Introduction
Acromegaly management is a significant challenge for endocrinologists. The Acromegaly Consensus Group developed several statements on the management of acromegaly and specifically on its medical treatment [1–3]. Acromegaly is a quite rare condition generally caused by a growth hormone (GH)-secreting pituitary adenoma [4]. Delayed diagnosis leads to prevalent presentation of the disease at the stage of macroadenoma (two-thirds of patients) and frequent persistence of active disease after surgery which remains in many patients the primary treatment option [5]. However, active acromegaly is potentially a life threatening condition due its severe systemic complications [6, 7] Therefore, elevated GH and insulin-like growth factor (IGF)-1 levels need to be strictly controlled after failure of surgery with medical or radiation treatments [8]. Furthermore, criteria for disease control may not be fulfilled in a considerable proportion of patients undergoing medical treatment with somatostatin receptor ligands (SRLs) after unsuccessful surgery [9, 10]. Accordingly, some acromegaly patients require the administration of GH antagonist Pegvisomant [11]. Pegvisomant has been introduced in clinical practice more than a decade ago as a medical therapy of acromegaly. However, specific guidelines for Pegvisomant use in acromegaly are lacking. Therefore, the Italian Society of Endocrinology constituted a task force with the objective of assessing the published literature and the clinical experience with Pegvisomant. This group involved endocrinologists recognized experts in the field of acromegaly management and their understanding of the data reported so far worldwide as well as their recommendations for Pegvisomant use in clinical practice are presented here. Biochemical and clinical results of Pegvisomant, indications, treatment modalities, combination therapies, safety and regulatory and cost/efficacy issues were evaluated. Evidences were graded with GRADE system [1–3, 12, 13] based on the quality of evidence as very low quality (VLQ; expert opinion with one or a small number of small uncontrolled studies in support), low quality (LQ; large series of small uncontrolled studies), moderate quality (MQ; one or a small number of large uncontrolled studies or meta-analyses), or high quality (HQ; controlled studies or large series of large uncontrolled studies with sufficiently long follow-up). Recommendations were defined discretionary (DR) if based on VLQ-LQ evidence, or strong (SR) if supported by MQ-HQ evidence.
What is Pegvisomant
Pegvisomant is a drug designed to block the GH receptor (GHR) and, therefore, GH action. The discovery of this GHR antagonist was made possible by the elucidation of the structure–function relationship of GH and its receptor [11, 14]. Growth hormone is a 22 kDa polypeptide with 191 amino acids, two disulphide bonds and four alpha helices synthesized in the anterior pituitary and central to regulation of growth and differentiation. It has many other biological actions including enhancement of protein synthesis, lipolysis and hyperglycemic effects. Although GH may have direct effects on peripheral tissues most of its growth promoting effects are mediated by IGF-1 [15–17]. Growth hormone has two distinct domains (sites one and two) that interact with preformed GHR dimer on plasma membrane triggering conformational changes required for signaling [18]. The affinity of GH binding site one for GHR is high whereas the affinity of site two is lower. After initial high affinity binding at site one, subsequent binding at site two produces functional receptor dimerization. After the GH/GHR interaction, a series of intracellular signaling systems is mobilized, resulting in the activation or inactivation of genes responsible for GH action [19].
Pegvisomant is a GH analog with a single-aminoacid substitution at position 120 that generates the antagonist. Additional changes include amino acid substitutions within binding site 1 and a further modification by the addition of polyethylene glycol moieties [20]. The GHR antagonist acts by failing to induce proper or functional GHR dimerization. The pegylated [polyethylene glycol (PEG)] counterpart Pegvisomant is generated by the conjugation of GHR antagonist with four or five moieties of PEG 5000; PEG molecule addition increases the size of the antagonist and its serum half life from ~30 min to more than 100 h, by reducing renal clearance and intravascular proteolysis, and reduces immunogenicity of the molecule [21]. Like GH, the GHR antagonist has a relatively small size (22 kDa), and is normally cleared via the kidneys and/or GHR internalization [22].
