) was compared to our standard prophylaxis (arm 1), which is in agreement with the slower development of donor T-cell chimerism. The lower incidence was due to a reduction in grade II acute GvHD, which explains why NRM remained no different from our standard prophylaxis (arm 1). However, given the low NRM overall, the study was not powered to detect differences at this level. A significant reduction in the use of systemic steroids and a lower incidence of CMV reactivation was also observed in patients treated with sirolimus. Whether the lower incidence of viral reactivation was due to the reduced steroid use, specific antiviral activity of sirolimus,22 or a combination of these factors is unknown. There was no increase in DLI use, relapse incidence or relapse-related mortality, even though donor T-cell chimerism developed significantly slower in the sirolimus-treated patients. Reports indicating that sirolimus has specific anti-neoplastic activity against lymphomas23 could not be confirmed. However, it cannot be ruled out that a potential anti-neoplastic effect of sirolimus was offset by an attenuated GvT response due to slower development of donor chimerism.
The addition of sirolimus to the prophylactic regimen was safe with no appreciable increase in adverse events; notably, no differences between arms were observed in the incidence of veno-occlusive disease of the liver, thrombocytic microangiopathy, hypercholesterolemia or hypertriglyceridemia, which previously have been associated with sirolimus in the setting of high-dose conditioning.24,25
The efficacy of sirolimus has been demonstrated in solid organ transplantation, but a definite role in allogeneic HCT has yet to be defined. In the setting of high-dose conditioning, the addition of sirolimus to calcineurin-based acute GvHD prophylaxis has been associated with lower levels of acute GvHD compared to historical data.15,26,27 In contrast, Furlong et al. reported excess toxicity without appreciable effect on GvHD in 2 smaller studies of sirolimus combined with a calcineurin inhibitor and methotrexate.28 More recently, in randomized trials, the addition of sirolimus to tacrolimus or as a substitute for methotrexate has been shown to reduce acute GvHD without affecting overall survival.29–31 To our knowledge, the current study is the first phase II randomized trial to investigate the efficacy of sirolimus as acute GvHD prophylaxis in truly non-myeloablative HCT. However, there has been some experience in the reduced-intensity conditioning setting, and there has been a recent preliminary abstract report from a randomized phase III trial comparing tacrolimus, methotrexate and sirolimus to conventional sirolimus-free regimens.32 Data from the trial yielded results similar to ours where the addition of sirolimus reduced grade II–IV acute GvHD with no effect on grade III–IV acute GvHD, chronic GvHD or survival.
Previous reports on the efficacy of sirolimus in the reduced intensity setting included between 23–91 patients transplanted with a variety of HLA-matched and -mismatched related or unrelated donors after reduced intensity conditioning with FLU combined with busulphan,33–35 melphalan,35–37 or cyclophosphamide.38 Sirolimus was administered as a single loading dose between 6–12 mg followed by daily doses between 2–4 mg and targeted at a plasma level between 3–14 ng/mL.33,34,36–38 Sirolimus was given in combination with tacrolimus with or without methotrexate and antithymocyte globulin. Grade II–IV acute GvHD rates were between 10–37% and chronic GvHD rates were between 40–74% at two years,33,34,36,37 with a trend towards better outcome in patients who did not receive methotrexate.33,34 Most of the studies did not clearly state for how long GvHD prophylaxis was planned, except for Claxton et al.;38 they discontinued sirolimus on Day 30 post transplantation and 43% of patients experienced grade II–IV acute GvHD and 77% chronic GvHD.
Perez-Simon et al. have compared a prospective cohort of 50 patients treated with sirolimus and tacrolimus with an equivalent retrospective cohort of 45 patients treated with CSP and MMF GvHD prophylaxis. Sirolimus was given until Day 180 post transplantion. Day 100 grade II–IV acute GvHD rates were similar in both groups at 45% and 49%, while a significant reduction in chronic GvHD from 90% to 50% was observed in sirolimus-treated patients which translated into 2-year NRM rates of 18% with no adverse impact on relapse rates.35
Direct comparisons between the studies using sirolimus after reduced-intensity conditioning transplantation are difficult due to heterogeneity in study populations. However, the overall experience has been positive with a favorable safety profile and efficacy in preventing both acute and chronic GvHD. Although it should be kept in mind that the previous publications were not based on randomized trials, acute GvHD rates were lower compared to the current trial, which could be attributed to their use of sirolimus loading doses and longer periods of administration.
