Abstract
Prostate cancer (PCA) is the 2nd leading cause of cancer-related deaths among men in the United States. Preventing or inhibiting metastasis-related events through non-toxic agents could be a useful approach for lowering high mortality among PCA patients. We have earlier reported that natural flavonoid silibinin possesses strong anti-metastatic efficacy against PCA however, mechanism/s of its action still remains largely unknown. One of the major events during metastasis is the replacement of cell-cell interaction with integrins-based cell-matrix interaction that controls motility, invasiveness and survival of cancer cells. Accordingly, here we examined silibinin effect on advanced human PCA PC3 cells' interaction with extracellular matrix component fibronectin. Silibinin (50-200 μM) treatment significantly decreased the fibronectin (5 μg/ml)-induced motile morphology via targeting actin cytoskeleton organization in PC3 cells. Silibinin also decreased the fibronectin-induced cell proliferation and motility but significantly increased cell death in PC3 cells. Silibinin also inhibited the PC3 cells invasiveness in Transwell invasion assays with fibronectin or cancer associated fibroblasts (CAFs) serving as chemoattractant. Importantly, PC3-luc cells cultured on fibronectin showed rapid dissemination and localized in lungs following tail vein injection in athymic male nude mice; however, in silibinin-treated PC3-luc cells, dissemination and lung localization was largely compromised. Molecular analyses revealed that silibinin treatment modulated the fibronectin-induced expression of integrins (α5, αV, β1 and β3), actin-remodeling (FAK, Src, GTPases, ARP2 and cortactin), apoptosis (cPARP and cleaved caspase 3), EMT (E-cadherin and β-catenin), and cell survival (survivin and Akt) related signaling molecules in PC3 cells. Furthermore, PC3-xenograft tissue analyses confirmed the inhibitory effect of silibinin on fibronectin and integrins expression. Together, these results showed that silibinin targets PCA cells' interaction with fibronectin and inhibits their motility, invasiveness and survival; thus further supporting silibinin use in PCA intervention including its metastatic progression.
Keywords: Prostate cancer, Metastasis, Fibronectin, Integrin, Silibinin, EMT
1. Introduction
Prostate cancer (PCA) is the most common non-cutaneous cancer, and is the second leading cause of cancer-related deaths in American men after lung cancer. According to the American Cancer Society reports, 238,590 new cases and 29,720 deaths from PCA were estimated in the United States in 2013 [1]. Patients with localized PCA have a very high 5-year survival rate; however, in patients with clinically detectable metastatic disease, median survival is mostly reduced to 12-15 months. Therefore, preventing or inhibiting metastasis through nontoxic agents could be a rationalized approach for lowering high mortality among PCA patients. Now, there are ample evidences that epithelial-to-mesenchymal transition (EMT) is a major event during metastasis in epithelial cancer cells [2-4]. During EMT, E-cadherin based cell-cell interaction is replaced by integrins-based cell-matrix interaction which then controls motility, invasiveness and survival of cancer cells, and also confers chemoresistance [2, 5]. Therefore, preventing EMT and/or inhibiting integrins-based cell-matrix interaction could be important towards lowering metastasis incidences as well as associated morbidity and mortality in PCA patients.
Integrins are transmembrane heterodimeric receptors composed of α and β subunits [6]. Extracellular domain of integrins interacts with extracellular matrix (ECM) components, while the cytoplasmic portion associates with the cytoskeleton and numerous signaling molecules [2]. Integrins recognize and bind specific ligands (such as fibronectin, vitornectin, collagen and laminin) resulting in clustering of integrins and recruitment and activation of signaling/adaptor molecules such as focal adhesion kinase (FAK), Src, integrin-linked kinase (ILK), PI3K/Akt, Ras/MAPK and Rho family of GTPases (Rac, Rho and Cdc42 etc) [2, 7-11]. These signaling cascades regulate a variety of cellular processes including cell adhesion, shape, EMT, migration, proliferation, apoptosis, etc. [2, 9, 11, 12]. Importantly, integrins are aberrantly expressed in several epithelial tumors including PCA, and promote disease progression and metastatic spread [8, 13-18]. Specific integrins (α subunits 4, 5, and v, and β subunits 1, 3 and 6) are known to interact with fibronectin, which is a multifunctional extracellular glycoprotein that is also considered of critical relevance for the process of cell survival and metastasis [19]. Therefore, it is expected that targeting integrins expression and their interaction with fibronectin as well as downstream signaling molecules related to cytoskeletal remodeling through non-toxic phytochemicals should prevent cancer cells motility and metastasis. In the present study, we analyzed the efficacy of a natural flavonoid silibinin on fibronectin-induced integrins expression as well as activation of downstream signaling pathways in human PCA PC3 cells.
Silibinin is the major bioactive constituent present in silymarin which is mainly isolated from milk thistle (Silybum marianum), and widely used as a hepatoprotective agent and has been marketed as a dietary supplement. Silibinin has shown strong efficacy against PCA cells both in vitro and in vivo, and is currently being tested in PCA patients [20-25]. Silibinin treatment has been reported to induce cell cycle arrest via activating cellular check points and cyclin dependent kinase inhibitors [20, 26, 27]; and to inhibit multiple signaling pathways such as androgen receptor, EGFR, IGF1R, IGFBP3, and NF-κB etc in human PCA cells [28-32]. In earlier studies, we have also reported that silibinin targets EMT-related molecules and inhibits PCA cells invasiveness and metastasis both in vitro and in vivo [3, 22, 23, 33]; however, the effect of silibinin treatment on PCA cells interaction with ECM component/s as well as integrin signaling remains unstudied. In the present study, for the first time, we examined the effect of silibinin treatment on advanced human PCA PC3 cells' interaction with ECM component fibronectin, and analyzed silibinin effect on fibronectin-induced motility, invasiveness and proliferation using PCA cell culture and animal models. Results clearly showed that silibinin targets fibronectin-integrins interaction as well as downstream signaling pathways, thereby inhibiting motility, invasiveness and survival of PC3 cells.
2. Materials & Methods
2.1 Cell lines and reagents
Human prostate carcinoma PC3 cells were obtained from the American Type Culture Collection (Manassas, VA) and cultured in RPMI1640 medium supplemented with 10% heat inactivated fetal bovine serum (FBS) and 100 U/ml penicillin G and 100 μg/ml streptomycin sulfate at 37°C in a humidified 5% CO2 incubator. PC3-luc cells (expressing luciferase gene) were from Applied Biological Materials (ABM, British Columbia, Canada) and cultured in Prigrow IV media (from ABM, British Columbia, Canada) supplemented with 10%FBS and 100 U/ml penicillin G and 100 μg/ml streptomycin. FBS, penicillin and streptomycin were from Gibco, Life Technologies (Grand Island, NY). Prostate cancer associated fibroblasts (CAFs) were obtained and cultured as described earlier [34]. Antibodies for β-catenin, Rac, MMP9 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies for E-cadherin, Cdc42, ARP2, Integrins (α5, αv, β1, and β3), pSrc-tyr416, total Src, pFAK-Tyr925, total FAK, pAkt-Ser473, total Akt, cPARP, cleaved caspase 3, and anti-rabbit peroxidase-conjugated secondary antibody were obtained from Cell Signaling (Beverly, MA). Survivin antibody was from Novus Biologicals (Littleton, CO). Fibronectin, DAPI (4′,6-diamidino-2-phenylindole), carboxymethylcellulose (CMC), Harris hematoxylin, silibinin, and β-actin antibody were from Sigma-Aldrich (St Louis, MO). ECL detection system and anti-mouse HRP-conjugated secondary antibody were from GE Healthcare (Buckinghamshire, UK). Antibody for α-tubulin was from Lab Vision Corporation (Fremont, CA). Rhodamine-tagged phalloidin was obtained from Life Technologies. Protein assay kit was from Bio-Rad Laboratories (Hercules, CA). ECL detection system and anti-mouse HRP conjugated secondary antibody were from GE Healthcare (Buckinghamshire, UK). All other reagents were obtained in their commercially available highest purity grade.
2.2 Morphological analyses
Cell culture plates were coated with BSA (5 μg/ml) or fibronectin (5 μg/ml) overnight and washed with phosphate-buffered saline (PBS) just before use. Silibinin stock solution was prepared in DMSO and stored at -20°C. An equal amount of DMSO (vehicle) was present in each treatment, including control; DMSO concentration did not exceed 0.1% (v/v) in any treatment. For morphological analyses, PC3 cells were plated on fibronectin coated plates along with DMSO or different concentrations of silibinin (50-200 μM in medium) for desired duration and examined under a light microscope. The number of attached cells with defined morphological features (flattened morphology with lamellipodia) were counted and compared (between DMSO treated control and silibinin-treatment groups). PC3 cells plated on BSA (5 μg/ml) coated or uncoated plates served as relevant controls. Photomicrographs were captured using a Canon Power Shot digital camera.
