Abstract
Macrophages are the primary cell type infected with HIV in the central nervous system, and infection of these cells is a major component in the development of neuropathogenesis and HIV-associated neurocognitive disorders. Within the brains of drug abusers, macrophages are exposed to increased levels of dopamine, a neurotransmitter that mediates the addictive and reinforcing effects of drugs of abuse such as cocaine and methamphetamine. In this study we examined the effects of dopamine on HIV entry into primary human macrophages. Exposure to dopamine during infection increased the entry of R5 tropic HIV into macrophages, irrespective of the concentration of the viral inoculum. The entry pathway affected was CCR5 dependent, as antagonizing CCR5 with the small molecule inhibitor TAK779 completely blocked entry. The effect was dose-dependent and had a steep threshold, only occurring above 108 M dopamine. The dopamine-mediated increase in entry required dopamine receptor activation, as it was abrogated by the pan-dopamine receptor antagonist flupenthixol, and could be mediated through both subtypes of dopamine receptors. These findings indicate that the effects of dopamine on macrophages may have a significant impact on HIV pathogenesis. They also suggest that drug-induced increases in CNS dopamine may be a common mechanism by which drugs of abuse with distinct modes of action exacerbate neuroinflammation and contribute to HIV-associated neurocognitive disorders in infected drug abusers.
Introduction
Human Immunodeficiency Virus type 1 (HIV) enters the central nervous system (CNS) within 8 days of initial infection [1] and leads to the development of HIV-associated neurological disorders (HAND) in 40–70% of individuals [2]–[6]. Macrophages and other cells of the monocytic lineage are the primary targets for HIV infection in the CNS [7]–[10], although HIV also infects astrocytes [8], [11], [12]. Macrophages are critical to HIV mediated neuropathogenesis [13]–[16] and may serve as viral reservoirs within the CNS [17], [18]. Macrophages also release inflammatory mediators and neurotoxic viral and host proteins, contributing to chronic neuroinflammation and neurotoxicity [16], [19], [20]. Thus, infection of CNS macrophages is central to HIV-associated neuroinflammation and neurocognitive dysfunction.
Macrophages in the CNS are exposed to dopamine, a catecholamine neurotransmitter that is increased by the use of illicit drugs such as cocaine and methamphetamine [21], [22], as well as by legal therapeutics such as Ritalin and some antidepressants [23], [24]. Studies in SIV-infected rhesus macaques show that increases in extracellular dopamine correlate with increased CNS viral loads [25], [26]. HIV-infected individuals show exacerbated neuropathology in regions of the brain with high levels of dopamine, such as the basal ganglia and substantia nigra [27]–[32]. Dopamine acts principally through dopamine receptors (DR), G-protein coupled receptors (GPCR) that are divided into D1-like DR (D1R and D5R) and D2-like DR (D2R, D3R and D4R) depending upon whether they activate (D1-like DR) or inhibit (D2-like DR) adenylyl cyclase [33]. Studies show that DR also activate alternative pathways, including mobilization of calcium from the endoplasmic reticulum [34]–[37]. The effects of dopamine on macrophage function, and the signaling pathways by which these effects are mediated, have not been studied extensively.
Our previous studies showed that dopamine increases HIV replication in human macrophages through activation of DR, increasing the total number of infected cells [38]. The mechanism(s) by which this occurred are unclear, but one possibility is by increasing HIV entry into macrophages. HIV entry is complex, and in macrophages, it is mediated by the interaction of the viral envelope protein gp120 with the surface receptor CD4 and co-receptor CCR5 [39]. In this study, we examined whether dopamine increases HIV entry and whether that increase was mediated by changes in CCR5 expression and/or activation of DR.
Our data showed that dopamine increased HIV entry into human primary monocyte-derived macrophages (MDM) by approximately 2-fold, and that the increased entry occurred at dopamine concentrations above 10−8 M. The increased entry required CCR5, but was not mediated through changes in the surface expression of this receptor. Increased entry also required activation of DR and was mediated by both D1-like and D2-like DR, suggesting that a common DR signaling mechanism mediates the increased entry. Using transfected HEK293 cells, we demonstrated that calcium mobilization resulting from activation of Gαq-coupled receptors, such as CCR5 [40], can be potentiated by both D1-like and D2-like DR. These data indicate that the dopamine-induced increase in macrophage HIV replication we previously reported is due, at least in part, to an increase in viral entry, and suggest that this could be a result of a dopamine-mediated increase in calcium mobilization.
Methods
Reagents
RPMI-1640, penicillin/streptomycin (P/S), 10X HEPES and 10X HBSS from Life Technologies (Carlsbad, CA). Human AB serum from Lonza (Basel, Switzerland). Fetal Bovine Serum from Lonza for MDM culture and from Gemini (West Sacramento, CA) for HEK293 culture. BSA, Acetylcholine, Probenecid, Dopamine, SKF81297, Sulpiride and SCH23390 from Sigma-Aldrich (St. Louis, MO). SKF38393 and Flupenthixol dihydrochloride from Tocris Biosciences (Minneapolis, MN). Quinpirole from Tocris or Sigma-Aldrich. All DR agonists and antagonists were resuspended in distilled H2O. TAK779 was obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH [41]. Macrophage colony stimulating factor (M-CSF) was from Peprotech (Rocky Hill, NJ), and was resuspended at 100 µM in distilled H2O.
Cell isolation and culture
Human peripheral blood mononuclear cells (PBMC) were separated from blood obtained from healthy donors (New York Blood Center, Long Island City, New York) by Ficoll-Paque (GE Healthcare, Piscataway, NJ) gradient centrifugation. Monocytes present in the PBMC were determined by flow cytometry of CD14+ cells in the PBMC using a FACS Canto II flow cytometer (Becton-Dickinson, Franklin Lakes, NJ). Monocyte derived macrophages (MDM) were obtained by adherence isolation, through culture in macrophage media (RPMI-1640 with 10% FCS, 5% human AB serum, 10 mM HEPES, 1% P/S, and 10 ng/mL M-CSF) for 3 days, washing and culturing another 3–5 days. After 6–8 total days in culture the cells were considered to be mature MDM. Flp-In T-Rex HEK 293 cells (HEK 293 cells, Life Technologies) were maintained in DMEM supplemented with 10% FBS and 2 mM L-glutamine (Life).
Generation of Viral Stocks
Viral DNA was prepared by isolating plasmids from bacterial stocks (HB101 cells, Life Technologies) transformed with viral DNA clones and purified using CsCl2 gradient ultracentrifugation. Stocks of HIVBaL and HIVBaL harboring Vpr-β-lactamase were prepared in parallel by co-transfecting 3 µg of HIVBaL DNA (obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: pWT/BAL), along with 15 µg of either pcDNA or pMM310 (a plasmid expressing Vpr-β-lactamase, obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: pMM310 (Cat#11444) from Dr. Michael Miller [42]) into HEK 293 cells using calcium phosphate precipitation as described by the manufacturer (Chemicon). A 100% HEK 293 cells were split 1:6 in a 10 cm2 plate 24 hours prior to transfection, with the goal of 30–40% confluency at the time of transfection. Media was changed at 16 hours post-transfection and collected 24 and 48 hours later. Supernatant was passed through a 0.45 µm cellulose acetate syringe filter (Corning, Tewksbury, MA), and treated with 20 U/ml DNAseI (Roche, Indianapolis, IN) for 30 minutes at 37°C. Viral stocks were purified and concentrated by passing the virus through 20% sucrose/PBS gradient centrifugation for 2 hr at 36,000 rpm, 4°C, and then viral pellets were resuspended in macrophage media and stored in aliquots at 70°C. To insure Vpr-β-lactamase virions maintained infectivity, they were tested by infecting MDM and compared to infections with non-Vpr-β-lactamase containing viral stock. Analysis of p24 production in response to infection showed no significant difference in infectivity of virus with and without Vpr-β-lactamase (data not shown). To Infectious units in each viral stock were determined by infection of Hi-5 GHOST cells (obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: GHOST Cell Transfectants - GHOST (3) Hi-5 from Dr. Vineet N. KewalRamani and Dr. Dan R. Littman [43]). Determination of the infectious units in each viral stock enabled inoculation using multiplicity of infection (MOI), insuring that all infections were performed with identical numbers of infectious virions per macrophage.
Viral Entry Assay
Entry assays were performed using the Geneblazer in vivo detection kit (Life Technologies) according to the manufacturers protocol, with optimization for use in primary macrophages as previously described [44]. Mature MDM cultured in flat, clear bottom 96 well black plates (ThermoFisher Scientific, Waltham, MA) were infected in quadruplicate with HIVBaL containing Vpr-β-lactamase, at a multiplicity of infection (MOI) of 0.002, 0.005 or 0.01, based on the CD14+ cells in the starting PBMC population. MDM were incubated for 2.5 or 4 hours at 37°C with 5% CO2. Dopamine or DR agonists were added concurrently with HIV, while the pan-DR antagonist flupenthixol was added 30 minutes prior to addition of virus. All DR agonists were used at 108 M, as our preliminary studies indicated that this was the concentration that induced a significant increase in entry in the greatest number of donors. After incubation, MDM were washed and incubated in the dark at RT for 6 hours in 100 µl of phenol red free macrophage media and 20 µL of 6X CCF2-AM loading solution (Loading solution ratios optimized for primary macrophages as follows; 0.04:0.36:0.25:1,935 A:B:D:C). CCF2-AM contains two fluorophores connected by a lactam ring, and generates green fluorescence by means of fluorescence resonance energy transfer (FRET) when the fluorophores are in close proximity. When the lactam ring is cleaved, the FRET interaction is disrupted and the fluorescence becomes blue. In this assay, uninfected cells fluoresce green because the lactam ring is intact and infected cells fluoresce blue because the β-lactamase enzyme contained within the infecting virus cleaves the lactam ring and separates the fluorophores [45].
After incubation at RT, 12 images of each infection condition were generated using a Zeiss IX70 inverted microscope (Zeiss) and an Olympus E-620 Live View DSLR (Olympus). Volocity (Perkin-Elmer) was used to enumerate the number of infected (blue) and uninfected (green) cells in each condition. Primary cells from different donors have varying susceptibility to HIV infection, therefore the number MDM infected with HIV was variable between experiments. There was also inter-experiment variation in the effects of different concentrations of dopamine on HIV entry, likely due to donor dependent differences in the response to dopamine. To evaluate changes in HIV entry induced by each concentration of dopamine, in each donor we compared the macrophages infected in the presence of each concentration of dopamine or agonist to the macrophages infected in control infections (those cultures infected with HIV alone). The mean difference in the number of cells infected is determined by the formula, 100x (Experimental – Control) / Control, and expressed as percent increase in macrophage infection relative to the control infection (HIV alone), which is defined as a 0% increase.
Flow Cytometry
CD14+ cells present in isolated PBMC were determined using anti-human CD14-PE (clone M5E2, BD Biosciences, Franklin Lakes, NJ) and an isotype matched negative control, IgG2a-PE (BD Biosciences). PBMC were stained for 30 minutes at 4°C, washed with 1% BSA in PBS and fixed with 2% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA). Immunopositive cells were analyzed by acquisition of 10,000 events on a FACS Canto II flow cytometer and analyzed using FlowJo (Treestar, Ashland OR). To analyze CD4 or CCR5 protein expression on the macrophage surface, MDM were detached from culture dishes with TrypLE Select (10X) for 30 minutes at 37°C, followed by gentle agitation and scraping. 1–2×105 MDM were stained for CCR5 or CD4 using anti-human CCR5-APC/Cy7 and CD4-FITC, with the isotype-matched negative controls, IgG1-APC/Cy7 or IgG1-FITC (BD Biosciences). Antibodies were titrated to determine the optimal concentration for staining PBMC and MDM. After subtracting out the background fluorescence from the isotype matched control, the mean fluorescence intensity (MFI) for each antigen was determined.