While the current trial did not meet the primary objective of reducing acute GvHD below 40%, one can conclude that the addition of sirolimus to tacrolimus and MMF is safe and efficacious, and may reduce incidence of acute GvHD, CMV reactivation, and use of systemic steroids. Although the addition of a third immunosuppressive agent resulted only in minor reductions in acute GvHD, sirolimus remains a promising drug in acute GvHD prophylaxis after HCT. However, a note of concern is the slow development of donor T-cell chimerism observed in the sirolimus arm, which could have a negative effect on relapse incidence, particularly in the context of a non-myeloablative conditioning regimen.39 To further investigate the role of sirolimus in the non-myeloablative setting, randomized phase III are trials needed to explore different treatment schemas. Currently a 2-arm phase III trial is ongoing using cyclosporine and MMF with and without sirolimus.
Acknowledgments
The authors would like to thank the patients who participated in the clinical trial. They also thank the members of the research staff, clinical staff, and referring physicians at all the participating sites.
Footnotes
The online version of this article has a Supplementary Appendix.
Funding
Research funding was provided by the National Institutes of Health, Bethesda, MD, grants, CA018029 and CA015704. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health nor its subsidiary Institutes and Centers. BK was supported by a fellowship from the Danish Cancer Society (DP08135), Frøken Amalie Jørgensens Mindelegat and Anders Hasselbalchs Fond. Research funding for LV was provided by the Danish Cancer Society (R56-A2960-12-S2), the Lundbeck Foundation (R32-A2730) and Rigshospitalet.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Thepot S, Zhou J, Perrot A, Robin M, Xhaard A, de Latour RP, et al. The graft-versus-leukemia effect is mainly restricted to NIH-defined chronic graft-versus-host disease after reduced intensity conditioning before allogeneic stem cell transplantation. Leukemia 2010;24(11):1852–8 [DOI] [PubMed] [Google Scholar]
- 2.Baron F, Maris MB, Sandmaier BM, Storer BE, Sorror M, Diaconescu R, et al. Graft-versus-tumor effects after allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning. J Clin Oncol 2005;23(9):1993–2003 [DOI] [PubMed] [Google Scholar]
- 3.Inamoto Y, Flowers MED, Lee SJ, Carpenter PA, Warren EH, Deeg HJ, et al. Influence of immunosuppressive treatment on risk of recurrent malignancy after allogeneic hematopoietic cell transplantation. Blood 2011;118(2):456–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Storb R, Gyurkocza B, Storer BE, Sorror ML, Blume K, Niederwieser D, et al. Graft-versus-host disease and graft-versus-tumor effects after allogeneic hematopoietic cell transplantation. J Clin Oncol 2013;31(12):1530–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Storb R, Yu C, Wagner JL, Deeg HJ, Nash RA, Kiem H-P, et al. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood 1997;89(8):3048–54 [PubMed] [Google Scholar]
- 6.Storb R, Raff RF, Appelbaum FR, Deeg HJ, Graham TC, Schuening FG, et al. DLA-identical bone marrow grafts after low-dose total body irradiation: the effect of canine recombinant hematopoietic growth factors. Blood 1994;84:3558–66 [PubMed] [Google Scholar]
- 7.Baron F, Sandmaier BM, Storer BE, Maris MB, Langston AA, Lange T, et al. Extended mycophenolate mofetil and shortened cyclosporine failed to reduce graft-versus-host disease after unrelated hematopoietic cell transplantation with nonmyeloablative conditioning. Biol Blood Marrow Transplant 2007;13(9):1041–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maris MB, Niederwieser D, Sandmaier BM, Storer B, Stuart M, Maloney D, et al. HLA- matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood 2003;102(6):2021–30 [DOI] [PubMed] [Google Scholar]
- 9.Maris MB, Sandmaier BM, Storer BE, Maloney DG, Shizuru JA, Agura E, et al. Unrelated donor granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cell transplantation after non-myeloablative conditioning: the effect of postgrafting mycophenolate mofetil dosing. Biol Blood Marrow Transplant 2006;12:454–65 [DOI] [PubMed] [Google Scholar]
