Abstract
Objective
To investigate the hypothesis that COMP can influence the morphology, stability and size of murine atherosclerotic lesions.
Methods
ApoE- and ApoE/COMP-knockout mice were fed a high-fat diet to develop atherosclerotic plaques at lesion sites of three different types; inflammatory and fibrous plaques induced in the carotid artery by low or oscillatory shear stress, respectively, and spontaneously developing plaques in the brachiocephalic artery. The localization of COMP in the plaques and the effect of COMP deficiency on plaque development were evaluated.
Results
COMP immunoreactivity was observed in about half of the investigated plaques from the ApoE null mice, mainly located along the intima-medial border. There were no significant differences in the size of inflammatory and fibrous carotid plaques between the genotypes. Plaques in the brachiocephalic artery from ApoE mice lacking COMP were increased in size with 54%. In these plaques the collagen content was also increased by 48%. There were no differences in relative collagen content in inflammatory and fibrous carotid plaques between genotypes. Polarized light microscopy showed that the increase in total collagen in brachiocephalic plaques was more than proportionally accounted for by an increase in thicker collagen fibrils.
Conclusion
We have shown that COMP deficiency has a significant impact on atherosclerotic plaque morphology and size. Our data also suggest that an altered collagen metabolism may be an important mechanism in this finding.
Keywords: Atherosclerosis, Plaque morphology, ApoE-KO mouse, COMP, Collagen fibre
Highlights
-
•
COMP is a common component of murine atherosclerotic plaques.
-
•
COMP-deficient ApoE-KO mice develop larger plaques in the brachiocephalic artery.
-
•
COMP-deficient ApoE-KO mice develop plaques with a higher collagen content.
-
•
Collagen fibres in COMP-deficient ApoE-KO mice are altered in size.
1. Introduction
Collagen fibres are common components of atherosclerotic plaques, and their ability to strengthen fibrous caps can be a decisive factor for stable or rupture-prone vulnerable lesions [1]. Cartilage oligomeric matrix protein (COMP: thrombospondin 5) is involved in the assembly of collagen fibres [2] and has been identified as a matrix component in human atherosclerotic and restenotic plaques [3], as well as in plaques from different murine atherosclerotic models [4].
ADAMTS7 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif-7) has been implicated in the degradation of COMP and has been identified as a novel genetic locus for coronary atherosclerosis [5–7]. Smooth muscle cells obtained from patients with a coronary artery disease-associated ADAMTS7 genotype show alteration in ADAMTS7 maturation, enhanced migration, and an increase in COMP degradation [8]. Ectopic over-expression of COMP by adenoviral infection has been shown to reduce the size of fibrotic, restenosis-like vascular lesions, and to attenuate ADAMTS7-facilitated cell migration [9]. COMP's apparent support of a differentiated smooth muscle phenotype has been attributed to its ability to serve as a ligand for α7β1 integrin [10]. COMP's ability to interact with the membrane proximal portion of the β1 chain has further been associated with its anti-apoptotic effect on cardiomyocytes [11].
To investigate the hypothesis that an alteration in COMP expression might influence the morphology and stability as well as size of atherosclerotic lesions, ApoE-mice with and without COMP were fed a high-fat diet. The brachiocephalic artery of fat-fed ApoE deficient mice develops complex plaques, which eventually spontaneously rupture [12]. Carotid lesions were induced by a shear stress modifying cast as described by Cheng et al. [13]. Under normal circumstances this location is a laminar flow region [14], but the cast alters the pattern of blood flow in the artery to reduce shearing forces at its proximal end and to induce oscillatory shear stress distal to it, so that lesions with an inflammatory phenotype develop proximal and a fibrous phenotype distal to the cast. By using this model we have recently shown that the size of inflammatory lesions decreases significantly in the absence of the collagen binding protein fibromodulin [15].
Thus, we assessed how COMP deficiency in Apo E knock out mice affects the development of inflammatory and fibrous carotid plaques and also spontaneously developing plaques in the brachiocephalic artery, with focus on collagen fibre formation.
2. Materials & methods
2.1. Animals and diet
All animal experiments were approved by the Malmoe/Lund regional ethical committee (Sweden). ApoE-null mice on a BL/6 background (B6.129P2-Apoetm1Unc/N11) purchased from Taconic (Lille Skensved, Denmark) and COMP knockout mice generated by targeted disruption [16] were crossed. From these resulting double heterozygous animals, new strains of ApoE/COMP double-null and ApoE-null mice were generated and female offspring were used in the experiments. At 16 weeks of age, mice were switched from normal chow diet to a high-fat diet containing 21% fat and 0.15% cholesterol (Special Diet Services, UK). Two weeks later surgery was performed, and the high-fat diet was continued for a further 12 weeks. At the age of 30 weeks mice were sacrificed and blood was taken for plasma cytokine and cholesterol analysis, which revealed no significant differences between the double and single knockout mice (Suppl. Tab. 1).
2.2. Periadventitial collar injury
Collar injury was performed as described previously by Cheng et al. [13]. To induce standardized changes in shear stress, a periadventitial cast was placed around the right carotid artery of ApoE-null and ApoE/COMP double-null 18-week-old female mice.
2.3. Tissue preparation
Mice were sacrificed and tissues embedded in paraffin as described [15]. Brachiocephalic sections for histological analysis were 3 μm and carotid sections 5 μm thick.
2.4. Morphometry
For carotid artery sections 'Accustain trichrome stain (Masson)' (Sigma–Aldrich) and BioPix iQ software (BioPix, Gothenburg) was used. Brachiocephalic sections were stained with Elastin Van Gieson for measuring the external and internal elastic lamellae, and lesion area using standard image analysis software (Image Pro Plus, MediaCybernetics).