Biochemical outcomes in trials and observational registries
Circulating GH values are not useful as biochemical marker of Pegvisomant effects in acromegaly both because endogenous GH secretion may increase during treatment due to negative feedback and, particularly, due to cross-reactivity of GH with Pegvisomant in most GH assays [21] (HQ). Therefore, GH should not be measured in monitoring Pegvisomant treatment (SR). Normalization of IGF-1 levels represents the main end point of Pegvisomant treatment (HQ) [23, 24] although sudden and remarkable GH increase during Pegvisomant therapy could be a marker of tumor re-growth [25] (VLQ). Many studies reported IGF-1 normalization or marked reduction in acromegaly patients treated with Pegvisomant [26] (HQ). In addition, improvement in quality of life was suggested even adding Pegvisomant in patients already effectively controlled by SRLs [27] (VLQ). However, reported effectiveness of Pegvisomant varied widely depending on the type of study (clinical trial vs. observational) as it happens with other medical therapies in acromegaly [3] (MQ). Indeed, serum IGF-1 levels normalized in more than 90 % of patients particularly in initial clinical trials [28–32], while the control rate was lower in studies performed in the clinical setting and based on the retrospective analysis of disease-specific databases [33–39] (Table 1). Inadequate dose titration, poor compliance to daily injections, suboptimal selection of patients and technical problems related to IGF-1 assay could justify a lower than expected efficacy in “real life” conditions (VLQ), since the existence of a true “biochemical resistance” to Pegvisomant, as observed with SRLs [40], has not been clearly documented yet (VLQ). Effectiveness of Pegvisomant may be inversely correlated to baseline IGF-1 levels and starting dose should be higher and dose titration more rapid in patients with a worse endocrine profile (VLQ) [26, 41]. Better efficacy of Pegvisomant was associated with male gender, leanness, lower baseline GH and/or IGF-1 levels, previous irradiation, and related to treatment duration and appropriate dose titration (LQ) [37, 38, 41]. The role of d3GHR polymorphism, which could modify receptor sensitivity to GH [42], in response to Pegvisomant is still controversial (VLQ) [43–45]. Availability of validated assays is crucial for monitoring appropriately effectiveness of treatment and dose titration (SR). For this reason, IGF-1 values should be measured with the same method over time in each patient (SR). At present, considerable differences exist among available assays, due to lack of standardization, use of different types of antibodies and interference of binding proteins (MQ) [46]. Moreover, specific age-related normative intervals are rarely obtained, as recommended by available general guidelines [10], in local populations by centralized laboratories (LQ). Finally, given the within-individual biological variation of IGF-1 assays caution should be also used in interpreting values close to reference limits even if obtained with the same method [47, 48] (DR).
Table 1.
Summary of biochemical results with Pegvisomant treatment in clinical trials and observational/retrospective studies in acromegaly
| Author | Primary end point | N. of patients | Disease control (%) | Dose of Pegvisomant | Duration of the study |
|---|---|---|---|---|---|
| Randomized clinical trials: | |||||
| Herman-Bonert et al. [28] | IGF-1 normalization | 3 | 100 | 30–80 mg/weekly | 6 weeks |
| 3 | 100 | 10–20 mg/day | 3 months | ||
| Trainer et al. [29] | Dose-related efficacy | 109 | 10 | placebo | 33 months |
| 38 | 10 mg/day | 3 months | |||
| 75 | 15 mg/day | 3 months | |||
| 82 | 20 mg/day | 3 months | |||
| van der Lely et al. [30] | IGF-1 normalization | 90 | 97 | – | 12 months |
| 62 | 92 | – | 18 months | ||
| Drake et al. [31] | IGF-1 normalization | 7 | 100 | 20 mg/day (median; range 15-40) | 24 months |
| Barkan et al. [32] | IGF-1 normalization | 49 | 78 | 16 mg/day (mean; range 5-40) | 8 months |
| Colao et al. [26] | IGF-1 normalization | 12 | 75 | 25 mg/day (median; range 10-40) | 12 months |
| Observational or retrospective studies: | |||||
| Schreiber et al. [33] | IGF-1 normalization | 147 | 64 | 16.5 mg/day (mean; range 10-50) | 6 months |
| 102 | 71 | 12 months | |||
| 39 | 76 | 24 months | |||
| Higham et al. [34] | IGF-1 normalization | 11 | 95 | 15 mg/day (median; range 10-60) | 91 months |
| Trainer [35] | IGF-1 normalization | 792 | 62 | 15 mg/day (median in controlled patients) | 60 months |
| 16 mg/day (median in not controlled patients) | |||||
| Buchfelder et al. [36] | IGF-1 normalization | 273 | 56 | 15 mg/day (median) | 6 months |
| 202 | 71 | 24 months | |||
| 133 | 71 | 36 months | |||
| 71 | 65 | 48 months | |||
| 24 | 58 | 60 months | |||
| Marazuela et al. [37] | IGF-1 normalization | 44 | 84 | 17 ± 7 mg/day in men 16 ± 8 mg/day in women | 23 months (mean) |
| Garsia Basavilbaso et al. [38] | Duration-related efficacy | 28 | 46 | 9.6 mg/day (mean) | 3 months |
| 59 | |||||
| 6 months | |||||
| van der Lely et al. [39] | Safety and efficacy | 1288 | 63 |
18 mg/day (mean in controlled patients) 20 mg/day (mean in uncontrolled patients) |
43 months (mean) |
Peripheral and tissue effects of Pegvisomant
Treatment with Pegvisomant improves clinical syndrome of acromegaly in a high percentage of patients (HQ), positively impacts glucose metabolism (MQ), quality of life (MQ) and cardiovascular and skeletal complications (MQ) [49] (Table 2).