- 10.Nash RA, Antin JH, Karanes C, Fay JW, Avalos BR, Yeager AM, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood 2000;96(6):2062–8 [PubMed] [Google Scholar]
- 11.Ratanatharathorn V, Nash RA, Przepiorka D, Devine SM, Klein JL, Weisdorf D, et al. Phase III study comparing methotrexate and tacrolimus (Prograf, FK506) with methotrexate and cyclosporine for graft-versus-host-disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood 1998;92(7):2303–14 [PubMed] [Google Scholar]
- 12.Hiraoka A, Ohashi Y, Okamoto S, Moriyama Y, Nagao T, Kodera Y, et al. Phase III study comparing tacrolimus (FK506) with cyclosporine for graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation. Japanese FK506 BMT Study Group. Bone Marrow Transplant 2001;28(2):181–5 [DOI] [PubMed] [Google Scholar]
- 13.Dumont FJ, Staruch MJ, Koprak SL, Melino MR, Sigal NH. Distinct mechanisms of suppression of murine T cell activation by the related macrolides FK-506 and rapamycin. J Immunol 1990;144(1):251–8 [PubMed] [Google Scholar]
- 14.Sehgal SN. Rapamune (RAPA, rapamycin, sirolimus): mechanism of action immunosuppressive effect results from blockade of signal transduction and inhibition of cell cycle progression. [Review]. Clin Biochem 1998;31(5):335–40 [DOI] [PubMed] [Google Scholar]
- 15.Cutler C, Li S, Ho VT, Koreth J, Alyea E, Soiffer RJ, et al. Extended follow-up of methotrexate-free immunosuppression using sirolimus and tacrolimus in related and unrelated donor peripheral blood stem cell transplantation. Blood 2007;109(7): 3108–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez R, Nakamura R, Palmer JM, Parker P, Shayani S, Nademanee A, et al. A phase II pilot study of tacrolimus/sirolimus GVHD prophylaxis for sibling donor hematopoietic stem cell transplantation using 3 conditioning regimens. [Erratum appears in Blood. 2010 May 27;115(21):4318] Blood 2010;115(5): 1098–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schulz KF, Altman DG, Moher DCONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petersdorf EW, Gooley TA, Anasetti C, Martin PJ, Smith AG, Mickelson EM, et al. Optimizing outcome after unrelated marrow transplantation by comprehensive matching of HLA class I and II alleles in the donor and recipient. Blood 1998;92(10): 3515–20 [PubMed] [Google Scholar]
- 19.Kahl C, Storer BE, Sandmaier BM, Mielcarek M, Maris MB, Blume KG, et al. Relapse risk among patients with malignant diseases given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood 2007;110(7):2744–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005;106(8):2912–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Kadhimi Z, Gul Z, Chen W, Smith D, Abidi M, Deol A, et al. High incidence of severe acute graft-versus-host disease with tacrolimus and mycophenolate mofetil in a large cohort of related and unrelated allogeneic transplantation patients. Biol Blood Marrow Transplant 2014;20(7):979–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marty FM, Bryar J, Browne SK, Schwarzberg T, Ho VT, Bassett IV, et al. Sirolimus-based graft-versus-host disease prophylaxis protects against cytomegalovirus reactivation after allogeneic hematopoietic stem cell transplantation: a cohort analysis. Blood 2007;110(2):490–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armand P, Gannamaneni S, Kim HT, Cutler CS, Ho VT, Koreth J, et al. Improved survival in lymphoma patients receiving sirolimus for graft-versus-host disease prophylaxis after allogeneic hematopoietic stem-cell transplantation with reduced-intensity conditioning. J Clin Oncol 2008;26(35):5767–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cutler C, Stevenson K, Kim HT, Richardson P, Ho VT, Linden E, et al. Sirolimus is associated with veno-occlusive disease of the liver after myeloablative allogeneic stem cell transplantation. Blood 2008;112(12): 4425–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cutler C, Henry NL, Magee C, Li S, Kim HT, Alyea E, et al. Sirolimus and thrombotic microangiopathy after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2005;11(7):551–7 [DOI] [PubMed] [Google Scholar]
- 26.Antin JH, Kim HT, Cutler C, Ho VT, Lee SJ, Miklos DB, et al. Sirolimus, tacrolimus, and low-dose methotrexate for graft-versus-host disease prophylaxis in mismatched related donor or unrelated donor transplantation. Blood 2003;102(5):1601–5 [DOI] [PubMed] [Google Scholar]
