Abstract
Sirtuins are class III deacetylases that regulate many essential processes, including cellular stress, genome stability, and metabolism. Although these NAD+-dependent deacetylases control adaptive cellular responses, identification of sirtuin-regulated signaling targets remain under-studied. Here, we demonstrate that acetylation of the mitogen-activated protein kinase kinase-1 (MEK1) stimulates its kinase activity, and that acetylated MEK1 is under the regulatory control of the sirtuin family members SIRT1 and SIRT2. Treatment of cells with sirtuin inhibitors, or siRNA knockdown of SIRT1 or SIRT2 proteins, increases MEK1 acetylation and subsequent phosphorylation of the extracellular signal-regulated kinase (ERK). Generation of an acetyl-specific MEK1 antibody demonstrates that endogenous acetylated MEK1 is extensively enriched in the nucleus following epidermal growth factor (EGF) stimulation. An acetyl-mimic of MEK1 increases inappropriate growth properties, suggesting that acetylation of MEK1 has oncogenic potential.
Keywords: MEK1, sirtuins, acetylation, growth promoting, oncogenic
INTRODUCTION
The Ras, Raf, mitogen activated protein kinase kinase (MEK), and extracellular signal regulated kinase (ERK) signaling pathway coordinates proliferation, cell cycle, and cell survival (1,2). Because of its importance, aberrant regulation of MEK/ERK pathways is one of the most common events in cancer (3–5). Mammals express two MEK proteins, MEK1/MAP2K1 and MEK2/MAP2K2. Together, these dual-specificity kinases phosphorylate both threonine (T) and tyrosine (Y) residues on ERK1/MAPK3 and ERK2/MAPK1, events essential for stimulating cell growth. Subsequently, activated ERK phosphorylates more than 80 known substrates, including the ELK1 transcription factor. Normally, MEK and ERK localize to the cytosol, but both proteins also have specialized functions as chromatin-associated kinases upon nuclear import (6).
Mammalian orthologs of the yeast Sir2 family of enzymes, known as sirtuins, mediate NAD+-dependent deacetylation and/or ADP-ribosyltransferase activity (7–9). The human genome encodes seven sirtuin proteins (SIRT1-7) that fulfill specialized deacetylase functions based on their cellular compartmentalization to the nucleus, cytoplasm, and the mitochondria (10,11). Sirtuins regulate gene expression by deacetylating histones H3 and H4, but also deacetylate numerous non-histone targets that control metabolism and cell fate. Although it remains controversial as to whether SIRT1 and SIRT2 function as tumor suppressors (10,12–14), both sirtuins are required to maintain genome integrity (15,16). Consistent with tumor suppressor properties, SIRT2-deficient male mice develop hepatocellular carcinomas (HCC) while female mice develop mammary tumors (15). Interestingly, human mammary and HCC express high levels of the p300/EP300 histone acetyltransferase, which correlate with tumor recurrence and poor clinical outcomes (17,18).
Since cellular adaptive responses regulated by NAD+-dependent deacetylases overlap with pathways that stimulate MEK/ERK, we examined whether MEK is regulated by sirtuins. Here, we provide evidence that p300 directly acetylates MEK1 to enhance its kinase activity. Knockdown of SIRT1 or SIRT2 heightens MEK1 acetylation indicating that MEK activity is controlled by these NAD+-dependent deacetylases. Furthermore, like phospho-mimic MEK1(S218/222D), acetyl-mimics of MEK1 promote inappropriate growth on soft agar. Thus, our work suggests that modulation of MEK1 acetylation by SIRT1 and SIRT2 negatively regulates MAPK pathways during pre-oncogenic events.
RESULTS
Activation of MEK1 by acetylation
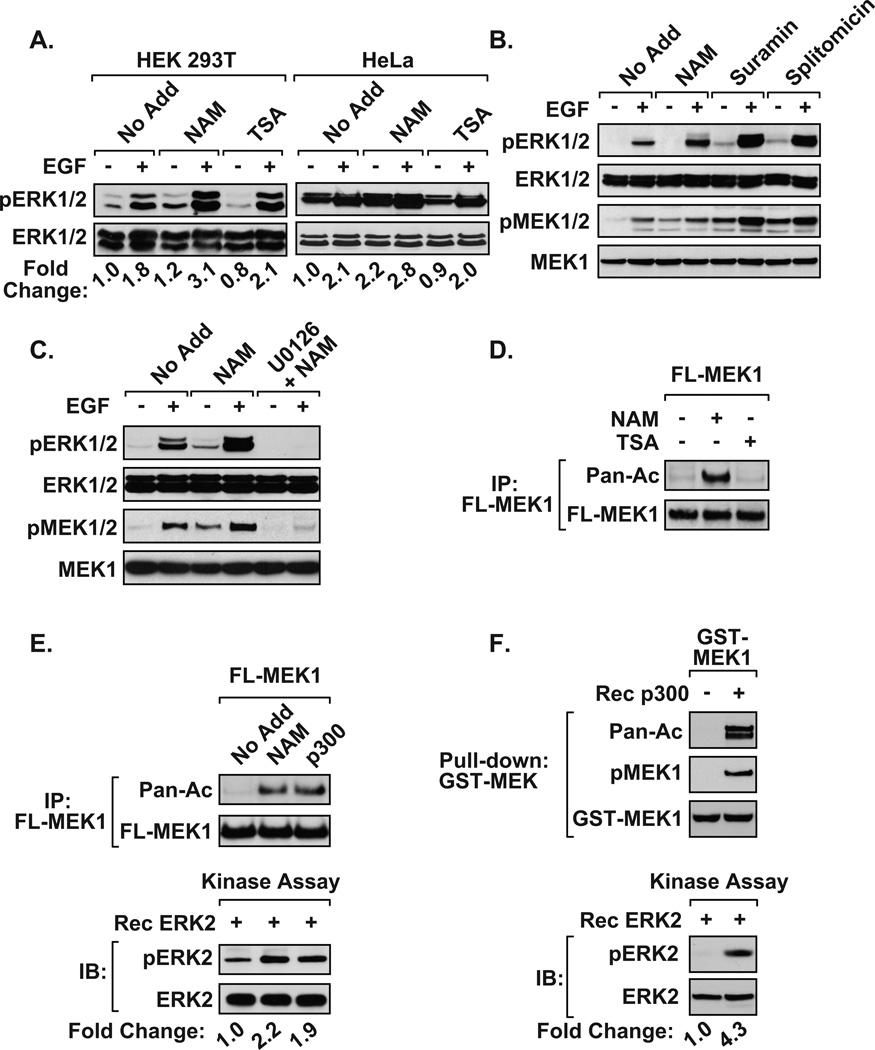