2.5. Immunohistochemistry and histology
The immunofluorescence and immunohistochemical detection with antibodies for COMP (kindly provided by Dick Heinegård), GEP (R&D Systems), α-smooth muscle actin (Sigma–Aldrich), ADAMTS7 (Biorbyt) and PCNA (Invitrogen/Abcam) and staining for collagen using picrosirius red is available in detail in the Supplementary Data.
Picrosirius red stained slides were viewed for total collagen content under normal transmitted light. To allow direct comparison between the proportions of collagen, the same sections in the same orientation were then viewed under polarized light, to view the birefringent properties of the collagen fibres; thicker, and therefore usually older, fibres show up red-orange, whereas thinner, usually newer, fibres show up yellow-green [17]. Analysis was performed using automated colour segmentation to select and count the area of the colours of interest, and the same segmentation was performed on all sections.
2.6. Statistical analysis
Values are expressed as mean ± standard error of the mean (SEM). Treatment group values were compared with their controls using the computer programs InStat and Prism (both GraphPad Software, San Diego, California, USA). For the comparison of group means, a check was first made for similar variances: if this was passed then an unpaired two sample two-tailed Student's t-test was carried out, or one-way analysis of variance if there were more than 2 groups. If the variances were significantly different, then an unpaired two sample two-tailed t-test with Welch's correction was used for 2 groups, and a Kruskal–Wallis test for more than 2 groups. Non-parametric tests were used where data were not normally distributed. In all cases, statistical significance was concluded where the two-tailed probability was less than 0.05.
3. Experimental results
3.1. Carotid arterial lesions
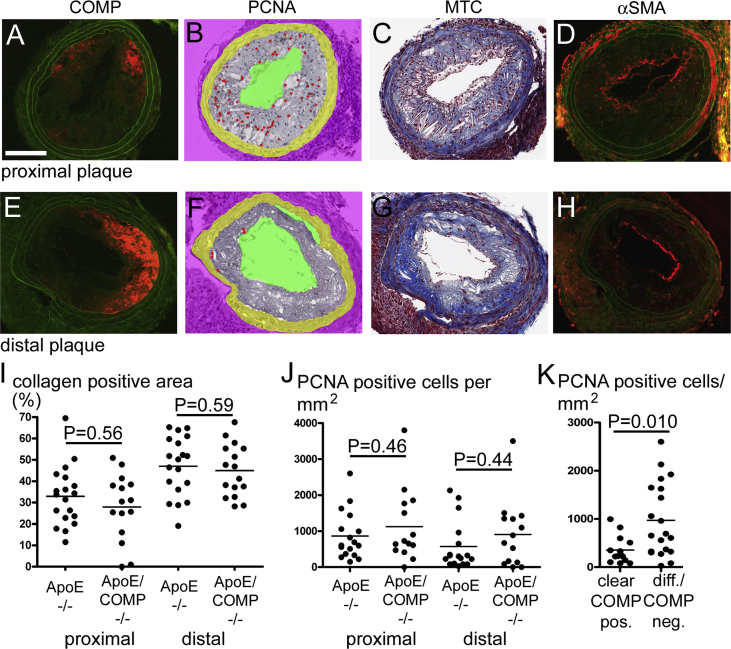
Standardized changes in shear stress induced carotid atherosclerotic lesions at the proximal and distal side of the cast. The proximal, inflammatory lesions exhibited an expected decrease in collagen content as compared to the distal, fibrous lesions (33 ± 3% vs. 47 ± 3%) (Fig. 1).
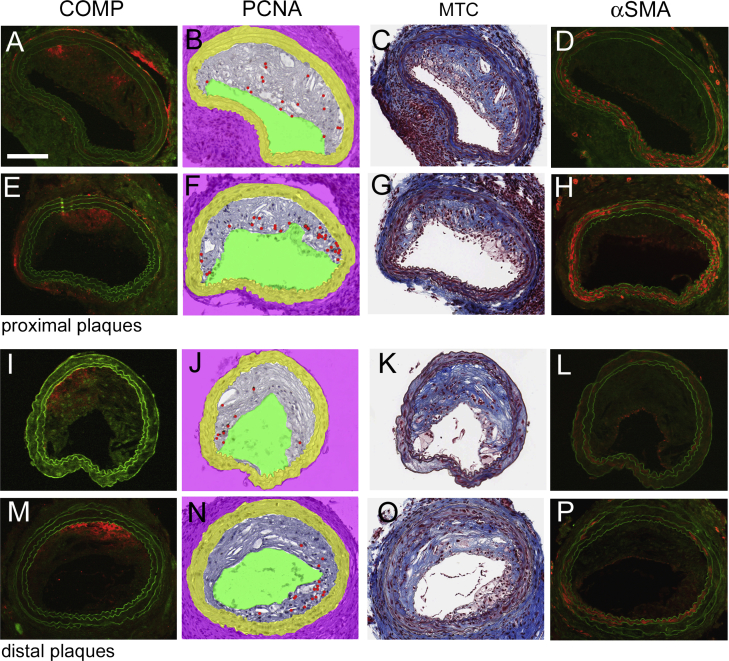
Fig. 1.
