Abstract
Wheat straw was fermented by Crinipellis sp. RCK-1, a lignin degrading fungus, under solid state fermentation conditions. The fungus degraded 18.38% lignin at the expense of 10.37% cellulose within 9 days. However, when wheat straw fermented for different duration was evaluated in vitro, the 5 day fungal fermented wheat straw called here “Biotech Feed” was found to possess 36.74% organic matter digestibility (OMD) and 5.38 (MJ/Kg Dry matter) metabolizable energy (ME). The Biotech Feed was also observed to be significantly enriched with essential amino acids and fungal protein by fungal fermentation, eventually increasing its nutritional value. The Biotech Feed upon in vitro analysis showed potential to replace 50% grain from concentrate mixture. Further, the calves fed on Biotech Feed based diets exhibited significantly higher (p<0.05) dry matter intake (DMI: 3.74 Kg/d), dry matter digestibility (DMD: 57.82%), total digestible nutrients (TDN: 54.76%) and comparatively gained 50 g more daily body weight.
India has approximately 600 million livestock, which requires almost 1000 million tons of hay or green fodder to sustain present level of productivity43. However, as per estimates nearly 230 million tons of green fodder is available and the livestock has to struggle with the devastating scarcity of approximately 800 million tons of green fodder. The deteriorating animal health and their sustainability could also pose a potential threat for human existence and their livelihood. This necessitates the use of certain alternative options such as the agricultural crop residues and grasses (lignocellulosic biomass) as feed sources. If these are utilized judiciously this may provide enough energy and nutrients to the animals. However, high lignin content and lower digestibility and protein content and poor palatability of crop residues and grasses discourage their use as the sole animal feed. Lignin, being a cementing material in plant cell wall restricts the fullest accessibility of carbohydrates, the energy reserve, to the microorganisms inside the gut of ruminating animals. Despite employing various physical, chemical and physicochemical methods to remove lignin from the plant residues, currently the research efforts are focused on biological alternatives, especially because they are environmentally benign and widely acceptable9,10,26,31,43,44,45.
Among various microorganisms known for lignin degradation, white- rot fungi (majorly basidiomycetes) have been adjudged most promising lignin degraders and have been largely studied for bioconversion of plant residues into nutritionally digestible animal feed under solid-state fermentation (SSF) conditions9,25,48. However, these white-rots have differential ability and mode to degrade lignin, either simultaneously or selectively. The white-rot fungi, which selectively degrade lignin without affecting much of the carbohydrates, and in turn expose protected and available carbohydrates to ruminants are prerequisite biological agent and especially considered well suited for animal feed development. Out of various selective lignin degrading fungi, only few have been studied in detail (Lentinus edodes, Pycnoporus cinnabarinus, Cereporiopsis subvermispora and Phlebia brevispora) at laboratory scale6,26,35,37. To the best of our knowledge except Zadrazil and coworkers53, who have demonstrated the ability of Pleurotus sp., to ferment straw in a 1.5 t capacity solid state bioreactor, large scale production of animal feed is scarcely been reported. However, they emphasized to conduct large scale animal feeding trials to further establish the suitability of the process of feed development.
Moreover, the majority of the white-rot fungi have been observed to degrade lignin considerably but at an expense of long fermentation period and thereby caused an undesirable reduction in carbohydrates and eventually digestibility of the fermented material35,36,44,47. This does not only hamper the commercialization but also affects the economic viability of the process. Therefore, it is imperative to employ a fast growing and selective lignin degrading fungus for cost effective, nutrient rich and digestible animal feed development. For any animal feed developed, it is imperative to test it in cattle and before designing large scale in vivo animal trials of fermented feed it becomes priority to assess the quality and nutritional value of the feed through in vitro testing37,44. Among different in vitro feed analyses, the gas production test hold specific importance, in which the amount of gas (CO2 and CH4) released, is measured when feeds are incubated in vitro with rumen liquor. The amount of gas produced is closely related to the digestibility and the energetic feed value of diets for ruminants28,43. The feeds with varied digestibility and nutrients are known to produce different volume of gases within a stipulated time. This fact is taken in to consideration while designing diets for in vivo animal feeding trials to ensure the nutritional balance and adequacy of diet for animals. Moreover, in certain extended efforts, attempts have been made to replace either feed concentrate or grains from traditional animal diets with fermented wheat straw, especially to bring down the cost of animal feed1,22. However, to the best of our knowledge none of the study could be transformed into a wholesome process or technology. Therefore, there is a need to develop a wholesome/fermentation based process or technology to transform crop residue (s) in to nutritionally rich and digestible cattle feed.
This paper deals with developing a process for bioconversion of wheat straw into a digestible and nutrient rich animal feed with a selective lignin degrading fungus, Crinipellis sp. RCK-1, grown under solid-state fermentation for 5 days. The fermented feed produced, called here “Biotech Feed” has been evaluated in vitro followed by in vivo testing in buffalo calves, which has found to replace 50% grains from feed concentrate mixture.
Results
Cell wall compositional changes of wheat straw
Crinipellis sp. RCK-1 grew luxuriantly on wheat straw under solid state fermentation (SSF) conditions, which could be because of homogenous distribution of fungal pellets as an inoculum. The unfermented (control) wheat straw found to have (% w/w) ADF; 53.74, NDF; 83.10, hemicellulose; 29.36, lignin; 10.53, cellulose; 39.50 ash; 3.71 and 2.95% of crude protein. At 100 g level SSF, Crinipellis sp. RCK-1 caused higher degradation in lignin (28.26%) till 15th day of fermentation and consumed lesser cellulose (15%) along with a 48% decrease in hemicellulose content (Table 1). The plant cell wall degradation profile of Crinipellis sp. RCK-1 clearly showed that the fungus degraded lignin at a faster rate than cellulose on 10th day. The percent SSF efficiency, which is a measure of amount of lignin degradation at the cost of carbohydrate content loss, was found maximally increased on 10th day (Table 1). The fungal fermentation also caused a significant increase of 14.31% in total crude protein content in the Biotech Feed till 10th day of incubation. More interestingly, upon scale up of Biotech Feed production process in Koji room (500 g substrate in each tray), Crinipellis sp. RCK-1 exhibited similar growth and substrate colonization and the substrate degradation was comparable to that was observed at 100 g substrate level SSF in smaller trays. However, a slight increase in carbohydrate (cellulose and hemicellulose) degradation was observed in scale up experiments, but lignin degradation remained almost same (16.06%) till 9th day with a maximal increase in crude protein by 40.81% (Table 1). Moreover, Crinipellis sp. RCK-1 degraded a fair amount of lignin (~7%) till 5 day irrespective of the scale of experiment while, preventive much of cellulose degradation and concurrently increased the crude protein (up to 15–18%).
Table 1. Cell wall composition (% w/w) of control and fungal treated wheat straw (Crinipellis sp. RCK-1) under solid state fermentation (SSF) condition.
| Fermentation level (Substrate weight) | Day | Hemi-cellulose | Lignin | Cellulose | % Eff. of SSF | Crude protein |
|---|---|---|---|---|---|---|
| Unfermented control | 0 | 29.36 ± 1.72 | 10.53 ± 0.013 | 39.50 ± 0.44 | - | 2.95 ± 0.116 |
| 100 g | 5 | 23.56 ± 1.55 (−19.76) | 9.84 ± 0.002 (−6.56) | 37.33 ± 1.15 (−5.48) | 8.67 | 3.418 ± 0.147 (+15.87) |
| 10 | 20.87 ± 2.27 (−28.93) | 8.60 ± 0.002 (−18.36) | 35.43 ± 0.47 (−10.31) | 15.38 | 3.372 ± 0.129 (+14.31) | |
| 15 | 15.24 ± 0.62 (−48.08) | 7.55 ± 0.014 (−28.26) | 33.21 ± 0.59 (−15.92) | 14.59 | 3.369 ± 0.201 (+14.19) | |
| 500 g | 5 | 23.74 ± 1.37 (−19.16) | 9.78 ± 0.007 (−7.16) | 36.71 ± 0.84 (−7.06) | 8.98 | 3.494 ± 0.198 (+18.43) |
| 7 | 21.49 ± 2.17 (−26.81) | 9.34 ± 0.006 − (11.26) | 33.94 ± 1.03 (−14.07) | 8.82 | 3.50 ± 0.126 (+18.64) | |
| 9 | 19.61 ± 1.36 (−33.2) | 8.84 ± 0.001 (−16.06) | 33.09 ± 1.57 (−16.22) | 10.46 | 4.154 ± 0.115 (+40.81) |
± Sign denotes the standard deviation (S.D.) and values in parenthesis represent % degradation of different components with respect to unfermented control (+% increase and – % decrease). % efficiency of SSF is a derived ratio from the values of these components ESSF = (loss of lignin/loss of hemicellulose + cellulose)* 100.