2.3 Confocal imaging
PC3 cells were grown over cover slips coated with fibronectin in the presence of either DMSO or silibinin (50-200 μM doses). After 1 hr, cells were fixed in 3.7% formaldehyde overnight at 4°C, permeabilized with 0.1% Triton X-100 for 15 min and thereafter blocking was done with 5% serum. Cells were washed with PBS containing 0.2% Tween 20 and incubated with rhodamine-tagged phalloidin and DAPI for 30 min. Cell images were captured at 1500× magnification on a Nikon inverted confocal microscope using 688/405 nm laser wavelengths to detect rhodamine-phalloidin (red) and DAPI (blue) emissions, respectively.
2.4 Cell viability assay
PC3 cells were cultured in RPMI media (with 0.5% FBS) for 24 hrs; thereafter cells were collected and re-plated on fibronectin (5 μg/ml) coated plates with or without silibinin treatment. After 24 hrs, cells were collected by brief trypsinization and washed twice with PBS. Thereafter, cell number and cell death were assessed by trypan blue dye using a haemocytometer under an inverted microscope.
2.5 Migration assay
The effect of silibinin treatment on fibronectin-induced migration was assessed in Transwell migration assay using Falcon 24-well cell culture inserts (BD Biosciences, San Jose, CA) with 8-μm pores. In this assay, the bottom chambers of Transwell were filled with RPMI1640 media containing 0.5% FBS, and in the top chambers, PC3 cells (5 × 104) were seeded in 500 μL RPMI (0.5% FBS) supplemented with FN (5 μg/ml) with or without silibinin (50-200 μM) treatment. RPMI media containing 0.5% FBS supplemented with BSA (5 μg/ml) in the upper chambers served as negative control. After 10 hrs, cells that did not migrate through the pores and therefore remained on the upper side of the filter membrane were gently removed by scrubbing and the migrated cells on the lower side of the filter membrane were fixed with ice cold methanol for 5 min followed by staining with hematoxylin/eosin and mounted. Images were captured using Cannon Power Shot A640 camera on a Zeiss inverted microscope and total number of migrated cells was counted.
2.6 Invasion assay
The effect of silibinin treatment on CAFs-induced PC3 cells invasiveness was assessed in matrigel invasion assay using BD BioCoat Matrigel invasion chambers (BD Biosciences, San Jose, CA). In this assay, CAFs were cultured in the bottom chambers of Transwell in stromal basal medium (supplemented with 0.5% FBS) with or without silibinin (100 μM), and in the top chambers, PC3 cells (2 × 104) were seeded in 500 μL RPMI (supplemented with 0.5% FBS) with or without silibinin (100 μM). Only stroma basal media (supplemented with 0.5% FBS) in the lower chambers served as negative control. After 10 hrs, non-invasive cells which remained on the upper side of the filter membrane were gently removed by scrubbing and the invasive cells on the lower side of the filter membrane were fixed with ice cold methanol for 5 min followed by staining with hematoxylin/eosin and mounted. Images were captured using Cannon Power Shot A640 camera on Zeiss inverted microscope and total number of invasive cells was counted. In another invasion assay, the bottom chambers of Transwell were filled with RPMI media containing 0.5% FBS supplemented with or without fibronectin (40 μg/ml), and in the top chambers PC3 cells (2 × 104) were seeded in 500 μL RPMI (0.5% FBS) with or without silibinin (50 and 100 μM). After 10 hrs, invasive cells were stained and quantified as detailed above. Only RPMI media containing 0.5% FBS in the lower chambers served as negative control in this experiment.
2.7 Tail vein injection and animal imaging
Athymic (nu/nu) male nude mice were purchased from NCI (Frederick, Bethesda, MD) and housed at the University of Colorado Denver (UCD) animal care facility. Protocols were approved by UCD Institutional Animal Care and Use Committee. Mice were administered via oral gavage either vehicle (200 μL of 0.5% CMC [w/v] in sterile water) or silibinin 200 mg/kg body weight (200 μL in 0.5% CMC) for one week before PC-3-luc cells injection. Silibinin dose (200 mg/kg body weight) was based upon our earlier completed study [35] and unpublished animal studies where we have observed biological effect of silibinin at this dose. PC-3-luc cells were cultured in Prigrow IV (with 0.5% FBS) for 24 hrs; thereafter cells were collected and re-plated on fibronectin (5 μg/ml) coated plates with or without silibinin treatment. PC3 cells plated on BSA (5 μg/ml) coated plates served as a control in this experiment. After 24 hrs, cells were harvested, counted and injected (7 × 105 cells/mouse) into nude mice (3-5 mice in each group) through tail vein injection. Six hrs after injection, PC3-luc cells dissemination in mice was analyzed by bioluminescence imaging. Briefly, mice were intraperitoneally injected with 100 μL of 30 mg/mL D-luciferin and imaged using Xenogen IVIS200 imaging system.
2.8 Western blotting
Whole-cell extracts were prepared and Western blotting was performed as previously described [36, 37]. Approximately, 50–70 μg of protein lysate per sample was denatured in 2× sample buffer and subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) on 6 or 12% Tris–glycine gel (as required based upon the protein molecular weight). The separated proteins were transferred on to nitrocellulose membrane followed by blocking with 5% non-fat milk powder (w/v) in Tris-buffered saline (10 mM Tris–HCl, pH 7.5, 100 mM NaCl, 0.1% Tween 20) for 1 h at room temperature. Membranes were probed for the protein levels of desired molecules using specific primary antibodies followed by the appropriate peroxidase-conjugated secondary antibody and visualized by ECL detection system. For certain proteins, membranes were also probed with appropriate secondary IR Dye-tagged antibodies and visualized using Odyssey infra-red imager (LI-COR Biosciences). Membranes were also stripped and re-probed again for protein of interest or with appropriate loading control. In each case, blots were subjected to multiple exposures on the film to make sure that the band density is in the linear range. The autoradiograms/bands were scanned with Adobe Photoshop 6.0 (Adobe Systems, San Jose, CA).
2.9 Immunohistochemistry (IHC)
Paraffin-embedded PC3 tumor tissue sections from an earlier completed PC3 xenograft study [38] were used to determine the in vivo effect of silibinin administration on the levels of integrins (αV, α5, and β1), fibronectin and MMP9 by IHC as described before [35, 38, 39]. Briefly, sections were incubated with specific primary antibody, followed by a specific biotinylated secondary antibody, and then conjugated HRP streptavidin and DAB working solution, and counterstained with hematoxylin. Stained sections were analyzed by Zeiss Axioscope 2 microscope and images captured by AxioCam MrC5 camera at 400× magnifications. Immunoreactivity (represented by brown staining) was scored as 0+ (no staining), 1+ (weak staining), 2+ (moderate staining), 3+ (strong staining), 4+ (very strong staining) as reported earlier [35, 39].
2.10 Statistical analysis
Statistical analysis was performed using SigmaStat 2.03 software (Jandel Scientific, San Rafael, CA). Data was analyzed using one way ANOVA (Tukey test) and a statistically significant difference was considered to be at p<0.05.