Generation of Dopamine Receptor Expressing HEK Cells
Stable cell lines were generated as previously described (Han et al. 2009). Briefly, Flp-In T-Rex 293 cells (Life Technologies) were transfected with either D1R or D2R expressing plasmids using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. Transfected HEK cells were selected and maintained in media with G418 (Mediatech, Manassas, VA) or Hygromycin B (Mediatech) for expression selection. Single colonies were isolated and screened for receptor expression using FACS analysis. D2s stable line refers to cells transfected with the D2R expressing plasmid SF-D2s, while D1 stable line refers to cells transfected for inducible expression of D1R expressing plasmid, 3xHA-D1. The D1-D2s double stable line refers to HEK cells stably transfected with 3xHA-D1 and SF-D2 plasmids. Cells with inducible receptors were incubated in 1 µg/ml tetracycline-containing medium overnight prior to experiments to induce expression. Generation of these cell lines enabled maintenance of consistent receptor expression levels for calcium flux assays.
Measurement of Calcium Flux
Calcium flux was measured using the Flipr Calcium 5 Assay kit (Molecular Devices, Sunnyvale, CA) and according to the manufacturers protocol. Briefly, cells were resuspended in HBSS buffer containing 20 mM HEPES and 2.5 mM probenecid and distributed in 40 µl volumes in 96 well plates (500,000 cells/well). Fifty µl Flipr5 dye (Molecular devices) was added to each well and plates were incubated in 37°C 5% CO2 for 1 hour. Plates containing 10x concentrated ligand were prepared in HBSS with 20 mM HEPES. During the calcium reading, performed on a Flexstation 3 (Molecular Devices), 10 µl ligand was injected to the well at the indicated times. Intracellular calcium levels were measured every 2 sec over the course of 220 sec. Data was analyzed by Softmax Pro 5.4 (Molecular Devices) and Prism 6.0 (Graphpad, La Jolla, CA).
Statistics
Statistics were determined using Prism 6.0. Entry assay and flow cytometry data were analyzed for normality using a D’Agostino and Pearson omnibus normality test. Normally distributed were analyzed using a two-tailed Student’s T-test and data that were not normally distributed were analyzed with Wilcoxen Matched Pairs Signed Rank Test. P < 0.05 was considered significant.
Results
Dopamine Increases HIV Entry into Primary Human Macrophages
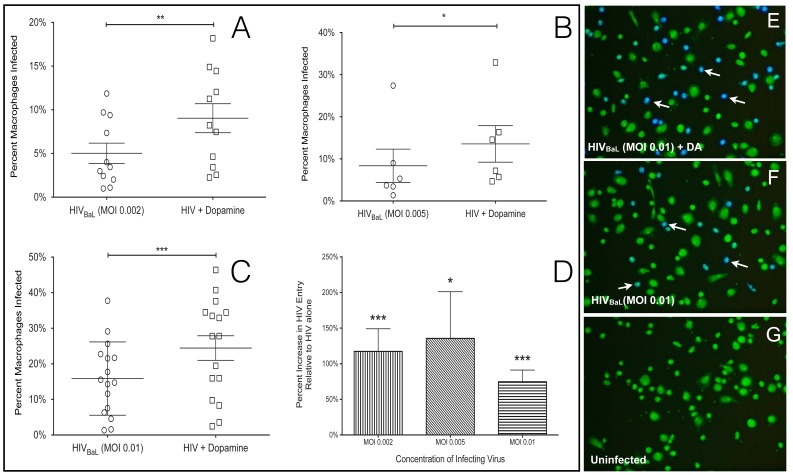
To determine whether dopamine-mediated increases in HIV replication in macrophages were due to an increase in viral entry, primary human MDM were infected with different concentrations of HIVBaL virions harboring an active β-lactamase enzyme (β-lac HIV). As described in the methods, entry of β-lac HIV into a macrophage causes that cell to fluoresce blue, while uninfected cells remain green. The percentage of viral entry was quantified by counting the number of blue and green cells and comparing the number of blue cells to the total cell number. Infection with β-lac HIV using a multiplicity of infection (MOI) of 0.002 for 4 hours resulted in infection of 1.0% to 11.9% of MDM, 1.3% to 27.4% using an MOI of 0.005 and 1.4% to 37.8% of cells using an MOI of 0.01 (Figure 1A, MOI 0.002, n = 11; 1B, MOI 0.005, n = 6; 1C, MOI 0.01, n = 16). The variation in the susceptibility of macrophages to infection with HIV demonstrates the heterogeneity of primary macrophages from different donors used in these studies. Treatment with dopamine significantly increased viral entry into MDM at all concentrations of HIV tested (MOI 0.002 (1A), 0.005 (1B) and 0.01(1C)). The magnitude of the dopamine-mediated increase in HIV entry resulting from infection with each concentration of HIV is shown in Figure 1D (MOI 0.002, 117% increase, n = 11, p = 0.001***; MOI 0.005, 135% increase, n = 6, p = 0.0313 *; MOI 0.01, 75% increase, n = 16, p = 0.0002 ***). These data indicate that the effect of dopamine on HIV entry is not dependent on the concentration of the infecting virus. In Figure 1D and subsequent figures showing a percent increase, the control infections (HIV alone) are defined as a 0% increase, as described in the Methods. A representative experiment showing MDM from a single donor infected with β-lac HIV at an MOI of 0.01 for 4 hours in the presence and absence of dopamine is shown (Figures 1E and 1F). Uninfected cultures showed no blue fluorescence, indicating that blue fluorescence was specifically induced by HIV entry (Figure 1G). Donor dependent differences among primary macrophages resulted in inter-experiment variation in baseline levels of HIV infection and in the magnitude of the increase in entry mediated by different concentrations of dopamine. To account for this variability, each infection was performed with 4 different concentrations of dopamine, 2×108 M, 2×107 M, 2×106 M and 2×105 M, to ensure that cells from each donor were treated with a concentration of dopamine that induced an optimal response. For each donor, the dopamine concentration that induced the maximal increase in entry was used for analysis.
Figure 1. Dopamine increases HIV entry into primary human macrophages.
Primary human monocyte derived macrophages were infected with 3 concentrations of β-lac HIV (MOI of 0.002, 0.005, 0.01) in the presence of either 2×108 M, 2×107 M, 2×10M or 2×105 M dopamine, and control cells were infected with HIV alone. In panels A-C each circle or square represents infection of macrophages from a single donor. Dopamine significantly increased viral entry in macrophages infected with all concentrations of HIV (A, MOI 0.002, n = 11, ** p = 0.0002), 0.005 (B, n = 6, * p = 0.0313) or 0.01 (C, MOI 0.01, n = 16, *** p = 0.001) after 4 hr incubation. Panel D shows the percent increase in entry mediated by dopamine in infections with each concentration of HIV relative to control (MOI 0.002 vertical lines, MOI 0.005 diagonal lines, MOI 0.01, horizontal lines). Panels E - G show representative images of cultures infected with 0.01 MOI β-lac HIV in the presence of (E) dopamine, (F) HIV alone, (G) and uninfected macrophages. White arrows indicate infected cells.
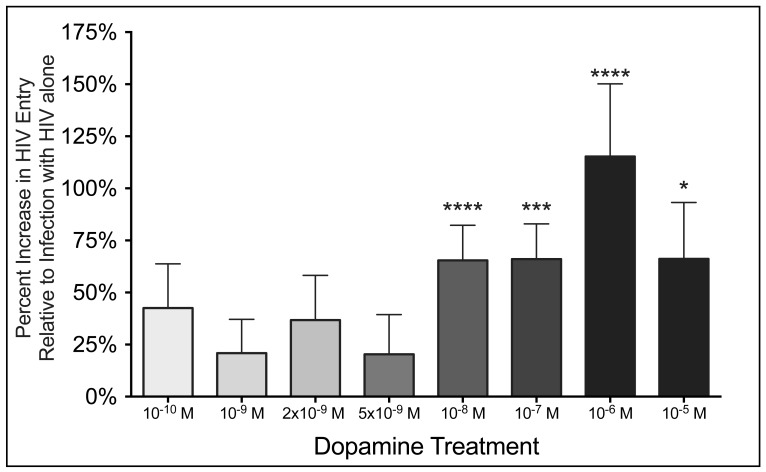
To characterize further the effects of dopamine on entry, β-lac HIV (MOI 0.01) was added to MDM for 2.5 hours in the presence of 8 different concentrations of dopamine, ranging from 1010 M to 105 M. Infection time was decreased to 2.5 hours to maximize the detection of the changes in entry, and MOI of 0.01 was used because this concentration provided the most consistent infection among donors. In experiments using cells derived from 32 different donors, addition of 0.01 MOI of β-lac HIV for 2.5 hours resulted in infection of 12.1 +/1.5% of MDM. The mean percentage of macrophages infected with HIV was significantly increased in the presence of the four highest concentrations of dopamine, 108, 107,106 and 105 M, (Figure 2, 108 M DA, 72% increase, n = 32, p < 0.0001 ****; 107 M DA, 73% increase, n = 21, p = 0.0005 ***; 106 M DA, 115% increase, n = 15, p < 0.0001 ****; 105 M DA, n = 13, p = 0.0171 *). Although a few donors showed increases in entry in response to concentrations of dopamine below 108 M dopamine, the mean amount of entry in MDM treated with less than 108 M dopamine was not significantly increased above the control infection with HIV alone (Figure 2, p > 0.05 for all conditions, 1010 M DA, n = 15; 109 M DA, n = 21; 2×109 M DA, n = 10; 5×109 M DA, n = 10). These data show that the dopamine-mediated increase in HIV entry into macrophages is dose dependent with a steep threshold, only occurring at concentrations of 108 M or greater of dopamine.
Figure 2. Increase in HIV entry requires a minimum threshold of dopamine.
MOI 0.01 β-lac HIV was added to macrophages in the presence of 1010, 109, 2×109, 5×109, 108, 107, 106 M and 105 M dopamine and control cells were infected with HIV alone. Relative to control infections, viral entry was significantly increased when MDM were infected with HIV in the presence of 108, 107, 106 M and 105 M dopamine (n = 1032, ** p < 0.01, *** p < 0.001 vs. HIV only) but not in the presence of 1010, 109, 2×109, or 5×109 M dopamine. There was no significant difference in the magnitude of the increase in entry among cultures infected the presence of 108, 107, 106 M and 105 M dopamine.