In vitro evaluation of fermented feed
In vitro gas production test was conducted to evaluate the changes in digestibility and nutrients of Biotech feed as an effect of fungal fermentation. The oven dried Biotech feed fermented for 5, 10 and 15 days along with the unfermented wheat straw (WS) were tested for in vitro gas production. True degradable organic matter (TDOM), microbial biomass production (MBP), organic matter digestibility (OMD) and metabolizable energy (ME) of 5 day Biotech Feed (BT5) was significantly higher (P<0.05) as compared to 10th (BT10) and 15th day (BT15) Biotech Feed. BT5 exhibited a significant improvement in OMD and ME of ~30% when compared with unfermented straw (Table 2). However, no significant difference was found in ME, OMD, MBP and TDOM of BT10 and BT15, which further suggested carrying out SSF of wheat straw till 5 day only. OMD and ME, however was found to be improved up to 10–12% in comparison to the unfermented wheat straw on 15 day of incubation, but 5 day fermented Biotech Feed (BT5) was found to be most suitable for in vivo animal testing due to its higher energy content and available carbohydrate based nutrients.
Table 2. In vitro gas production profile of fermented Biotech Feed.
| Sample | TDOM (mg) | MBP (mg) | OM digestibility (%) | ME (MJ/kgDM) |
|---|---|---|---|---|
| Wheat straw | 136.82a | 107.11a | 28.05a | 4.06a |
| 5 days old Biotech Feed (BT5) | 162.15b | 110.95a | 36.74b | 5.38b |
| 10 days old Biotech Feed (BT10) | 135.84a | 98.07b | 31.31a | 4.56a |
| 15 days old Biotech Feed (BT15) | 138.70a | 101.80b | 30.98a | 4.50a |
| SEM ± | 3.34 | 2.68 | 1.27 | 0.378 |
Values bearing different superscripts (a–b) in a column differ significantly (P < 0.05), while same superscripts denotes non significant difference. Overall SEM ± has been given by first calculating sample mean then calculating overall mean in a column (↓) for an individual parameter in different groups.
TDOM- True Degradable Organic Matter; MBP- Microbial Biomass Production; OM-Organic Matter digestibility; ME- Metabolizable Energy.
The animal diets were also characterized to assess their nutritional quality in terms of their adequacy to meet out the animal maintenance and potential to replace certain amount of grains from traditional feed concentrates. Keeping in view the nutrient balance, four diets i.e. T1 control (WS + concentrate mixture), T2 (5 day Biotech Feed + concentrate mixture), T3 (5 day Biotech feed + 50% grain replaced from concentrate mixture), T4 (5 day Biotech Feed + 100% grain replaced from concentrate mixture) were tested. The organic matter digestibility was significantly higher in T2 and T3 diets as compared to T1 and T4. A same trend was found for gas production (GP), TDOM and MBP. While, T2 had maximum ME (MJ/kg DM) followed by T3, T4 and T1 and the difference among the four treatments was found significantly differing (Table 3). The in vitro analysis suggested that 50% grain can be replaced from concentrate mixture in Biotech Feed based diet (T3), without compromising with the energy and nutrients availability of the diet. However, replacement of 100% grains from concentrate (T4 diet) reduced OMD 40.65% and ME by 5.47 MJ/kg DM and hence was not included in in vivo digestibility trial further. The partitioning factor (PF) was found reduced in diets as compared to control which in turn exhibits improved available energy in fermented diets (Table 3).
Table 3. Effect of grain replacement from concentrate mixture in Biotech Feed based diets.
| Sample | IVGP/200mg | TDOM/200mg | TDOM% | OMD% | MBP/200mg | EMP | PF | ME (MJ/kg DM) |
|---|---|---|---|---|---|---|---|---|
| T1 Control (WS + Conc. mixture) | 25.27b | 106.72d | 53.36b | 40.21b | 57.37c | 53.76b | 4.25a | 5.45b |
| T2 (Biotech Feed + Conc. mixture) | 30.05a | 120.22a | 60.11a | 44.09a | 65.69a | 54.64a,b | 4.01b | 6.00a |
| T3 (Biotech Feed + 50% Grain replaced from conc. mixture) | 29.53a | 118.41c | 59.20a | 43.54a | 65.06a | 54.94a,b | 4.01b | 5.91a |
| T4 (Biotech Feed + 100% grain replaced from conc. mixture) | 26.10b | 108.28b | 54.14a | 40.65b | 59.83b | 55.24b | 4.15a | 5.47b |
| SEM ± | 0.54 | 1.55 | 0.81 | 0.45 | 0.91 | 0.20 | 0.04 | 0.07 |
Values bearing different superscripts (a–d) in a column differ significantly (P < 0.05), while same superscripts (a–d, ab) denotes non significant difference. Overall SEM ± has been given by first calculating sample mean then calculating overall mean in a column (↓) for an individual parameter in different groups.
IVGP- in vitro gas production; TDOM- True Degradable Organic Matter; MBP- Microbial Biomass Production; OMD-Organic Matter digestibility, ME- Metabolizable Energy; EMP- efficiency of microbial production; PF- partitioning factor.
Amino acid analysis of fermented wheat straw
Amino acid analysis of unfermented and Crinipellis sp RCK-1 5 day fermented feed (BT5) revealed that fungal fermentation has considerably improved the individual amino acid content. Among various essential amino acids, Arg, Thr, Ileu and Leu were found increased up to 33.33, 34.30, 31.58 and 30.77%, respectively (Table 4). Since there was an increase in total crude protein content, an increase in non essential amino acids was also noticed. Cys was not present in unfermented wheat straw and was probably added through fungal fermentation. Total amino acid content of BT5 increased by 4.28% compared to unfermented wheat straw (Table 4).
Table 4. Amino acid content of unfermented and fungal fermented wheat straw.
| g/100 gds | Control | CR 5 | % Increase |
|---|---|---|---|
| Non-essential amino acids | |||
| Asp | 0.207 | 0.260 | 20.51 |
| Glu | 0.480 | 0.653 | 26.53 |
| Ser | 0.120 | 0.187 | 35.71 |
| Gly | 0.127 | 0.287 | 55.81 |
| Ala-pro | 0.167 | 0.233 | 28.57 |
| Tyr | 0.100 | 0.073 | - |
| Cys | 0.000 | 0.040 | 100.00 |
| Essential amino acids | |||
| Val | 0.133 | 0.127 | - |
| Met | 0.267 | 0.080 | - |
| Ileu | 0.087 | 0.127 | 31.58 |
| Leu | 0.060 | 0.087 | 30.77 |
| Phe | 0.000 | 0.000 | 0.00 |
| Lys | 5.800 | 5.613 | - |
| His | 0.013 | 0.013 | 0.00 |
| Arg | 0.067 | 0.100 | 33.33 |
| Thr | 0.153 | 0.233 | 34.29 |
| Total AA | 7.780 | 8.113 | 4.28 |
gds- gram dry substrate.
Control – Unfermented wheat straw.
CR5- Crinipellis sp. RCK-1, 5 day fermented wheat straw.
Analysis of fungal biomass enrichment through ergosterol estimation
Crinipellis sp RCK-1 5 day fermented feed (BT5) was found to have significantly higher amount of fungal cell mass. Fungal biomass was quantified in term of total ergosterol content in unfermented and BT5 straw. Unfermented wheat straw contained 19.44 µg/gram dry substrate (gds) of ergosterol content, while BT5 was found to be maximally enriched with ergosterol content (292.17 µg/gds).