Table 2.
Clinical and comorbidity outcomes of Pegvisomant therapy in acromegaly
| Endpoints | Results | References |
|---|---|---|
| Glucose metabolism | ||
| Fasting glucose levels |
|
[52–54] |
| Glucose tolerance |
|
[53, 58] |
| HbA1c % |
|
[33, 53] |
| Insulin sensitivity |
|
[52, 55–57] |
| HOMA index |
|
[52, 55] |
| Lipid metabolism | ||
| Total cholesterol |
|
[59, 60] / [26, 61] |
| LDL cholesterol |
|
[59, 60] / [26, 61] |
| Triglyceride |
|
[59, 60] / [26, 61] |
| Lipoprotein (a) |
|
[59, 60] |
| Cardiovascular complications | ||
| Cardiac mass |
|
[63] |
| Systolic and diastolic function |
|
[63] |
| Rhythm disturbances |
|
[64] |
| Blood pressure |
|
[26, 61] |
| Framingham risk score |
|
[61] |
| Carotid arteries wall thickness |
|
[65] |
| Brachial arteries vascular function |
|
[65] |
| Skeletal complications | ||
| Bone turn-over |
|
[71, 72] |
| BMD |
|
[73] |
* Denote significant change
Glucose and lipid metabolism
In acromegaly, abnormal glucose tolerance, insulin resistance, hyperinsulinemia and diabetes mellitus are frequently observed [50] (HQ). Medical treatment of acromegaly may variably influence glucose metabolism. It is known that SRLs inhibit insulin secretion, inducing a possibly negative impact on glucose homeostasis (MQ) [51], whereas Pegvisomant improves insulin sensitivity likely by ameliorating IGF-1 excess and its effect on insulin resistance (MQ) [33, 52–57]. Several studies demonstrated that Pegvisomant monotherapy induced a significant decrease in fasting glucose levels and HbA1c [33, 52–54, 58] also in patients with diabetes mellitus and impaired glucose tolerance (MQ). A positive impact of Pegvisomant on peripheral insulin sensitivity was also demonstrated [52, 55–57] (MQ). However, a substantial proportion of patients included in these studies were resistant to SRLs; therefore, improved glucose metabolism could derive from better biochemical control and/or to removed inhibitory effect of SRLs on insulin secretion [58] (VLQ). Variable results were observed on lipid metabolism after Pegvisomant. An increase in total and LDL cholesterol with unchanged triglyceride levels and a significant decline in lipoprotein (a) levels was observed [59, 60], whereas other authors [24, 61] reported that lipid profile did not change during Pegvisomant therapy (LQ).
Cardiovascular and skeletal complications
Acromegaly is associated with a specific cardiomyopathy, characterized by biventricular hypertrophy and complicated by initial diastolic dysfunction and late systolic dysfunction, potentially leading to heart failure (HQ) [62]. Furthermore, systemic arterial hypertension, frequently associated with the disease, contributes to worsening acromegalic cardiomyopathy [62]. Long-term (18 months) treatment with Pegvisomant induced a significant reduction of cardiac mass and significant improvement of diastolic and systolic function in patients with acromegaly mostly resistant to SRLs (LQ) [63]. Treatment with Pegvisomant could also exert beneficial effects on rhythm disorders and hyperkinetic syndrome (LQ) [64]. Moreover, 12 months of Pegvisomant therapy were associated with improved blood pressure, particularly of diastolic values, in hypertensive patients [24, 61] (LQ). IGF-I normalization significantly lowered predicted cardiovascular risk, calculated with the Framingham risk score [61] (LQ). On Pegvisomant slight reduction of carotid arteries wall thickness and significant improvement of brachial arteries vascular function in patients with acromegaly resistant to SRLs were reported (VLQ) [65].
Growth hormone and IGF-I play a significant role in the regulation of bone metabolism [66, 67] (HQ). Acromegaly increases risk of vertebral fractures not necessarily associated with reduced bone mass (MQ) [68–70] but with increased bone turn-over which normalized during 6 months of Pegvisomant treatment [71, 72] (LQ). Long-term treatment with Pegvisomant also induced a significant increase of bone mineral density in active acromegaly (LQ) [73]. Although Pegvisomant use was weakly associated with an increased rate of fractures this has been attributed to global increased severity of the disease in treated patients [70] (LQ).