Dopamine-Mediated Increase in HIV Entry Requires CCR5
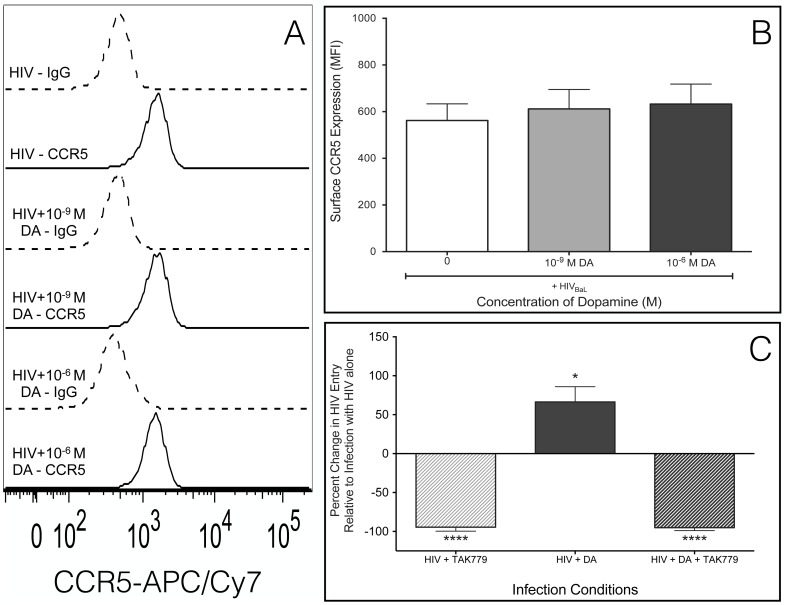
In macrophages, HIV entry is generally mediated by binding of gp120 to CD4 and CCR5 on the plasma membrane, which initiates fusion of the viral membrane with the cell membrane [39]. Expression of CCR5 correlates with macrophage susceptibility to HIV [46]–[48] and increased surface CCR5 can mediate increased HIV infection [49]–[51]. Therefore, dopamine-treated macrophages were evaluated for changes in surface CCR5 by flow cytometry. MDM were treated with dopamine for 2.5 hours in the presence or absence of HIV infection, and MDM not treated with dopamine were used as controls. Dopamine concentrations of 106 M and 109 M were selected as representative concentrations that did or did not increase HIV entry, respectively. Neither concentration of dopamine increased surface CCR5 in HIV-infected MDM (Figures 3A, 3B, n = 7) or in uninfected MDM, and HIV infection alone did not increase CCR5 expression (data not shown). These data indicate that dopamine does not increase HIV entry into macrophages by increasing surface CCR5. Macrophages were also examined by flow cytometry for dopamine-mediated changes in surface CD4. However, CD4 receptor expression on MDM was low and varied among donors, and we were not able to quantify reliably these data (data not shown).
Figure 3. CCR5 is necessary for dopamine-mediated increase in HIV entry.
Macrophages were infected with HIVBaL in the presence of 109 M or 106 M dopamine and analyzed by flow cytometry for CCR5. (A) A representative histogram of CCR5 surface expression in a single donor in response to HIV alone (IgG – dashed grey lines, CCR5 – solid grey lines) is shown. (B) The mean MFI of CCR5 on the surface of macrophages from seven donors infected with HIV alone (white), HIV + 109 M DA (light gray) or HIV + 106 M DA (dark gray) relative HIV alone. Neither concentration of dopamine-induced significant changes in the expression of this protein on the cell surface relative to the macrophages infected with HIV alone. (C) Macrophages were pretreated with 2×107 M TAK779, and then an MOI of 0.01 β-lac HIV was added in the presence or absence of 108 M dopamine. As controls, both macrophages pretreated with TAK779 and macrophages not pretreated were infected with HIV alone. Dopamine significantly increased HIV entry (n = 6, p = 0.0194 *), and pretreatment with 2×107 M of the CCR5 inhibitor TAK779 blocked HIV entry into macrophages infected in both the presence and absence of dopamine (HIV + TAK779, p = 0.0001 **, HIV + TAK779 + DA, p = 0.0003 ***).
In the CNS, where macrophages could be exposed to high concentrations of dopamine, R5-tropic viruses predominate [52]–[54]. However, in addition to CCR5-mediated entry, HIV can enter macrophages through alternative pathways such as the endocytic pathway or through interaction with the co-receptor CXCR4 or minor co-receptors including CCR3 [53], [55]–[60]. To determine whether dopamine increases viral entry by enabling HIV to use a CCR5-independent entry mechanism, we examined dopamine-mediated changes in entry in the presence of a CCR5 inhibitor. Macrophages were pretreated with the CCR5 inhibitor TAK779 (2 ×107 M) for 1 hour, then TAK779 treated and untreated MDM were infected with 0.01 MOI of β-lac HIV in the presence or absence of 108 M dopamine. Dopamine treatment significantly increased HIV entry relative to cells infected with HIV alone (Figure 3C, n = 6,108 M DA, 66.4% increase, p = 0.0194 *). Blocking CCR5 with TAK779 abrogated HIV entry in pretreated cultures infected with both HIV alone and with HIV + dopamine (Figure 3C, n = 6, HIV + TAK779, 94.5% decrease, p = 0.0001 **, HIV + TAK779 + DA, 95.5% decrease, p = 0.0003 ***). These data demonstrate that the dopamine-mediated increase in entry requires CCR5 and suggest that dopamine does not act through a CCR5-independent entry pathway.
Increase in HIV Entry is Specifically Mediated by Activation of Dopamine Receptors
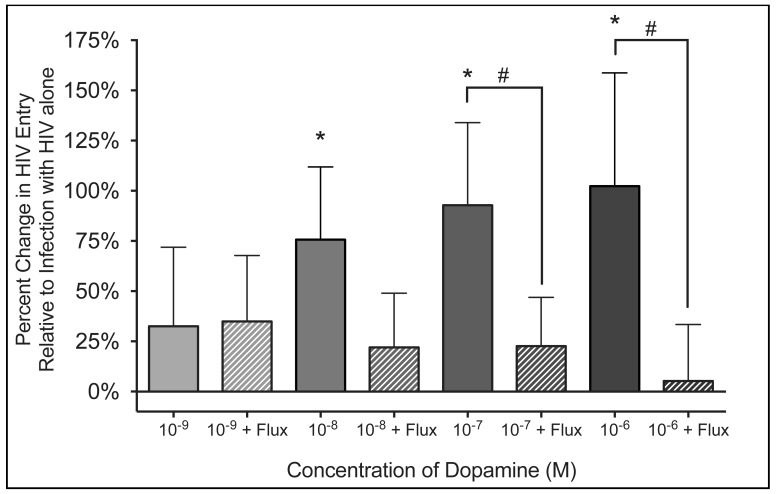
Studies in rodents show that dopamine can activate other types of receptors in addition to DR [61], [62]. To determine whether the dopamine-induced increase in HIV entry is specifically mediated by activation of DR, we examined entry in the presence of the pan-DR antagonist flupenthixol. This molecule binds to all DR with high affinity (Ki < 8×108 M for all DR, [63]–[65]), and should interfere with the binding of dopamine to these receptors.
Macrophages were infected with of β-lac HIV (0.01 MOI) and concurrently treated with 109 M, 108 M, 107 M and 106 M dopamine. Infections were performed in presence or absence of 106 M flupenthixol, added 30 minutes prior to the addition of β-lac HIV and dopamine. The presence of 108 M, 107 M and 106 M dopamine significantly increased viral entry (Figure 4, n = 7 for all concentrations of dopamine, 108 M DA 76% increase, p = 0.0156 *; 107 M DA 93% increase, p = 0.0469; 106 M DA, 102% increase, p = 0.0156 *). Pretreatment with flupenthixol abrogated this effect, significantly reducing HIV entry in the presence of 107 M (p = 0.0313 *) and 106 M dopamine (p = 0.0313 *). Fluxpenthixol did not significantly decrease the effect of 108 M dopamine, although infections in the presence of 108 M dopamine showed a large decrease in entry that trended toward significance (p = 0.0781). Flupenthixol treatment did not affect viral entry in MDM not treated with dopamine. These data demonstrate that activation of DR is necessary for the dopamine-mediated increase in HIV entry.
Figure 4. Activation of dopamine receptors required for the dopamine-mediated increase in HIV entry.
Macrophages were pretreated with the pan-dopamine receptor (DR) antagonist flupenthixol (flux, 106 M) and then MOI 0.01 β-lac HIV was added concurrently with either 109, 108, 107 and 106 M dopamine. As controls, both vehicle treated and flupenthixol treated cells were infected with HIV alone. Solid columns represent infections in the presence of dopamine, while hatched columns to the right of each solid column of the same color represent infections in presence of an identical concentration of dopamine as well as flupenthixol. There was a significant increase in viral entry into macrophages infected with HIV the presence of 108, 107 and 106 M dopamine (n = 7 for all concentrations of dopamine, * p < 0.05 vs. HIV only). This increase was blocked by flupenthixol treatment, and entry was significantly reduced in the presence of 107 and 106 M dopamine (n = 7, # p < 0.05 vs. identical concentration of dopamine without flupenthixol).
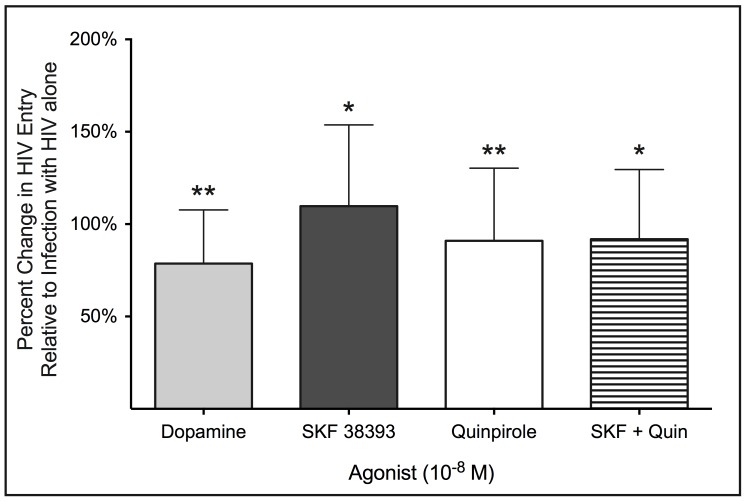
Our previous data showed that activation of D2-like DR increased viral replication [38], and more recent experiments demonstrated that D1-like DR activation did so as well (P.J. Gaskill and J.W. Berman, unpublished data). To determine the subtypes of DR that mediate the effects of dopamine on HIV entry, MDM were infected with 0.01 MOI of β-lac HIV in the presence of either dopamine, SKF 38393 (D1-like DR agonist), Quinpirole (D2-like DR agonist) or both SKF and Quinpirole together. Dopamine and all agonists were added at a concentration of 108 M. Dopamine, SKF 38393 and Quinpirole each significantly increased HIV entry into macrophages (Figure 5, n = 10 for all agonists, Dopamine, 79% increase, p = 0.0039 **; SKF 38393 110% increase, p = 0.0342 *; Quinpirole, 91% increase, p = 0.0098 **). Infection in the presence of agonists for both D1-like and D2-like DR together also significantly increased entry, but did not enhance the increase in entry beyond that seen for each agonist individually (Figure 5, n = 10, SKF 38393 + Quin, 92% increase, p = 0.0375 *). The magnitude of the increase in entry did not differ significantly among any of these DR ligands. These experiments demonstrate that the activation of either D1-like or D2-like receptors mediates increased HIV entry into macrophages. The lack of an additive or synergistic effect could indicate that the two types of DR act through a common signaling pathway to increase HIV entry.
Figure 5. Activation of both D1-like and D2-like dopamine receptors is capable of mediating increased HIV entry.
Macrophages were infected with β-lac HIV (MOI 0.01) in the presence of either 108 M dopamine (light gray), a D1-like DR agonist (108 M SKF 38393, dark gray), a D2-like DR agonist (108 M Quinpirole, white), both SKF 38393 and Quinpirole together (horizontal lines). Macrophages infected with HIV alone served as controls. Dopamine, SKF 38393, Quinpirole and SKF 383893 + Quinpirole all significantly increased viral entry relative to infections with HIV alone (n = 10, * p > 0.05, ** p > 0.01 vs. HIV alone).