Structural characterization of fungal fermented feed by electron microscopy
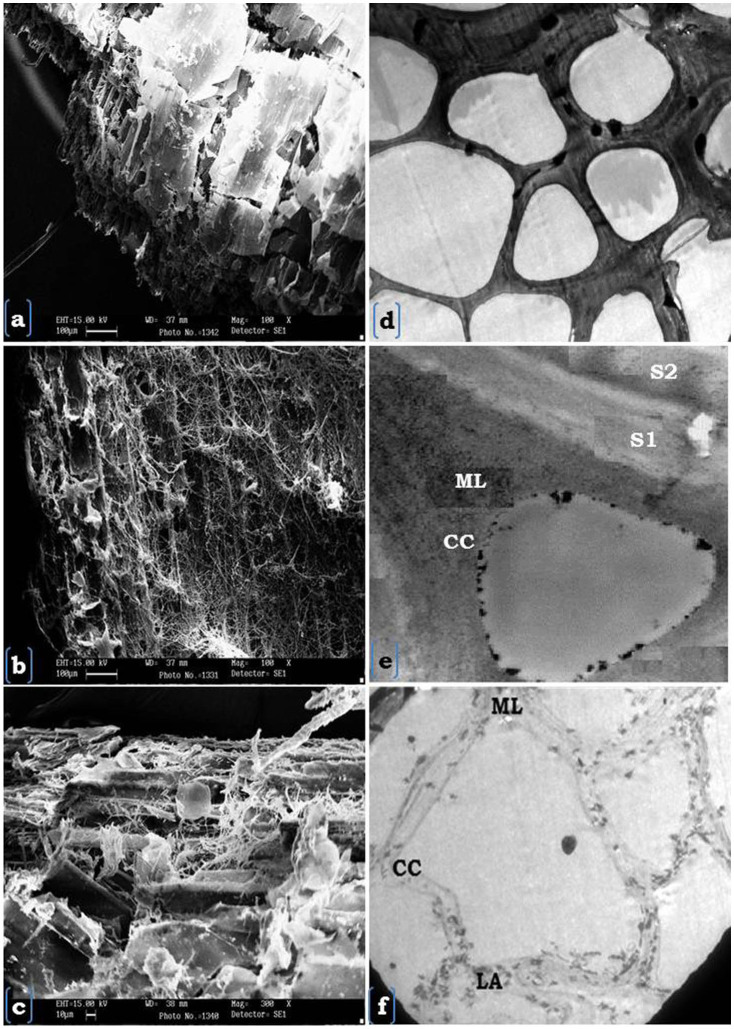
Structural transformation in wheat straw due to fermentation was studied by SEM and TEM. Scanning electron micrographs of unfermented and fermented wheat straw revealed that there was a heavy fungal attack on wheat straw surface and extended to vascular bundles and adjoining cells i.e. parenchymatic cells (Fig. 1 a, b). The structural matrix of wheat straw was observed to be completely distorted and fungal hyphae were clearly visible within the fermented wheat straw (Fig. 1b). The hyphae of Crinipellis sp. RCK-1 appeared to be penetrated deep within the wheat straw and dismantled the original structure (Fig. 1c). Whereas, SEM pictures of unfermented wheat straw showed clear visible organized structure of cell lumina and intact vascular bundles (Fig. 1a).
Figure 1. Scanning electron micrograph of unfermented (a- 100 µm) and Crinipellis sp RCK-1 5 day fermented wheat straws (b) at 100 µm (c) 10 µm and transmission Electron Micrograph showing selective lignin degradation, (d–e) unfermented and (f) Crinipellis sp RCK-1, 5 day fermented wheat straw. Abbreviations: CC- Cell corner, S1-S2- layers, ML- Middle lamellae.
TEM figures of unfermented wheat straw possessed intact cell wall structure as electron dense region and an organized cork like structural arrangement of cells (Fig. 1 d). While, fungal degraded wheat straw cell walls have shown either complete degradation of lignin- rich middle lamellae, between S1 and S2 layers or selective degradation of lignin from cell walls of wheat straw (Fig. 1e, f). A clear reduction in electron dense region was observed within the cell walls and aggregates of lignin residuals were seen in cellular skeleton. Moreover, fungal hyphae were also observed in cell lumina (Fig. 1f).
In vivo digestibility trial in buffalo calves
Chemical composition of feed and fodder
All the three diets were balanced for their nutritional value i.e. crude protein and organic matter. The ingredients of diets i.e. wheat straw, Biotech Feed, concentrate mixture and green fodder were analyzed for their proximate composition. The crude protein of the Biotech Feed increased from 3.15 to 3.65%, while neutral detergent fiber (NDF) and Acid detergent fiber (ADF) content in Biotech Feed were decreased from 83.42, 56.86 to 78.40, and 53.60%, respectively, as compared to unfermented wheat straw. The ash content of Biotech Feed was also found increased from 7.40 to 10.20% compared to control wheat straw.
Voluntary intake of nutrients
Dietary group T2 recorded highest dry matter intake in terms of kg body weight (BW)% and g kg−1 metabolic body weight (W 0.75) followed by T1 and T3. The values of digestible dry matter and digestible organic matter intake (kg BW% and g kg −1 W 0.75) were significantly higher in Biotech Feed based diets T2 and T3 in terms of kg BW% and g kg −1 W 0.75 (Table 5).
Table 5. Voluntary intake of different nutrients in calves under different treatments.
| Treatments | ||||
|---|---|---|---|---|
| Particulars | T-1 | T-2 | T-3 | SEM± |
| DMI (kg day −1) | 3.27a | 3.74a | 2.97a | 0.184 |
| DMI (kg BW%) | 2.80a | 2.95a | 2.92a | 0.046 |
| DMI (g kg −1 W 0.75 ) | 91.95a | 98.21b | 93.03a | 1.188 |
| OMI (kg day −1) | 2.99a | 3.38a | 2.65a | 0.169 |
| OMI (kg BW%) | 2.57a | 2.66a | 2.61a | 0.040 |
| OMI (g kg −1 W 0.75 ) | 84.29a | 88.82a | 82.99a | 1.090 |
| DDMI (kg day −1) | 1.71a | 2.16a | 1.71a | 0.110 |
| DDMI (kg BW%) | 1.46c | 1.70a | 1.68a,b | 0.042 |
| DDMI (g kg −1 W 0.75 ) | 47.91c | 56.79a | 53.47a,b | 1.275 |
| DOMI (kg day −1) | 1.64a | 2.04a | 1.59a | 0.105 |
| DOMI (kg BW%) | 1.40c | 1.61a | 1.56a,b | 0.039 |
| DOMI (g kg −1 W 0.75 ) | 40.07a | 45.03a,b | 48.87b,c | 2.700 |
Values bearing different superscripts (a–c, bc) in a row differ significantly (P < 0.05), while same or common superscripts (a–c, ab) denotes non significant difference. Overall SEM ± has been given by first calculating sample mean then calculating overall mean in a row (→) for an individual parameter in different groups.
DMI, dry matter intake; OMI, organic matter intake; DDMI, digestible dry matter intake; DOMI, digestible organic matter intake; BW, body weight; W 0.75, metabolic body weight.
Digestibility coefficients of nutrients
Total tract digestibility was significantly (P<0.05) improved among calves fed on Biotech feed based diets (T2 and T3) (Table 6). The DM and OM digestibility were 52.07 and 54.52%, respectively, in the wheat straw based diet T1, whereas they were found as 57.82 and 60.54% in T2; 57.47 and 59.83% in T3 diets, respectively. The digestibility of NDF, ADF, ether extract (EE) and roughage were significantly affected by the treatments except crude protein digestibility, which was found not significantly differing. The straw dry matter digestibility (DMD) was significantly higher in Biotech Feed based diets (T2 and T3) than with control group. The average values of roughage dry matter digestibility were 32.22, 42.61 and 39.16% in T1, T2 and T3, respectively.
Table 6. Digestibility of different nutrients (%) in calves under different treatments.
| Treatment (diets) | DM | Roughage | OM | CP | EE | NDF | ADF |
|---|---|---|---|---|---|---|---|
| T1 | 52.07c | 32.22c | 54.52c | 68.96b | 72.36b | 45.81c | 36.69c |
| T2 | 57.82a | 42.61a | 60.54a | 74.72a | 75.15a | 53.33a,b | 51.42a |
| T3 | 57.47a,b | 39.16b | 59.83a,b | 69.97b | 67.25c | 53.47a | 47.42a,b |
| SEM± | 0.848 | 1.492 | 0.862 | 2.154 | 1.115 | 1.1198 | 2.031 |
Values bearing different superscripts (a–c) in a column differ significantly (P < 0.05), while same or common superscripts (a–c, ab) denotes non significant difference. Overall SEM ± has been given by first calculating sample mean then calculating overall mean in a column (↓) for an individual parameter in different groups.
DM, dry matter; OM, organic matter; CP, crude protein; EE, ether extract; NDF, neutral detergent fiber; ADF, acid detergent fiber.
Nutrient density
The total digestible nutrient is known as the sum of the digestible fiber, protein, lipid, and carbohydrate components of a feedstuff or diet, which is directly related to digestible energy. The total digestible nutrient (TDN) % in Biotech Feed diet based groups (T2 and T3) was found significantly (P<0.05) higher (Table 7). Whereas, the digestible crude protein (DCP) is largely determined by subtracting unavailable protein from crude protein. The digestible crude protein content was found to be improved in T2 and T3 diets non significantly higher when compared with the control diet (T1).