Indications
Pegvisomant is traditionally indicated for treatment of acromegaly patients with inadequate response to pituitary adenomectomy or radiation therapy, or for those intolerant or resistant to SRLs (HQ). However, a clear-cut definition of resistance to SRLs is missing (VLQ) [74]. In fact, during SRL therapy biochemical control is defined as random basal GH lower than 1 mcg/liter and IGF-1 levels below the upper limit of normal range for age (MQ) [3]. Using these strict criteria [10] normalization of biochemical activity in unselected patients with acromegaly after long-term (>6–12 months) treatment with maximal SRL doses occurs approximately in 25–50 % of cases [3, 75–77] (MQ). Non-responders to SRL therapy (minimal effect on GH and IGF-I levels and on tumor shrinkage) should be switched to Pegvisomant (SR). In partial responders to SRLs, Pegvisomant monotherapy or combination therapy with Pegvisomant and SRL should be considered (DR). Tumor shrinkage quite frequently (around 50 % of treated patients) occurs during therapy with SRLs often but not necessarily together with biochemical normalization [78–80] (MQ). Interestingly, in patients with acromegaly and McCune Albright syndrome surgery and even radiation therapy often can not be performed [81] and SRLs have very low chances to be effective [81] (LQ). In these patients, Pegvisomant can be considered as primary treatment (DR). Moreover, primary post-surgical medical treatment with Pegvisomant should be considered in patients already proven to be resistant to SRLs as those who underwent a sufficiently long (>3–6 months) trial of pre-surgical SRL treatment which demonstrated to be ineffective in controlling GH and IGF-1 (unless a > 75 % surgical debulking is achieved [82]) (DR). Primary post-surgical Pegvisomant treatment can be considered in patients after irradiation in whom elevated IGF-1 levels may persist for long time but likelihood of tumor regrowth is modest [1] (DR) and in patients with poorly controlled diabetes mellitus in whom SRLs may potentially worsen glucose metabolism [51–54] (DR).
Treatment modalities
Pegvisomant is administered by subcutaneous injections. Ten, 15, and 20 mg per vial are available dosages. Initially, treatment regimens contemplated a 40–80 mg loading dose. In clinical practice this procedure has not proven to be useful and has been abandoned (LQ) [23]. Daily administration is the most effective because it achieves higher serum Pegvisomant concentrations with a lower dose of drug (MQ) [29, 83]. The target of therapy is to achieve serum IGF-I in the middle of age-related reference range (MQ) [11]. Starting dose is usually 10 mg/day and maximum maintenance dose which currently can be administered based on regulatory indications is 30 mg daily (LQ) [84]. For patients who require a dose >20 mg daily, Pegvisomant treatment is more inconvenient due to daily multiple injections (VLQ) [85]. After treatment start, serum IGF-I levels fall within 2 weeks and then reach a plateau after 4 weeks (HQ) [29]. Consequently, it is suggested to measure IGF-I 4 to 6 weeks after beginning treatment and after every change of dose until biochemical control is reached (DR). Once serum IGF-I levels are normalized, they should be monitored every 3–6 months [38] since Pegvisomant dose may require up- or down-titration in the same individual during treatment (DR) [3].
Combination therapies
Dopamine agonists
Cabergoline, a dopamine receptor agonist, has limited activity when used as monotherapy in acromegaly (MQ) [86, 87]. However, its combination with SRLs was shown to be effective in some patients (LQ) [86]. Few data are available regarding the combination of cabergoline and Pegvisomant. However, it was reported that addition of Pegvisomant to cabergoline as well as of cabergoline to Pegvisomant may result in improved IGF-1 control (LQ) [88, 89]. A better response was associated with baseline IGF-1 levels not higher than 160 % of ULN. No correlation was found with baseline prolactin levels. The combined treatment was well tolerated and safe (LQ).
Somatostatin receptor ligands
When compared with monotherapy, combination treatment with SRLs may require a lower dose (even in only 1 weekly administration) of Pegvisomant to obtain similar efficacy (MQ) (Table 3) [90–93]. This is due to different mechanisms, including elevation of serum Pegvisomant levels [93], reduced insulin concentration in the portal vein, which decreases the number of available liver GH receptors [94] and reduced endogenous GH levels (LQ). In all reported trials, combination treatment was generally well tolerated (LQ). However, transient liver function test abnormalities were observed in a variable percentage of cases (11–38 %), apparently higher when compared with monotherapy. Significant tumor shrinkage during combined treatment was observed in 13–19 % of patients [95]. Glucose metabolism was not substantially affected [96].
Table 3.