Activation of Both D1-like and D2-like Dopamine Receptors Potentiates Calcium Mobilization Triggered by Prior Activation of Gαq-Coupled GPCR
That HIV entry is increased by both D1-like and D2-like DR suggests the possibility that they do so through a common signaling pathway. Although D1-like and D2-like receptors have opposite effects on cAMP production, they have both been reported to cause calcium mobilization. Calcium is important for HIV infection; it is induced by binding of gp120 to CCR5 on macrophages [66], [67], and calcium mobilization mediated by activation of the inositol triphosphate receptor (IP3R) in the endoplasmic reticulum is required for HIV entry [68]. Both subtypes of DR have been reported to mediate calcium release from the endoplasmic reticulum through a potentiation mechanism, D1-like DR through activation of protein kinase A [69], and D2-like DR through interaction with phospholipase C (PLC) and calcyon [70]. In this process, activation of DR evokes an increase in Ca2+ mobilization following an initial Ca2+ release mediated by activation of a Gαq-coupled receptor, such as CCR5. In addition, in cells heterologously expressing both D1R and D2R, coactivation of both receptor subtypes can initiate calcium release through Gβγ-mediated signaling [35]. Thus, a common pathway through which both D1-like and D2-like DR could mediate calcium flux is Gβγ activation of PLCβ, as activation of both subtypes of DR release Gβγ subunits. This would enable activation of both subtypes of DR to increase HIV entry by potentiating the Ca2+ mobilization required for the entry process. To determine whether both D1-like and D2-like DR could increase calcium mobilization through potentiation of Gαo signaling, we examined calcium flux in HEK293 cells stably transfected with D1R, D2R, or both dopamine receptors.
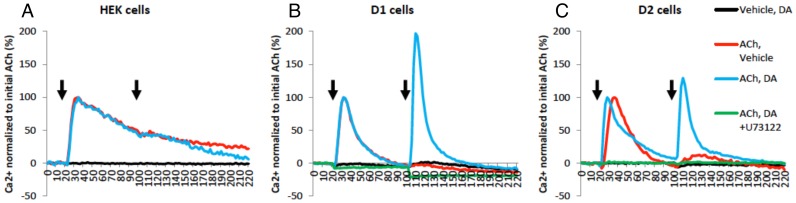
To determine whether DR activation in the absence of Gαo activation induced calcium flux, parental HEK cells or cells transfected with either D1R or D2R were treated with 105 M dopamine (Figure 6, black curves). These experiments showed that dopamine alone did not induce any calcium release. The Gαq-mediated calcium response was tested using 105 M acetylcholine (ACh) to stimulate endogenous Gαq-coupled muscarinic receptors (M3) expressed in HEK cells [71] (Figure 6, red curves). Treatment with acetylcholine resulted in a clear pattern of prolonged (> 60 secs to subside) internal calcium release, with kinetics consistent with IP3R-mediated calcium mobilization [72]. This ACh-induced calcium flux was specific to muscarinic receptor activation as it was blocked by the muscarinic receptor-specific antagonist pirenzepine (data not shown).
Figure 6. Dopamine receptors potentiate Gαq-receptor mediated calcium flux through phospholipase C.
Intracellular calcium levels were measured every 2 seconds for 220 seconds and plotted against time. Ca2+ level is shown in percentage normalized to the initial 105 M acetylcholine (ACh) added at 20 seconds. Vehicle (red curve), 105 M dopamine (DA, blue curve), or 105 M DA with 105 M U73122 (PLC inhibitor, green curve) was added at 100 seconds following 105 M ACh at 20 secs, indicated by arrows. As a negative control, vehicle followed by 105 M DA was also measured (black curve). Experiments were performed in parental (A) HEK cells, (B) D1R expressing cells, and (C) D2R expressing cells. Traces are representatives of n = 3 experiments.
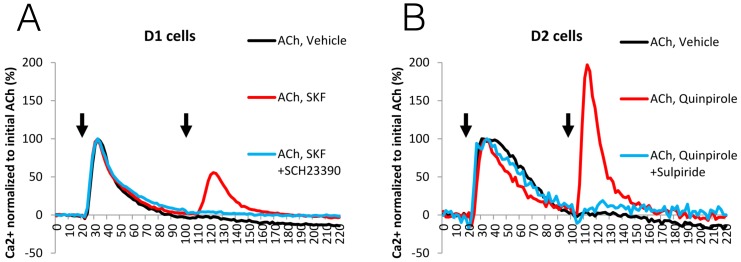
Once Gαq-mediated calcium release was defined, we determined whether 105 M dopamine, which was inactive alone, could potentiate the effect of 105 M ACh if added 80 seconds later. Treatment with dopamine following Gαq stimulation induced a second calcium response in cell lines expressing either D1R or D2R, but not in the control parental HEK cells (Figure 6, blue curves). In all of the cell lines, pretreatment with 105 M U73122, a potent inhibitor of PLC [73], abrogated the ACh-mediated calcium mobilization, as well as the subsequent dopamine-mediated potentiation effect. (Figure 6, green curves). To validate the involvement of D1R and D2R in calcium release, selective agonists as well as antagonists were used. Using HEK cells expressing either D1R or D2R, the potentiation of Gαq-mediated calcium release was triggered and blocked by their cognate agonists and antagonists (105 M SKF81297 and SCH23390 for D1R and 105 M Quinpirole and Sulpiride for D2R) respectively (Figure 7A, B). These results demonstrate the existence of specific D1-like and D2-like DR-dependent mechanisms for potentiation of Gαq-mediated calcium mobilization through PLC activation.
Figure 7. Potentiation of Gαq-mediated calcium flux is induced specifically by activation of D1-like and D2-like dopamine receptors.
Intracellular calcium levels were measured every 2 seconds and plotted against time. Ca2+ level is shown in percentage normalized to the initial 105 M acetylcholine (ACh) added at 20 secs. (A) In D1 stable cells, either vehicle control (black curve), 105 M SKF81297 (D1 agonist, red curve), or 105 M SKF81297 with 105 M SCH23390 (D1 antagonist, blue curve) was added at 100 secs following 105 M ACh at 20 secs, indicated by arrows. (B) In D2 stable cells, either vehicle control (black curve), 105 M Quinpirole (D2 agonist, red curve), or 105 M Quinpirole with 105 M Sulpiride (D2 antagonist, blue curve) was added at 100 secs following 105 M ACh at 20 secs, indicated by arrows. Traces are representatives of n = 3 experiments.
Discussion
Increases in CNS dopamine and damage to the dopaminergic system correlate with HIV-associated neuropathogenesis and HAND [31], [32], but the mechanisms underlying these correlations are not well understood. Prior to the use of combinatorial anti-retroviral therapy (cART), increased amounts of HIV DNA [74] and increased neuropathology were found in regions of the brain innervated by dopaminergic neurons, especially the basal ganglia [27], [28], [30], [75]. In SIV-infected macaques, treatment with Selegiline, L-DOPA or methamphetamine, all of which elevate CNS dopamine, increased viral replication in the CNS and exacerbated neuropathology [25], [26], [76]. These data indicate strong connection between the dopaminergic system and the development of neuropathology and HAND. Our laboratory showed that dopamine increases HIV infection in macrophages, and alters macrophage production of inflammatory cytokines [38], [77]. Although astrocytes can be infected with HIV [8], [11], [12], macrophages and other cells of the monocytic lineage are the primary target cells for the virus in the CNS [7]–[10]. This indicates that the impact of dopamine on HAND may be mediated, at least in part, by its effects on macrophage infection [78]. Thus, defining the mechanism by which dopamine increases HIV infection of these cells is important to understanding the effects of drug abuse on the development HAND.
This study demonstrates that dopamine significantly increases the entry of HIV into primary human macrophages across a range of concentrations of the infecting virus. This effect showed a very steep concentration dependence such that at and above 108 M dopamine the increase in entry was of similar magnitude. The mechanism(s) underlying this steep dose dependence is unclear. The concentration of dopamine that CNS macrophages may be exposed to in the human brain is difficult to determine, but based on studies in animal models, basal dopamine levels in the CNS are estimated to be in the low nanomolar range, although some studies suggest they may be higher, particularly during active neurotransmission [79]–[82]. Dopamine concentrations also vary regionally within the CNS [83], [84], and the amount of dopamine to which macrophages could be exposed depends on the area of the brain in which they reside and the type of stimulation initiating the dopamine release [85]–[87].
The use of illicit drugs, alcohol, and certain therapeutic agents significantly increases dopamine [88]–[95]. The greatest elevation of dopamine concentrations occur within the basal ganglia, specifically in the striatum and nucleus accumbens, as well as in the ventral tegmental area and substantia nigra, which contain the dopaminergic cell bodies [87], [96]. Dopaminergic communication is mediated by volume transmission, causing dopamine to suffuse the tissue and extracellular space surrounding the releasing neurons [97]–[99]. Depending on the region, neurotransmission can evoke dopamine concentrations above 108 M at substantial distance from the synapse at which the dopamine was released. Using animal models, this distance is estimated to be 7–20 µm in the striatum and 6–10 µm in the nucleus accumbens [80], [97], [99]–[102]. Thus, CNS macrophages in these regions could encounter dopamine as it effluxes from the synapse or when it is released extrasynaptically [80], [85], [87], [103], [104]. Use of drugs that increase CNS dopamine concentrations significantly expand the radius of dopamine diffusion [85]. Thus, in the CNS of drug abusers, the increased volume of tissue containing higher concentrations of dopamine would expose greater numbers of macrophages to dopamine as it diffuses into a larger area surrounding dopaminergic neurons affected by drugs of abuse.
The concentration of dopamine released in response to different drugs depends on the mechanism of action of the drug [94], [105], the age of the user [106], and the nature of the drug use, as there are large differences in dopamine response between chronic drug abusers and intermittent or naive users [107]–[109]. Some studies show that chronic drug use decreases drug-induced dopamine release [91], [107], [110], [111], suggesting that macrophages in the CNS of chronic drug users are less likely to be exposed to dopamine levels high enough to increase viral entry. However, acute and intermittent drug use could expose CNS macrophages to dopamine concentrations greater than the threshold at which dopamine increases HIV entry. Overall, these data suggest elevation of CNS dopamine may be a common mechanism by which different types of drugs increase HIV infection of macrophages and thereby contribute to neuropathogenesis and the development of HAND.
HIV infection is initiated by the entry of the virus into the target cell. In macrophages, viral entry is mediated by the interaction of the HIV envelope protein, gp120, with CD4 and a chemokine receptor, generally CCR5 [39]. The majority of viruses in the CNS, where macrophages would encounter the higher concentrations of dopamine induced by drug abuse, use CCR5 [53], [54], [112], [113], although it has been suggested that X4 viruses may infect macrophages in the later stages of HIV neuropathogenesis [52]. Increases in surface CCR5 can increase HIV infection [49], [50], [114], but neither 109 M nor 106 M dopamine significantly altered surface expression of CCR5. These results indicate that dopamine does not mediate its effects on entry by increasing the expression of CCR5, and suggest the possibility that dopamine may increase entry into macrophages through a CCR5-independent entry pathway, such as use of alternate co-receptors or endocytosis [55]–[58]. However, treatment with the CCR5 inhibitor TAK779 abrogated viral entry in both the presence and absence of dopamine.