3. Results
3.1 Silibinin inhibits fibronectin-induced motile morphology in PC3 cells
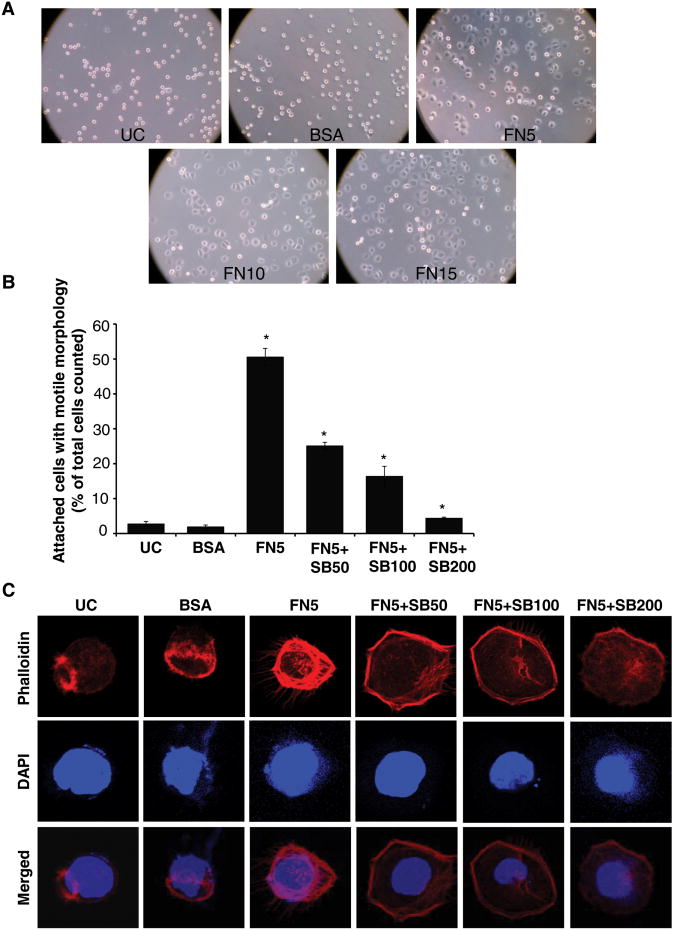
First, we analyzed the effect of fibronectin on the attachment and morphology of the human PCA PC3 cells. PC3 cell were plated on fibronectin (5-15 μg/ml) coated plates. After 1 hr, cells were examined for attachment and morphology under a light microscope. PC3 cells plated on BSA (15 μg/ml) coated or uncoated (UC) plates served as relevant controls in this experiment. PC3 cells barely attached and most of the cells were round in shape without any morphological distinction on uncoated and BSA coated wells (Figure 1A). However, cells attached and showed a flattened morphology with lamellipodia-like structures suggesting a ‘motile morphology’ in fibronectin coated plates at all the concentrations of fibronectin used (Figure 1A). From this experiment, we selected a 5 μg/ml fibronectin dose for subsequent experiments, as this dose was sufficient for attachment and morphological distinction by PC3 cells.
Figure 1. Silibinin inhibits fibronectin-induced motile morphology in PC3 cells.
(A) PC3 cells were plated on cell culture plates coated with different concentrations of fibronectin (5-15 μg/ml). After 1 hr, cells were examined under a light microscope (at 100× magnification). PC3 cells plated on BSA (15 μg/ml) coated or uncoated plates served as relevant controls. (B) PC3 cells (1.5 × 104 per well) were plated on fibronectin coated culture plates (5 μg/ml fibronectin) along with DMSO or different concentrations of silibinin (50-200 μM). After 1 hr, attached cells with defined motile morphology were counted under a light microscope. PC3 cells plated on BSA (5 μg/ml) coated or uncoated plates served as relevant control. Data shown in bar diagram is mean±SEM of three samples. (D) PC3 cells (1.5 × 104 per well) were plated on fibronectin (5 μg/ml) coated cover slips along with DMSO or different concentrations of silibinin (50-200 μM). After 1 hr, cells were fixed and analyzed for F-actin distribution through confocal microscopy detailed in ‘Materials and methods’. In confocal pictures shown (at 1500×), rhodamine-phalloidin (in red) represents cellular F-actin distribution, while DAPI (in blue) stains nuclei. Abbreviations: UC: Uncoated; BSA: Bovine serum albumin; FN: Fibronectin; SB: Silibinin. *, p≤0.001
Next, we examined effect of silibinin treatment on fibronectin-induced attachment and cell morphology. Silibinin (50-200 μM) treatment strongly inhibited the fibronectin (5 μg/ml)-induced attachment and motile morphology in PC3 cells in a dose-dependent manner (Figure 1B). Since actin cytoskeleton is critical for the motile morphology, we next examined the effect of silibinin treatment on fibronectin-induced actin reorganization by analyzing F-actin distribution using rhodamine-tagged phalloidin. As shown in Figure 1C, PC3 cells plated over uncoated and BSA coated wells were round in shape after 1 hr of plating with an F-actin filament ring at one end of the cells. However, PC3 cells plated over fibronectin (5 μg/ml) coated plate showed robust actin cytoskeleton with F-actin filaments bundles forming lamellipodia and several filopodial like protrusions (Figure 1C). Silibinin treatment disrupted the fibronectin-induced strong actin filaments organization as well as filopodial protrusions (Figure 1C).
3.2 Silibinin inhibits fibronectin-induced proliferation and motility in PC3 cells
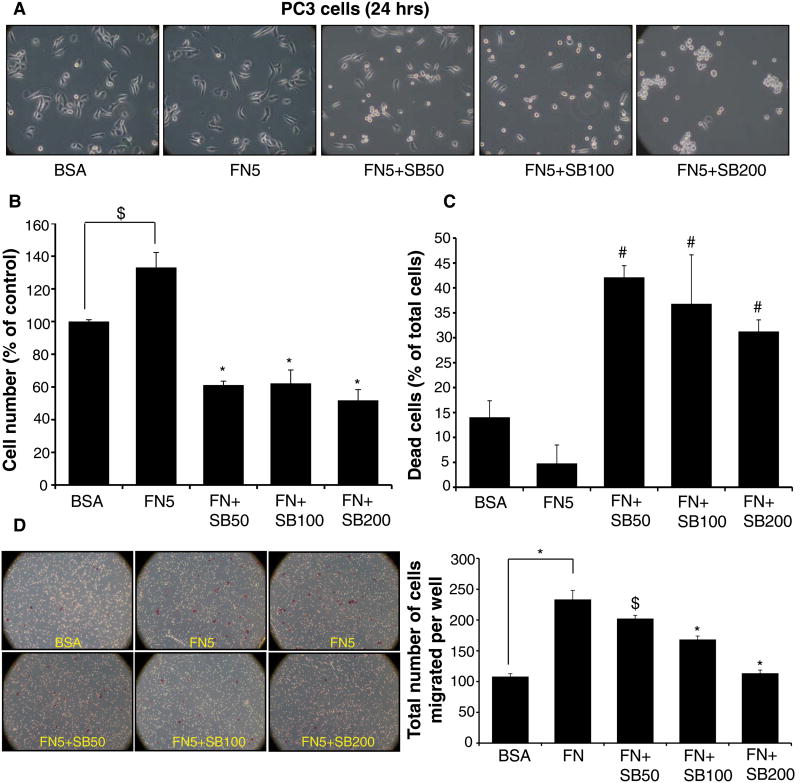
Fibronectin is known to enhance cell survival and motility in cancer cells, so next we examined silibinin effect on fibronectin-induced proliferation and motility in human PCA PC3 cells. The high serum levels found in supplemented growth media may obscure fibronectin-mediated cell proliferation; therefore, for these studies, PC3 cells were first cultured in RPMI media supplemented with 0.5% FBS for 24 hrs; thereafter, cells were collected and re-plated on fibronectin (5 μg/ml) coated plates with or without silibinin under low serum condition (0.5% FBS). PC3 cells plated on BSA (5 μg/ml) coated plates served as a control in this experiment. Under these culture conditions, 24 hrs after plating, PC3 cells were much elongated on fibronectin coated plates compared with BSA, and silibinin treatment resulted in rounding of the cells (Figure 2A). Importantly, with silibinin treatment especially at a dose of 200 μM, PC3 cells were not only rounded but also adhered to each other (Figure 2A). Silibinin (50-200 μM) treatment also significantly decreased the total cell number induced by fibronectin, while significantly increased the death in PC3 cells (Figure 2B and 2C). Silibinin effect on cell number and cell death was not dose-dependent at this time-point; still, in terms of its effect on cell morphology it is clear that higher doses of silibinin do have a better effect (Figure 2A).
Figure 2. Silibinin inhibits fibronectin-induced proliferation and motility in PC3 cells.
PC3 cells were cultured in RPMI media (with 0.5% FBS) for 24 hrs; thereafter cells were collected and re-plated on fibronectin (5 μg/ml) coated plates with or without silibinin (50-200 μM). PC3 cells plated on BSA (5 μg/ml) coated plates served as relevant control. After 24 hrs, cells were analyzed for (A) morphology under a microscope; and (B-C) total cell number and dead cells (% of total cells). (D) Effect of silibinin treatment on fibronectin-induced motility was analyzed in a Transwell migration assay. Data shown in bar diagram is mean±SEM of three samples.
Abbreviations: BSA: Bovine serum albumin; FN: Fibronectin; SB: Silibinin. *, p≤0.001; #, p≤0.01; $, p≤0.05
Next, we examined the effect of silibinin on fibronectin-induced PC3 cells motility in a Transwell migration assay. Silibinin treatment (50-200 μM) for 10 hrs significantly decreased the fibronectin-induced motility of PC3 cells in a dose-dependent manner (Figure 2D-pictures and bar diagram).