To determine whether cellular acetylation regulates the MEK/ERK pathway, serum and glucose starved cells were treated with deacetylase inhibitors overnight then acutely stimulated with epidermal growth factor (EGF). MEK1 activation was determined by measuring the levels of MEK1/2 and ERK1/2 phosphorylation by immunoblot. Treatment of HEK 293T or HeLa cells with the class III HDAC inhibitor, nicotinamide (NAM), increased ERK1/2 phosphorylation in response to EGF stimulation. However, ERK activity was not enhanced to the same level in the presence of the class I/II HDAC inhibitor trichostatin A (TSA) (Figure 1A). NAM treatment enhanced phosphorylated MEK1 and ERK activity in a dose-dependent manner (Supplementary Figure 1). The addition of other sirtuin inhibitors, suramin or splitomicin, also enhanced EGF-induced ERK phosphorylation (Figure 1B). Interestingly, sirtuin inhibitors increased phosphorylated MEK1/2 levels, suggesting that they increased EGF-induced MEK activity (Figure 1B). Moreover, treatment of HEK 293T cells with the pharmacological MEK inhibitor (U0126) prevented ERK phosphorylation in the response to NAM exposure (Figure 1C). To determine whether acetyltransferases directly target MEK1, we exposed HEK 293T cells to deacetylase inhibitors, and subsequently immunoblotted MEK1 immunoprecipitates with a pan-acetyl antibody. Detection of an acetylated MEK1 protein was confirmed in NAM-treated, but not TSA-treated, cell extracts (Figure 1D). Re-probing the blot with Flag antibody confirmed that the acetylated band was exogenously expressed MEK1. Collectively, these results are consistent with the observation that MEK1 is deacetylated predominantly by sirtuins rather than class I/II deacetylases.
Figure 1. Activation of MEK1 by acetylation.
A) NAM-treated cells display heightened ERK activity. HEK 293T, and HeLa cells were maintained as described (24). Glucose and serum deprived HEK 293T and HeLa cells were left untreated (No Add) or treated overnight with nicotinamide (NAM, 500 µM) or trichostatin A (TSA, 500 nM). EGF (100 ng/ml) was added for 15 min, and immunoblotting was performed on whole-cell extracts. B) Immunoblots demonstrate that the class III HDAC inhibitors, NAM, suramin (35 µM), and splitomycin (60 µM), enhance EGF-mediated phosphorylated MEK and ERK in HEK 293T cells. C) The MEK inhibitor U0126 blocked the ability of NAM to potentiate MEK-mediated activation of ERK. HEK 293T cells treated with U0126 (20 µM) for 2 hrs, followed by 15 min EGF stimulation, were harvested and immunoblotted. D) The inhibition of class III HDAC activity, but not class I/II activity, increases MEK1 acetylation. HEK 293T cells transfected with Flag-MEK1 (FL-MEK1) were treated overnight with NAM or TSA. Flag-MEK1 proteins were immunoprecipitated and blots were analyzed for acetylated MEK1 using pan-acetyl antibody (24,25). E) Acetylation activates MEK1. Glucose and serum deprived HEK 293T cells transfected with Flag-MEK1 were cultured in the absence (No Add) or presence of NAM. Alternatively, cells were co-transfected with Flag-MEK1 and p300. Half of the immunoprecipitated Flag-MEK1 was assayed by immunoblotting (top panel), and the remaining half was used in in vitro kinase assays (bottom panel). Immunoprecipitated Flag-MEK1 material was resuspended in 20 µl kinase buffer (20mM MOPS, pH7.2, 25mM β-glycerol phosphate, 5mM EGTA, 1mM sodium orthovanadate, 1mM dithiothreitol) containing 1 µg inactive ERK2 and 200 µM ATP Immunoblots detect phosphorylated ERK. F) Immunoblots demonstrate that in vitro p300-mediated acetylation activates recombinant MEK1 to phosphorylate ERK2. For A, E and F, the fold change in ERK phosphorylation was determined following densitometric analysis of three independent experiments. Densitometry data was normalized to pan ERK loading control and the fold ERK phosphorylation changes were log2 transformed and a one-tailed Student’s t-test was performed. The following reagents were purchased: NAD+ and EGF (Sigma-Aldrich), splitomicin, TSA, and U0126 (Calbiochem), suramin (Biomol), recombinant GST-MEK1 and p300/HAT (Millipore), and ERK2 (Cell Signaling). Antibodies used include: M2 Flag (Sigma), pan acetyl-lysine, MEK1/2(pS218/222), MEK1, ERK1/2 (pT202/Y204), and ERK1/2 (Cell Signaling).