Table 7. Nutrient density (%), growth performance and blood biochemical profile of calves fed under different treatments.
| Nutritive Value (%) | T1 | T2 | T3 | SEM ± |
|---|---|---|---|---|
| No. of Animals | 4 | 4 | 4 | - |
| Experimental period (days) | 90 + 7 = 97 | 90 + 7 = 97 | 90 + 7 = 97 | - |
| Total digestible nutrient | 49.97b | 54.76a | 53.38a,c | 0.665 |
| Digestible crude protein | 8.42a | 8.77a | 8.80a | 0.254 |
| Initial live body weight (kg) | 84.50a | 85.00a | 82.25a | 4.029 |
| Final live body weight (kg) | 116.53a | 121.53a | 112.98a | 4.503 |
| Total gain (kg) | 32.02a | 36.52a | 30.72a | 1.111 |
| Av. Daily Gain (g/d) | 355.83a | 405.83a | 341.39a | 12.364 |
| Feed Conversion (kg DMI/kg gain) | 9.16a | 9.18a | 8.76a | 0.340 |
| PCV (Packed cell volume) | 33.00a | 33.25a | 34.75a | 0.742 |
| Haemoglobin (mg/dl) | 9.75a | 10.25a | 10.13a | 0.875 |
| Total protein (g/dl) | 7.45a | 7.95a | 7.83a | 0.160 |
| Albumin (g/dl) | 3.70a | 3.80a | 3.73a | 0.064 |
| BUN (blood urea nitrogen) (mg/dl) | 7.62a | 16.83b | 16.25b,c | 1.483 |
| Serum creatinine (mg/dl) | 1.10a | 1.13a | 1.15a | 0.059 |
| Alkaline phosphatase (U/L) | 325.00a | 293.75a | 241.50a | 34.675 |
| Cholesterol (mg/dl) | 153.43a | 133.23a,b | 93.43c | 8.991 |
Values bearing different superscripts (a–c, ac, bc, ab) in a row differ significantly (P < 0.05), while same or common superscripts (a–c) denotes non significant difference. Overall SEM ± has been given by first calculating sample mean then calculating overall mean in a row (→) for an individual parameter in different groups.
Growth performance
Average daily gain (ADG) and feed conversion of the Biotech Feed based diet studies revealed that average daily gain were higher in the T2 group in comparison to the T1 and T3 groups (Table 7). ADG (g/d) was observed to be 405.83 in case of Biotech Feed based T2 diet, which was significantly higher from the control diet (T1, 355.83 g/d).
Blood biochemical analysis
Blood biochemical analysis showed that there were non-significant differences in packed cell volume (PCV), haemoglobin (Hb), total protein, albumin and serum creatinine, but significant difference were found in blood urea nitrogen (BUN) and cholesterol level (Table 7). Low levels of cholesterol and alkaline phosphatase in T3 group is an indicative of better excretory function by calves fed Biotech Feed based diet. Higher level of BUN in the T2 and T3 groups indicated the higher content of crude protein in the diet.
Discussion
Crinipellis sp. RCK-1 was found to degrade lignin in wheat straw faster on 5th day than cellulose and hemicellulose. However, the hemicellulose content, which was observed to degrade at a slightly higher rate, is known to play only a minor role in supplying nutrients in the cattle rumen48. Earlier, various fungi i.e. Pleurotus sp., T. versicolor, C. subvermispora, Ganoderma sp., B. adusta, L.edodes and P. brevispora have been studied for bioconversion of wheat straw to digestible animal feed. But the majority of them took quite a long period in degrading either similar or higher amounts of lignin compared to Crinipellis sp. RCK-1, i.e. Pleurotus sp., (~37% in 30 days) T. versicolor (~31% in 30 days), C. subvermispora (62% in 49 days), Ganoderma sp. (~11% in 49 days), B. adusta (42% in 49 days), L.edodes (58.9% in 49 days) and P. brevispora (45.4% in 49 days)5,44,48. Prolonged incubation periods have already been reported to be found associated with simultaneous degradation of cellulose and hemicellulose eventually causing an undesirable decrease in dry matter digestibility of the fermented substrate. Since the selective ligninolysis is characterized by a higher positive correlation between total organic matter loss and lignin loss as compared to polysaccharides degradation43. Hence, the present study advocated carrying out the SSF for shorter period to prevent much of the cellulose and hemicellulose losses14,36,44,47. Moreover, the increase in crude protein in the Biotech Feed could be explained by capturing of excess nitrogen during fungal fermentation30.
The findings of the in vitro gas production test were consistent with our earlier observations, where ME and OMD in P. ostreatus and T. versicolor fermented straws were observed to be declined after 20th and10th days, respectively, even after a continuous decrease in lignin content44. The decline in TDOM, OMD and ME after a certain period of SSF is normally attributed to the addition of degradable nitrogen compound to fiber rich feeds at the later stages of fermentation. Alternatively, it may have also resulted due to the improved capturing of nutrients, thereby causing higher production of microbial protein instead of gas and the diversion of carbon source for producing microbial protein than gas28. The present findings are instrumental with our earlier studies and other workers where longer fermentation periods caused either no change or an undesired decrease in OMD, ME and short chain fatty acids (SCFAs)44,45,48. Contrary to that, consistent increase in digestibility with increase in fungal degradation of lignocellulosic components during prolonged incubations (30–60 days) has also been reported by several workers6,35,36. In vitro analysis of Biotech feed based diets depicted that even after reduction of 50% grains, which in turn reduced 0.34 Mcal/kg dry matter from concentrate mixture, no significant decrease was observed in OM digestibility, metabolizable energy and microbial biomass production. This clearly indicated that Biotech feed has the potential to replace 50% grains from traditional feed concentrates and paved the way for in vivo evaluation of feed.
However, based on various studies it is widely accepted that the extent of lignin degradation and increase in in vitro digestibility largely depend on the fungus and incubation conditions and hence can not be generalized47,48. Recently, Tuyen et al48 have also shown that removing lignin alone does not always improve the in vitro gas production in the glass syringe test or in vitro digestibility and a weak correlation (r = 0.47) has been observed between them. Moreover, some studies have also reported a strong and significant correlation between the increase in gas production and lignin to cellulose ratio (percent SSF efficiency in present study) and inclusion of hemicellulose loss in the analysis has shown to increase the goodness of fit of the equation48. Furthermore, reduced partitioning factor (PF) in diets as compared to control is also a good sign of improved available energy in fermented diets.
Amino acids are required for protein synthesis for maintenance, growth and productivity of animal43. Essential and non-essential amino acids are generally discriminated on the basis of metabolic capability of animal to synthesize it or not. Rumen microbes are capable of synthesizing amino acids for microbial protein synthesis only when sufficient carbon source (majorly from dietary carbohydrate), non- protein nitrogen (from inorganic supplementation) and inorganic sulphur are available7. It was thereby inferred, that fermentation of wheat straw by Crinipellis sp. RCK-1 provided sufficient nutrients to support microbial protein synthesis, eventually causing an increase in essential amino acids as well. The better assimilation of nutrients and adequate supply of amino acids supported not only the maintenance but also resulted in an improved body weight gain among animals fed on fermented diets. Supplementation of the essential amino acid is already been emphasized to achieve a low cost feed formulation43
Analysis of ergosterol as fungal biomass index holds specific importance majorly because of the rare 5–7 double bonding based sensitive assay in UV range in neutral lipid extracts since this bonding is rarely reported in major sterols of plants32. Fungal biomass estimation is a major problem while determining fungal growth especially in solid state fermented products, since the biomass grows and remains trapped inside the substrate. Ergosterol content has been established a reliable indicator of fungal growth under SSF conditions and it majorly represents the live biomass19. The fungal biomass could have been determined in terms of chitin, however, this method has faced a number of criticism specially, the tendency of the chitin content in mycelium to vary with age and the substrate specific interference with the assay27,52. While, ergosterol, the predominant sterol of most fungi, found almost exclusively in membranes of living fungal cells, is not produced in significant quantities by green plants and hence can be used as an index of fungal colonization32,51. Moreover, the ergosterol method is more sensitive and easier and takes only 5–6 h and has the tenacity to be scaled up to cope with large numbers of samples within the same time scale42. Therefore, fungal biomass was estimated employing ergosterol based method. Enrichment of fermented feed with enormous fungal biomass displays development of an efficient bioprocess of wheat straw bioconversion, providing an environment for a luxuriant growth of the fungus within substrate eventually producing more digestible and nutrient rich cattle feed.
Within 5 days of incubation, Crinipellis sp. RCK-1 efficiently colonized and degraded straw as has been clearly demonstrated through surface micrographs of the unfermented and fermented wheat straw. The SEM micrographs were also taken at the edges of wheat straw to provide a better view of degradation and to capture the sites of distortion. Middle lamella and S2 layer showed a clear separation of tissues as has been observed by Berrocal et al11 during biological upgrading of wheat straw under SSF conditions by Streptomyces cyaneus. The degradation of lignin rich middle lamella and cell corners have supported visually that the lignin was degraded in the wheat straw11. Presence of fungal hyphae inside the cell lumina further confirmed the deep penetration by the fungus and its progressive degradation of the wheat straw. However, unlike various other studies, Crinipellis sp. RCK-1 has shown the potential to selectively degrade lignin irrespective of the tissue ranging from most lignified xylem and sclerenchyma to less lignified parenchyma2,11. This observation clearly demonstrated the robust nature of Crinipellis sp. RCK-1 and its potential to convert poor quality wheat straw into nutritive animal feed without affecting much of the cellulose and hemicellulose portions of the cell walls. The electron dense residuals of lignin termed here as lignin aggregates have also been demonstrated earlier by many workers and they are considered to be a common characteristic of an advanced stage of white rot decay8,11.