Studies investigating the efficacy and safety of adding PEG to SRLs in patients with uncontrolled acromegaly
| Design of the study | N. of patients | Length of the study, median (range) | Mean age (SD) | SRL treated patients (%) | IGF-1 at baseline, mean (SD) | PEG dose, median (range) | IGF-1 at EOS, mean (SD) | Patients with normal IGF-1 at EOS (%) | TLEE (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Feenstra et al. [90] | Prospective | 19 | 42 weeks | 51 years (12.6) |
Lanreotide ATG 120 mg/4 weeks (81 %) Octreotide LAR 30 mg/4 weeks (19 %) |
510 ng/ml (229) | 60 mg weekly (40–80 mg) | 187 (92) | 95 | 38 |
| Neggers et al. [91] | Prospective | 32 | 138 weeks (35–149) | 53 years (12.8) |
Lanreotide ATG 120 mg/4 weeks (69 %) Octreotide LAR 30 mg/4 weeks (31 %) |
428 ng/ml (220) | 60 mg, weekly or biweekly (40–160) | 137 ng/ml (47) | 100 | 34 |
| Van der Lely. [92] | Prospective | 57 | 28 weeks | 51.6 years (12.7) | Lanreotide ATG 120 mg/4 weeks (100 %) | NA | 60 mg, weekly or biweekly (40–120) | NA | 57.9 | 11 |
| Jorgensen et al. [93] | Prospective | 11 | 12 weeks | 46 years (NA) | Octreotide LAR 30 mg/2-4 weeks (100 %) | 458 ng/ml (67) | 15 mg daily | 195 ng/ml (24) | 91 | NA |
| Bianchi et al. [95] | Retrospective | 27 | 30 weeks (6–72) | 31 years (median age at diagnosis) |
Lanreotide ATG 120 mg/4 weeks (63 %) Octreotide LAR 30 mg/4 weeks (37 %) |
661 ng/ml (162) | 20 mg daily (10–40) | 372 ng/ml (216) | 55.5 | 11.1 |
SRLs somatostatin receptor ligands, PEG Pegvisomant, EOS end of study, TLEE transient liver enzyme elevation
General and tumor growth safety
General safety
In clinical trials, Pegvisomant has been shown to be generally safe and well tolerated [29, 30] (HQ). In a global non-interventional surveillance study (1,288 subjects, mean duration 3.7 years) Pegvisomant-related adverse events (AE) (changes in tumor size, increase in liver enzymes, and injection site reactions) were recorded in 9.6 % of subjects [39]. In all studies, mortality was not related to Pegvisomant use (MQ). Injection-site reactions were initially reported with a frequency up to 11 % and were generally mild, erythematous, self-limited and did not require treatment [29, 30]. Lipodistrophy during Pegvisomant therapy was sporadically reported likely due to local lypolitic GH inhibition (LQ). Frequent rotation of injection sites could prevent local reactions and patients should be carefully monitored and trained [97, 98] (SR). Surveillance studies [33, 98] reported an elevation of liver transaminase levels > 3 times ULN in about 5–8 % of patients mainly previously treated with SRLs. Transaminase level elevations during Pegvisomant treatment were often mild and transient, did not appear to be dose-related (idiosyncratic drug toxicity?) and occurred within the first year of treatment (MQ). Rare cases of drug-induced hepatitis (but not liver failure) were reported (VLQ) [99]. When Pegvisomant was combined with SRLs, transient liver enzyme elevations seemed to be 2–3 times more frequent (MQ) [33, 39, 82, 99–103]. Controversial is the correlation between diabetes mellitus and elevated transaminase levels (VLQ) [33, 92, 99, 101]. A common polymorphism found in Gilbert’s syndrome was associated with Pegvisomant-induced liver injury [104]. Biliary complications may arise from restitution to normal of gallbladder motility after cessation of SRL treatment [10]. We recommend not to start Pegvisomant if there is a liver dysfunction (SR). Liver function should be evaluated monthly for at least 6 months after initiating therapy, quarterly for next 6 months, and then semi-annually (SR). If transaminases increase >5 times ULN or >3 times ULN with increased serum bilirubin Pegvisomant must be discontinued (SR). If transaminases increase < 3 times ULN without signs or symptoms of liver failure Pegvisomant could be continued (DR), but they must be monitored weekly (SR) [24, 29, 33]. Since Pegvisomant may improve glucose tolerance, glucose levels should be monitored particularly in first months of treatment and anti-diabetic drugs adjusted if necessary (DR) [30, 33].