3.3 Silibinin inhibits fibronectin- and CAFs-induced invasiveness of PC3 cells
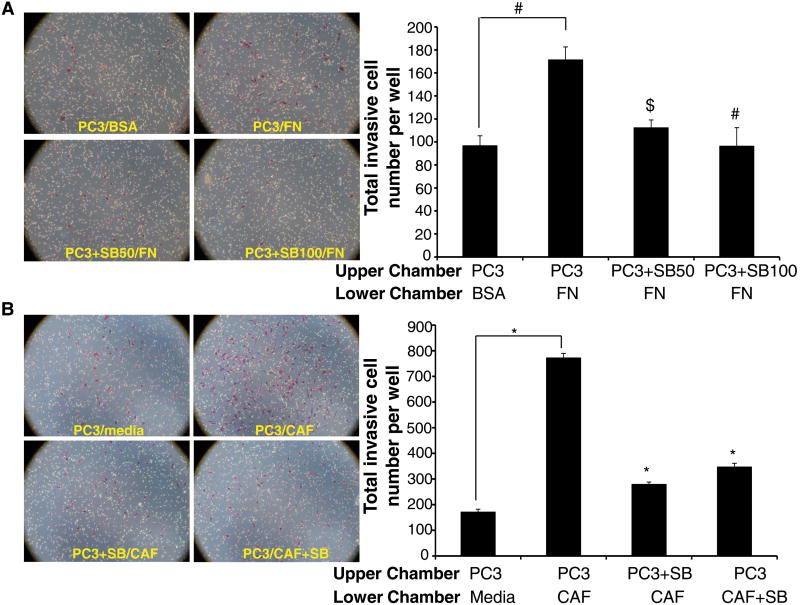
Next we examined the effect of silibinin treatment on the invasiveness of PC3 cells in Transwell invasion assay with fibronectin (40 μg/ml) used as a chemoattractant in the lower chambers. As shown in Figure 3A, fibronectin significantly enhanced the invasiveness of PC3 cells through the matrigel layer compared with BSA control, while silibinin (50 and 100 μM) treatment strongly reduced the invasiveness of PC3 cells.
Figure 3. Silibinin inhibits fibronectin- and CAFs-induced invasiveness in PC3 cells.
(A) The bottom chambers of Transwell were filled with RPMI media containing 0.5% FBS supplemented with BSA or fibronectin (40 μg/ml), and in the top chambers PC3 cells (2 × 104) were seeded in RPMI media containing 0.5% FBS with or without silibinin (50 and 100 μM). After 10 h, PC3 cells invasiveness was measured. (B) CAF cells were grown in the bottom chambers of Transwell with or without silibinin (100 μM), and PC-3 cells (2 × 104) were seeded in the top chambers with or without silibinin (100 μM). After 10 hrs, PC3 cells invasiveness was measured. Cell invasion data shown are means±SEM of three samples. Representative photomicrographs are shown at 100×. Abbreviations: BSA: Bovine serum albumin; FN: Fibronectin; SB: Silibinin; CAF: Cancer-associated fibroblasts. *, p≤0.001; #, p≤0.01; $, p≤0.05
In the tumor microenvironment, CAFs release ECM components including fibronectin and are involved in ECM remodeling, facilitating movement of cancer and endothelial cells. Therefore, next we analyzed the effect of CAFs presence in the lower chambers as chemoattractant on the invasiveness of PC3 cells cultured in the upper chambers of Transwells. As shown in Figure 3B, CAFs strongly induced the invasiveness of PC3 cells while silibinin presence either in the upper chamber (with PC3 cells) or in the lower chambers (with CAFs) significantly reduced the invasiveness of PC3 cells.
3.4 Silibinin inhibits fibronectin-induced dissemination of PC3-luc cells in vivo
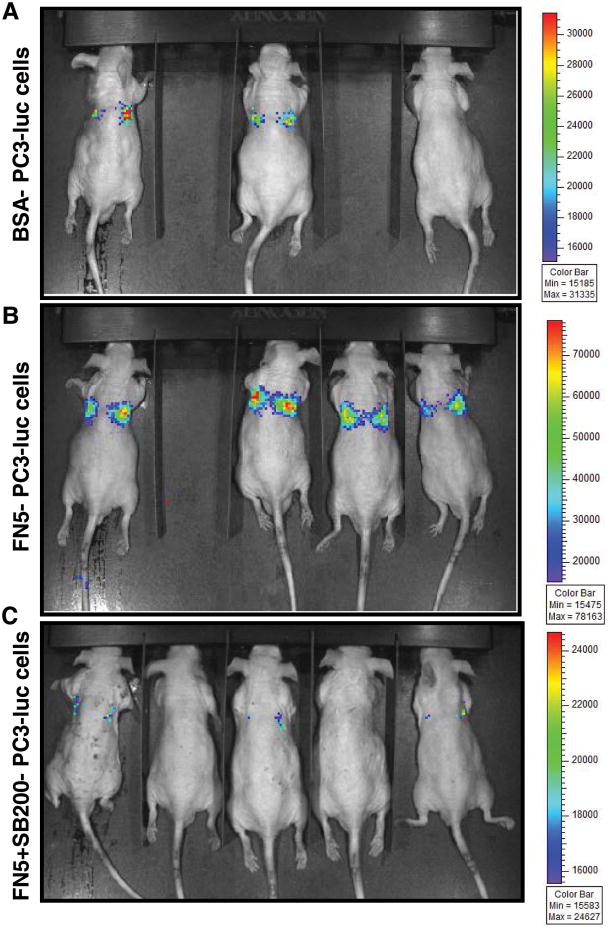
Activated integrin signaling (following fibronectin exposure) protects the cells during metastatic spread in the blood through conferring anoikis resistance and play a critical role in intravasation at distant metastatic sites. We next employed tail vein injection metastatic model, mimicking the hematogenous dissemination of cancer cells, to understand the in vivo capability of PCA cells to survive and colonize at the metastatic site when cultured on fibronectin coated plates with or without silibinin treatment. For this study, we used PC3-luc cells (expressing luciferase gene) to non-invasively image and monitor the dissemination of cells when injected in mice. PC3-luc cells were cultured in RPMI media supplemented with 0.5% FBS for 24 hrs; thereafter cells were collected and re-plated on fibronectin (5 μg/ml) coated plates with or without silibinin (200 μM) treatment. PC3 cells plated on BSA (5 μg/ml) coated plates served as a control in this experiment. After 24 hrs, cells were harvested, and 7 × 105 cells were injected in each male nude mouse through tail vein. Thereafter, mice were imaged 6 hrs later by Xenogen IVIS200 imaging system. As shown in the Figure 4A, 2/3 mice showed faint bioluminescence signal in mice injected with PC3-luc cells cultured on BSA-coated plates; however, 4/4 animals showed strong bioluminescence signal and lung localization in mice injected with PC3-luc cells cultured on fibronectin-coated plates (Figure 4B). Importantly, in mice injected with PC3-luc cells cultured on fibronectin-coated plates along with silibinin treatment, 2/5 mice showed no bioluminescence signal and other 3/5 mice showed extremely dim bioluminescence signal (Figure 4C).
Figure 4. Silibinin inhibits fibronectin-induced dissemination of PC3-luc cells in athymic nude mice.