COMP, PCNA, collagen and smooth muscle α-actin in carotid plaques. A–H: Consecutive sections from a proximal (A–D) and a distal (E–H) carotid plaque stained for COMP (A,E), PCNA (B,F), collagen (C,G) and α-SMA (D,H). COMP and α-SMA are represented by red immunofluorescence, while green colour represents autofluorescence in the respective images. PCNA positive cells, individually revealed by evaluation of the DAB-developed original slide under the microscope, are indicated by red dots in the morphometrically-processed images. Collagen appears blue in the masson-trichrome (MTC) processed images. Scale bar: 100 μm. I + J: Analysis of proximal and of distal carotid plaques of either ApoE-deficient (ApoE−/−) or ApoE and COMP deficient (ApoE/COMP−/−) animals. I: Collagen contents in % of the total plaque area as determined by masson-trichrome. (ApoE−/−: n = 19; proximal ApoE/COMP−/−: n = 15; distal ApoE/COMP−/−: n = 16). J: Number of PCNA positive cells per mm2. (ApoE−/−: n = 17; proximal ApoE/COMP−/−: n = 14; distal ApoE/COMP−/−: n = 15). K: Analysis of PCNA positive cells per mm2 in plaques showing either clear COMP immunoreactivity (clear COMP pos., n = 14) or very weak and diffuse or no COMP immunoreactivity (diff./COMP neg., n = 20). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.1.1. COMP immunoreactivity
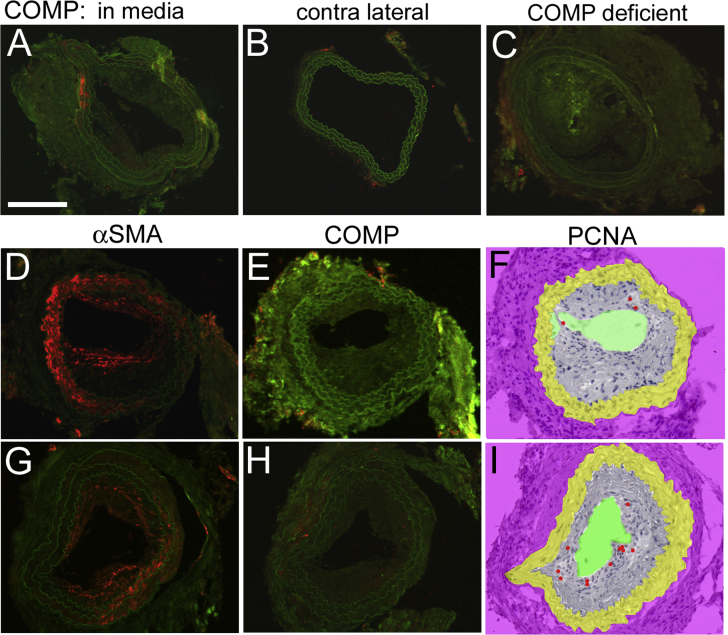
The presence of COMP was analyzed by fluorescence immunohistochemistry in sections obtained from the central area of each inflammatory and fibrous carotid plaque (Fig. 1, Suppl. Figs. 1 and 2). 63% of the ApoE single knockout mice exhibited clearly detectable areas of distinct COMP immunoreactivity in inflammatory and/or fibrous plaques. These COMP positive plaques were equally distributed between inflammatory and fibrous plaques and represented 45% of the totally investigated lesions (n = 38), while 55% of the plaques showed either very weak and diffuse or no detectable COMP immunoreactivity. COMP was mainly located along the intima-medial border and occasionally COMP immunoreactivity was also observed in the media. No COMP expression was found in the contra lateral arteries (without cast) or in arteries from the ApoE/COMP double knockout mice (Suppl. Fig. 2).
3.1.2. COMP co-localizes with collagen but not with proliferative activity
By comparing serial sections stained for COMP and collagen, we can conclude that COMP generally co-localizes with collagen in the plaque tissue (detected by masson-trichrome-staining) (Fig. 1, Suppl. Fig. 1). Comparison of the extension of collagen-positive (blue) areas in inflammatory and fibrous carotid plaques in relation to the total area of the plaque showed that there were no significant differences in the relative collagen content between plaques from ApoE mice with and without COMP expression (Fig. 1).
COMP has been demonstrated to support the contractile phenotype of smooth muscle cells [10], which is associated with a low proliferation rate [18]. To investigate the effect of COMP deficiency on cell proliferation, serial sections were stained for the proliferative cell nuclear antigen (PCNA) (Fig. 1, Suppl. Fig. 1). Consecutive sections of carotid plaques demonstrate that the proliferative activity appeared to be reduced or absent in areas, which were clearly COMP-positive. However, there was not a significant difference in the number of neointimal PCNA-positive cells between the ApoE- and ApoE/COMP-null genotypes (Fig. 1, Suppl. Fig. 1). The plaques from the ApoE deficient mice were divided into two categories, the first with clear positive COMP immunoreactivity and the second with plaques exhibiting very weak and diffuse or no immunoreactivity at all. Clear COMP immunoreactivity (in 45% of the total number of plaques) associated with a lower number of proliferating cells in the plaques in comparison to plaques with very weak or no COMP immunoreactivity (55% of the total plaque number) (Fig. 1).
3.1.3. COMP is not associated with differentiated smooth muscle cells
The contractile phenotype of smooth muscle cells is characterized by the expression of high amounts of α-smooth muscle actin (α-SMA), which is a differentiation marker for this cell type. To investigate the role of COMP on the expression of α-SMA, plaque material from ApoE mice with and without COMP expression were stained with a α-SMA antibody (Fig. 1, Suppl. Fig. 1). α-SMA immunofluorescence was mainly present along the intima-luminal border in the plaque. Thus, its occurrence was in marked contrast to the presence of COMP, which was mostly associated with the intima-medial border. Similar to COMP, α-SMA immunofluorescence showed strong individual variations. Strong α-SMA immunofluorescence was present around the lumen in plaques from both genotypes. In plaques from the ApoE single knock out mouse the α-SMA and the COMP immunoreactivity was rather complementary than overlapping (Suppl. Fig. 2).