Next, MEK1 was expressed along with the p300 histone acetyltransferase, MEK1 was immunoprecipitated and blots were probed with a pan-α-acetyl antibody. p300 effectively acetylated MEK1, while NAM potentiated MEK1 acetylation in the absence of ectopically expressed p300 (Figure 1E). Importantly, the use of these immunoprecipitates for in vitro kinase assays revealed that acetylation of MEK1 stimulated its ability to phosphorylate inactive ERK2 (Figure 1E). Moreover, in vitro acetylation by recombinant p300 resulted in elevated auto-phosphorylation of MEK1 and phosphorylation of ERK2 (Figure 1F). These findings demonstrate that acetylation of MEK1 is sufficient to activate its kinase activity.
SIRT1 and SIRT2 regulate MEK1 acetylation
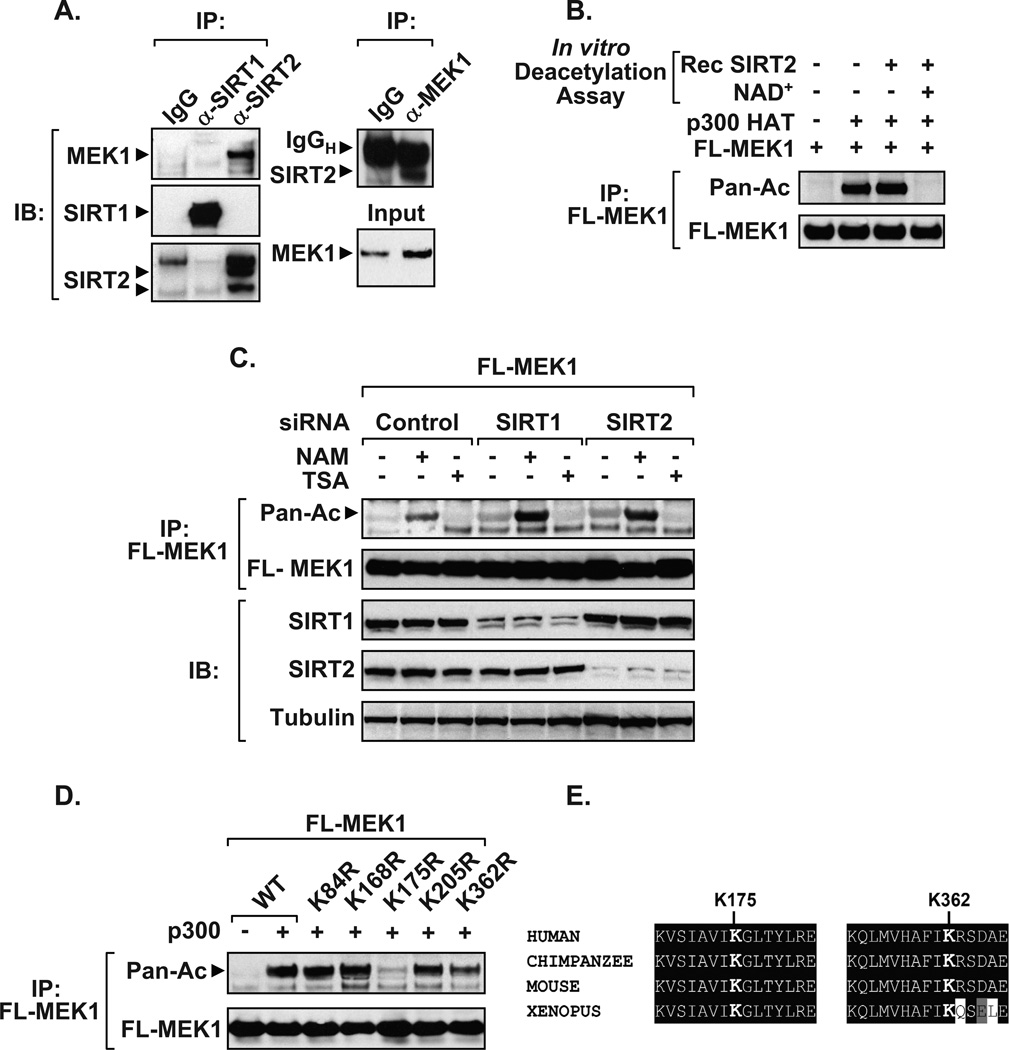
Since MEK activity was upregulated by sirtuin inhibitors and because MEK is localized in both the cytosol and nucleus, co-immunoprecipitations were undertaken to elucidate whether endogenous MEK1 physically interacts with SIRT1 or SIRT2. We found that MEK1 interacted with SIRT2, and a reverse-immunoprecipitations confirmed this protein-protein interaction using whole cell lysates (Figure 2A). Interestingly, SIRT1 did not pull down with MEK1. Subsequent in vitro assays demonstrated that SIRT2 directly and effectively deacetylates MEK1 in an NAD+-dependent manner (Figure 2B). Although no interaction between SIRT1 and MEK1 was observed in Figure 2A, knockdown of either SIRT1 or SIRT2 increased acetylated MEK1 following NAM treatment (Figure 2C). Similarly, ectopic expression of either SIRT1 or SIRT2 inhibits the ability of NAM to increase phosphorylated MEK1 or ERK levels (Supplementary Figure 2). Next, we performed site-directed mutagenesis on each individual lysine (K) residue within human MEK1 to determine which sites are acetylated. Only two of the lysine to arginine (K→R) mutations in MEK1, K175R and K362R, showed reduced acetylation (Figure 2D). Both sites are evolutionarily conserved from Homo sapiens to Xenopus, suggesting that these residues are important for MEK1 function (Figure 2E).