- 27.Cutler C, Kim HT, Hochberg E, Ho V, Alyea E, Lee SJ, et al. Sirolimus and tacrolimus without methotrexate as graft-versus-host disease prophylaxis after matched related donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplant 2004;10(5):328–36 [DOI] [PubMed] [Google Scholar]
- 28.Furlong T, Kiem H-P, Appelbaum FR, Carpenter PA, Deeg HJ, Doney K, et al. Sirolimus in combination with cyclosporine or tacrolimus plus methotrexate for prevention of graft-versus-host disease following hematopoietic cell transplantation from unrelated donors. Biol Blood Marrow Transplant 2008;14(5):531–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cutler C, Logan BR, Nakamura R, Johnston L, Choi SW, Porter DL, et al. Tacrolimus/sirolimus vs. tacrolimus/methotrexate for graft-vs.-host disease prophylaxis after HLA-matched, related donor hematopoietic stem cell transplantation: results of Blood and Marrow Transplant Clinical Trials Network Trial 0402. Blood. 2012;120(21):Abstract 739 [Google Scholar]
- 30.Pidala J, Kim J, Jim H, Kharfan-Dabaja MA, Nishihori T, Fernandez HF, et al. A randomized phase II study to evaluate tacrolimus in combination with sirolimus or methotrexate after allogeneic hematopoietic cell transplantation. Haematologica 2012;97(12):1882–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pulsipher MA, Langholz B, Wall DA, Schultz KR, Bunin N, Carroll WL, et al. The addition of sirolimus to tacrolimus/methotrexate GVHD prophylaxis in children with ALL: a phase III COG/PBMTC trial. Blood 2014; February 6 [Epub ahead of print 2014] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armand P, Kim HT, Sainvil MM, Bachanova V, Devine SM, Waller EK, et al. The addition of sirolimus to the GVHD prophylaxis regimen in reduced intensity allogeneic stem cell transplantation for lymphoma: a multicenter randomized trial. Blood. 2013;122(21):Abstract 704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alyea EP, Li S, Kim HT, Cutler C, Ho V, Soiffer RJ, et al. Sirolimus, tacrolimus, and low-dose methotrexate as graft-versus-host disease prophylaxis in related and unrelated donor reduced-intensity conditioning allogeneic peripheral blood stem cell transplantation. Biol Blood Marrow Transplant 2008;14(8):920–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ho VT, Aldridge J, Kim HT, Cutler C, Koreth J, Armand P, et al. Comparison of tacrolimus and sirolimus (Tac/Sir) versus tacrolimus, sirolimus, and mini-methotrexate (Tac/Sir/MTX) as acute graft-versus-host disease prophylaxis after reduced-intensity conditioning allogeneic peripheral blood stem cell transplantation. Biol Blood Marrow Transplant 2009;15(7):844–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perez-Simon JA, Martino R, Parody R, Cabrero M, Lopez-Corral L, Valcarcel D, et al. The combination of sirolimus plus tacrolimus improves outcome after reduced-intensity conditioning, unrelated donor hematopoietic stem cell transplantation compared with cyclosporine plus mycofenolate. Haematologica 2013;98(4):526–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura R, Palmer JM, O’Donnell MR, Stiller T, Thomas SH, Chao J, et al. Reduced intensity allogeneic hematopoietic stem cell transplantation for MDS using tacrolimus/sirolimus-based GVHD prophylaxis. Leuk Res 2012;36(9):1152–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khaled SK, Palmer J, Stiller T, Senitzer D, Maegawa R, Rodriguez R, et al. A phase II study of sirolimus, tacrolimus and rabbit anti-thymocyte globulin as GVHD prophylaxis after unrelated-donor PBSC transplant. Bone Marrow Transplant 2013;48(2):278–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Claxton DF, Ehmann C, Rybka W. Control of advanced and refractory acute myelogenous leukaemia with sirolimus-based non-myeloablative allogeneic stem cell transplantation. Br J Haematol 2005;130(2):256–64 [DOI] [PubMed] [Google Scholar]
- 39.Kornblit B, Maloney DG, Storb R, Storek J, Hari P, Vucinic V, et al. Fludarabine/2 Gy TBI is superior to 2 Gy TBI as conditioning for HLA-matched related HCT: A phase III randomized trial. Biol Blood Marrow Transplant 2013;19(9):1340–7 [DOI] [PMC free article] [PubMed] [Google Scholar]