3.1.4. Morphometric comparisons
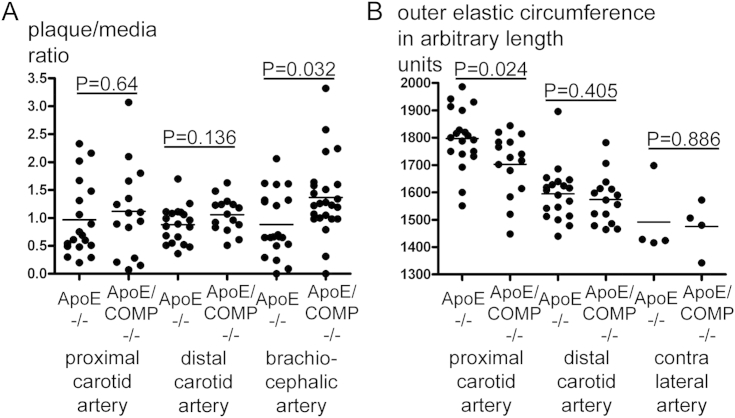
There was a tendency to larger carotid plaques in ApoE mice lacking COMP in comparison to plaques from the ApoE mice, however, the differences where not significant (Fig. 2). However, the outer circumference (external elastic lamina) around the inflammatory lesions in the ApoE single knockout mice was significantly wider compared with the outer circumference around inflammatory ApoE/COMP double knockout lesions (Fig. 2).
Fig. 2.
Morphometric analysis of carotid and brachiocephalic plaques. A: Plaque/media ratios of inflammatory (proximal) and fibrous (distal) carotid (CA) and brachiocephalic (BCA) lesions of either ApoE-deficient (ApoE−/−) or ApoE and COMP deficient (ApoE/COMP−/−) animals. BCA ratios show significant differences between genotypes (proximal and distal CA ApoE−/−: n = 19; proximal CA ApoE/COMP−/−: n = 15; distal CA ApoE/COMP−/−: n = 16; BCA ApoE−/−: n = 20; BCA ApoE/COMP−/−: n = 23). B: Outer circumferences of inflammatory (proximal) and fibrous (distal) carotid lesions and contra lateral carotid arteries of either ApoE-deficient (ApoE−/−) or ApoE and COMP deficient (ApoE/COMP−/−) animals. Circumferences are expressed in arbitrary length units calculated by the program used to analyze the scanned images. Around inflammatory lesions outer circumferences show significant differences between genotypes (proximal and distal CA ApoE−/−: n = 19; proximal and distal CA ApoE/COMP−/−: n = 15; contra lateral CA: n = 4).
3.1.5. Collagen fibre characterization
To enable determination of the proportions of collagen fibre thickness, carotid plaque sections were processed with picrosirius red and subsequently analyzed with normal light for total collagen content, and with polarized light for their birefringent properties. In this analysis between genotypes neither significant differences in collagen content nor in collagen structure of either inflammatory or fibrous lesions could be detected (Suppl. Fig. 3).
3.2. Brachiocephalic atherosclerotic lesions
3.2.1. COMP immunoreactivity
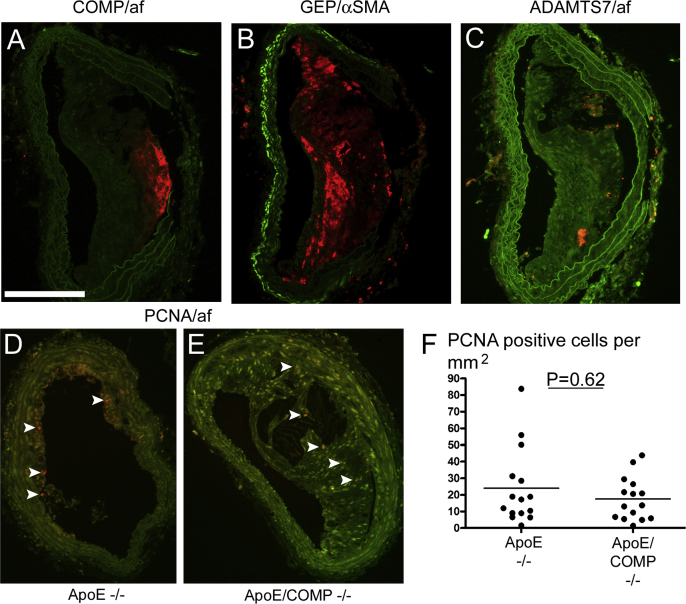
Analysis of brachiocephalic arterial plaques for the presence of COMP by fluorescence immunohistochemistry showed a similar wide variation as observed in carotid plaques. COMP immunoreactivity was also in these plaques mainly located along the intima-medial border (Fig. 3).
Fig. 3.
COMP, GEP, ADAMTS7 and PCNA immunofluorescence in brachiocephalic plaques. A–E: Brachiocephalic sections stained fluorescence-immunohistochemically for COMP (A), GEP (B), ADAMTS7 (C), and PCNA (D,E), all represented by red colour. Green colour represents autofluorescence, except for particularly bright green fluorescence in (B), which indicates α-SMA. Sections A-D are from ApoE-deficient mice, while section E is from an ApoE and COMP deficient mouse. Arrowheads point to PCNA positive cells. Scale bar: 200 μm. F: Number of PCNA positive cells per mm2 (ApoE−/−: n = 15; ApoE/COMP−/−: n = 15). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2.2. Morphometric comparisons
In brachiocephalic atherosclerotic lesions the cross-sectional area of the plaque was increased by 54% in ApoE/COMP double knockout mice compared with ApoE single knockout controls (p = 0.039, Mann–Whitney test) (Table 1, Fig. 2). In comparison, the COMP deficient plaques from the carotid arteries were also increased in size, however, these changes were not statistically significant (Fig. 2). Since the increase in cross-sectional area in the brachiocephalic arteries from COMP null mice was not accompanied by a concomitant increase of the outer circumference, the lumenal cross-sectional area was decreased by 28% (p = 0.001, Mann–Whitney test) (Table 1, Fig. 4).
Table 1.