Tumor growth safety
Only 1 out of 43 subjects treated with Pegvisomant for 29 months and monitored for 58 months, showed an increase in pituitary tumor volume [105]. In the German Pegvisomant Observational Study [106] in 18 out of 307 (5.9 %) patients treated with Pegvisomant for an average of 86 weeks tumor size increased; however, after centralized image re-evaluation, tumor progression was confirmed in only eight patients (3 %). Among 61 patients observed by Buhk et al. [107], in 3 (4.9 %) increased tumor volume >25 % during the first year of therapy was reported. Marazuela et al. [37] observed significant increased tumor size in 6.7 % of subjects (5 of 75), followed for 29 ± 20 months; absence of previous irradiation and shorter duration of pre-Pegvisomant SRL therapy were associated with increased risk of growth (LQ). In the global surveillance study [39] incidence of increased pituitary tumor size was 7.2 % (67 of 936) in the local MRI reading, while again it was only 3.2 % (45 of 936) in the central reading. Thus, a careful serial evaluation of all available images is necessary to avoid misinterpretations (SR) [39, 106]. Therefore, tumor growth, observed more frequently during the first year of treatment, may prevalently reflect the disease natural history [24, 30] or the consequence of SRL discontinuation [106]. On the contrary, irradiation seems to be associated with a reduction in tumor size [24, 105, 108]. All patients treated with Pegvisomant should undergo regular sellar MRI to screen for potential tumor growth (SR). A more intensive MRI follow-up protocol should be followed in non-irradiated patients (DR).
Regulatory and cost/efficacy issues
Regulatory issues
Pegvisomant was licensed for the treatment of acromegaly in 2002 by EMA (EU, European Medicines Agency) and in 2003 by FDA (US, Food and Drug Administration). Label indications in EU limit use of Pegvisomant to patients with acromegaly with inadequate response to surgery and/or radiation therapy and in whom medical treatment with SRLs did not normalize IGF-I or was not tolerated (third line therapy). Label indications in US indicate Pegvisomant in acromegaly patients with inadequate response to surgery and/or radiation therapy and/or other medical therapies, or for whom these therapies are not appropriate (first/second line therapy in specific cases) better reflecting available guidelines (MQ) [1–3]. Pegvisomant should be prescribed by doctors with expertise in acromegaly management (MQ). National and regional regulatory agencies provide largely variable criteria to allow centers for prescription (VLQ). First injection of Pegvisomant should be done under close medical supervision (SR) and specific warnings about systemic hypersensitivity reactions were recently added in the package leaflet. Injections less frequently than daily normalize IGF-I levels in some patients [108] and in Acrostudy [39] 12 % of clinicians did not use daily injections (VLQ). Combination therapy Pegvisomant + SRLs is not recommended by EMA though the Agency recognized the interest for the complementary actions of these drugs. Pegvisomant in combination therapy is considered an “off-label” use by some local regulatory agencies. Pegvisomant should not be used during pregnancy unless clearly necessary according to EMA and FDA (MQ) (pregnancy class B). In fact, there are only few reports about its safety in pregnancy [109].
Cost/efficacy analysis
Pegvisomant is an effective but expensive drug (MQ). Certainly, the direct costs of neurosurgery, dopaminergic agents, SRLs and radiotherapy are lower than lifelong Pegvisomant treatment, but standard therapies do not provide biochemical normalization in some patients (HQ). On the other hand, control of disease is associated with normalized mortality rate and improvement of comorbidities (HQ) [1–3]. In addition, burden of direct and indirect (associated comorbidities and loss of working days) costs is higher in patients with acromegaly not controlled by standard therapies (MQ) [110, 111]. Therefore, if Pegvisomant is prescribed according to licensed use it may be cost-effective considering relative rarity of acromegaly (MQ). Nevertheless, according to a pharmacoeconomic model [112] the best cost-effectiveness ratio could be reached with Pegvisomant price reduced by about one-third (VLQ).
Summary of recommendations
Place of Pegvisomant in guidelines
Primary treatment
Pegvisomant cannot be recommended as primary treatment of the general acromegaly population (SR). In fact, surgery (performed by an experienced neurosurgeon) remains the primary treatment option in patients with acromegaly with totally resectable tumor (SR). Moreover, SRLs are primary medical treatment if surgery is contraindicated, not accepted by the patient or in case of poor likelihood of total surgical resection (SR). When surgery and radiation therapy cannot be performed and SRL are unlikely to be, or may not be, effective as in patients with acromegaly and McCune Albright syndrome or empty sella [113] Pegvisomant could be considered as primary treatment option (DR).
First-line (post-surgery) pharmacologic treatment
SRLs are primary first-line therapy after surgery (SR). Primary postsurgical therapy with cabergoline may be considered particularly in patients with relatively mild disease [114] (DR). There are at least three circumstances in which primary postsurgical medical treatment with Pegvisomant could be considered (DR): (1) patients who underwent a sufficiently long (>3–6 months) trial of pre-surgical SRL treatment [3] that was ineffective in controlling GH and IGF-1 and in whom mass effect of residual tumor is not an issue; (2) patients with residual tumor in whom radiation treatment is given as second option: in fact, after radiation elevated IGF-1 levels may persist for long time but likelihood of tumor regrowth is modest [1]; (3) patients with poorly controlled diabetes mellitus in whom SRL treatment may potentially worsen glucose metabolism [51–54].