These results indicate that the increase in entry is not mediated through a CCR5-independent entry pathway, as increased entry by means of this alternative pathway would not have been blocked by inhibition of CCR5. Thus, dopamine may induce other changes that enhance the entry process. Macrophage-tropic HIV from the CNS can exhibit conformational changes in gp120 that enable these viruses to infect more efficiently cells with low surface CD4 [53], [113], [115], such as macrophages and microglia [116], [117]. Increases in surface CD4 also increase viral replication [49], [114]. Therefore, a dopamine-mediated increase in this receptor could increase HIV entry. We examined CD4 expression on MDM and found that surface CD4 was low and varied in different donors. While we were unable to quantify these data reliably, they did suggest that dopamine does not increase surface CD4.
Another possibility is that dopamine-mediated changes in CCR5 could increase viral entry. CCR5 is present on the cell surface in multiple conformational states [118], and studies show different conformations of this receptor increase the binding affinity or accessibility of CCR5 to HIV, changing the efficiency of entry or fusion [119]–[121]. In macrophage-tropic CNS viruses, changes in the interaction of gp120 with the 1st and 2nd extracellular loop regions and the N-Terminus increased the efficiency of HIV infection [120]. Thus, dopamine-mediated changes in the expression or abundance of specific conformations of CCR5 on the macrophage surface could be responsible for the increase in HIV entry. Our study used a single virus strain derived from the lung, HIVBaL, so it is unclear whether dopamine-mediated changes in the conformation CCR5 could affect the entry of other strains of HIV. However, we found previously that dopamine increases the replication of HIVADA and HIVYU2 [38], viruses that were derived from the blood and brain, respectively [122], [123], suggesting that dopamine-mediated changes in CCR5 could increase HIV entry of R5 viruses derived from many different compartments.
In addition to CCR5, the dopamine-mediated increase in HIV entry requires DR activation and is mediated by D1-like and D2-like DR, suggesting a signaling pathway common to both subtypes of DR. The major pathway activated by DR is the modulation of cAMP, D1-like DR activating adenylyl cyclase through Gαs/olf, and D2-like DR inhibiting it through Gαi/o [33], [34]. However, D1-like and D2-like dopamine receptors have opposing effects on the regulation of cAMP activity, making it less likely that this pathway is mediating the change in HIV entry. One signaling mechanism common to both D1-like and D2-like dopamine receptors is calcium mobilization, although it is unclear if calcium flux occurs through monomeric DR or heteromeric D1R–D2R complexes [35], [124]. The interaction of gp120 with CCR5 induces calcium mobilization [66], [67], [125], [126], and CCR5 activation is mediated by Gαq [40]. In the U87 astrocyte cell line, calcium release induced by Gαq-mediated activation of PLC was required for successful infection [68], suggesting that a dopamine-mediated increase in calcium may increase HIV entry.
The primary pathway by which DR mediate increased calcium release from the endoplasmic reticulum is a “potentiation” mechanism. In this mechanism, stimulation of a Gαq-coupled receptor, such as CCR5, initiates calcium release and then calcium flux is potentiated through a second pathway. Studies show that D1R-induced Gαs- and PKA-mediated activation of IP3R [69] or D2R-induced PLC- and calcyon-mediated activation of IP3R [70] can potentiate Gαq-mediated calcium release. Thus, it is possible that both D1-like and D2-like DR increase entry through calcium release mediated by distinct pathways, as both of these mechanisms were shown to be specific to one DR subtype. However, the data show no additive or synergistic effect on entry resulting from activation of both D1-like and D2-like DR, and if the two types of DR were acting on distinct signaling pathways, the effects on entry might be more than for either pathway alone as both pathways acted to increase entry. This suggests that the DR induced effects on HIV entry may be mediated by a common signaling pathway.
One possible common mechanism for mediating calcium release through activation of both D1-like and D2-like DR is Gβγ subunit-mediated PLCβ activation [127], which could be initiated by both subtypes of DR. Using our transfected HEK293 cells, we demonstrated that both D1R and D2R potentiate calcium mobilization initiated by Gαq activation, and that these effects were specifically mediated by activation of PLC. Thus, activation of either D1-like or D2-like DR could potentiate the Gαq-mediated calcium flux initiated by gp120-CCR5 binding, and the resulting increase in calcium could increase HIV entry. Although this mechanism addresses the involvement of both dopamine receptor subtypes and CCR5 in the increase in HIV entry, DR signaling in primary human macrophages is poorly defined and entry could also be increased by a separate effect of DR activation. For example, DR have been reported to form oligomers with other GPCRs that alter receptor pharmacology, trafficking and signaling [128]–[132]. If DR-CCR5 heteromers were formed, this could alter the conformation or trafficking of CCR5 to enhance its ability to bind viral particles or increase the efficiency of the membrane fusion process. Thus, there are several possible mechanisms by which these receptors could mediate the increase in HIV entry. Regardless of these possibilities, our data demonstrate that dopamine alters a stage of the replication cycle between viral attachment and uncoating, the beginning and end of the entry process, respectively [133]. Studies are ongoing examining dopaminergic changes in the expression and function of host and viral proteins involved in the entry process.
Dopamine-mediated increases in HIV infection of macrophages could have a substantial impact on early HIV infection in the CNS, where most viruses are R5-tropic [112]. During acute infection, before most individuals know they are infected and/or begin therapy, infection of a greater number of CNS macrophages could increase the amount of HIV in the CNS, accelerating both the spread of infection and the developing neuroinflammation present early after CNS invasion [134], [135]. This possibility is supported by a recent study showing that methamphetamine is associated with increased neurocognitive impairment in early stage HIV infection [136]. A high percentage of HIV-positive individuals are drug users [137]–[140] and often have poor adherence to medication [141]–[145]. Therefore, an increase in viral entry into macrophages could also be significant during the course of infection with each subsequent use of a drug during a medication hiatus.
The adverse effects of dopamine on HIV replication are not limited to HIV-infected drug abusers but could also impact individuals prescribed legal therapeutics that alter dopamine levels. These drugs include L-DOPA or direct acting DR agonists for Parkinson’s disease [146], Ritalin [24], and antidepressants and psychiatric drugs such as Bupropion, a norepinephrine-dopamine reuptake inhibitor [23] or Abilify, a partial D2-like DR agonist [147]. There is a high prevalence of psychiatric disorders among HIV infected people [148]–[152]. These individuals are more likely to be prescribed drugs that modulate the dopaminergic system and thereby might increase the progression of HIV infection in the CNS.
In addition to drug-induced increases in dopamine, numerous studies have found that HIV infection by itself can alter the CNS dopaminergic system. Some studies show dopamine and dopamine metabolites are reduced in postmortem brains from HIV infected individuals [153]–[155] and in the CSF of HIV+ individuals with late stage disease [156]–[159]. However, another study showed increased CNS dopamine in the CSF of therapy-naive, HIV-infected people in early stage disease [160], [161]. Postmortem data from individuals with HIV encephalitis also suggested increased dopaminergic tone in the striatum [162]. Additionally, some studies show CNS hypermetabolism in subcortical regions such as the basal ganglia in earlier stages of infection, and subcortical hypometabolism in later stage disease [163]–[166]. Thus, HIV infection may have different effects on CNS metabolism at different stages of disease, and may potentially increase CNS dopamine in subcortical structures in early stage disease before reducing CNS dopamine during more advanced infection. A study examining changes in cerebral metabolism in drug abusers showed that intravenous drug use may have a synergistic effect with HIV on cerebral metabolism, increasing subcortical hypermetabolism and inducing the premature emergence of cortical hypometabolism relative to HIV infected non-drug users [166]. Thus, use of drugs of abuse or other substances that increase CNS dopamine during early stage infection could increase the already elevated levels of dopamine that may occur in early HIV infection of the CNS, increasing the number of macrophages exposed to higher levels of dopamine. Conversely, in late stage disease, HIV+ individuals may be less susceptible to a dopamine-mediated increase in macrophage infection due to the lower dopaminergic tone in the CNS.
The data in this study show that the number of macrophages infected with HIV is greater in the presence of increased dopamine. Elevated dopamine may significantly impact the onset and initial progression of HIV CNS infection by enabling the virus to enter more macrophages early in infection. This would increase the amount of virus in the CNS, contributing to the development of inflammation and the formation of viral reservoirs. Thus, increases in CNS dopamine may be a common mechanism by which different drugs of abuse modulate the development of HAND. Overall, these data contribute to our understanding of HIV infection of the CNS and the development of HAND in HIV-infected drug abusers and may help to guide the use of therapeutic interventions in HIV-infected drug abusers.
Acknowledgments
We would like to thank all of the members of Dr. Joan W. Berman’s laboratory at Einstein, especially Dr. Tina Calderon, for important discussions, and Dr. Jennifer Cano Granit, a former student in the Kalpana Laboratory, for help with the optimization of the entry assay. We would also like to thank the Flow Cytometry Facility, especially Lydia Tesfa, and the Einstein Center For AIDS Research (AI-051519) for their assistance, especially Xuhong Wu and the CFAR Virology Core, for invaluable assistance generating the viral stocks and developing the entry assay.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All data are included within the paper.