In vitro gas production (IVGP) has been reported to be advantageous over in vivo methods for estimation of nutrient availability in cattle feed, being less expensive, less time consuming, requiring small amounts of samples allowing a better quantification of nutrient utilization and accuracy in describing digestibility. However, to ensure the suitability of fermented feed, in vivo digestion trials are essential and hence it was attempted to establish the nutritive potential of fermented straw as cattle feed through in vivo feeding28,40,45. Such a long period of 90 days was chosen to ensure the consistency in animal performance, minimizing the fluctuation in feed intake and to determine any refusal by the calves. Only after the 90 days when, feed intake, palatability and left over (if any) became constant, 7 days digestion trial was carried out.
The results were instrumental with various in vivo animal feeding trials carried out under similar conditions. In accordance to the present study, an increase in DMI (% of body weight/day) has been observed in lambs by Calazada et al13, which were fed with P. sajor caju fermented wheat straw. In a similar feeding trial using Murrah male buffalo calves, an attempt was made to replace wheat straw/rice straw (in control diet) with Pleurotus treated spent wheat straws at a rate of 0% (T1), 50% (T2) and 100% (T3)22. It was observed that DMI (kg/100 kg body weight) was found to be increased as 1.63 kg/100 kg body weight in T2 diet fed calves when compared with T1 (1.56 kg/100 kg body weight) in case of wheat straw, while it remained unchanged in animal fed with rice straw based diets. Similarly, during an in vivo feeding of Simmenthal heifers, an attempt was made to replace 26% (group II) and 44% (group III) maize grains with P. ostreatus mushroom compost wheat straw, but a sudden decrease in average daily gain was observed from 1150 g/d in control group (I) to 1140 and 990 g/d, respectively1. Likewise, regular fluctuations have also been noticed by Fazaeli et al.17 in parameters such as DMI (g/d), OMI (g/d), DMI (g/kg BW0.75), OMI (g/kg BW0.75), digestible dry matter intake (DDMI) and digestible organic matter intake (DOMI) g/d and DDMI and DOMI (g/kg BW0.75), when male sheep were fed with fermented wheat straw treated with different Pleurotus strains.
In this study, the highest values for nutrients digestibility were observed in T2, which was significantly higher than other treatments. Similar observation for nutrient digestibility has been observed by many other workers while feeding fungal fermented diets to the monogastric animals and ruminants i.e. lambs, goats, buffalo calves, Simmenthal heifers, sheep and Hanwoo steers13,17,21,22,34,37. However, non-significant improvement in in vivo digestibility for certain nutrients i.e. crude protein and ether extract has also been reported by Okano et al.37 and Shrivastava et al45. However, in contrast to our findings a decrease in TDN was observed when chemically (urea) treated (UTRS) and fungal treated (FTRS) rice straw were fed to cross bred goats i.e. 51.28 and 38.38%, respectively21. This decrease was linked with a higher dry matter loss and loss of potential energy materials by Coprinus fimetarius (currently known as coprinopsis cinerea) during prolonged fungal fermentations. The increase in TDN content in our study is a clear indicator of improved and intact availability of energy substances in Biotech Feed.
The gradual increase in DCP in the T2 and T3 dietary groups represented an improved assimilation of nitrogen by ruminants as has been reported by Kakkar and Dhanda22, where replacement of 50 and 100% plain wheat straw with Pleurotus sp. fermented straw in diets (T2 and T3, respectively) improved DCP. Interestingly, in the present study the T3 group, where 50% grains from concentrate mixture have been replaced by Biotech Feed, exhibited highest DCP as 8.8% and improved TDN, which eventually advocated the possibility of using T3 diet as a sole ration to the ruminants in future. Our present results are consistent with our earlier report, where TDN and DCP were found increased upon feeding Ganoderma sp. rckk02 fermented wheat straw based diet to crossbred goats45. Growth rate in terms of average daily gain (ADG) of calves was also found effectively improved upon feeding Biotech Feed. In a similar study Dey et al15 have reported improvement in ADG, when Orpinomyces sp., an anaerobic gut fungus belonging to the phylum Neocallimastigomycota, was administered at the rate of 106 CFU/ml/calf/week (diet T2) in addition to wheat straw + concentrate feed based diet (T1). The improved ADG among calves fed on diet T2 (709 g/d) compared to diet T1 (614 g/d) was attributed to the better utilization of fermented wheat straw because of the availability of more digestible carbohydrates15,45. Likewise, Salman et al41 have also shown that feeding of fermented sugar beet pulp supplemented diet at the rate of 0.6% (T3) and 0.9% (T4) to goats, significantly improved ADG up to 95.25 and 104.83 gram/hour/day (g/h/d), respectively, compared to a control diet (T1, unsupplemented) i.e. 88.58 (g/h/d). However, their study also clearly revealed that at the lower rate of supplementation i.e. 0.3% (T2), ADG could not be improved, as in case of our study.
In vivo feeding trials pertaining to grain replacement have not been much successful in past and a decrease in ADG has also been reported, which could largely be due to the problem of palatability, reduced feed conversion and animal refusal1. Moreover, different to the findings of Adamovic et al1, where grain replaced (44%) diet when fed to the calves resulted in a significant reduction in ADG, in our study a non significant decrease in ADG was noticed in calves fed with T3 diet (50% grain replaced). Data of feed conversion in the present study also revealed the best value with T3 followed by control (T1) and T2, which were found almost similar. These findings were in accordance with a previous report and indicated that daily gain, feed intake and feed conversion were improved in T3 diet41. Decreased feed conversion is undesirable and is known to found associated with depressed average daily gain by ruminants1. Moreover, elevated levels of parameters representing blood urea nitrogen (BUN) utilization, total protein, serum creatinine and albumin are good indicators of improved protein utilization in calves in T2 and T3 diet groups34. The levels of these indicators in blood of fermented straw fed groups (T2 and T3) were found within normal range and thereby did not have any adverse effect on health of calves. The present study showed that no major and significant differences were noticed in blood metabolites among different dietary groups. Similar findings were reported by Kholif et al23, where replacement of 50% of Egyptian berseem clover with spent rice straw of Pleurotus ostreatus in goat rations improved their productive performance without marked effect on metabolic indicators of health. It is apparent thereby, that Biotech Feed based diets can replace the conventional diets and grains in it without causing any adverse effects.
In summary, the present study has clearly demonstrated that the fungus Crinipellis sp. RCK-1 has potential in degrading lignin and not affecting much of cellulose and can therefore improve the nutritional quality of crop residues like wheat straw. Crinipellis sp. RCK-1, owing to its fast growing and selective lignin degrading nature, proved to be a potential candidate for effective solid state bioconversion of wheat straw into digestible and nutrient rich animal feed (Biotech Feed). The production of Biotech Feed, capable of replacing 50% grains, needs further interventions to make it a commercially viable product.
Methods
Microorganism
Crinipellis sp. RCK-1 obtained from fungal culture collection of Lignocellulose Biotechnology Laboratory, University of Delhi South Campus, New Delhi, grown and maintained on malt extract agar (MEA) containing (g/l): malt extract 20.0, KH2PO4 0.5, MgSO4.7H2O 0.5, Ca (N03)2.4H20 0.5, pH 5.4 and at 30°C16,50. This fungal culture has been deposited with International Depository Authority (IDA) at Microbial Type Culture Collection (MTCC- WDCM773), Institute of Microbial Technology (IMTECH), Chandigarh, India (Accession no. MTCC 5722).
Inoculum development
Preparation of pellets in fungal shake flask (250 ml)
The 250 ml Erlenmeyer flasks, each containing 50 ml of malt extract broth (MEB) containing (g/L) malt extract 20, KH2PO4 0.5, MgSO4.7H2O 0.5, Ca (N03)2.4H20 0.5 and pH 5.4, were sterilized at 121°C (15 psi)16,50. Each flask was inoculated with four mycelial discs (8 mm diameter each) taken from the growing edges of 4 day old fungal cultures. The flasks were incubated in an incubator shaker (Innova 44R, New Brunswick Scientific, Enfield, CT, 06082-4444 USA) at 150 rpm and 30°C for 3 days. The fungal pellets so developed were used as seed inoculum for further large scale inoculum production.
Preparation of fungal pellets in shake flask (2 L)
Large scale inoculum production was carried out in 2 L shake flasks, each having 1L of MEB sterilized at 121°C (15 psi) and inoculated with pellets developed as described above as seed inoculum (10% v/v). The flasks were incubated at 150 rpm and 30°C for 72 hours and pellets thus developed were used as inoculum for large scale (500 g wheat straw in each tray) development of Biotech Feed.
Solid State Fermentation (SSF) of wheat straw
Laboratory scale
Each enamel tray (30 × 25 × 5 cm) containing 100 g (dry weight) of wheat straw (~5 mm size) moistened with 200 ml (1:2) of micronutrient solution was autoclaved at 121°C (15 psi) for 30 min. The trays were inoculated with fungal pellet inoculum with 1% (w/w) of the substrate on dry weight basis. A total no. of 6 trays were used in parallel in the lab scale experiment for each incubation period i.e. 5, 10 and 15 days. The trays were incubated at 30°C for 5, 10 and 15 days. The trays without fungal pellets were used as control. The trays harvested after various fermentation periods were oven dried until it achieved constant weight and processed further for estimation of cell wall composition, proximate content and in vitro digestibility.