Figure 2. SIRT1 and SIRT2 deacetylate MEK1.
A) Endogenous interaction between SIRT2 and MEK1. Whole cell extracts from HEK 293T were immunoprecipitated using SIRT1, SIRT2, or rabbit IgG control antibodies and blotted using a MEK1 antibody (left panel). Re-probed blots show that SIRT1 and SIRT2 were effectively immunoprecipitated. Reverse immunoprecipitations confirm interaction between endogenous MEK1 and SIRT2 (right panel). B) Immunoprecipitated Flag-MEK1 is effectively deacetylated in vitro by recombinant SIRT2 enzyme in the presence of the NAD+ co-factor. Deacetylation assays were carried out as previously described (24). C) Knockdown of SIRT1 or SIRT2 potentiates MEK1 acetylation in response to NAM treatment. HEK 293T cells transfected with Flag-MEK1 and siRNAs were treated overnight with NAM or TSA and acetylation assays performed. Immunoblots performed on inputs confirm the knockdown of SIRT1 or SIRT2 compared to α-Tubulin. D) Lysine resides 175 and 362 are required for MEK1 acetylation. HEK 293T cells were co-transfected with plasmids encoding wild-type Flag-MEK1 (WT), or site-directed (K→R) mutants, along with vector control (−) or p300 (+). The acetylation status of immunoprecipitated epitope-tagged MEK1 was detected using an acetyl antibody, compared to immunoprecipitated Flag-MEK1 levels. E) ClustalW sequence alignment of MEK1 highlights the conservation of K175 and K362 across various species. Data presented in Figure 2 are representatives of at least three independent experiments. Small interfering RNAs to SIRT1, SIRT2, or control were purchased from Dharmacon. Additional antibodies used in Figure 2 include, α-Tubulin (Sigma), SIRT1 (Biomol), and SIRT2 (Abcam).
Acetyl-mimic MEK1 displays elevated kinase activity
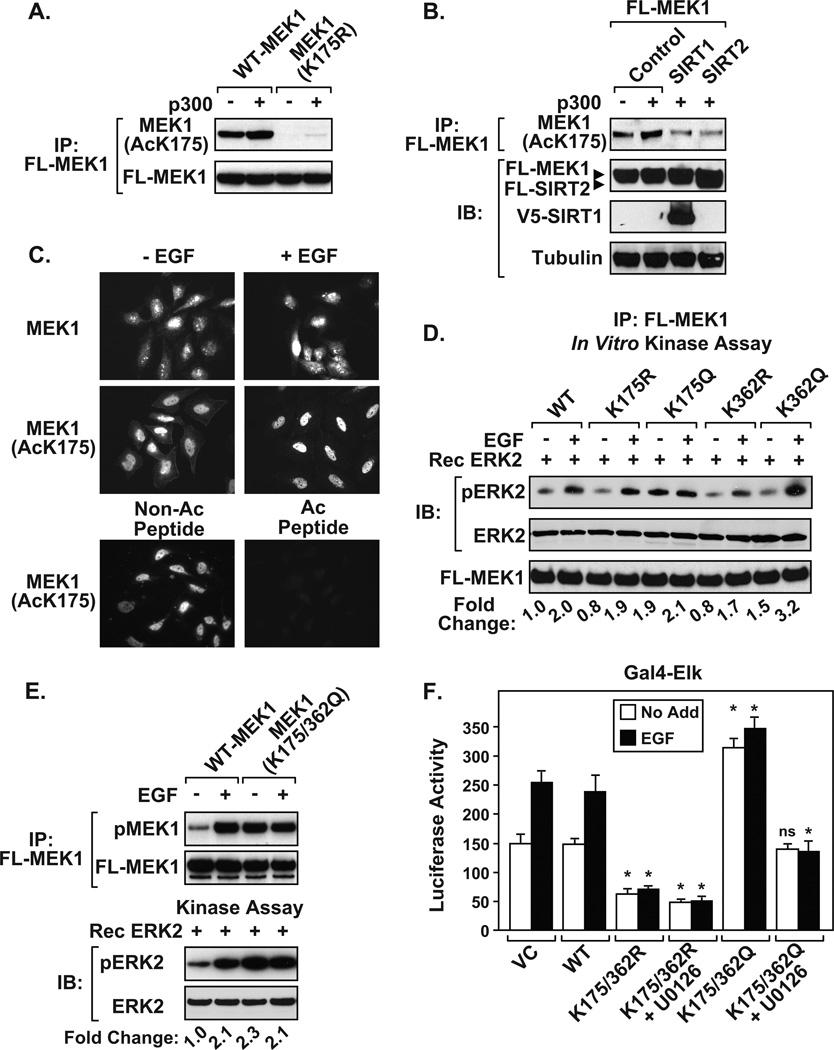
To examine acetylated MEK1, we developed an antibody directed towards K175Ac. We chose K175 because of its specificity to MEK1 and not MEK2. Immunoblot of immunoprecipitated MEK1 showed that the MEK1(AcK175) antibody specifically recognizes acetylated wild-type MEK1, but not MEK1(K175R) (Figure 3A). Exogenous expression of p300 increased Flag-MEK1 K175 acetylation, which was reduced by co-expressing SIRT1 or SIRT2 (Figure 3B). Next, we used immunofluorescence (IF) on HeLa cells to assess the intracellular localization of MEK1. Unstimulated HeLa cells displayed plasma membrane, cytoplasmic, and nuclear staining using the MEK1(AcK175) antibody (Figure 3C). However, cells displayed exclusively nuclear MEK1(AcK175) staining following EGF stimulation. Immunofluorescence staining with the pan α-MEK1 antibody confirmed that MEK1 was expressed in the cytoplasm and nucleus of HeLa cells. The MEK1(AcK175) antibody was specific since pretreatment with acetyl-MEK1(K175) peptide abolished IF staining, compared to the non-acetylated blocking peptide (Figure 3C).