(A-C) PC3 cells were cultured in RPMI media (with 0.5% FBS) for 24 hrs; thereafter cells were collected and re-plated on fibronectin (5 μg/ml) coated plates with or without silibinin (200 μM). PC3 cells plated on BSA (5 μg/ml) coated plates served as relevant control. After 24 hrs, cells were harvested, and 7 × 105 cells were injected in each male athymic nude mouse. Mice were imaged 6 hrs later as described in the ‘Materials and methods’. Representative mice images as well as bioluminescence intensity scale bar for each image are presented. Abbreviations: BSA: Bovine serum albumin; FN: Fibronectin; SB: Silibinin
3.4 Silibinin targets multiple signaling pathways down-stream of fibronectin-integrin in PC3 cells
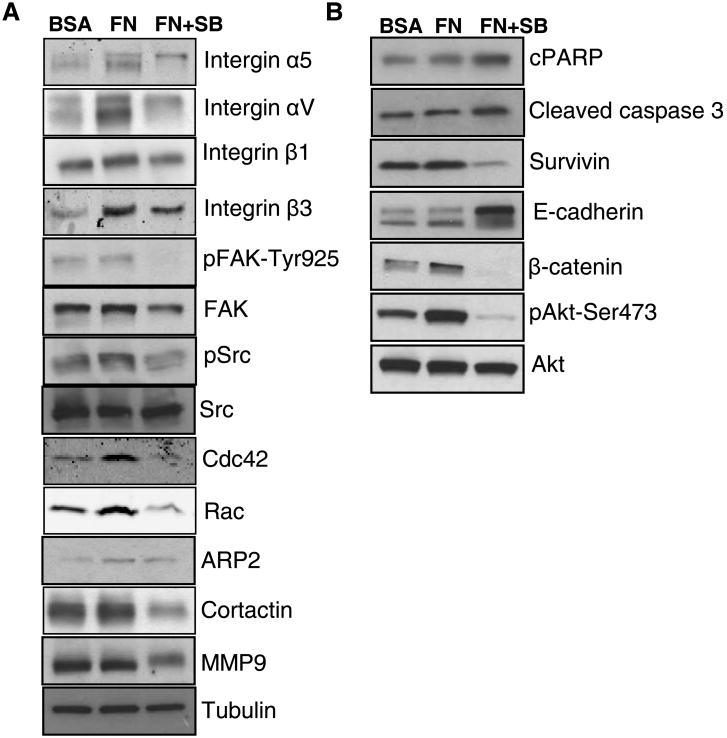
Fibronectin-integrin interaction is known to activate and/or modulate several signaling pathways regulating adhesion, motility, invasiveness, survival, anoikis as well as chemoresistance of cancer cells [9, 13, 19, 40]. Our results revealed that silibinin (200 μM) treatment for 24 hrs decreased the fibronectin-induced expression of integrins (α5, αV, β1 and β3) in PC3 cells (Figure 5A). One of the key molecules recruited downstream of integrins is non receptor tyrosine kinase FAK, which is autophosphorylated, and then binds and activates Src, that in turn phosphorylate FAK at tyrosine residue [2, 9]. As shown in Figure 5A, silibinin treatment decreased the phosphorylation of FAK at Tyr-925 site, while total FAK level was only slightly affected by silibinin treatment. Silibinin treatment also reduced the fibronectin-induced phosphorylation of Src kinase without affecting the total Src level (Figure 5A). Integrins clustering on plasma membrane also activates Rho family of small GTPases that play a critical role in actin cytoskeleton remodeling and formation of various motile structures such as lamellipodia and filopodia [9-11, 41]. Our results also showed that fibronectin activated while silibinin reduced the expression of GTPases Cdc42 and Rac (Figure 5A). Rho family GTPases are known to activate ARP2/3 and cortactin, and both of these molecules (ARP2/3 and cortactin) play an important role in actin polymerization and the branching that is required for the formation of motile structures such as lamellipodia, filopodia, etc [42-45]. Importantly, silibinin treatment also decreased the levels of ARP2 and cortactin (Figure 5A). Silibinin treatment also reduced the level of matrix metalloproteinase 9 (MMP9) that belongs to family of zinc and calcium dependent endopeptidase involved in ECM degradation and considered important during invasion and cancer metastasis [46].
Figure 5. Silibinin targets multiple signaling pathways down-stream of fibronectin-integrin in PC3 cells.
(A-B) PC3 cells were cultured in RPMI media (with 0.5% FBS) for 24 hrs; thereafter cells were collected and re-plated on fibronectin (5 μg/ml) coated plates with or without silibinin (200 μM). PC3 cells plated on BSA (5 μg/ml) coated plates served as relevant control. After 24 hrs, cells were harvested and whole cell lysates were prepared and analyzed for Integrins (α5, αV, β1 and β3), phosphorylated and total FAK and Src, Cdc42, Rac, ARP2, cortactin, MMP9, cPARP, cleaved caspase 3, survivin, E-cadherin, β-catenin, and phosphorylated and total Akt. Tubulin was used as a loading control
Next, we examined the effect of silibinin treatment on apoptosis and survival related signaling molecules as fibronectin-integrins interaction is known to promote survival and proliferation as well as prevents cell death [2, 6, 12]. As shown in Figure 5B, silibinin treatment slightly increased the level of cPARP and cleaved caspase 3, molecular markers of apoptosis, compared with PC3 cells cultured over fibronectin coated plates alone. Importantly, silibinin treatment strongly decreased the fibronectin-induced survivin level, which is an established negative regulator of apoptosis and is also reported to promote metastasis [47].
Integrin based signaling is also known to provide cells a mesenchymal phenotype associated with increased invasiveness [2]. Our results showed that silibinin treatment resulted in higher E-cadherin expression (Figure 5B), an important adherens junction protein, which also correlates with the round morphology and greater adhesion between cells by silibinin treatment as shown in Figure 2A. Silibinin treatment also reduced the fibronectin-induced β-catenin expression in PC3 cells (Figure 5B). Next, we examined silibinin effect on fibronectin-induced Akt phosphorylation which is known to play an important role in promoting cell survival, proliferation, EMT, and inhibiting apoptosis [2, 48]. Importantly, Akt phosphorylation at Ser-473 site was significantly increased in the presence of fibronectin while silibinin treatment inhibited that without affecting the total Akt level in PC3 cells (Figure 5B).
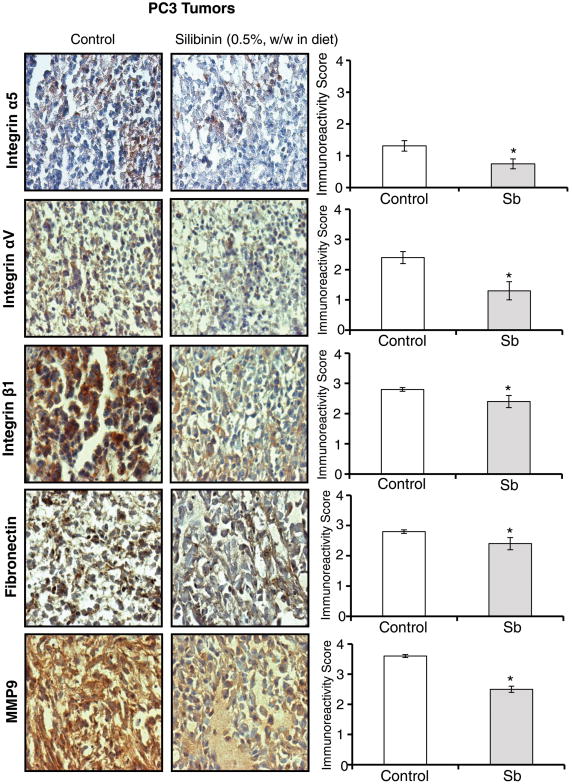
3.5 Silibinin feeding inhibits integrins, fibronectin and MMP9 expression in PC3 tumors
Next, we confirmed our cell culture results in vivo by analyzing PC3 xenograft tissues from a completed study [38]. Earlier, we have reported that silibinin feeding (0.5%, w/w in diet) strongly inhibited the growth of PC3 tumors in nude mice [38]. IHC analyses of PC3 tumor tissues showed that silibinin feeding moderately decreased the integrins α5 and αV expression while only slightly but significantly decreased the integrin β1 expression (Figure 6). Furthermore, we observed a slight but significant decrease in fibronectin level, and a moderate decrease in MMP9 level in PC3 tumor tissues (Figure 6). These in vivo results further support our cell culture findings that silibinin targets fibronectin-integrin signaling in PCA cells.
Figure 6. Effect of silibinin feeding on the expression of integrins, fibronectin and MMP9 in PC3 tumors.
PC3 tumor tissues were analyzed by IHC to determine silibinin's effects on the expression of integrins (α5, αV, and β1), fibronectin and MMP9. Immunoreactivity (represented by brown staining) of these biomarkers was scored as 0+ (no staining), 1+ (weak staining), 2+ (moderate staining), 3+ (strong staining), and 4+ (very strong staining). Data shown in bar diagram represent mean ± SEM of 6-9 samples for each group. SB: Silibinin. *, p≤0.001
4. Discussion
Integrin-ECM interaction as well as associated signaling cascades help cancer cells in acquiring invasiveness and motility, survival in the circulation as well as extravasation at metastatic sites [2, 13, 19]. In the past, several studies in pre-clinical models have shown that blocking integrin/ECM interaction could be effective in inhibiting cancer growth and metastasis [6, 8, 17, 19]. However, serious doubts have been raised over the selectivity of agents targeting integrins or integrins-ECM interaction, as these proteins and their interactions are also involved in several cellular activities essential for normal cellular functions [8]. Therefore, there is a need to develop agent/s targeting integrins, ECM components and/or their interactions specifically in the tumors. One attractive option could be to test the efficacy of non-toxic natural agents to target integrins, ECM and their interaction in cancer cells to prevent their growth, invasiveness and metastasis. In this direction, here we tested the efficacy of a natural non-toxic dietary supplement silibinin against PCA cells' interaction with fibronectin. Results are encouraging as silibinin inhibited the fibronectin-induced motile morphology, migration, invasion, and dissemination in vivo as well as inhibited multiple fibronectin-induced signaling pathways in human PCA PC3 cells.