Second-line pharmacologic treatment
Partial (GH and IGF-I decreased but not normalized) or no response (minimal changes in GH and IGF-1) to SRLs may be observed (HQ) [3]. Patients with no response after an adequately long (6–12 months) period of treatment with maximal doses of SRL should be switched to Pegvisomant monotherapy (SR). If biochemical control is not achieved Pegvisomant dose should be increased (SR) and/or combination treatment with dopamine agonists should be given (DR). In patients who do not achieve biochemical control of the disease [7] but have documented partial response to SRLs (>50 % reduction of GH and IGF-1 vs. baseline and/or tumor shrinkage >20 %) either switching to Pegvisomant monotherapy or combination therapy Pegvisomant + SRL should be considered (DR). If SRL + Pegvisomant combination is not effective a possible alternative could be association of Pegvisomant with dopamine agonists (DR) [3] (Fig. 1). Patients seldom do not tolerate SRL treatment for gastrointestinal side effects (LQ) [115]: these subjects should be switched to Pegvisomant monotherapy regardless biochemical efficacy of SRL (taking into account potential mass effect) (SR).
Fig. 1.
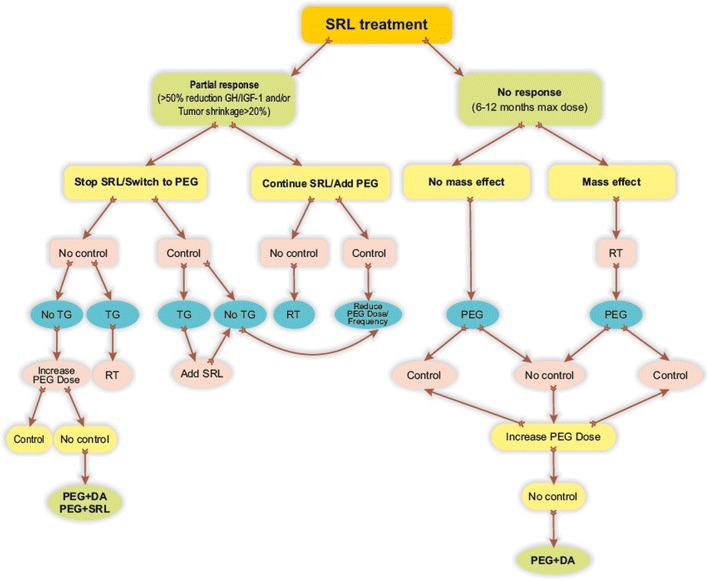
Proposed algorithm for the use of Pegvisomant in acromegaly patients partially or not responder to SRLs. SRL somatostatin receptor ligands, PEG Pegvisomant, RT radiation therapy, TG tumor growth, DA dopamine agonists
Dose, efficacy and safety monitoring
Individual optimal dose of Pegvisomant may vary according to anthropometric and genetic characteristics (VLQ). Recommended starting dose is 10 mg/day s.c. (DR). An initial load dose of Pegvisomant is not recommended (DR). Doses of Pegvisomant exceeding 30 mg/day are not recommended although in biochemically and clinically persistently active disease with no other treatment choice a further dose increase to 40 mg/day could be considered (DR).
Growth hormone should not be measured to assess effects of Pegvisomant (SR). Goal of Pegvisomant treatment is to normalize circulating IGF-1 levels (SR). Biochemical effects of Pegvisomant should be checked in laboratories with experience in IGF-1 measurement which give reference values divided by decade of age (SR). Patients with deranged glucose homeostasis on SRLs should be switched to Pegvisomant (DR). SRL treatment is known to counteract myocardial hypertrophy in patients with acromegaly [116] (HQ). Pegvisomant was also associated with positive cardiovascular effects and acromegaly cardiopathy does not contraindicate Pegvisomant (SR). Pegvisomant is the only treatment which was shown to normalize bone turnover in acromegaly [71] and prevalent vertebral fractures do not contraindicate Pegvisomant (DR).
Patients with known liver dysfunction should not be initiated with Pegvisomant (SR). Liver function should be evaluated periodically during therapy (SR). Injection-site reactions, such as lipodystrophy or lipohypertrophy may rarely occur and frequent rotation of injection sites is recommended (SR). Unlike SRLs [78–80] Pegvisomant treatment does not target tumor (HQ). Therefore, regular MRI monitoring is required (SR).