Funding Statement
These studies were funded by grants from the National Institutes of Drug Abuse, DA029476 (PJG), K05DA022413 (JAJ) and DA025567 (JWB), the National Institutes of Allergy and Infectious Disease, AI095171-01 (GVK) and the National Institutes of Mental Health, R01 MH54137 (JAJ), MH090958 (JWB) and MH075679 (JWB). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Valcour V, Chalermchai T, Sailasuta N, Marovich M, Lerdlum S, et al. (2012) Central Nervous System Viral Invasion and Inflammation During Acute HIV Infection. J Infect Dis 206: 275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cysique LA, Maruff P, Brew BJ (2004) Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: a combined study of two cohorts. J Neurovirol 10: 350–357. [DOI] [PubMed] [Google Scholar]
- 3. Heaton RK, Clifford DB, Franklin DR Jr, Woods SP, Ake C, et al. (2010) HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 75: 2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, et al. (2011) HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 17: 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Simioni S, Cavassini M, Annoni JM, Rimbault Abraham A, Bourquin I, et al. (2010) Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS 24: 1243–1250. [DOI] [PubMed] [Google Scholar]
- 6. Tozzi V, Balestra P, Serraino D, Bellagamba R, Corpolongo A, et al. (2005) Neurocognitive impairment and survival in a cohort of HIV-infected patients treated with HAART. AIDS Res Hum Retroviruses 21: 706–713. [DOI] [PubMed] [Google Scholar]
- 7. Koenig S, Gendelman HE, Orenstein JM, Dal Canto MC, Pezeshkpour GH, et al. (1986) Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science 233: 1089–1093. [DOI] [PubMed] [Google Scholar]
- 8. Takahashi K, Wesselingh SL, Griffin DE, McArthur JC, Johnson RT, et al. (1996) Localization of HIV-1 in human brain using polymerase chain reaction/in situ hybridization and immunocytochemistry. Ann Neurol 39: 705–711. [DOI] [PubMed] [Google Scholar]
- 9. Williams KC, Corey S, Westmoreland SV, Pauley D, Knight H, et al. (2001) Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J Exp Med 193: 905–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams DW, Veenstra M, Gaskill PJ, Morgello S, Calderon TM, et al. (2014) Monocytes Mediate HIV Neuropathogenesis: Mechanisms that Contribute to HIV Associated Neurocognitive Disorders. Curr HIV Res. [DOI] [PMC free article] [PubMed]
- 11. Eugenin EA, Berman JW (2007) Gap junctions mediate human immunodeficiency virus-bystander killing in astrocytes. J Neurosci 27: 12844–12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Churchill MJ, Wesselingh SL, Cowley D, Pardo CA, McArthur JC, et al. (2009) Extensive astrocyte infection is prominent in human immunodeficiency virus-associated dementia. Ann Neurol 66: 253–258. [DOI] [PubMed] [Google Scholar]
- 13. Koppensteiner H, Brack-Werner R, Schindler M (2012) Macrophages and their relevance in Human Immunodeficiency Virus Type I infection. Retrovirology 9: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yadav A, Collman RG (2009) CNS inflammation and macrophage/microglial biology associated with HIV-1 infection. J Neuroimmune Pharmacol 4: 430–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hult B, Chana G, Masliah E, Everall I (2008) Neurobiology of HIV. Int Rev Psychiatry 20: 3–13. [DOI] [PubMed] [Google Scholar]
- 16. Williams KC, Hickey WF (2002) Central nervous system damage, monocytes and macrophages, and neurological disorders in AIDS. Annu Rev Neurosci 25: 537–562. [DOI] [PubMed] [Google Scholar]
- 17. Churchill M, Nath A (2013) Where does HIV hide? A focus on the central nervous system. Curr Opin HIV AIDS 8: 165–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thompson KA, Cherry CL, Bell JE, McLean CA (2011) Brain cell reservoirs of latent virus in presymptomatic HIV-infected individuals. Am J Pathol 179: 1623–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brabers NA, Nottet HS (2006) Role of the pro-inflammatory cytokines TNF-alpha and IL-1beta in HIV-associated dementia. Eur J Clin Invest 36: 447–458. [DOI] [PubMed] [Google Scholar]
- 20. Kraft-Terry SD, Buch SJ, Fox HS, Gendelman HE (2009) A coat of many colors: neuroimmune crosstalk in human immunodeficiency virus infection. Neuron 64: 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carboni E, Imperato A, Perezzani L, Di Chiara G (1989) Amphetamine, cocaine, phencyclidine and nomifensine increase extracellular dopamine concentrations preferentially in the nucleus accumbens of freely moving rats. Neuroscience 28: 653–661. [DOI] [PubMed] [Google Scholar]
- 22.Olive MF, Taylor Lewis (2013) The neurocircuitry of illicit psychostimulant addiction: acute and chronic effects in humans. Substance Abuse and Rehabilitation: 29. [DOI] [PMC free article] [PubMed]
- 23. Papakostas GI (2006) Dopaminergic-based pharmacotherapies for depression. Eur Neuropsychopharmacol 16: 391–402. [DOI] [PubMed] [Google Scholar]
- 24. Volkow ND, Wang G, Fowler JS, Logan J, Gerasimov M, et al. (2001) Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci 21: RC121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Czub S, Koutsilieri E, Sopper S, Czub M, Stahl-Hennig C, et al. (2001) Enhancement of central nervous system pathology in early simian immunodeficiency virus infection by dopaminergic drugs. Acta Neuropathol 101: 85–91. [DOI] [PubMed] [Google Scholar]
- 26. Czub S, Czub M, Koutsilieri E, Sopper S, Villinger F, et al. (2004) Modulation of simian immunodeficiency virus neuropathology by dopaminergic drugs. Acta Neuropathol 107: 216–226. [DOI] [PubMed] [Google Scholar]
- 27. Aylward EH, Brettschneider PD, McArthur JC, Harris GJ, Schlaepfer TE, et al. (1995) Magnetic resonance imaging measurement of gray matter volume reductions in HIV dementia. Am J Psychiatry 152: 987–994. [DOI] [PubMed] [Google Scholar]
- 28. Aylward EH, Henderer JD, McArthur JC, Brettschneider PD, Harris GJ, et al. (1993) Reduced basal ganglia volume in HIV-1-associated dementia: results from quantitative neuroimaging. Neurology 43: 2099–2104. [DOI] [PubMed] [Google Scholar]
- 30. Reyes MG, Faraldi F, Senseng CS, Flowers C, Fariello R (1991) Nigral degeneration in acquired immune deficiency syndrome (AIDS). Acta Neuropathol 82: 39–44. [DOI] [PubMed] [Google Scholar]
- 31. Berger JR, Arendt G (2000) HIV dementia: the role of the basal ganglia and dopaminergic systems. J Psychopharmacol 14: 214–221. [DOI] [PubMed] [Google Scholar]
- 32. Purohit V, Rapaka R, Shurtleff D (2011) Drugs of abuse, dopamine, and HIV-associated neurocognitive disorders/HIV-associated dementia. Mol Neurobiol 44: 102–110. [DOI] [PubMed] [Google Scholar]
- 33. Missale C, Nash SR, Robinson SW, Jaber M, Caron MG (1998) Dopamine receptors: from structure to function. Physiol Rev 78: 189–225. [DOI] [PubMed] [Google Scholar]
- 34. Beaulieu JM, Gainetdinov RR (2011) The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 63: 182–217. [DOI] [PubMed] [Google Scholar]
- 35. Chun LS, Free RB, Doyle TB, Huang XP, Rankin ML, et al. (2013) D1-D2 dopamine receptor synergy promotes calcium signaling via multiple mechanisms. Mol Pharmacol 84: 190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hasbi A, Fan T, Alijaniaram M, Nguyen T, Perreault ML, et al. (2009) Calcium signaling cascade links dopamine D1-D2 receptor heteromer to striatal BDNF production and neuronal growth. Proc Natl Acad Sci U S A 106: 21377–21382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rashid AJ, So CH, Kong MM, Furtak T, El-Ghundi M, et al. (2007) D1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc Natl Acad Sci U S A 104: 654–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gaskill PJ, Calderon TM, Luers AJ, Eugenin EA, Javitch JA, et al. (2009) Human immunodeficiency virus (HIV) infection of human macrophages is increased by dopamine: a bridge between HIV-associated neurologic disorders and drug abuse. Am J Pathol 175: 1148–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Berger EA, Murphy PM, Farber JM (1999) Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol 17: 657–700. [DOI] [PubMed] [Google Scholar]
- 40. Arai H, Charo IF (1996) Differential regulation of G-protein-mediated signaling by chemokine receptors. J Biol Chem 271: 21814–21819. [DOI] [PubMed] [Google Scholar]
- 41. Baba M, Nishimura O, Kanzaki N, Okamoto M, Sawada H, et al. (1999) A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc Natl Acad Sci U S A 96: 5698–5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tobiume M, Lineberger JE, Lundquist CA, Miller MD, Aiken C (2003) Nef does not affect the efficiency of human immunodeficiency virus type 1 fusion with target cells. J Virol 77: 10645–10650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Morner A, Bjorndal A, Albert J, Kewalramani VN, Littman DR, et al. (1999) Primary human immunodeficiency virus type 2 (HIV-2) isolates, like HIV-1 isolates, frequently use CCR5 but show promiscuity in coreceptor usage. J Virol 73: 2343–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hazleton JE, Berman JW, Eugenin EA (2012) Purinergic receptors are required for HIV-1 infection of primary human macrophages. J Immunol 188: 4488–4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cavrois M, De Noronha C, Greene WC (2002) A sensitive and specific enzyme-based assay detecting HIV-1 virion fusion in primary T lymphocytes. Nat Biotechnol 20: 1151–1154. [DOI] [PubMed] [Google Scholar]
- 46. Li W, Yu M, Bai L, Bu D, Xu X (2006) Downregulation of CCR5 expression on cells by recombinant adenovirus containing antisense CCR5, a possible measure to prevent HIV-1 from entering target cells. J Acquir Immune Defic Syndr 43: 516–522. [DOI] [PubMed] [Google Scholar]
- 47. Naif HM, Li S, Ho-Shon M, Mathijs JM, Williamson P, et al. (1997) The state of maturation of monocytes into macrophages determines the effects of IL-4 and IL-13 on HIV replication. J Immunol 158: 501–511. [PubMed] [Google Scholar]
- 48. Tuttle DL, Harrison JK, Anders C, Sleasman JW, Goodenow MM (1998) Expression of CCR5 increases during monocyte differentiation and directly mediates macrophage susceptibility to infection by human immunodeficiency virus type 1. J Virol 72: 4962–4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang J, Crawford K, Yuan M, Wang H, Gorry PR, et al. (2002) Regulation of CC chemokine receptor 5 and CD4 expression and human immunodeficiency virus type 1 replication in human macrophages and microglia by T helper type 2 cytokines. J Infect Dis 185: 885–897. [DOI] [PubMed] [Google Scholar]
- 50. Sozzani S, Ghezzi S, Iannolo G, Luini W, Borsatti A, et al. (1998) Interleukin 10 increases CCR5 expression and HIV infection in human monocytes. J Exp Med 187: 439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sartori E, Calistri A, Salata C, Del Vecchio C, Palu G, et al. (2011) Herpes simplex virus type 2 infection increases human immunodeficiency virus type 1 entry into human primary macrophages. Virol J 8: 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gabuzda D, Wang J (1999) Chemokine receptors and virus entry in the central nervous system. J Neurovirol 5: 643–658. [DOI] [PubMed] [Google Scholar]
- 53. Peters PJ, Bhattacharya J, Hibbitts S, Dittmar MT, Simmons G, et al. (2004) Biological analysis of human immunodeficiency virus type 1 R5 envelopes amplified from brain and lymph node tissues of AIDS patients with neuropathology reveals two distinct tropism phenotypes and identifies envelopes in the brain that confer an enhanced tropism and fusigenicity for macrophages. J Virol 78: 6915–6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schnell G, Joseph S, Spudich S, Price RW, Swanstrom R (2011) HIV-1 replication in the central nervous system occurs in two distinct cell types. PLoS Pathog 7: e1002286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, et al. (1996) The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell 85: 1135–1148. [DOI] [PubMed] [Google Scholar]