Fibronectin expression has been reported to be increased in several types of malignancies including PCA, and its expression positively correlates with an invasive and metastatic phenotype [49-51]. Similarly, integrins are aberrantly expressed in several cancers including PCA [16, 52, 53]. Therefore, fibronectin-integrin axis has been suggested as a target for preventive and therapeutic approaches against cancer progression [17-19, 54, 55]. Results from present study showed that silibinin inhibited the fibronectin-induced expression of integrins as well as downstream signaling molecules including FAK/Src/GTPases/Akt/MMP9. There have been several reports highlighting the important role of these signaling molecules in survival, anoikis resistance, invasiveness and metastasis of cancer cells [2, 7-9]. For example, Lee et al reported that integrin β1 is upregulated in human PCA cells which is associated with higher FAK auto-phosphorylation and correlated with anoikis resistance and metastasis [8]. Earlier, Zeng et al have also reported the important role of FAK and PI3K in integrin-fibronectin mediated MMP1-dependent invasion of metastatic PCA cells [7]. Therefore, silibinin mediated inhibition of fibronectin-induced motility, invasiveness and survival as well as in vivo dissemination could be through inhibition of integrins and downstream signaling pathways.
In earlier studies, we and others have reported that silibinin targets multiple signaling pathways associated with PCA invasiveness and metastasis [3, 33]. Silibinin inhibited the migration and invasiveness of human PCA cells through enhancing E-cadherin expression [56]. In other cell culture studies, silibinin was also reported to decrease migratory/invasive potential and to inhibit vimentin and increase cytokeratin-18 expression in PCA cells [57-59]. We have also reported in transgenic adenocarcinoma of the mouse prostate (TRAMP) model that silibinin feeding strongly reduces the expression of fibronectin corresponding to a significant decrease in the metastatic progression of the disease [22]. Importantly, in the same study we also observed that silibinin feeding decreased the MMPs (2, 3 and 9) and increased the E-cadherin expression in the prostate tissues [22]. In the present study, we showed for the first time that silibinin treatment inhibited the fibronectin-induced integrins expression while enhanced the E-cadherin expression, and significantly reduced the fibronectin-induced motile morphology in PC3 cells. Supporting these results, Handorean et al have earlier reported that silibinin (at 100 μM dose) inhibited the human PCA PC3M cells adhesion to fibronectin [60]. Silibinin treatment also inhibited the high-glucose mediated increase in matrix-associated peri-cellular fibronectin as well as soluble fibronectin in human mesangial cells, suggesting that silibinin could inhibit fibronectin production or enhance its degradation [61]. Based upon previous studies and results from the present study, it seems that silibinin targets multiple signaling including the integrins, fibronectin and their interaction, thus promoting epithelial characteristics and inhibiting invasiveness of PCA cells.
Fibronectin is primarily secreted by fibroblasts and plays an important role in wound healing. Similarly, in the tumor microenvironment, CAFs promote cancer cells survival and metastasis as well as angiogenesis through secreting ECM components including fibronectin as well as growth factors and cytokines [62]. Importantly, increased fibronectin level is an important event during preparation of pre-metastatic niches, supporting its important role in facilitating metastasis [63]. In the present study, we observed that CAFs or fibronectin in the Transwell assays acted as a strong chemoattractant and significantly enhanced the invasiveness of PC3 cells. Importantly, silibinin treatment inhibited the CAF-induced PC3 invasiveness, probably through targeting the CAFs ability to secret fibronectin or other ECM components. Our unpublished data also support this notion as silibinin treatment strongly reduced the fibronectin secretion in the media by CAFs. Also, we have recently reported that silibinin treatment inhibits the capacity of PCA cells to induce a CAF-like phenotype in prostate stromal cells by acting on PCA cells' capacity to secrete TGFβ2 [34]. Also, PC3 tumor tissues from silibinin-fed mice exhibited significantly reduced TGFβ2 expression as well as decreased CAF biomarkers such as α-SMA, vimentin and FAP expression [34]. Analyses of tumor tissues from the same study also suggested that fibronectin expression was also decreased with silibinin treatment, which could be due to inhibition of CAFs activation by silibinin in these tumors. Overall, in prostate tumor microenvironment, silibinin could target PCA cells ability to activate CAFs and/or inhibit CAFs ability to secrete fibronectin; thereby, silibinin could inhibit the survival and invasiveness of PCA cells.
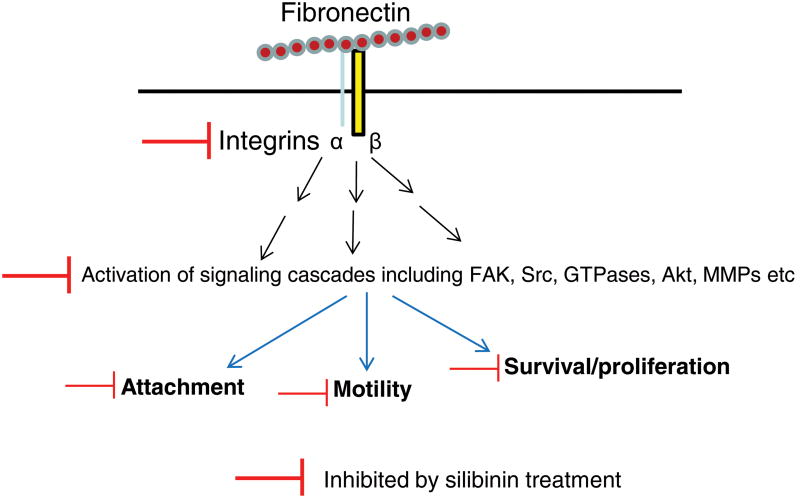
In conclusion, our results showed that silibinin inhibits fibronectin-induced integrin signaling as well as motility and invasiveness of PCA cells (Figure 7). Further studies are warranted to understand the mechanism through which silibinin targets fibronectin-integrin interaction and decreases fibronectin expression in vivo. Since silibinin was also effective in lowering the fibronectin-induced survival in PCA cells (Figure 7), silibinin should be further tested for its potential to overcome chemotherapeutic resistance. Overall, these completed studies suggest that non-toxic [3, 24, 64] silibinin could be useful in preventing and inhibiting PCA metastatic progression.
Figure 7. A summary of silibinin effect on integrin signaling in prostate cancer cells.
Silibinin targets the fibronectin induced integrin signaling together with several additional signaling cascades, thereby inhibiting the attachment, motility and proliferation of prostate cancer cells.
Highlights.
Silibinin inhibits fibronectin-induce motile morphology in PC3 cells
Silibinin inhibits fibronectin-induced migration and invasion in PC3 cells
Silibinin targets fibronectin-induced integrins and downstream signaling molecule
Acknowledgments
This work was supported by NCI RO1 grant CA102514 (to RA). Authors also acknowledge the CCSG P30CA046934 grant for supporting the UCD Shared Resources used in this study. Funding sources had no involvement in study design; or in the collection, analyses and interpretation of the data; or in writing this manuscript; or in the decision to submit this manuscript for publication.