Scale up of solid state fermentation (SSF)
Biotech Feed production was carried out by fermenting 50 kg of wheat straw in stainless steel trays (50.80 × 50.80 cm) inoculated with fungal pellet inoculum 1% (w/w on dry weight basis) and incubated for 5 days in a Koji room (Lignocellulose Biotechnology Lab, University of Delhi South Campus New Delhi, India). Koji room (3.04 × 3.04 Meter) is an especially designed fermentation facility equipped with controlled humidity (70 ± 5%), temperature (30 ± 2°C) and sterility through sterile air circulation system with HEPA filters (0.3 μm). Each single batch of solid state fermentation was comprised of 100 trays and each tray contained 500 g of wheat straw. Thus, each batch of SSF contained 50.0 kg of wheat straw and in all five such batches of fermentation were carried out in a month, which resulted in production of ~250 Kg/Month (0.25 ton). The production was carried out for >10 months and in total 2.5 ton of fungal treated straw was produced for carrying out feeding trial in calves. The Biotech Feed was harvested and oven dried at 60°C for 24 hour followed by sun drying. The dried Biotech Feed was packed in airtight plastic bags and stored for in vivo animal feeding trial. A random sampling among different batches of Biotech Feed was also done to ensure the consistency and homogeneity of the resulted fermented feed.
In vitro gas production test of Biotech Feed
The in vitro and in vivo trials were performed strictly as per NRC guidelines and with due permission of the Institutional Ethical committee. The in vitro digestibility of Biotech Feeds was analyzed at Indian Veterinary Research Institute (IVRI), Barielly, UP, India. Two male buffaloes of 1-1 ½ years of age were surgically operated for fixing rumen fistula and maintained on standard diet for collecting fresh rumen liquor for studying in vitro gas production. The degradability of dry matter, organic matter and neutral detergent fibre was estimated following the method of Menke and Steingass28. The samples were introduced into a calibrated glass syringe with buffered rumen liquor medium and incubated at 39°C. The gas produced was recorded from the glass syringe at the end of 24 hour incubation. Other parameters like organic matter digestibility (OMD), metabolizable energy (ME), short chain fatty acids (SCFAs), partitioning factor (PF) and microbial biomass (MBP) were calculated as described earlier18,28. The PF is the amount (mg) of truly digested organic matter (TDOM) divided by the amount (ml) of gas produced in vitro12. It is generally taken as an index of partitioning of TDOM between microbial biomass and fermentation gases produced during fermentation. Thus, the higher PF means that a higher proportion of TDOM is used for synthesis of microbial biomass. This has implications in ruminant feeding.
Amino acid analysis of control and fermented wheat straw
Amino acid profile of control and fermented wheat straw was carried out using standard AOAC method3. Feed sample (75 mg) was taken in duplicate in 30 ml capacity vials and added with 10.0 ml 6N HCl. The vials were sealed under constant supply of nitrogen gas and were kept at 110°C for 24 hour for complete hydrolysis of protein38. At the time of analysis, the seal was broken and the content was filtered through Whatman no. 1 filter paper and again the filtrate was filtered through 0.45 µ Millipore filter paper (Sigma-Aldrich Corp.St. Louis, MO, USA). Thereafter, an aliquot of 5 µl of protein hydrolysate was taken in a reaction vial and mixed with 20 µl redrying solution. It was dried under vacuum and several additional drying were carried out. After drying, 20 µl of derivatization solution was added and mixed gently. The tube was kept at room temperature for 20 minutes and then again dried under vacuum using PICO tag assembly (Waters Corp 34 Maple Street, Milford, MA 01757 U.S.A). Thereafter sample were diluted to 100 µl using sample diluents and analysed for amino acids.
Analysis of fungal biomass (ergosterol estimation)
Extraction and estimation of ergosterol was carried out with slight modification in the method described by Gessner et al19. Wheat straw sample (200–250 mg) was placed in chilled centrifuge tube. The sample was extracted with 5 ml methanol for 2 min using laboratory blender (Model HL 1632/00, Philips India Limited, Gurgaon- 122002, India.) and the crude extract was centrifuged at 4500 rpm in a Falcon tube for 5 minutes. The supernatant was then transferred to a 100 ml round bottom flask. The pellet was re-extracted again in 10 ml methanol by shaking at 350 rpm for 10 min followed by a third extraction step. 1.6 g KOH and 4 ml ethanol was then added to the combined supernatants and refluxed for 30 min in a water bath at 80°C. The saponified solution was filtered by cellulose filters to remove any precipitates and collected in separatory funnels. Before extraction, 8 ml distilled water was added and rotated manually for 1 min with three 20-ml portions of petroleum ether (b.p. 35–60°C). The pooled etheric phases were evaporated to about 2 ml in a water bath maintained at 30°C. They were then transferred to small glass tubes and evaporated to complete dryness. The dried sample was re-dissolved in methanol and benzene before estimation using HPLC. The solution was briefly centrifuged, and a volume of 10 µl was injected into a Waters HPLC system having fixed wavelength absorbance detector operating at 282 nm (Waters Corporation 34 Maple Street Milford, MA 01757 508.478.2000, USA). The column was a reversed-phase, C18 and was protected by a guard column having methanol as mobile phase at a flow rate of 1.5 ml/min. During the entire procedure, samples were protected from direct sunlight.
Structural characterization of fungal fermented wheat straw by Electron Microscopy
SEM and TEM of the fermented wheat straw samples were carried out at Electron Microscope Facility, All India Institute of Medical Science (AIIMS), New Delhi. Small pieces of wet straw were cut and fixed in 2.5% glutaraldehyde and 2% paraformaldehyde in 0.1 M sodium phosphate buffer (pH 7.3) for 12 hours at 4°C. After washing it in buffer, the samples were post-fixed in 1% OsO4 for 2 hour at 4°C. The samples were dehydrated in an ascending grade of acetone, critical-point dried (Critical point dryer, Polaron Quorum Technologies Ltd, Judges House Lewes Road Laughton Lewes East Sussex BN8 6BN) and mounted on aluminium stubs for SEM. They were sputter-coated (SCD 050 Super Cool Sputter System, Baltec Technology, Raudondvario pl. 148, LT-47175 Kaunas, Lithuania) with colloidal gold and observed under a Leo 435 VP scanning electron microscope (Cambridge, UK) at an operating voltage 15 kV. Images were digitally acquired by using a CCD camera attached to the microscope. For TEM, samples after washing and dehydration in an ascending grade of acetone, samples were infiltrated and embedded in araldite CY 212 (TAAB Laboratories Equipment Ltd 3 Minerva House, Calleva Park Aldermaston, Berks, RG7 8NA, England). Thin sections of grey-silver colour interference (70–80 nm) were cut and mounted onto 300 mesh- copper grids. Sections were stained with alcoholic uranyl acetate and alkaline lead citrate, washed gently with distilled water and observed under a Morgagni 268 D transmission electron microscope (Fei Company, Achtseweg Noord 55651 GG Eindhoven The Netherland) at an operating voltage 80 kV. Images were digitally acquired by using a CCD camera (Megaview III, Fei Company) attached to the microscope.
In vivo digestibility trial
In vivo animal feeding of fermented feed based diets were carried out after ethical clearance from the Institutional Ethical Committee. Twelve buffalo calves (average body weight 80–85 kg) were purchased locally from Gohana Town, Sonepat, Haryana, India. These calves were divided into three groups and allotted three different treatments/diets. In Treatment I (T1): Wheat straw fed ad libitum with concentrate mixture for maintenance and growth requirement, Treatment II (T2): Biotech Feed fed ad libitum with concentrate requirements for maintenance and growth requirement and Treatment III (T3): Biotech Feed fed ad libitum with concentrate in which 50% grains are reduced. These 50% grains were replaced with Biotech Feed in T3 group, which resulted in 0.34 Mcal less Metabolizable energy/kg Dry Matter in concentrate mixture compared to T1 and T2 groups. Concentration mixture comprised of wheat bran (47.50%) + soybean cake (23.50%) + maize (26%) + mineral mixture (2%) + salt (1%).
Housing of Experimental Calves
The calves with identification number on their ears were shifted to a well-ventilated shed having cemented floor. The sheds were equipped to protect the animals from cold and avoid the mixing of feed and residual material of one animal with other. The calves were dewormed before in vivo growth trial.
Feeding of Calves
The calves were fed for 90 days for their adaptation with unfermented and fermented wheat straw based diets. Their feeding regime, intake, output, weight gain, and all other parameters were recorded. However, after completion of 90 days, they were further fed for 7 more days and during these 7 days the nutrient digestibility of feed in animals were evaluated. The weighed diets containing either unfermented or fermented wheat straws (Biotech Feed) were fed ad libitum twice a day around 8 A.M. in the morning and 2.30 P.M. in the evening. The concentrate mixture was given according to the specification of National Research Council33 and on the basis of their growth pattern. The feed offered to and residue left by individual animal were weighed and recorded on daily basis. The animals were freed every day between 6–8 AM in an open area to have exercise and access to running fresh drinking water. The animals were also offered fresh water twice a day.