Figure 3. Requirement of K175 and K362 for MEK1 activity.
A) Newly developed MEK1(AcK175) antibody detects acetylated Flag-MEK1, but not Flag-MEK1(K175R) in HEK 293T cells. The MEK1 (AcK175) polyclonal antibody was developed by conjugating keyhole limpet hemocyanin antigen to a 12 amino acid peptide corresponding to human MEK1 acetylated at position K175. Antisera from immunized rabbits was passed through a column coupled with the unacetylated peptide to remove antibody that reacted with non-acetylated MEK1. B) Ectopic expression of either SIRT1 or SIRT2 dampens MEK1(K175) acetylation. C) HeLa cells display enriched endogenous acetylated MEK1(K175) in the nucleus following EGF stimulation. Overnight serum-deprived HeLa cells were left untreated or treated with EGF, fixed with paraformaldehyde, and analyzed by IF. Endogenous MEK1 was detected using pan MEK1 antibody or MEK1(AcK175) antibody. Pre-incubation of the MEK1(AcK175) antibody with acetylated MEK1 peptide (2 µg), but not the non-acetylated peptide (2 µg), blocked detection of acetylated MEK1 (bottom panels). D) K175 and K362 are important for regulating MEK1-dependent phosphorylation of ERK. Glucose and serum deprived HEK 293T cells expressing Flag-MEK1 wild-type (WT), or site-directed mutants (K175R, K175Q, K362R, K362Q) were left untreated or stimulated with EGF. Immunoprecipitated MEK1 proteins were used in in vitro kinase assays as described in Figure 1E. E) Immunoprecipitated acetyl-mimic MEK1(K175/362Q) displays elevated kinase activity. For D and E, the fold change in ERK phosphorylation was determined as described in Figure 1. Similar fold changes in ERK phosphorylation were obtained in three individual experiments. F) The acetyl-mimic MEK1(K175/362Q) effectively stimulates ELK transcriptional activity. Glucose and serum deprived HEK 293T cells transiently expressing the Gal4-luciferase reporter, Gal4-ELK, and Flag tagged wild-type (WT), or MEK1 mutants were either left alone (No Add) or treated with U0126, followed by EGF stimulation for 8 hrs prior to harvesting. Data are a calculated mean ± S.D. P-values were calculated relative to No Add or EGF stimulated wild-type MEK1 (WT), *p <0.05, N = 3, (ns) not significant.
To determine whether acetylated MEK has increased activity, MEK1 mutants were constructed in which K residues were changed to glutamine (Q) to act as acetylation “mimics”. Cells expressing MEK1 constructs (WT, K→R, or K→Q) were either left alone or stimulated with EGF, and immunoprecipitated Flag-tagged MEK1 proteins were then used in in vitro kinase assays. MEK1(K362R), but not K175R, showed reduced activity following EGF stimulation, MEK1(K175Q) displayed constitutive activity independent of EGF treatment, and MEK1(K362Q) showed elevated EGF-induced activity (Figure 3D). The MEK1 double mutant (K175/362Q) displayed constitutive MEK1 phosphorylation and elevated kinase activity, compared to wild-type MEK1 (Figure 3E). Consistent with a loss-of-function phenotype, cells expressing MEK1(K175/362R) displayed reduced basal Gal4-ElK transcriptional activity, which remained unresponsive to EGF treatment (Figure 3F). In contrast, MEK1(K175/362Q) expressing cells showed elevated Gal4-ELK activity independent of EGF stimulation. The ability of the MEK1(K175/362Q) protein to stimulate the transactivation potential of ELK remained sensitive to U0126. These results highlight the importance of K175 and K362 for activation of MEK1 independent of upstream kinases.