Footnotes
Conflict of Interest Statement: The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Guarino M, Rubino B, Ballabio G. The role of epithelial-mesenchymal transition in cancer pathology. Pathology. 2007;39:305–318. doi: 10.1080/00313020701329914. [DOI] [PubMed] [Google Scholar]
- 3.Deep G, Agarwal R. Antimetastatic efficacy of silibinin: molecular mechanisms and therapeutic potential against cancer. Cancer metastasis reviews. 2010;29:447–463. doi: 10.1007/s10555-010-9237-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer research. 2006;66:8319–8326. doi: 10.1158/0008-5472.CAN-06-0410. [DOI] [PubMed] [Google Scholar]
- 5.Zutter MM. Integrin-mediated adhesion: tipping the balance between chemosensitivity and chemoresistance. Advances in experimental medicine and biology. 2007;608:87–100. doi: 10.1007/978-0-387-74039-3_6. [DOI] [PubMed] [Google Scholar]
- 6.Millard M, Odde S, Neamati N. Integrin targeted therapeutics. Theranostics. 2011;1:154–188. doi: 10.7150/thno/v01p0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeng ZZ, Jia Y, Hahn NJ, Markwart SM, Rockwood KF, Livant DL. Role of focal adhesion kinase and phosphatidylinositol 3′-kinase in integrin fibronectin receptor-mediated, matrix metalloproteinase-1-dependent invasion by metastatic prostate cancer cells. Cancer research. 2006;66:8091–8099. doi: 10.1158/0008-5472.CAN-05-4400. [DOI] [PubMed] [Google Scholar]
- 8.Lee YC, Jin JK, Cheng CJ, Huang CF, Song JH, Huang M, Brown WS, Zhang S, Yu-Lee LY, Yeh ET, McIntyre BW, Logothetis CJ, Gallick GE, Lin SH. Targeting constitutively activated beta1 integrins inhibits prostate cancer metastasis. Molecular cancer research : MCR. 2013;11:405–417. doi: 10.1158/1541-7786.MCR-12-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canel M, Serrels A, Frame MC, Brunton VG. E-cadherin-integrin crosstalk in cancer invasion and metastasis. Journal of cell science. 2013;126:393–401. doi: 10.1242/jcs.100115. [DOI] [PubMed] [Google Scholar]
- 10.Rathinam R, Berrier A, Alahari SK. Role of Rho GTPases and their regulators in cancer progression. Front Biosci (Landmark Ed) 2011;16:2561–2571. doi: 10.2741/3872. [DOI] [PubMed] [Google Scholar]
- 11.DeMali KA, Wennerberg K, Burridge K. Integrin signaling to the actin cytoskeleton. Current opinion in cell biology. 2003;15:572–582. doi: 10.1016/s0955-0674(03)00109-1. [DOI] [PubMed] [Google Scholar]
- 12.Morgan M, Saba S, Gower W. Fibronectin influences cellular proliferation and apoptosis similarly in LNCaP and PC-3 prostate cancer cell lines. Urologic oncology. 2000;5:155–159. doi: 10.1016/s1078-1439(99)00058-7. [DOI] [PubMed] [Google Scholar]
- 13.Knowles LM, Gurski LA, Engel C, Gnarra JR, Maranchie JK, Pilch J. Integrin alphavbeta3 and fibronectin upregulate Slug in cancer cells to promote clot invasion and metastasis. Cancer research. 2013;73:6175–6184. doi: 10.1158/0008-5472.CAN-13-0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pal S, Ganguly KK, Chatterjee A. Extracellular matrix protein fibronectin induces matrix metalloproteinases in human prostate adenocarcinoma cells PC-3. Cell communication & adhesion. 2013;20:105–114. doi: 10.3109/15419061.2013.833193. [DOI] [PubMed] [Google Scholar]
- 15.Ganguly KK, Pal S, Moulik S, Chatterjee A. Integrins and metastasis. Cell adhesion & migration. 2013;7:251–261. doi: 10.4161/cam.23840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mizejewski GJ. Role of integrins in cancer: survey of expression patterns. Proc Soc Exp Biol Med. 1999;222:124–138. doi: 10.1177/153537029922200203. [DOI] [PubMed] [Google Scholar]
- 17.Park CC, Zhang H, Pallavicini M, Gray JW, Baehner F, Park CJ, Bissell MJ. Beta1 integrin inhibitory antibody induces apoptosis of breast cancer cells, inhibits growth, and distinguishes malignant from normal phenotype in three dimensional cultures and in vivo. Cancer research. 2006;66:1526–1535. doi: 10.1158/0008-5472.CAN-05-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fornaro M, Manes T, Languino LR. Integrins and prostate cancer metastases. Cancer metastasis reviews. 2001;20:321–331. doi: 10.1023/a:1015547830323. [DOI] [PubMed] [Google Scholar]
- 19.Malik G, Knowles LM, Dhir R, Xu S, Yang S, Ruoslahti E, Pilch J. Plasma fibronectin promotes lung metastasis by contributions to fibrin clots and tumor cell invasion. Cancer research. 2010;70:4327–4334. doi: 10.1158/0008-5472.CAN-09-3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deep G, Singh RP, Agarwal C, Kroll DJ, Agarwal R. Silymarin and silibinin cause G1 and G2-M cell cycle arrest via distinct circuitries in human prostate cancer PC3 cells: a comparison of flavanone silibinin with flavanolignan mixture silymarin. Oncogene. 2006;25:1053–1069. doi: 10.1038/sj.onc.1209146. [DOI] [PubMed] [Google Scholar]
- 21.Singh RP, Raina K, Deep G, Chan D, Agarwal R. Silibinin suppresses growth of human prostate carcinoma PC-3 orthotopic xenograft via activation of extracellular signal-regulated kinase 1/2 and inhibition of signal transducers and activators of transcription signaling. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:613–621. doi: 10.1158/1078-0432.CCR-08-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raina K, Rajamanickam S, Singh RP, Deep G, Chittezhath M, Agarwal R. Stage-specific inhibitory effects and associated mechanisms of silibinin on tumor progression and metastasis in transgenic adenocarcinoma of the mouse prostate model. Cancer research. 2008;68:6822–6830. doi: 10.1158/0008-5472.CAN-08-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh RP, Raina K, Sharma G, Agarwal R. Silibinin inhibits established prostate tumor growth, progression, invasion, and metastasis and suppresses tumor angiogenesis and epithelial-mesenchymal transition in transgenic adenocarcinoma of the mouse prostate model mice. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:7773–7780. doi: 10.1158/1078-0432.CCR-08-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flaig TW, Gustafson DL, Su LJ, Zirrolli JA, Crighton F, Harrison GS, Pierson AS, Agarwal R, Glode LM. A phase I and pharmacokinetic study of silybin-phytosome in prostate cancer patients. Invest New Drugs. 2007;25:139–146. doi: 10.1007/s10637-006-9019-2. [DOI] [PubMed] [Google Scholar]
- 25.Flaig TW, Glode M, Gustafson D, van Bokhoven A, Tao Y, Wilson S, Su LJ, Li Y, Harrison G, Agarwal R, Crawford ED, Lucia MS, Pollak M. A study of high-dose oral silybin-phytosome followed by prostatectomy in patients with localized prostate cancer. The Prostate. 2010;70:848–855. doi: 10.1002/pros.21118. [DOI] [PubMed] [Google Scholar]
- 26.Deep G, Agarwal R. New combination therapies with cell-cycle agents. Curr Opin Investig Drugs. 2008;9:591–604. [PMC free article] [PubMed] [Google Scholar]
- 27.Roy S, Kaur M, Agarwal C, Tecklenburg M, Sclafani RA, Agarwal R. p21 and p27 induction by silibinin is essential for its cell cycle arrest effect in prostate carcinoma cells. Molecular cancer therapeutics. 2007;6:2696–2707. doi: 10.1158/1535-7163.MCT-07-0104. [DOI] [PubMed] [Google Scholar]
- 28.Dhanalakshmi S, Singh RP, Agarwal C, Agarwal R. Silibinin inhibits constitutive and TNFalpha-induced activation of NF-kappaB and sensitizes human prostate carcinoma DU145 cells to TNFalpha-induced apoptosis. Oncogene. 2002;21:1759–1767. doi: 10.1038/sj.onc.1205240. [DOI] [PubMed] [Google Scholar]
- 29.Singh RP, Dhanalakshmi S, Tyagi AK, Chan DC, Agarwal C, Agarwal R. Dietary feeding of silibinin inhibits advance human prostate carcinoma growth in athymic nude mice and increases plasma insulin-like growth factor-binding protein-3 levels. Cancer research. 2002;62:3063–3069. [PubMed] [Google Scholar]
- 30.Zi X, Zhang J, Agarwal R, Pollak M. Silibinin up-regulates insulin-like growth factor-binding protein 3 expression and inhibits proliferation of androgen-independent prostate cancer cells. Cancer research. 2000;60:5617–5620. [PubMed] [Google Scholar]
- 31.Zi X, Agarwal R. Silibinin decreases prostate-specific antigen with cell growth inhibition via G1 arrest, leading to differentiation of prostate carcinoma cells: implications for prostate cancer intervention. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:7490–7495. doi: 10.1073/pnas.96.13.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh RP, Agarwal R. Prostate cancer prevention by silibinin. Current cancer drug targets. 2004;4:1–11. doi: 10.2174/1568009043481605. [DOI] [PubMed] [Google Scholar]