- 56. Jiang C, Parrish NF, Wilen CB, Li H, Chen Y, et al. (2011) Primary infection by a human immunodeficiency virus with atypical coreceptor tropism. J Virol 85: 10669–10681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Miyauchi K, Kim Y, Latinovic O, Morozov V, Melikyan GB (2009) HIV enters cells via endocytosis and dynamin-dependent fusion with endosomes. Cell 137: 433–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rana S, Besson G, Cook DG, Rucker J, Smyth RJ, et al. (1997) Role of CCR5 in infection of primary macrophages and lymphocytes by macrophage-tropic strains of human immunodeficiency virus: resistance to patient-derived and prototype isolates resulting from the delta ccr5 mutation. J Virol 71: 3219–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Verani A, Pesenti E, Polo S, Tresoldi E, Scarlatti G, et al. (1998) CXCR4 is a functional coreceptor for infection of human macrophages by CXCR4-dependent primary HIV-1 isolates. J Immunol 161: 2084–2088. [PubMed] [Google Scholar]
- 60. Gorry PR, Bristol G, Zack JA, Ritola K, Swanstrom R, et al. (2001) Macrophage tropism of human immunodeficiency virus type 1 isolates from brain and lymphoid tissues predicts neurotropism independent of coreceptor specificity. J Virol 75: 10073–10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hasko G, Szabo C, Nemeth ZH, Deitch EA (2002) Dopamine suppresses IL-12 p40 production by lipopolysaccharide-stimulated macrophages via a beta-adrenoceptor-mediated mechanism. J Neuroimmunol 122: 34–39. [DOI] [PubMed] [Google Scholar]
- 62. Lin Y, Quartermain D, Dunn AJ, Weinshenker D, Stone EA (2008) Possible dopaminergic stimulation of locus coeruleus alpha1-adrenoceptors involved in behavioral activation. Synapse 62: 516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Seeman P, Corbett R, Van Tol HH (1998) Dopamine D4 receptors may alleviate antipsychotic-induced parkinsonism. Adv Pharmacol 42: 478–482. [DOI] [PubMed] [Google Scholar]
- 64. Sunahara RK, Guan HC, O’Dowd BF, Seeman P, Laurier LG, et al. (1991) Cloning of the gene for a human dopamine D5 receptor with higher affinity for dopamine than D1. Nature 350: 614–619. [DOI] [PubMed] [Google Scholar]
- 65. Toll L, Berzetei-Gurske IP, Polgar WE, Brandt SR, Adapa ID, et al. (1998) Standard binding and functional assays related to medications development division testing for potential cocaine and opiate narcotic treatment medications. NIDA Res Monogr 178: 440–466. [PubMed] [Google Scholar]
- 66. Arthos J, Rubbert A, Rabin RL, Cicala C, Machado E, et al. (2000) CCR5 signal transduction in macrophages by human immunodeficiency virus and simian immunodeficiency virus envelopes. J Virol 74: 6418–6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lee C, Liu QH, Tomkowicz B, Yi Y, Freedman BD, et al. (2003) Macrophage activation through CCR5- and CXCR4-mediated gp120-elicited signaling pathways. J Leukoc Biol 74: 676–682. [DOI] [PubMed] [Google Scholar]
- 68. Harmon B, Ratner L (2008) Induction of the G q Signaling Cascade by the Human Immunodeficiency Virus Envelope Is Required for Virus Entry. Journal of Virology 82: 9191–9205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dai R, Ali MK, Lezcano N, Bergson C (2008) A crucial role for cAMP and protein kinase A in D1 dopamine receptor regulated intracellular calcium transients. Neurosignals 16: 112–123. [DOI] [PubMed] [Google Scholar]
- 70. Fregeau MO, Carrier M, Guillemette G (2013) Mechanism of dopamine D2 receptor-induced Ca(2+) release in PC-12 cells. Cell Signal 25: 2871–2877. [DOI] [PubMed] [Google Scholar]
- 71. Atwood BK, Lopez J, Wager-Miller J, Mackie K, Straiker A (2011) Expression of G protein-coupled receptors and related proteins in HEK293, AtT20, BV2, and N18 cell lines as revealed by microarray analysis. BMC Genomics 12: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Berridge MJ, Irvine RF (1989) Inositol phosphates and cell signalling. Nature 341: 197–205. [DOI] [PubMed] [Google Scholar]
- 73. Bleasdale JE, Thakur NR, Gremban RS, Bundy GL, Fitzpatrick FA, et al. (1990) Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophils. J Pharmacol Exp Ther 255: 756–768. [PubMed] [Google Scholar]
- 74. Fujimura RK, Goodkin K, Petito CK, Douyon R, Feaster DJ, et al. (1997) HIV-1 proviral DNA load across neuroanatomic regions of individuals with evidence for HIV-1-associated dementia. J Acquir Immune Defic Syndr Hum Retrovirol 16: 146–152. [DOI] [PubMed] [Google Scholar]
- 75. Itoh K, Mehraein P, Weis S (2000) Neuronal damage of the substantia nigra in HIV-1 infected brains. Acta Neuropathol 99: 376–384. [DOI] [PubMed] [Google Scholar]
- 76. Marcondes MC, Flynn C, Watry DD, Zandonatti M, Fox HS (2010) Methamphetamine increases brain viral load and activates natural killer cells in simian immunodeficiency virus-infected monkeys. Am J Pathol 177: 355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gaskill PJ, Carvallo L, Eugenin EA, Berman JW (2012) Characterization and function of the human macrophage dopaminergic system: implications for CNS disease and drug abuse. J Neuroinflammation 9: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gaskill PJ, Calderon TM, Coley JS, Berman JW (2013) Drug Induced Increases in CNS Dopamine Alter Monocyte, Macrophage and T Cell Functions: Implications for HAND. J Neuroimmune Pharmacol. [DOI] [PMC free article] [PubMed]
- 79. Wightman RM, Robinson DL (2002) Transient changes in mesolimbic dopamine and their association with ‘reward’. J Neurochem 82: 721–735. [DOI] [PubMed] [Google Scholar]
- 80. Garris PA, Ciolkowski EL, Pastore P, Wightman RM (1994) Efflux of dopamine from the synaptic cleft in the nucleus accumbens of the rat brain. J Neurosci 14: 6084–6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Garris PA, Wightman RM (1994) In vivo voltammetric measurement of evoked extracellular dopamine in the rat basolateral amygdaloid nucleus. J Physiol 478 ( Pt 2): 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Spuhler IA, Hauri A (2013) Decoding the dopamine signal in macaque prefrontal cortex: a simulation study using the Cx3Dp simulator. PLoS One 8: e71615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wightman PAGRM (1995) Voltammetric Methods in Brain Systems. Neuromethods. Totowa, New Jersey: Humana Press. 349.
- 84. Girault JA, Greengard P (2004) The neurobiology of dopamine signaling. Arch Neurol 61: 641–644. [DOI] [PubMed] [Google Scholar]
- 85. Venton BJ, Zhang H, Garris PA, Phillips PEM, Sulzer D, et al. (2003) Real-time decoding of dopamine concentration changes in the caudate-putamen during tonic and phasic firing. Journal of Neurochemistry 87: 1284–1295. [DOI] [PubMed] [Google Scholar]
- 86. Ford CP, Gantz SC, Phillips PE, Williams JT (2010) Control of extracellular dopamine at dendrite and axon terminals. J Neurosci 30: 6975–6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Rice ME, Patel JC, Cragg SJ (2011) Dopamine release in the basal ganglia. Neuroscience 198: 112–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Di Chiara G, Imperato A (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A 85: 5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Koob GF (1992) Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci 13: 177–184. [DOI] [PubMed] [Google Scholar]
- 90. Urban NB, Kegeles LS, Slifstein M, Xu X, Martinez D, et al. (2010) Sex differences in striatal dopamine release in young adults after oral alcohol challenge: a positron emission tomography imaging study with [(1)(1)C]raclopride. Biol Psychiatry 68: 689–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Urban NB, Slifstein M, Thompson JL, Xu X, Girgis RR, et al. (2012) Dopamine release in chronic cannabis users: a [11c]raclopride positron emission tomography study. Biol Psychiatry 71: 677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Xi ZX, Kleitz HK, Deng X, Ladenheim B, Peng XQ, et al. (2009) A single high dose of methamphetamine increases cocaine self-administration by depletion of striatal dopamine in rats. Neuroscience 161: 392–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zachek MK, Takmakov P, Park J, Wightman RM, McCarty GS (2010) Simultaneous monitoring of dopamine concentration at spatially different brain locations in vivo. Biosens Bioelectron 25: 1179–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Luscher C, Malenka RC (2011) Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron 69: 650–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sulzer D (2011) How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron 69: 628–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Volkow ND, Wang GJ, Fowler JS, Tomasi D (2012) Addiction circuitry in the human brain. Annu Rev Pharmacol Toxicol 52: 321–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Cragg SJ, Nicholson C, Kume-Kick J, Tao L, Rice ME (2001) Dopamine-mediated volume transmission in midbrain is regulated by distinct extracellular geometry and uptake. J Neurophysiol 85: 1761–1771. [DOI] [PubMed] [Google Scholar]
- 98. Fuxe K, Borroto-Escuela DO, Romero-Fernandez W, Zhang WB, Agnati LF (2013) Volume transmission and its different forms in the central nervous system. Chin J Integr Med 19: 323–329. [DOI] [PubMed] [Google Scholar]
- 99. Lee T, Cai LX, Lelyveld VS, Hai A, Jasanoff A (2014) Molecular-level functional magnetic resonance imaging of dopaminergic signaling. Science 344: 533–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Stamford JA, Kruk ZL, Palij P, Millar J (1988) Diffusion and uptake of dopamine in rat caudate and nucleus accumbens compared using fast cyclic voltammetry. Brain Res 448: 381–385. [DOI] [PubMed] [Google Scholar]
- 101. Cragg SJ, Rice ME (2004) DAncing past the DAT at a DA synapse. Trends Neurosci 27: 270–277. [DOI] [PubMed] [Google Scholar]
- 102. Rice ME, Cragg SJ (2008) Dopamine spillover after quantal release: rethinking dopamine transmission in the nigrostriatal pathway. Brain Res Rev 58: 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Vizi ES, Fekete A, Karoly R, Mike A (2010) Non-synaptic receptors and transporters involved in brain functions and targets of drug treatment. Br J Pharmacol 160: 785–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Trueta C, De-Miguel FF (2012) Extrasynaptic exocytosis and its mechanisms: a source of molecules mediating volume transmission in the nervous system. Front Physiol 3: 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F (2011) Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci U S A 108: 15037–15042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Abi-Dargham A, Kegeles LS, Martinez D, Innis RB, Laruelle M (2003) Dopamine mediation of positive reinforcing effects of amphetamine in stimulant naı̈ve healthy volunteers: results from a large cohort. European Neuropsychopharmacology 13: 459–468. [DOI] [PubMed] [Google Scholar]
- 107. Volkow ND, Wang GJ, Tomasi D, Kollins SH, Wigal TL, et al. (2012) Methylphenidate-elicited dopamine increases in ventral striatum are associated with long-term symptom improvement in adults with attention deficit hyperactivity disorder. J Neurosci 32: 841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zhang L, Dong Y, Doyon WM, Dani JA (2012) Withdrawal from chronic nicotine exposure alters dopamine signaling dynamics in the nucleus accumbens. Biol Psychiatry 71: 184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Grace AA (1995) The tonic/phasic model of dopamine system regulation: its relevance for understanding how stimulant abuse can alter basal ganglia function. Drug Alcohol Depend 37: 111–129. [DOI] [PubMed] [Google Scholar]
- 110. Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, et al. (2007) Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry 164: 622–629. [DOI] [PubMed] [Google Scholar]
- 111. Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, et al. (1997) Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature 386: 830–833. [DOI] [PubMed] [Google Scholar]
- 112. Gabuzda D, Wang J (2000) Chemokine receptors and mechanisms of cell death in HIV neuropathogenesis. J Neurovirol 6 Suppl 1S24–32. [PubMed] [Google Scholar]