Morphometrical and histochemical parameter of brachiocephalic plaques (n = 20 for ApoE and 23 for ApoE/COMP deficient animals).
| KO | Plaque/media ratio | Lumen area (mm2 × 103) | Vessel area (mm2 × 103) | Buried fibrous caps (mm−2) | Plaque elastin % | Cartilaginous metaplasia % | Plaque collagen % |
||
|---|---|---|---|---|---|---|---|---|---|
| Thin | Thick | Total | |||||||
| ApoE | 0.88 ± 0.13 | 136.6 ± 9.8 | 302.2 ± 12.8 | 5.1 ± 1.5 | 1.8 ± 0.2 | 0.29 ± 0.16 | 1.3 ± 0.2 | 10.4 ± 1.7 | 37.3 ± 3.7 |
| ApoE/COMP | *1.36 ± 0.15 | *98.9 ± 7.1 | 298.3 ± 15.2 | 3.8 ± 1.2 | 2.3 ± 0.1 | *0.58 ± 0.17 | 1.2 ± 0.2 | *15.9 ± 1.5 | *55.2 ± 3.0 |
Values are expressed as mean ± SEM. *p < 0.05 versus ApoE single knockout.
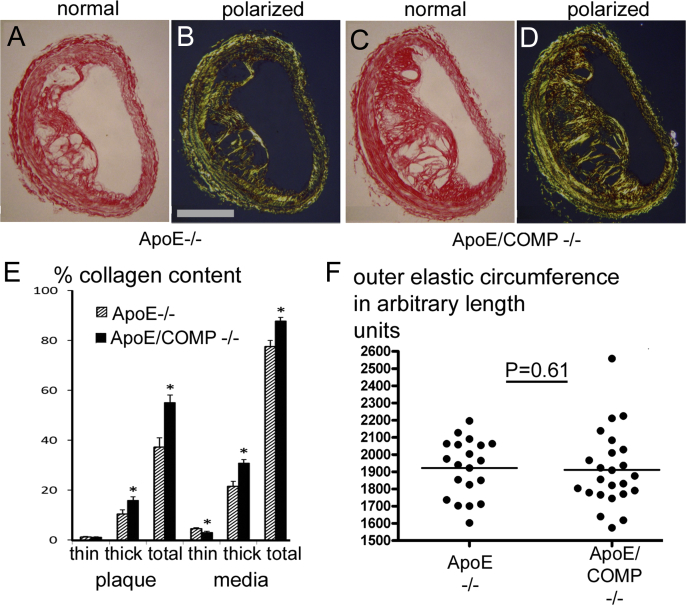
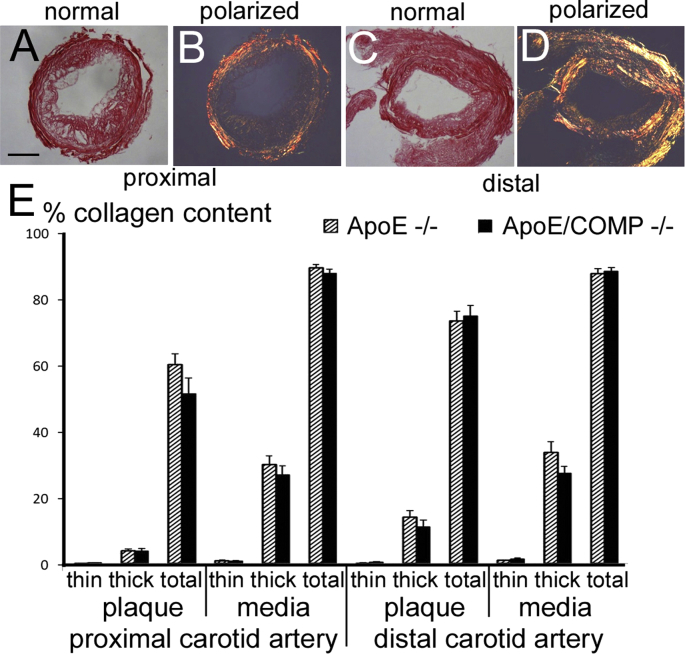
Fig. 4.
Collagen content and fibre size and circumference of picrosirius red stained brachiocephalic lesions. A-D Examples of picrosirius red stained brachiocephalic plaques observed under normal (A,C) and polarized (B,D) light. Scale bar: 200 μm. E: Total collagen content and fibre size associated collagen content in plaque and media. Values showing significant differences (p < 0.05) between ApoE single KO's (striped bars) and ApoE/COMP double-KO's (filled bars) are indicated by stars. F: Outer elastic circumferences of the brachiocephalic lesions of either ApoE-deficient (ApoE−/−; n = 20) or ApoE and COMP deficient (ApoE/COMP−/−; n = 23) animals.
3.2.3. Comparison of proliferative activity
Although the average number of PCNA-positive cells per plaque was slightly higher in plaques of ApoE/COMP deficient animals than of ApoE deficient mice, the larger size of plaques of ApoE/COMP deficient animals led to a lower number per area. However, the difference was not significant (Fig. 3).
3.2.4. COMP deficiency does not affect the occurrence of buried fibrous caps
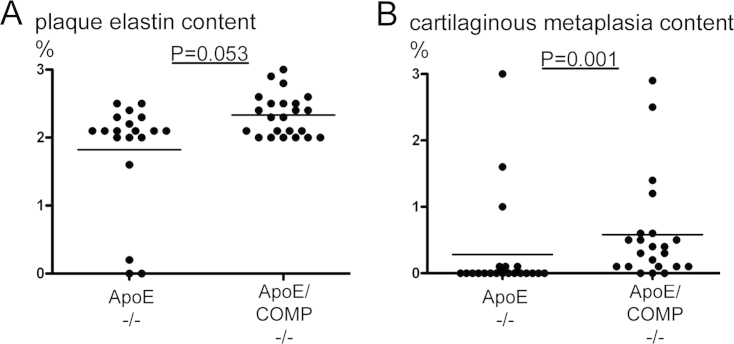
The brachiocephalic artery of fat-fed ApoE deficient mice develops complex plaques, which eventually spontaneously rupture [12]. To analyze the effect of COMP deficiency on plaque rupture, arteries were stained with van Gieson. The results demonstrate that COMP deficiency did neither affect the number of buried fibrous caps nor the elastin content. However, we found that the proportion of elastin in the media was significantly increased in arteries from COMP null mice (Table 1,Suppl. Tab. 2, Suppl. Fig. 4).