Acetyl-mimic MEK1 promotes inappropriate proliferation
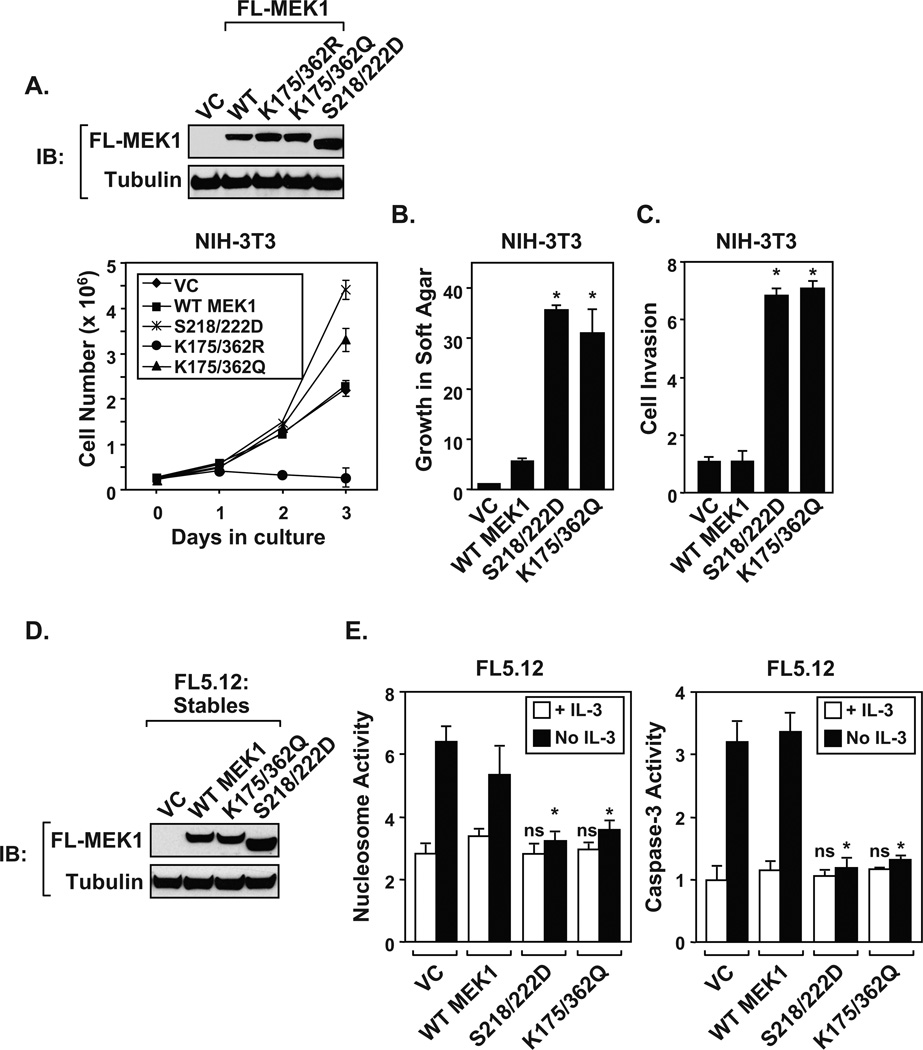
Since the acetyl-mimic MEK1(K175/362Q) shows enhanced kinase activity, experiments were undertaken to elucidate whether MEK1 mutants promoted cellular proliferation. Retroviral-transduced NIH-3T3 cells expressing vector control (VC), epitope-tagged wildtype (WT) or mutant MEK1 proteins (K175/362R, K175/362Q, S218/222D) were assayed for proliferation and viability. Expression levels were measured by immunoblot (Figure 4A, top). Cells expressing acetyl-mimic MEK1(K175/362Q) displayed similar enhanced proliferation rates as cells expressing constitutively active phospho-mimic MEK1(S218/222D) (Figure 4A, bottom). In contrast, cells expressing the acetylation-defective MEK1(K175/362R) mutant showed a loss of proliferation over a three day period, compared to wild-type MEK1 or vector control. Importantly, a stable cell line that expresses MEK1(K175/362R) could not be generated, suggesting that mutating these residues altered cell viability. Moving forward, cells stably expressing MEK1(K175/362Q) displayed increased invasion through matrigel and growth in soft agar in a similar manner as constitutively active phospho-mimic MEK1(S218/222D) (Figure 4B and 4C). As predicted, cells expressing wild-type MEK1 or vector control failed to invade or grow in soft agar.
Figure 4. The MEK1 acetyl-mimic promotes inappropriate growth control.
A) Immunoblots demonstrate the expression of MEK1 proteins following retroviral transduction in NIH-3T3 cells. The MEK1 acetyl-mimic promotes cell proliferation. NIH-3T3 cells were seeded at 3 × 105 cells per 6-well plate. At each time point, cells were trypsinized and viable cells counted. B) The MEK1 acetyl-mimic stimulates growth in soft agar. NIH-3T3 cells stably expressing MEK1 wild-type (WT), constitutively active (S218/222D), acetyl-mimic (K175/362Q) proteins, or vector control (VC) were assayed for growth in soft agar. The colonies (≥ 30 µm) were numerated after 4 weeks in culture. C) The MEK1 acetyl-mimic promotes invasion to a similar degree as the MEK1 phospho-mimic. NIH-3T3 cells stably expressing MEK1 (WT), constitutively active (S218/222D), or acetyl-mimic (K175/362Q) proteins were assayed for invasion through matrigel. D) Immunoblots demonstrate the stable expression of MEK1 proteins in the IL-3-depedent FL5.12 myeloid cell line. FL5.12 were maintained as described (19). F) Expression of the MEK1 acetyl-mimic overcomes apoptosis following IL-3 withdrawal. FL5.12 cells stably expressing (WT), constitutively active (S218/222D), or acetyl-mimic MEK1(K175/362Q) were cultured in either the presence (+ IL-3) or absence (No IL-3) for 16 hrs prior to harvesting for nucleosome and capase-3 assays (24). Data shown in each panel of Figure 4 are a calculated mean ± S.D, ns = not significant compared to values obtained for wild-type MEK1 (WT), *p <0.05, N = 3. The NIH3T3 and FL5.12 stable clones were created by retroviral transduction (19). NIH3T3 and FL5.12 stable clones were selected using 2.5 or 2 µg/ml puromycin (Sigma-Aldrich), respectively. Following selection and subcloning, five individual clones were pooled and used for further characterization. The following reagents were purchased: matrigel (BD Biosciences) and CytoSelect™ Soft Agar Colony Formation kit (Cell Biolabs).