- 113. Gorry PR, Taylor J, Holm GH, Mehle A, Morgan T, et al. (2002) Increased CCR5 Affinity and Reduced CCR5/CD4 Dependence of a Neurovirulent Primary Human Immunodeficiency Virus Type 1 Isolate. Journal of Virology 76: 6277–6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D (1998) Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol 72: 2855–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Martin-Garcia J, Cao W, Varela-Rohena A, Plassmeyer ML, Gonzalez-Scarano F (2006) HIV-1 tropism for the central nervous system: Brain-derived envelope glycoproteins with lower CD4 dependence and reduced sensitivity to a fusion inhibitor. Virology 346: 169–179. [DOI] [PubMed] [Google Scholar]
- 116. Lewin SR, Sonza S, Irving LB, McDonald CF, Mills J, et al. (1996) Surface CD4 is critical to in vitro HIV infection of human alveolar macrophages. AIDS Res Hum Retroviruses 12: 877–883. [DOI] [PubMed] [Google Scholar]
- 117. Dick AD, Pell M, Brew BJ, Foulcher E, Sedgwick JD (1997) Direct ex vivo flow cytometric analysis of human microglial cell CD4 expression: examination of central nervous system biopsy specimens from HIV-seropositive patients and patients with other neurological disease. AIDS 11: 1699–1708. [DOI] [PubMed] [Google Scholar]
- 118. Blanpain C, Vanderwinden JM, Cihak J, Wittamer V, Le Poul E, et al. (2002) Multiple active states and oligomerization of CCR5 revealed by functional properties of monoclonal antibodies. Mol Biol Cell 13: 723–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. de Voux A, Chan MC, Folefoc AT, Madziva MT, Flanagan CA (2013) Constitutively active CCR5 chemokine receptors differ in mediating HIV envelope-dependent fusion. PLoS One 8: e54532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Salimi H, Roche M, Webb N, Gray LR, Chikere K, et al. (2012) Macrophage-tropic HIV-1 variants from brain demonstrate alterations in the way gp120 engages both CD4 and CCR5. J Leukoc Biol. [DOI] [PMC free article] [PubMed]
- 121. Flanagan CA (2014) Receptor Conformation and Constitutive Activity in CCR5 Chemokine Receptor Function and HIV Infection. Adv Pharmacol 70: 215–263. [DOI] [PubMed] [Google Scholar]
- 122. Gendelman HE, Orenstein JM, Martin MA, Ferrua C, Mitra R, et al. (1988) Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med 167: 1428–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Li Y, Kappes JC, Conway JA, Price RW, Shaw GM, et al. (1991) Molecular characterization of human immunodeficiency virus type 1 cloned directly from uncultured human brain tissue: identification of replication-competent and -defective viral genomes. J Virol 65: 3973–3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Hasbi A, O’Dowd BF, George SR (2011) Dopamine D1-D2 receptor heteromer signaling pathway in the brain: emerging physiological relevance. Mol Brain 4: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Melar M, Ott DE, Hope TJ (2007) Physiological levels of virion-associated human immunodeficiency virus type 1 envelope induce coreceptor-dependent calcium flux. J Virol 81: 1773–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Liu QH, Williams DA, McManus C, Baribaud F, Doms RW, et al. (2000) HIV-1 gp120 and chemokines activate ion channels in primary macrophages through CCR5 and CXCR4 stimulation. Proc Natl Acad Sci U S A 97: 4832–4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Stehno-Bittel L, Krapivinsky G, Krapivinsky L, Perez-Terzic C, Clapham DE (1995) The G protein beta gamma subunit transduces the muscarinic receptor signal for Ca2+ release in Xenopus oocytes. J Biol Chem 270: 30068–30074. [DOI] [PubMed] [Google Scholar]
- 129. Han Y, Moreira IS, Urizar E, Weinstein H, Javitch JA (2009) Allosteric communication between protomers of dopamine class A GPCR dimers modulates activation. Nat Chem Biol 5: 688–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Guo W, Urizar E, Kralikova M, Mobarec JC, Shi L, et al. (2008) Dopamine D2 receptors form higher order oligomers at physiological expression levels. EMBO J 27: 2293–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Fuxe K, Canals M, Torvinen M, Marcellino D, Terasmaa A, et al. (2007) Intramembrane receptor-receptor interactions: a novel principle in molecular medicine. J Neural Transm 114: 49–75. [DOI] [PubMed] [Google Scholar]
- 132. Franco R, Lluis C, Canela EI, Mallol J, Agnati L, et al. (2007) Receptor-receptor interactions involving adenosine A1 or dopamine D1 receptors and accessory proteins. J Neural Transm 114: 93–104. [DOI] [PubMed] [Google Scholar]
- 133. Arhel N (2010) Revisiting HIV-1 uncoating. Retrovirology 7: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Spudich S, Gisslen M, Hagberg L, Lee E, Liegler T, et al. (2011) Central nervous system immune activation characterizes primary human immunodeficiency virus 1 infection even in participants with minimal cerebrospinal fluid viral burden. J Infect Dis 204: 753–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Gray F, Scaravilli F, Everall I, Chretien F, An S, et al. (1996) Neuropathology of early HIV-1 infection. Brain Pathol 6: 1–15. [DOI] [PubMed] [Google Scholar]
- 136. Weber E, Morgan EE, Iudicello JE, Blackstone K, Grant I, et al. (2013) Substance use is a risk factor for neurocognitive deficits and neuropsychiatric distress in acute and early HIV infection. J Neurovirol 19: 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Substance Abuse and Mental Health Services Administration CfBHSaQ (2010) The NSDUH Report: HIV/AIDS and Substance Use. Rockville, MD.
- 138. Beyrer C, Wirtz AL, Baral S, Peryskina A, Sifakis F (2010) Epidemiologic links between drug use and HIV epidemics: an international perspective. J Acquir Immune Defic Syndr 55 Suppl 1S10–16. [DOI] [PubMed] [Google Scholar]
- 139. Mathers BM, Degenhardt L, Phillips B, Wiessing L, Hickman M, et al. (2008) Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet 372: 1733–1745. [DOI] [PubMed] [Google Scholar]
- 140. Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, et al. (2001) Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry 58: 721–728. [DOI] [PubMed] [Google Scholar]
- 141. Moore DJ, Blackstone K, Woods SP, Ellis RJ, Atkinson JH, et al. (2012) Methamphetamine use and neuropsychiatric factors are associated with antiretroviral non-adherence. AIDS Care 24: 1504–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Hinkin CH, Barclay TR, Castellon SA, Levine AJ, Durvasula RS, et al. (2007) Drug use and medication adherence among HIV-1 infected individuals. AIDS Behav 11: 185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Arnsten JH, Demas PA, Grant RW, Gourevitch MN, Farzadegan H, et al. (2002) Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J Gen Intern Med 17: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Berg KM, Wilson IB, Li X, Arnsten JH (2012) Comparison of antiretroviral adherence questions. AIDS Behav 16: 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Rosen MI, Black AC, Arnsten JH, Goggin K, Remien RH, et al. (2013) Association between use of specific drugs and antiretroviral adherence: findings from MACH 14. AIDS Behav 17: 142–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Meissner WG, Frasier M, Gasser T, Goetz CG, Lozano A, et al. (2011) Priorities in Parkinson’s disease research. Nat Rev Drug Discov 10: 377–393. [DOI] [PubMed] [Google Scholar]
- 147. Burris KD (2002) Aripiprazole, a Novel Antipsychotic, Is a High-Affinity Partial Agonist at Human Dopamine D2 Receptors. Journal of Pharmacology and Experimental Therapeutics 302: 381–389. [DOI] [PubMed] [Google Scholar]
- 148. Rosenberg SD, Goodman LA, Osher FC, Swartz MS, Essock SM, et al. (2001) Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness. Am J Public Health 91: 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Himelhoch S, McCarthy JF, Ganoczy D, Medoff D, Dixon LB, et al. (2007) Understanding associations between serious mental illness and HIV among patients in the VA Health System. Psychiatr Serv 58: 1165–1172. [DOI] [PubMed] [Google Scholar]
- 150. Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, et al. (2011) HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 17: 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Brown GR, Rundell JR, McManis SE, Kendall SN, Zachary R, et al. (1992) Prevalence of psychiatric disorders in early stages of HIV infection. Psychosom Med 54: 588–601. [DOI] [PubMed] [Google Scholar]
- 152. Blank MB, Mandell DS, Aiken L, Hadley TR (2002) Co-occurrence of HIV and serious mental illness among Medicaid recipients. Psychiatr Serv 53: 868–873. [DOI] [PubMed] [Google Scholar]
- 153. Sardar AM, Czudek C, Reynolds GP (1996) Dopamine deficits in the brain: the neurochemical basis of parkinsonian symptoms in AIDS. Neuroreport 7: 910–912. [DOI] [PubMed] [Google Scholar]
- 154. Kumar AM, Fernandez JB, Singer EJ, Commins D, Waldrop-Valverde D, et al. (2009) Human immunodeficiency virus type 1 in the central nervous system leads to decreased dopamine in different regions of postmortem human brains. J Neurovirol 15: 257–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Kumar AM, Ownby RL, Waldrop-Valverde D, Fernandez B, Kumar M (2011) Human immunodeficiency virus infection in the CNS and decreased dopamine availability: relationship with neuropsychological performance. J Neurovirol 17: 26–40. [DOI] [PubMed] [Google Scholar]
- 156. Larsson M, Hagberg L, Forsman A, Norkrans G (1991) Cerebrospinal fluid catecholamine metabolites in HIV-infected patients. J Neurosci Res 28: 406–409. [DOI] [PubMed] [Google Scholar]
- 157. Berger JR, Kumar M, Kumar A, Fernandez JB, Levin B (1994) Cerebrospinal fluid dopamine in HIV-1 infection. AIDS 8: 67–71. [DOI] [PubMed] [Google Scholar]
- 158. di Rocco A, Bottiglieri T, Dorfman D, Werner P, Morrison C, et al. (2000) Decreased homovanilic acid in cerebrospinal fluid correlates with impaired neuropsychologic function in HIV-1-infected patients. Clin Neuropharmacol 23: 190–194. [DOI] [PubMed] [Google Scholar]
- 159. Koutsilieri E, ter Meulen V, Riederer P (2001) Neurotransmission in HIV associated dementia: a short review. J Neural Transm 108: 767–775. [DOI] [PubMed] [Google Scholar]
- 160. Scheller C, Arendt G, Nolting T, Antke C, Sopper S, et al. (2010) Increased dopaminergic neurotransmission in therapy-naive asymptomatic HIV patients is not associated with adaptive changes at the dopaminergic synapses. J Neural Transm 117: 699–705. [DOI] [PubMed] [Google Scholar]
- 161. Horn A, Scheller C, du Plessis S, Arendt G, Nolting T, et al. (2013) Increases in CSF dopamine in HIV patients are due to the dopamine transporter 10/10-repeat allele which is more frequent in HIV-infected individuals. J Neural Transm 120: 1411–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Gelman BB, Spencer JA, Holzer CE 3rd, Soukup VM (2006) Abnormal striatal dopaminergic synapses in National NeuroAIDS Tissue Consortium subjects with HIV encephalitis. J Neuroimmune Pharmacol 1: 410–420. [DOI] [PubMed] [Google Scholar]
- 163. Rottenberg DA, Moeller JR, Strother SC, Sidtis JJ, Navia BA, et al. (1987) The metabolic pathology of the AIDS dementia complex. Ann Neurol 22: 700–706. [DOI] [PubMed] [Google Scholar]
- 164. van Gorp WG, Mandelkern MA, Gee M, Hinkin CH, Stern CE, et al. (1992) Cerebral metabolic dysfunction in AIDS: findings in a sample with and without dementia. J Neuropsychiatry Clin Neurosci 4: 280–287. [DOI] [PubMed] [Google Scholar]
- 165. Rottenberg DA, Sidtis JJ, Strother SC, Schaper KA, Anderson JR, et al. (1996) Abnormal cerebral glucose metabolism in HIV-1 seropositive subjects with and without dementia. J Nucl Med 37: 1133–1141. [PubMed] [Google Scholar]
- 166. Georgiou MF, Gonenc A, Waldrop-Valverde D, Kuker RA, Ezuddin SH, et al. (2008) Analysis of the effects of injecting drug use and HIV-1 infection on 18F-FDG PET brain metabolism. J Nucl Med 49: 1999–2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All data are included within the paper.