3.2.5. Comparison of collagen content
Brachiocephalic plaque sections were stained with picrosirius red to assess changes in collagen content, which in the plaque and media in lesions from ApoE/COMP double knockouts was increased by 48% (p = 0.001) and 13% (p = 0.004), respectively (Table 1, Suppl. Tab. 2, Fig. 4).
3.2.6. Cartilage metaplasia is increased in COMP deficient plaques
Brachiocephalic arteries of fat-fed ApoE knockout mice often show cartilaginous changes, sometimes dubbed cartilaginous metaplasia, which may also manifest in the media of advanced atherosclerotic lesions. COMP is abundantly expressed in normal cartilage and therefore we wanted to investigate presence of cartilage formation in plaques with and without COMP expression. Our results demonstrate that in the lesions the COMP deficiency rather favoured the extent of cartilaginous metaplasia: there was a 100% increase in the plaque (p = 0.001) and a 177% increase in the media of ApoE/COMP double knockouts compared to ApoE knockout mice (p = 0.003) (Table 1, Suppl. Tab. 2, Suppl. Fig. 4).
3.2.7. GEP, ADAMTS7 and αSMA immunoreactivity
COMP has been implicated in a protein network with the TNF-α-antagonist granulin–epithelin-precursor (GEP) and the metalloprotease ADAMTS7 by binding to either molecule and being a competitive substrate [19]. Therefore, consecutive plaque sections were immunohistochemically investigated for the presence of these molecules (Fig. 3). While GEP-immunofluorescence could be abundantly observed in the investigated brachiocephalic plaques, ADAMTS7-immunofluorescence was more sparsely distributes in the plaque tissue. However, strong ADAMTS7 immunofluorescence could eventually be observed in the adventitia. α-SMA immunoreactivity was located along the intima-luminal border of the plaque. There was no overlap between COMP and GEP immunofluorescence; their distribution rather appeared complementary.
3.2.8. COMP deficiency is associated with increased thicker collagen fibrils
The use of picrosirius red to stain for collagen enables a determination of the proportions of highly cross-linked thicker and likely older collagen fibres, which appear orange-red in polarized light, and less cross-linked thinner, likely more recently synthesized fibrillar collagen, which appears green-yellow (Fig. 4). Polarized light microscopy showed that the increase in total collagen in brachiocephalic plaques in the double knockouts was more than proportionally accounted for by an increase in thicker collagen fibrils. In the plaque, the increase in the ratio of older to total collagen was 96% (p = 0.004). In the media an increase of 179% (p < 0.001) was noted (Table 1, Suppl. Tab. 2, Fig. 4).
4. Discussion
The present investigation reveals that COMP is expressed in arterial atherosclerotic lesions from mice, thereby extending previous observations in human plaques by Riessen et al. (2001) and in mouse plaques by Ström et al. (2004) to a more comprehensive picture [3,4]. However, the fact that COMP is not being present in all investigated plaques at the time point when the mice were sacrificed does not necessarily mean that it never had an influence on the development of the plaque at earlier time points. COMP has been shown to have a catalytic function on collagen-microfibril assembly by bringing collagen molecules together without actually binding to the developing fibril, and then dissociating from the mature fibrils [2]. At very high levels of COMP relative to collagen molecules fibrillogenesis can actually even be impaired [20].
In the brachiocephalic arteries of the COMP-deficient ApoE knockout mice the relative collagen contents of the plaques was increased by 48%. Much of this increase was accounted for by a greater accumulation of red-orange appearing collagen fibres visualized by polarized light. Birefringence in these colours indicates not only that these were thick, but also tightly packed and well-aligned collagen fibres [21], thus, likely normally matured older, highly cross-linked fibres uncompromised in their structural stability. ApoE single knockout plaques contained old:new collagen in the ratio 9.0:1, but in the ApoE/COMP double knockouts this increased by 96% to 17.7:1. This may suggests that COMP could actually be involved in the control of collagen fibre size.
Electron microscopy has shown that COMP immunoreactivity is associated with anchoring plaques in the sub-epidermal papillary layer of the dermis [22]. Collagen fibres in cartilage, the most prominent COMP-expressing tissue, are similar to collagen fibres found in the papillary layer of the dermis characterized by a comparatively thin diameter. Thin collagen fibres, termed microtendons, are also observed at myotendinous junctions [23], structures specifically affected by myopathy in COMP T585M mutant mice [24]. Thus, COMP expression appears to coincide in various tissues with small collagen fibres, which support our finding that atherosclerotic plaques of COMP-deficient animals contained a higher proportion of thick collagen fibres. Therefore the changes in morphology in brachiocephalic artery plaques lacking COMP could be a consequence of altered collagen metabolism. This hypothesis is strengthened by the fact that we could not detect significant differences neither in collagen fibre diameter nor in the size of the carotid inflammatory and fibrous lesions between the two genotypes.
Brachiocephalic arteries from fat-fed ApoE knockout mice show a remarkable capacity to outwardly remodel in response to plaque growth, such that they maintain the lumen of the vessel within quite narrow limits [25]. This homoeostatic mechanism appears to be damaged in animals lacking COMP, because they suffered a 28% reduction in lumen area while no compensatory differences in vessel area were seen. Putting this finding together with the shorter circumference of inflammatory carotid lesions in COMP deficient mice suggests that outward remodelling, which requires active collagen turnover, may be compromised in the absence of COMP. Interestingly, no changes were seen in the elastin content of brachiocephalic plaques. However, there was a significant increase in the proportion of elastin in the media of brachiocephalic arteries of mice lacking COMP, which, coupled with the increase in collagen, may lead to decreased vessel compliance, and prevent the outward remodelling that normally occurs with increased plaque burden.