Weight gain/weight loss measurement
Body weight of each animal was recorded at fortnight intervals for three consecutive days at around 8.0 A.M., i.e. before offering the feed and water, and the mean weights were calculated to find out gain or loss in body weight. The calves were weighed on an ‘Avery Make’ bridge type balance (Avery Weigh-Tronix) Ballabgarh, Haryana, India with a capacity of 500 Kg.
Digestibility trial
Following the 90 days adaptation phase, a 7 days digestion trial was conducted on all the 12 calves used in experimental study. A weighed quantity of diets was offered twice a day at 8.0 A.M. in the morning and 2.30 P.M. in evening. The calves were offered water twice a day. During the digestibility trial, samples of unfermented straw, fermented straw (Biotech Feed), green fodder and concentrate mixture were collected daily at the time of feeding. All the samples were collected after thorough mixing of the feed. The residues were also sampled next morning after thoroughly mixing and weighing, 100 g of samples were kept in a hot air oven maintained at 70°C for 24 hour or till the weight became constant. The dried samples were ground in a laboratory Wiley mill (Thomas Scientific, NJ 08085 U.S.A.) using 1 mm sieves, mixed well and stored in clean and labeled polythene bags for further biochemical analysis.
Collection and sampling of feces
The feces of each calf were collected individually and carefully to avoid the mixing of feces with urine. After 24 hour collection, feces of individual calves were weighed and mixed manually on cemented floor. Aliquots of 300–400 g were withdrawn and taken to laboratory for dry matter and biochemical analysis. Out of it, an aliquot equal to 1/50 to 1/100th (the proportion was fixed according to the quantity of feces voided by the individual calves) part of the total feces voided were taken for dry matter determination. For estimation of nitrogen, an aliquot of daily collection equal to 1/500 to 1/1000th of the feces voided by the calves were thoroughly mixed with about 1–2 ml of 25% sulphuric acid solution and kept in a wide mouthed glass bottle with a tight lid. At the end of digestibility trial, the bulk samples were ground in a laboratory Willey mill using 1 mm sieve and stored in polythene bags for further biochemical analysis.
Compositional and proximate analysis
Loss in dry matter were estimated by weighing the wheat straw before and after fungal fermentation. The weight loss was calculated by subtracting the dry weight of fermented feed from the weight of unfermented (control) wheat straw24. Oven dried wheat straw (control and fermented ones, diets and feces were ground (1 mm) and sieved (30 mesh) in a moisture free environment and stored in zip lock bags to avoid any moisture gain. These samples were analyzed for proximate principles and cell wall components following standard methods4,20. Neutral detergent fiber (NDF) using sodium sulfite but not amylase and acid detergent fiber (ADF) were determined according to Van Soest et al.49. Lignin was determined by solubilization of cellulose with sulphuric acid39. Percent efficiency of SSF process is expressed as the loss of lignin compared to carbohydrate (cellulose + hemicellulose) breakdown29. Analytical grade chemical reagents were used for all the analyses. All the analyses were carried out in triplicates unless otherwise stated.
Statistical Analysis
The data for in vivo animal feed trial was analyzed using Simple Randomized Block Design (RBD) with three treatments and four replicates as per the procedure suggested by Snedecor and Cochran46. Differences in various treatments were tested by one way analysis of variance (ANOVA) following Bonferroni's posthoc test (P<0.05) SYSTATE (6.0.1) software.
Author Contributions
Conceived and designed the experiments: B.S., K.K.J. and R.C.K. Performed the experiments: B.S., K.K.J., R.C.K. and A.K. Analyzed the data: B.S., K.K.J. and R.C.K. Contributed reagents/materials/analysis tools/Animal Facility: R.C.K. and A.K. Wrote the manuscript: B.S., K.K.J. and R.C.K. All authors prepared the manuscript.
Acknowledgments
The authors are grateful to the Department of Biotechnology, Ministry of Science and Technology, Government of India, New Delhi, India for the financial grant (22/01/2007 (BT/PR7510/AAQ/01/289/2006)). The authors wholeheartedly acknowledge the constant advice and moral support from Prof. P. N. Bhat, chairman World Buffalo Trust, India. The authors also wish to express their gratefulness to the director of IVRI, Izatnagar, Bareilly (U.P) for providing their facilities for In vitro digestibility analyses. Bhuvnesh Shrivastava and Kavish Kumar Jain express their indebtedness to the Council of Scientific and Industrial Research, New Delhi, for the award of a Senior Research Fellowships [(9/45(1079)/2011-EMR-I) & (09/045(1115)/2011-EMR-I)] during the course of this work. The authors acknowledge the help from Ms. Urvashi Kuhad, Dept. of Modern Indian Languages and Comparative Literature, University of Delhi, Delhi for editing the manuscript.
References
- Adamovic M. et al. The biodegradation of wheat straw by Pleurotus ostreatus mushrooms and its use in cattle feeding. Anim Feed Sci Technol 71, 357–362 (1998). [Google Scholar]
- Akin D. E. et al. Alterations in the structure, chemistry, and biodegradation of grass lignocellulose treated with white rot fungi Ceriporiopsis subvermispora and Cyathus stercoreus. Appl. Environ. Microbiol. 61, 591–1598 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amino Acid Profile without Tryptophan (AAP-T) AOAC Official Method 982.30 E (a,b), chp. 45.3.05 (2006).
- AOAC Official Methods of Analysis 17th ed. Association of Official Analytical Chemists, Gaithersburg, MD. (1990).
- Arora D. S. & Sharma R. K. Effect of different supplements on bioprocessing of wheat straw by Phlebia brevispora: changes in its chemical composition, in vitro digestibility and nutritional properties. Bioresour. Technol. 102, 8085–8091 (2011). [DOI] [PubMed] [Google Scholar]
- Arora D. S., Sharma R. K. & Priyanka C. Biodelignification of wheat straw and its effect on in vitro digestibility and antioxidant properties. Int. Biodet. Biodeg. 65, 352–358 (2011). [Google Scholar]
- Atasoglu C., Valde's C., Walker N. D., Newbold C. J. & Wallace R. J. De novo synthesis of amino acids by the ruminal bacteria Prevotella bryantii B14, Selenomonas ruminantium HD4, and Streptococcus bovis ES1. Appl. Environ. Microbiol. 64, 2836–2843 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrasa J. M., Camarero S., Martínez A. T. & Ruel K. Ultrastructural aspects of wheat straw degradation by Phanerochaete chrysosporium and Trametes versicolor. Appl. Microbiol. Biotechnol. 43, 766–770 (1995). [Google Scholar]
- Basu S., Gaur R., Gomes J., Sreekrishnan T. R. & Bisaria V. S. Effect of seed culture on solid state bioconversion of wheat straw by Phanerochaete chrysosporium for animal feed production. J. Biosci. Bioeng. 93, 25–30 (2002). [PubMed] [Google Scholar]
- Berikashvili V. et al. The comparison of white-rot basidiomycetes lignocellulolytic potential in wheat straw solid-state Fermentation. Ecological Eng. Environ. Protection. 1, 69–74 (2014). [Google Scholar]
- Berrocal M. J., Rodríguez A. S., Ball, Pérez-Leblic M. I. & Arias M. E. Solubilisation and mineralisation of 14C lignocellulose from wheat straw by Streptomyces cyaneus CECT 3335 during growth in solid-state fermentation. Appl. Microbiol. Biotechnol. 48, 379–384 (1997). [Google Scholar]
- Blümmel M., Makkar H., Chisanga G., Mtimuni J. & Becker K. The prediction of dry matter intake of temperate and tropical roughages from in vitro digestibility/gas production data, and the dry matter intake and in vitro digestibility of African roughages in relation to ruminant live weight. Anim. Feed Sci. Technol. 69, 131–141 (1997). [Google Scholar]
- Calzada J. F., Franco L. F., De Arriola M. C., Rolz C. & M. A. Acceptability, body weight changes and digestibility of spent wheat straw after harvesting of Pleurotus sajor-caju. Biol. Wastes 22, 303–309 (1987). [Google Scholar]
- Chen J., Fales S. L., Varga G. A. & Royse D. J. Biodegradation of cell wall components of maize stover colonized by white-rot fungi and resulting impact on in vitro digestibility. J. Sci. Food Agric. 68, 91–98 (1995). [Google Scholar]
- Dey A., Sehgal J. P., Puniya A. K. & Singh K. Influence of anaerobic fungal culture (Orpinomycessp.) administration on growth rate, ruminal fermentation and nutrient digestion in calves. Asian-Austr. J. Anim. Sci. 17, 820–824 (2004). [Google Scholar]
- Dhawan S. & Kuhad R. C. Effect of amino acids and vitamins on laccase production by the bird's nest fungus. Cyathus bulleri. Bioresour. Technol. 84, 35–38 (2002). [DOI] [PubMed] [Google Scholar]
- Fazaeli H., Azizi A. & Amile M. Nutritive value index of treated wheat straw with Pleurotus fungi fed to sheep. Pak. J. Biol. Sci. 9, 2444–2449 (2006). [Google Scholar]
- Gatachew G., Makkar H. P. S. & Becker K. Tropical browse: contents of phenolic compounds, in vitro gas production and stoichiometric relationship between short chain fatty acid and in vitro gas production. J. Agric. Sci. 139, 341–352 (2002). [Google Scholar]
- Gessner M. O., Bauchrowitz M. A. & Escautier M. Extraction and quantification of ergosterol as a measure of fungal biomass in leaf litter. Microb. Ecol. 22, 285–291 (1991). [DOI] [PubMed] [Google Scholar]
- Goering H. K. & Van Soest P. J. Forage Fiber Analysis Agricultural Handbook, vol. 379, USDA, Washington, DC. (1970).