To confirm that MEK1(K175/362Q) displays similar growth promoting properties in other cell types, MEK1 proteins were expressed in the hematopoietic interleukin-3 (IL-3)-dependent cell line FL5.12 (Figure 4D). FL5.12 cells were chosen because constitutively active MEK1 is known to overcome IL-3-dependent growth (19). To determine whether MEK1(K175/362Q) could overcome cell death following IL-3 withdrawal, FL5.12 cells expressing MEK1 proteins were assayed for the induction of apoptosis using nucleosome and caspase-3 assays. FL5.12 cells expressing either MEK1(S218/222D) or MEK1(K175/362Q) proteins were resistant to apoptosis following IL-3 withdrawal, compared to control or wild-type MEK1 expressing cells (Figure 4E). Experiments performed in NIH-3T3 and FL5.12 cells indicate that the acetyl-mimic MEK1(K175/362Q) displays growth promoting properties similar to phospho-mimic MEK1(S218/222D).
DISCUSSION
Our work indicates that p300-induced MEK1 acetylation increases its kinase activity for ERK. Pharmacological inhibition of sirtuins and siRNA knockdown of SIRT1 or SIRT2 induces acetylation of MEK1 within the cell. Subsequent generation of single and double acetyl-mimics of MEK1 at K175 and K362 display elevated basal and EGF-inducible activity, indicating biological relevance of MEK1 acetylation. Furthermore, expression of the MEK1 double acetyl-mimic promotes inappropriate cell proliferation, invasion and growth in soft agar, in a manner similar as the constitutively active phospho-mimic MEK1(S218/222D).
MEK1 knockout mice, unlike MEK2, are embryonic lethal, suggesting that MEK1 serves a unique function that cannot be compensated for by MEK2 (20,21). Since MEK1 and MEK2 display considerable conservation at the amino acid level (~80%), it has been hypothesized that post-translational modifications within MEK1 account for differences between these two kinases. Consistent with this idea, MEK1, but not MEK2, is phosphorylated at S298 by the p21-activated kinase (PAK)-1, an event that promotes MEK1 autophosphorylation in the activation loop (22). In a similar manner, we find that human MEK1 is acetylated at K175, yet MEK2 contains an arginine rather than a lysine at the equivalent site (R179). Thus, neutralization of the lysine charge at K175 by acetylation specifically and uniquely alters MEK1, but not MEK2, kinase activity. Mechanistically, it is not clear how acetylation alters MEK1 activity, since K175 and K362 are contained in α-helies that reside on a surface away from the active site. One possibility is that acetylation governs allosteric changes in MEK1 to promote autophosphorylation in the activation loop, similar to proposed mechanisms by which vMOS- and PAK1-dependent phosphorylation activates MEK1 (22,23). Consistent with this hypothesis, we have observed: 1) increase in autophosphorylation upon acetylation of MEK1 by recombinant p300 in vitro, 2) elevation in phosphorylated MEK1(S218/222) following treatment with sirtuin inhibitors, and 3) constitutive MEK1 phosphorylation upon expression of MEK1(K175/362Q), which remains sensitive to U0126. Moreover, NAM loses its ability to potentiate MEK1 activity in cells treated with the pharmacological inhibitor U0126. Similar to our observations, PAK1-mediated allosteric regulation of MEK1 is also inhibited by U0126 (22). Collectively, our results support the hypothesis that acetylated MEK1 promotes phosphorylation in the activation loop, an event that remains sensitive to the U0126 inhibitor.
Although numerous proteins are known to be deacetylated by sirtuins, few have been shown to be preferentially regulated by this class of deacetylases. Our work identifies MEK1 as one of the unique sirtuin-regulated substrates, linking these two important families of enzymes to changes in cellular metabolism, growth factor activation, and stress responses. Cells expressing MEK1(K175/362Q) display a growth advantage, indicating that an acetyl-mimic of MEK1 inappropriately signals to promote growth in immortalized cells. This raises the question as to whether SIRT2 levels in a cancer cell can regulate MEK/ERK activity. Interestingly, in an accompanying manuscript in this issue, Bernards and colleagues identify SIRT2 as an important molecule that confers resistance to therapies targeting the MAP Kinase signaling pathway. Thus, work by the Bernards laboratory and our own suggests that SIRT2 acts as a key deacetylase that regulates inappropriate MEK1 acetylation and subsequent activation. However, additional work is required to elucidate whether inappropriate acetylation of MEK1 promotes Ras-mediated transformation in human cancers.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Drs. JS. Smith, PT. Stukenberg, and JJ. Wamsley, and Ms. A. Sherman for scientific and editorial review. HA-MEK1, HA-MEK1(S218/222D), and Flag-ERK1 were gifts from Dr. M. Weber, University of Virginia. p300 was provided by Dr. D. Livingston, Dana-Farber Cancer Institute. Gal4-ELK1 was a gift from Dr. C. Der, University of North Carolina. V5-SIRT1 and Flag-SIRT2 were gifts from E. Verdin, Gladstone Institute. The pBabe-puro vector was provided by Dr. Robert Weinberg, Whitehead Institute, MIT. Work was supported by the National Cancer Institute (CA132580 & CA104397) to MWM, and CA110552 awarded to F.Y.
Footnotes
CONFLICTS OF INTEREST:
The authors declare no competing financial interest in relation to the work described.