Studies have shown that COMP associates with ADAMTS7 and GEP in a protein–protein interaction network [19]. GEP, which has been found in the shoulder regions of human atherosclerotic plaques [26], may as an antagonist to TNF-α [27] be considered anti-inflammatory. On the other hand, can GEP undergo proteolytic processing to produce small peptides called granulins that may be pro-inflammatory mediators, thus can potentially play a role in plaque growth. There is evidence that GEP inhibits the degradation of COMP by ADAMTS proteases as ADAMTS7, and speculation that COMP in turn inhibits the degradation of GEP into pro-inflammatory granulins [19]. However, we observed barely any ADAMTS7 immunoreactivity and no apparent overlap of COMP- and GEP immunoreactivity in plaque tissue. This may suggest that COMP and GEP are independently active in different regions of the atherosclerotic plaque, although it is not excluding possible interactions during the early stages of plaque development. Plaque growth appears to depend on sufficient collagen deposition, which requires the migration of smooth muscle cells, which is supported by ADAMTS7, which is induced by TNF-α, which is inhibited by GEP, which in turn may be protected by COMP from degradation. In other words: in the absence of COMP, smooth muscle cells may migrate more efficiently. The more extensive plaque development in the larger brachiocephalic arteries might be more dependent on an efficient migration of smooth muscle cells, in comparison to plaques developing in the carotids. These considerations based on other studies could be another possible explanation for the observation of more obvious differences in plaque size in the larger brachiocephalic artery between genotypes.
Our results indicate that COMP can have a role in collagen fibre assembly and may thereby affect the structure and growth of atherosclerotic lesions. We have recently shown that the size of inflammatory lesions with defective collagen assembly decreases significantly [15]. These and previous observations, which, for example show that degradation of collagen fibres in the vessel wall increases the risk of atherosclerotic plaque rupture [28], confirm that changes in collagen fibre structure can have profound functional consequences for the plaque tissue. This renders our observation relevant to both basic and also clinical aspects of atherosclerosis. This is also supported by our observations that COMP expression in plaque tissue from mice corresponds with the study by Canfield et al. (2002). This study shows that COMP expression in post mortem human diseased vessels is mainly localized in fibrous tissue adjacent to the media, underlying the lipid core, and not in the fibrous cap [29]. It is conceivable that like in normal tissue, in plaques there also exist specialized areas with different requirements for the optimal design of collagen fibres.
In conclusion, this study is presenting evidence that COMP, in its capacity as collagen fibrillogenesis-affecting molecules, can have significant effects on atherosclerotic plaque development.
Sources of funding
Swedish Heart-Lung Foundation, Swedish Research Council, Swedish Foundation for Strategic Research, British Heart Foundation, Higher Education Funding Council for England, Alfred Österlund Foundation, Crafoord Foundation, Thelma Zoegas Foundation, Greta och Johan Kocks Foundation, Vinnova.
Conflict of interest
None declared.
Acknowledgements
The authors wish to thank Åke Oldberg for providing COMP deficient mice and Gunnel Roos for expert technical assistance.
Contributor Information
Andrew R. Bond, Email: andrew.bond@bristol.ac.uk.
Anna Hultgårdh-Nilsson, Email: anna.hultgardh@med.lu.se.
Anki Knutsson, Email: anki.knutsson@med.lu.se.
Christopher L. Jackson, Email: chris.jackson@bristol.ac.uk.
Uwe Rauch, Email: uwe.rauch@med.lu.se.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Jackson C.L., Bennett M.R., Biessen E.A., Johnson J.L., Krams R. Assessment of unstable atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2007;27:714–720. doi: 10.1161/01.ATV.0000261873.86623.e1. [DOI] [PubMed] [Google Scholar]
- 2.Halasz K., Kassner A., Morgelin M., Heinegard D. COMP acts as a catalyst in collagen fibrillogenesis. J Biol Chem. 2007;282:31166–31173. doi: 10.1074/jbc.M705735200. [DOI] [PubMed] [Google Scholar]
- 3.Riessen R., Fenchel M., Chen H., Axel D.I., Karsch K.R. Cartilage oligomeric matrix protein (thrombospondin-5) is expressed by human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2001;21:47–54. doi: 10.1161/01.atv.21.1.47. [DOI] [PubMed] [Google Scholar]
- 4.Strom A., Ahlqvist E., Franzen A., Heinegard D., Hultgardh-Nilsson A. Extracellular matrix components in atherosclerotic arteries of Apo E/LDL receptor deficient mice: an immunohistochemical study. Histol Histopathol. 2004;19:337–347. doi: 10.14670/HH-19.337. [DOI] [PubMed] [Google Scholar]
- 5.Dickinson S.C., Vankemmelbeke M.N., Buttle D.J., Rosenberg K., Heinegard D. Cleavage of cartilage oligomeric matrix protein (thrombospondin-5) by matrix metalloproteinases and a disintegrin and metalloproteinase with thrombospondin motifs. Matrix Biol. 2003;22:267–278. doi: 10.1016/s0945-053x(03)00034-9. [DOI] [PubMed] [Google Scholar]