- 33.Deep G, Agarwal R. Targeting Tumor Micro environment with Silibinin: Promise and Potential for a Translational Cancer Chemopreventive Strategy. Current cancer drug targets. 2013 doi: 10.2174/15680096113139990041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ting HJ, Deep G, Jain AK, Cimic A, Sirintrapun J, Romero LM, Cramer SD, Agarwal C, Agarwal R. Silibinin prevents prostate cancer cell-mediated differentiation of naive fibroblasts into cancer-associated fibroblast phenotype by targeting TGF beta2. Molecular carcinogenesis. 2014 doi: 10.1002/mc.22135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deep G, Raina K, Singh RP, Oberlies NH, Kroll DJ, Agarwal R. Isosilibinin inhibits advanced human prostate cancer growth in athymic nude mice: comparison with silymarin and silibinin, International journal of cancer. Journal international du cancer. 2008;123:2750–2758. doi: 10.1002/ijc.23879. [DOI] [PubMed] [Google Scholar]
- 36.Kavitha CV, Agarwal C, Agarwal R, Deep G. Asiatic Acid inhibits pro-angiogenic effects of VEGF and human gliomas in endothelial cell culture models. PloS one. 2011;6:e22745. doi: 10.1371/journal.pone.0022745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zi X, Feyes DK, Agarwal R. Anticarcinogenic effect of a flavonoid antioxidant, silymarin, in human breast cancer cells MDA-MB 468: induction of G1 arrest through an increase in Cip1/p21 concomitant with a decrease in kinase activity of cyclin-dependent kinases and associated cyclins. Clinical cancer research : an official journal of the American Association for Cancer Research. 1998;4:1055–1064. [PubMed] [Google Scholar]
- 38.Singh RP, Deep G, Blouin MJ, Pollak MN, Agarwal R. Silibinin suppresses in vivo growth of human prostate carcinoma PC-3 tumor xenograft. Carcinogenesis. 2007;28:2567–2574. doi: 10.1093/carcin/bgm218. [DOI] [PubMed] [Google Scholar]
- 39.Deep G, Gangar SC, Rajamanickam S, Raina K, Gu M, Agarwal C, Oberlies NH, Agarwal R. Angiopreventive efficacy of pure flavonolignans from milk thistle extract against prostate cancer: targeting VEGF-VEGFR signaling. PloS one. 2012;7:e34630. doi: 10.1371/journal.pone.0034630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas F, Holly JM, Persad R, Bahl A, Perks CM. Fibronectin confers survival against chemotherapeutic agents but not against radiotherapy in DU145 prostate cancer cells: involvement of the insulin like growth factor-1 receptor. The Prostate. 2010;70:856–865. doi: 10.1002/pros.21119. [DOI] [PubMed] [Google Scholar]
- 41.Hall A. The cytoskeleton and cancer. Cancer metastasis reviews. 2009;28:5–14. doi: 10.1007/s10555-008-9166-3. [DOI] [PubMed] [Google Scholar]
- 42.Kirkbride KC, Sung BH, Sinha S, Weaver AM. Cortactin: a multifunctional regulator of cellular invasiveness. Cell adhesion & migration. 2011;5:187–198. doi: 10.4161/cam.5.2.14773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bershadsky A. Magic touch: how does cell-cell adhesion trigger actin assembly? Trends in cell biology. 2004;14:589–593. doi: 10.1016/j.tcb.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 44.Gunst SJ. Actions by actin: reciprocal regulation of cortactin activity by tyrosine kinases and F-actin. The Biochemical journal. 2004;380:e7–8. doi: 10.1042/BJ20040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weed SA, Parsons JT. Cortactin: coupling membrane dynamics to cortical actin assembly. Oncogene. 2001;20:6418–6434. doi: 10.1038/sj.onc.1204783. [DOI] [PubMed] [Google Scholar]
- 46.Moroz A, Delella FK, Lacorte LM, Deffune E, Felisbino SL. Fibronectin induces MMP2 expression in human prostate cancer cells. Biochemical and biophysical research communications. 2013;430:1319–1321. doi: 10.1016/j.bbrc.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 47.Mehrotra S, Languino LR, Raskett CM, Mercurio AM, Dohi T, Altieri DC. IAP regulation of metastasis. Cancer cell. 2010;17:53–64. doi: 10.1016/j.ccr.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng GZ, Park S, Shu S, He L, Kong W, Zhang W, Yuan Z, Wang LH, Cheng JQ. Advances of AKT pathway in human oncogenesis and as a target for anti-cancer drug discovery. Current cancer drug targets. 2008;8:2–6. [PubMed] [Google Scholar]
- 49.Suer S, Sonmez H, Karaaslan I, Baloglu H, Kokoglu E. Tissue sialic acid and fibronectin levels in human prostatic cancer. Cancer letters. 1996;99:135–137. doi: 10.1016/0304-3835(95)04084-6. [DOI] [PubMed] [Google Scholar]
- 50.Sonmez H, Suer S, Karaarslan I, Baloglu H, Kokoglu E. Tissue fibronectin levels of human prostatic cancer, as a tumor marker. Cancer biochemistry biophysics. 1995;15:107–110. [PubMed] [Google Scholar]
- 51.Isisag A, Nese N, Ermete M, Lekili M, Ayhan S, Kandiloglu AR. Col IV and Fn distribution in prostatic adenocarcinoma and correlation of 67LR, MMP-9 and TIMP-1 expression with Gleason score. Analytical and quantitative cytology and histology/the International Academy of Cytology [and] American Society of Cytology. 2003;25:263–272. [PubMed] [Google Scholar]
- 52.Aoudjit F, Vuori K. Integrin signaling in cancer cell survival and chemoresistance. Chemotherapy research and practice. 2012;2012:283181. doi: 10.1155/2012/283181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cantor D, Slapetova I, Kan A, McQuade LR, Baker MS. Overexpression of alphavbeta6 integrin alters the colorectal cancer cell proteome in favor of elevated proliferation and a switching in cellular adhesion that increases invasion. Journal of proteome research. 2013;12:2477–2490. doi: 10.1021/pr301099f. [DOI] [PubMed] [Google Scholar]
- 54.Stachurska A, Elbanowski J, Kowalczynska HM. Role of alpha5beta1 and alphavbeta3 integrins in relation to adhesion and spreading dynamics of prostate cancer cells interacting with fibronectin under in vitro conditions. Cell biology international. 2012;36:883–892. doi: 10.1042/CBI20110522. [DOI] [PubMed] [Google Scholar]
- 55.Cress AE, Rabinovitz I, Zhu W, Nagle RB. The alpha 6 beta 1 and alpha 6 beta 4 integrins in human prostate cancer progression. Cancer metastasis reviews. 1995;14:219–228. doi: 10.1007/BF00690293. [DOI] [PubMed] [Google Scholar]
- 56.Deep G, Gangar SC, Agarwal C, Agarwal R. Role of E-cadherin in antimigratory and antiinvasive efficacy of silibinin in prostate cancer cells. Cancer Prev Res (Phila) 2011;4:1222–1232. doi: 10.1158/1940-6207.CAPR-10-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mokhtari MJ, Motamed N, Shokrgozar MA. Evaluation of silibinin on the viability, migration and adhesion of the human prostate adenocarcinoma (PC-3) cell line. Cell biology international. 2008;32:888–892. doi: 10.1016/j.cellbi.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 58.Wu KJ, Zeng J, Zhu GD, Zhang LL, Zhang D, Li L, Fan JH, Wang XY, He DL. Silibinin inhibits prostate cancer invasion, motility and migration by suppressing vimentin and MMP-2 expression. Acta pharmacologica Sinica. 2009;30:1162–1168. doi: 10.1038/aps.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu K, Zeng J, Li L, Fan J, Zhang D, Xue Y, Zhu G, Yang L, Wang X, He D. Silibinin reverses epithelial-to-mesenchymal transition in metastatic prostate cancer cells by targeting transcription factors. Oncol Rep. 2010;23:1545–1552. [PubMed] [Google Scholar]
- 60.Handorean AM, Yang K, Robbins EW, Flaig TW, Iczkowski KA. Silibinin suppresses CD44 expression in prostate cancer cells. Am J Transl Res. 2009;1:80–86. [PMC free article] [PubMed] [Google Scholar]
- 61.Wenzel S, Stolte H, Soose M. Effects of silibinin and antioxidants on high glucose-induced alterations of fibronectin turnover in human mesangial cell cultures. The Journal of pharmacology and experimental therapeutics. 1996;279:1520–1526. [PubMed] [Google Scholar]
- 62.Kalluri R, Zeisberg M. Fibroblasts in cancer, Nature reviews. Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 63.Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, Le QT, Giaccia AJ. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kidd P, Head K. A review of the bioavailability and clinical efficacy of milk thistle phytosome: a silybin-phosphatidylcholine complex (Siliphos) Altern Med Rev. 2005;10:193–203. [PubMed] [Google Scholar]