REFERENCES
- 1.Kyriakis JM, Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol Rev. 2012;92:689–737. doi: 10.1152/physrev.00028.2011. [DOI] [PubMed] [Google Scholar]
- 2.Keshet Y, Seger R. The MAP kinase signaling cascades: a system of hundreds of components regulates a diverse array of physiological functions. Methods Mol Biol. 2010;661:3–38. doi: 10.1007/978-1-60761-795-2_1. [DOI] [PubMed] [Google Scholar]
- 3.Duffy A, Kummar S. Targeting mitogen-activated protein kinase kinase (MEK) in solid tumors. Target Oncol. 2009;4:267–273. doi: 10.1007/s11523-009-0125-x. [DOI] [PubMed] [Google Scholar]
- 4.Huang P, Han J, Hui L. MAPK signaling in inflammation-associated cancer development. Protein Cell. 2010;1:218–226. doi: 10.1007/s13238-010-0019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 6.Lawrence MC, McGlynn K, Shao C, Duan L, Naziruddin B, Levy MF, et al. Chromatin-bound mitogen-activated protein kinases transmit dynamic signals in transcription complexes in β-cells. Proceedings of the National Academy of Sciences. 2008;105:13315–13320. doi: 10.1073/pnas.0806465105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 8.Landry J, Sutton A, Tafrov ST, Heller RC, Stebbins J, Pillus L, et al. The silencing protein SIR2 and its homologs are NAD-dependent protein deacetylases. Proc Natl Acad Sci U S A. 2000;97:5807–5811. doi: 10.1073/pnas.110148297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith JS, Brachmann CB, Celic I, Kenna MA, Muhammad S, Starai VJ, et al. A phylogenetically conserved NAD+dependent protein deacetylase activity in the Sir2 protein family. Proc Natl Acad Sci U S A. 2000;97:6658–6663. doi: 10.1073/pnas.97.12.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010;5:253–295. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verdin E, Hirschey MD, Finley LW, Haigis MC. Sirtuin regulation of mitochondria: energy production, apoptosis, and signaling. Trends Biochem Sci. 2010;35:669–675. doi: 10.1016/j.tibs.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brooks CL, Gu W. How does SIRT1 affect metabolism, senescence and cancer? Nat Rev Cancer. 2009;9:123–128. doi: 10.1038/nrc2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng CX. SIRT1, is it a tumor promoter or tumor suppressor? Int J Biol Sci. 2009;5:147–152. doi: 10.7150/ijbs.5.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saunders LR, Verdin E. Sirtuins: critical regulators at the crossroads between cancer and aging. Oncogene. 2007;26:5489–5504. doi: 10.1038/sj.onc.1210616. [DOI] [PubMed] [Google Scholar]
- 15.Kim HS, Vassilopoulos A, Wang RH, Lahusen T, Xiao Z, Xu X, et al. SIRT2 maintains genome integrity and suppresses tumorigenesis through regulating APC/C activity. Cancer Cell. 2011;20:487–499. doi: 10.1016/j.ccr.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang RH, Sengupta K, Li C, Kim HS, Cao L, Xiao C, et al. Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer Cell. 2008;14:312–323. doi: 10.1016/j.ccr.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li M, Luo R, Chen J, Cao Y, Lu J, He J, et al. High expression of transcriptional coactivator p300 correlates with aggressive features and poor prognosis of hepatocellular carcinoma. Journal of Translational Medicine. 2011;9:5. doi: 10.1186/1479-5876-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao X, Cai M, Chen J, Guan X, Kung H, Zeng Y, et al. High expression of p300 in human breast cancer correlates with tumor recurrence and predicts adverse prognosis. Chin J Cancer Res. 2011;23:201–207. doi: 10.1007/s11670-011-0201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blalock WL, Pearce M, Steelman LS, Franklin RA, McCarthy SA, Cherwinski H, et al. A conditionally-active form of MEK1 results in autocrine tranformation of human and mouse hematopoietic cells. Oncogene. 2000;19:526–536. doi: 10.1038/sj.onc.1203337. [DOI] [PubMed] [Google Scholar]
- 20.Giroux S, Tremblay M, Bernard D, Cardin-Girard JF, Aubry S, Larouche L, et al. Embryonic death of Mek1-deficient mice reveals a role for this kinase in angiogenesis in the labyrinthine region of the placenta. Curr Biol. 1999;9:369–372. doi: 10.1016/s0960-9822(99)80164-x. [DOI] [PubMed] [Google Scholar]
- 21.Catalanotti F, Reyes G, Jesenberger V, Galabova-Kovacs G, de Matos Simoes R, Carugo O, et al. A Mek1–Mek2 heterodimer determines the strength and duration of the Erk signal. Nat Struct Mol Biol. 2009;16:294–303. doi: 10.1038/nsmb.1564. [DOI] [PubMed] [Google Scholar]
- 22.Park ER, Eblen ST, Catling AD. MEK1 activation by PAK: A novel mechanism. Cell Signal. 2007;19:1488–1496. doi: 10.1016/j.cellsig.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Resing KA, Mansour SJ, Hermann AS, Johnson RS, Candia JM, Fukasawa K, et al. Determination of v-Mos-catalyzed phosphorylation sites and autophosphorylation sites on MAP kinase kinase by ESI/MS. Biochemistry. 1995;34:2610–2620. doi: 10.1021/bi00008a027. [DOI] [PubMed] [Google Scholar]
- 24.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allison DF, Wamsley JJ, Kumar M, Li D, Gray LG, Hart GW, et al. Modification of RelA by O-linked N-acetylglucosamine links glucose metabolism to NF-kappaB acetylation and transcription. Proc Natl Acad Sci U S A. 2012;109:16888–16893. doi: 10.1073/pnas.1208468109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.