- 6.Guo F., Lai Y., Tian Q., Lin E.A., Kong L. Granulin-epithelin precursor binds directly to ADAMTS-7 and ADAMTS-12 and inhibits their degradation of cartilage oligomeric matrix protein. Arthritis Rheum. 2010;62:2023–2036. doi: 10.1002/art.27491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reilly M.P., Li M., He J., Ferguson J.F., Stylianou I.M. Identification of ADAMTS7 as a novel locus for coronary atherosclerosis and association of ABO with myocardial infarction in the presence of coronary atherosclerosis: two genome-wide association studies. Lancet. 2011;377:383–392. doi: 10.1016/S0140-6736(10)61996-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pu X., Xiao Q., Kiechl S., Chan K., Ng F.L. ADAMTS7 cleavage and vascular smooth muscle cell migration is affected by a coronary-artery-disease-associated variant. Am J Hum Genet. 2013;92:366–374. doi: 10.1016/j.ajhg.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L., Zheng J., Bai X., Liu B., Liu C.J. ADAMTS-7 mediates vascular smooth muscle cell migration and neointima formation in balloon-injured rat arteries. Circ Res. 2009;104:688–698. doi: 10.1161/CIRCRESAHA.108.188425. [DOI] [PubMed] [Google Scholar]
- 10.Wang L., Zheng J., Du Y., Huang Y., Li J. Cartilage oligomeric matrix protein maintains the contractile phenotype of vascular smooth muscle cells by interacting with alpha(7)beta(1) integrin. Circ Res. 2010;106:514–525. doi: 10.1161/CIRCRESAHA.109.202762. [DOI] [PubMed] [Google Scholar]
- 11.Huang Y., Xia J., Zheng J., Geng B., Liu P., Yu F. Deficiency of cartilage oligomeric matrix protein causes dilated cardiomyopathy. Basic Res Cardiol. 2013;108:374. doi: 10.1007/s00395-013-0374-9. [DOI] [PubMed] [Google Scholar]
- 12.Bond A.R., Jackson C.L. The fat-fed apolipoprotein E knockout mouse brachiocephalic artery in the study of atherosclerotic plaque rupture. J Biomed Biotechnol. 2011;2011:379069. doi: 10.1155/2011/379069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng C., Tempel D., van Haperen R., van der Baan A., Grosveld F. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation. 2006;113:2744–2753. doi: 10.1161/CIRCULATIONAHA.105.590018. [DOI] [PubMed] [Google Scholar]
- 14.Chiu J.J., Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91:327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shami A., Gustafsson R., Kalamajski S., Krams R., Segers D. Fibromodulin deficiency reduces low-density lipoprotein accumulation in atherosclerotic plaques in apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol. 2013;33:354–361. doi: 10.1161/ATVBAHA.112.300723. [DOI] [PubMed] [Google Scholar]
- 16.Svensson L., Aszodi A., Heinegard D., Hunziker E.B., Reinholt F.P. Cartilage oligomeric matrix protein-deficient mice have normal skeletal development. Mol Cell Biol. 2002;22:4366–4371. doi: 10.1128/MCB.22.12.4366-4371.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rich L., Whittaker P. Collagen and picrosirius red staining: a polarized light assessment of fibrillar hue and spatial distribution. Braz J Morphol Sci. 2005;22:97–104. [Google Scholar]
- 18.Rzucidlo E.M., Martin K.A., Powell R.J. Regulation of vascular smooth muscle cell differentiation. J Vasc Surg. 2007;45:25A–32A. doi: 10.1016/j.jvs.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Lin E.A., Liu C.J. The role of ADAMTSs in arthritis. Protein Cell. 2010;1:33–47. doi: 10.1007/s13238-010-0002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinegard D. Proteoglycans and more–from molecules to biology. Int J Exp Pathol. 2009;90:575–586. doi: 10.1111/j.1365-2613.2009.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dayan D., Hiss Y., Hirshberg A., Bubis J.J., Wolman M. Are the polarization colors of picrosirius red-stained collagen determined only by the diameter of the fibers? Histochem. 1989;93:27–29. doi: 10.1007/BF00266843. [DOI] [PubMed] [Google Scholar]
- 22.Agarwal P., Zwolanek D., Keene D.R., Schulz J.N., Blumbach K. Collagen XII and XIV, new partners of cartilage oligomeric matrix protein in the skin extracellular matrix suprastructure. J Biol Chem. 2012;287:22549–22559. doi: 10.1074/jbc.M111.335935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore M.J. The dual connective tissue system of rat soleus muscle. Muscle Nerve. 1983;6:416–422. doi: 10.1002/mus.880060604. [DOI] [PubMed] [Google Scholar]
- 24.Pirog K.A., Jaka O., Katakura Y., Meadows R.S., Kadler K.E. A mouse model offers novel insights into the myopathy and tendinopathy often associated with pseudoachondroplasia and multiple epiphyseal dysplasia. Hum Mol Genet. 2010;19:52–64. doi: 10.1093/hmg/ddp466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson C.L. Is there life after plaque rupture? Biochem Soc Trans. 2007;35:887–889. doi: 10.1042/BST0350887. [DOI] [PubMed] [Google Scholar]
- 26.Kojima Y., Ono K., Inoue K., Takagi Y., Kikuta K. Progranulin expression in advanced human atherosclerotic plaque. Atherosclerosis. 2009;206:102–108. doi: 10.1016/j.atherosclerosis.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 27.Tang W., Lu Y., Tian Q.Y., Zhang Y., Guo F.J. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science. 2011;332:478–484. doi: 10.1126/science.1199214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang X., Xenos M., Alemu Y., Rambhia S.H., Lavi I. Biomechanical factors in coronary vulnerable plaque risk of rupture: intravascular ultrasound-based patient-specific fluid-structure interaction studies. Coron Artery Dis. 2013;24:75–87. doi: 10.1097/MCA.0b013e32835bbe99. [DOI] [PubMed] [Google Scholar]
- 29.Canfield A.E., Farrington C., Dziobon M.D., Boot-Handford R.P., Heagerty A.M. The involvement of matrix glyscoproteins in vascular calcification and fibrosis: an immunohistochemical study. J Pathol. 2002;196:228–234. doi: 10.1002/path.1020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.