- Gupta B. N., Walli T. K., Rai S. N. & Singh K. Effect of feeding fungal treated wheat straw on dry matter consumption and nutrient utilization in crossbred goats. Ind. J. Anim. Nutr. 5, 222–227 (1988). [Google Scholar]
- Kakkar V. K. & Dhanda S. Comparative evaluation of wheat and paddy straws for mushroom production and feeding residual straws to ruminants. Bioresour. Technol. 66, 175–177 (1998). [Google Scholar]
- Kholif A. E. Khattab H. M., El-Shewy A. A., Gado H. M. & Mariezcurrena M. D. Nutrient digestibility, ruminal fermentation activities, serum parameters and milk production and composition of lactating goats fed diets containing rice straw treated with Pleurotus ostreatus. Asian Australas. J. Anim. Sci. 27, 357–364 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhad R. C. & Johri B. N. Production of Cyathus stercoreus fruit bodies in cultures. Ind. J. Microbiol. 24, 45–56 (1984). [Google Scholar]
- Kuhad R. C., Singh A. & Eriksson K-E. l. Microorganisms and enzymes involved in the degradation of plant fiber cell walls. Adv. Biochem. Eng. Biotechnol. 57, 45–125 (1997). [DOI] [PubMed] [Google Scholar]
- Kuhar S., Nair L. M. & Kuhad R. C. Pretreatment of lignocellulosic material with fungi capable of higher lignin degradation and lower carbohydrate degradation improves substrate acid hydrolysis and the eventual conversion to ethanol. Can. J. Microbiol. 54, 305–313 (2008). [DOI] [PubMed] [Google Scholar]
- Matcham S. E., Jordan B. R. & Wood D. A. Estimation of fungal biomass in a solid substrate by three independent methods. Appl. Microbiol. Biotechnol. 21, 108–112 (1985). [Google Scholar]
- Menke K. H. & Steingass H. Estimation of the energetic feed value obtained from chemical analyses and in vitro production using rumen fluid. Anim. Res. Dev. 28, 7–55 (1988). [Google Scholar]
- Moyson E. & Verachtert H. Growth of higher fungi on wheat straw and their impact on the digestibility of the substrate. Appl. Microbiol. Biotechnol. 36, 42–424 (1991). [Google Scholar]
- Nasehi M., Torbatinejad N. M., Zerehdaran S. & Safaei A. R. Effect of (Pleurotus florida) Fungi on Chemical Composition and Rumen Degradability of Wheat and Barley Straw. Iranian J App. Animal Sci. 4, 257–261 (2014). [Google Scholar]
- Neifar M. et al. Improving the nutritive value of olive cake by solid state cultivation of the medicinal mushroom Fomes fomentarius. Chemosphere 91, 110–114 (2013). [DOI] [PubMed] [Google Scholar]
- Newell S. Y., Arsuffi T. L. & Fallon R. D. Fundamental procedures for determining ergosterol content of decaying plant material by liquid chromatography. Appl. Environ. Microbiol. 54, 1876–1879 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC Nutrient Requirements of Dairy Cattle, 6th Revised edition. National Research Council, National Academy of Science, Washington, DC. (1989).
- Oh Y. K. et al. Effects of spent mushroom substrates supplementation on rumen fermentation and blood metabolites in Hanwoo steers. Asian-Aust. J. Anim. Sci. 23, 1608–1613 (2010). [Google Scholar]
- Okano K. et al. Comparison of in vitro digestibility and chemical composition among sugarcane bagasses treated by four white-rot fungi. Anim. Sci. J. 77, 308–313 (2006). [Google Scholar]
- Okano K., Kitagawa M., Sasaki Y. & Watanabe T. Conversion of Japanese red cedar (Cryptomeria japonica) in to a feed for ruminants by white rot basidiomycetes. Anim. Feed. Sci. Technol. 120, 235–243 (2005). [Google Scholar]
- Okano K., Ohkoshi N., Nishiyama A., Usagawa T. & Kitagawa M. Improving the nutritive value of madake bamboo, Phyllostachys bambusoides, for ruminants by culturing with the white-rot fungus Ceriporiopsis subvermispora. Anim. Feed. Sci. Technol. 152, 278–285 (2009). [Google Scholar]
- Roach D. & Gehrke C. W. The gas-liquid chromatography of amino acids. J. Chromatogr. 43, 303 (1969). [DOI] [PubMed] [Google Scholar]
- Robertson J. B. & Van Soest P. J. The detergent system of analysis. In: James, WPT Theander, O (Eds.), The analysis of dietary fiber in food. Marcel Dekker, New York, NY, USA, pp. 123–158 (Chapter 9) (1981).
- Sallam S. M. A. Nutritive value assessment of the alternative feed resources by gas production and rumen fermentation in vitro. Res. J. Agric. Biol. Sci. 1, 200–209 (2005). [Google Scholar]
- Salman F. M. et al. Biologically treated sugar beet pulp as a supplement in goat rations. Int. J. Agric. Biol. 10, 412–416 (2008). [Google Scholar]
- Seitz l. M., Sauer D. B., Burroughs R., Mohr H. E. & Hubbard j. D. Ergosterol as a measure of fungal growth. Phytopathol 69, 1202–1203 (1979). [Google Scholar]
- Sharma R. K. & Arora D. S. Fungal degradation of Lignocellulosic residues: an aspect of improved nutritive quality. Crit Rev Microbiol. 1-9, 10.3109/1040841X.2013.791247 (2013). [DOI] [PubMed] [Google Scholar]
- Shrivastava B. et al. White-rot fungal conversion of wheat straw to energy rich cattle feed. Biodegradation. 22, 823–831 (2011). [DOI] [PubMed] [Google Scholar]
- Shrivastava B. et al. Solid state bioconversion of wheat straw into digestible and nutritive ruminant feed by Ganoderma sp. rckk02. Bioresour. Technol. 107, 347–351 (2012). [DOI] [PubMed] [Google Scholar]
- Snedecor G. W. & Cochran W. G. Statistical Methods, eighth ed. Iowa State Univ. Press, Ames, IA, USA [Snedecor, G. W. (ed) (1–503)] (1989).
- Tripathi J. P. & Yadav J. S. Optimization of solid substrate fermentation of wheat straw into animal feed by Pleurotus ostreatus: a pilot effort. Anim. Feed. Sci. Technol. 37, 59–72 (1992). [Google Scholar]
- Tuyen V. D., Cone J. W., Baars J. J. P., Sonnenberg A. S. M. & Hendriks W. H. Fungal strain and incubation period affect chemical composition and nutrient availability of wheat straw for rumen fermentation. Bioresour. Technol. 111, 336–342 (2012). [DOI] [PubMed] [Google Scholar]
- Van Soest P. J., Robertson J. B. & Lewis B. A. Methods for dietary fiber, neutral detergent fiber and non-starch polysaccharides in relation to animal nutrition. J. Dairy. Sci. 74, 3583–3597 (1991). [DOI] [PubMed] [Google Scholar]
- Vasdev K. & Kuhad R. C. Decolorization of poly R-478 (Polyvinylamie sulphonate Anthrapyridone) by Cyathus bulleri. Fol. Microbiol. 39, 61–64 (1994). [Google Scholar]
- Wallander H. et al. Evaluation of methods to estimate production, biomass and turnover of ectomycorrhizal mycelium in forests soils – A review. Soil Biol Biochem 57, 1034–1042 (2013). [Google Scholar]
- Whipps J. M. & Lewis D. H. Methodology of a chitin assay. Trans Br Mycol Soc. 74, 416–417 (1980). [Google Scholar]
- Zadrazil F., Kamra D. N., Isikhuemhen O. S., Schuchardt F. & Flachowsky G. Bioconversion of Lignocellulose into Ruminant Feed with White Rot Fungi—Review of Work Done at the FAL, Braunschweig. J. Appl. Anim. Res. 10, 105–124 (1996). [Google Scholar]