Acknowledgments
The authors are indebted to the following colleagues for their assistance in the preparation of manuscript as well as for the fruitful discussion: Donato Iacovazzo (Rome), Elena Malchiodi (Milano), Gherardo Mazziotti (Brescia), Nunzia Prencipe (Torino), Soraya Puglisi (Messina), Paola Sartorato (Montebelluna) and Ludovica Francesca Stella Grasso (Naples). The authors would like to thank SIE President and Guidelines Committee for their helpful support.
Conflict of interest
A.Giustina is consultant for Ipsen, Novartis and Pfizer; F. Bogazzi is member of Acrostudy Board and recipient of research grant from Pfizer; S. Cannavo' is speaker and member of scientific board for Eli Lilly, Italfarmaco and Viropharma; E. De Menis is speaker for Ipsen and Pfizer; S. Grottoli is recipient of research grant and support from Ipsen, Italfarmaco, Novartis and Pfizer; R. Pivonello has been principal investigator of research studies from Novartis, is recipient of research grants from Novartis, Pfizer, Viropharma and IBSA, is consultant for Novartis, Ipsen, Pfizer, Viropharma, Ferring and Italfarmaco, and speaker for Novartis. M.R. Ambrosio, P. Beck Peccoz, L. De Marinis have nothing to disclose.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- 1.Melmed S, Colao A, Barkan A, Molitch M, Grossman AB, Kleinberg D, Clemmons D, Chanson P, Laws E, Schlechte J, Vance ML, Ho K, Giustina A, Acromegaly Consensus Group Guidelines for acromegaly management: an update. J Clin Endocrinol Metab. 2009;94:1509–1517. doi: 10.1210/jc.2008-2421. [DOI] [PubMed] [Google Scholar]
- 2.Melmed S, Casanueva F, Cavagnini F, Chanson P, Frohman LA, Gaillard R, Ghigo E, Ho K, Jaquet P, Kleinberg D, Lamberts S, Laws E, Lombardi G, Sheppard MC, Thorner M, Vance ML, Wass JA, Giustina A. Consensus statement: medical management of acromegaly. Eur J Endocrinol. 2005;153:737–740. doi: 10.1530/eje.1.02036. [DOI] [PubMed] [Google Scholar]
- 3.Giustina A, Chanson P, Kleinberg D, Bronstein MD, Clemmons DR, Klibanski A, van der Lely AJ, Strasburger CJ, Lamberts SW, Ho KK, Casanueva FF, Melmed S. A consensus on the medical treatment of acromegaly. Nat Rev Endocrinol. 2014;10:243–248. doi: 10.1038/nrendo.2014.21. [DOI] [PubMed] [Google Scholar]
- 4.Melmed S. Acromegaly pathogenesis and treatment. J Clin Invest. 2009;119:3189–3202. doi: 10.1172/JCI39375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giustina A, Bronstein MD, Casanueva FF, Chanson P, Ghigo E, Ho KK, Klibanski A, Lamberts S, Trainer P, Melmed S. Current management practices for acromegaly: an international survey. Pituitary. 2011;14:125–133. doi: 10.1007/s11102-010-0269-9. [DOI] [PubMed] [Google Scholar]
- 6.Giustina A, Casanueva FF, Cavagnini F, Chanson P, Clemmons D, Frohman LA, Gaillard R, Ho K, Jaquet P, Kleinberg DL, Lamberts SW, Lombardi G, Sheppard M, Strasburger CJ, Vance ML, Wass JA, Melmed S, Pituitary Society and the European Neuroendocrine Association Diagnosis and treatment of acromegaly complications. J Endocrinol Invest. 2003;26:1242–1247. doi: 10.1007/BF03349164. [DOI] [PubMed] [Google Scholar]
- 7.Melmed S, Casanueva FF, Klibanski A, Bronstein MD, Chanson P, Lamberts SW, Strasburger CJ, Wass JA, Giustina A. A consensus on the diagnosis and treatment of acromegaly complications. Pituitary. 2013;16:294–302. doi: 10.1007/s11102-012-0420-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holdaway IM, Bolland MJ, Gamble GD. A meta-analysis of the effect of lowering serum levels of GH and IGF-I on mortality in acromegaly. Eur J Endocrinol. 2008;159:89–95. doi: 10.1530/EJE-08-0267. [DOI] [PubMed] [Google Scholar]
- 9.Giustina A, Barkan A, Casanueva FF, Cavagnini F, Frohman L, Ho K, Veldhuis J, Wass J, Von Werder K, Melmed S. Criteria for cure of acromegaly: a consensus statement. J Clin Endocrinol Metab. 2000;85:526–529. doi: 10.1210/jcem.85.2.6363. [DOI] [PubMed] [Google Scholar]
- 10.Giustina A, Chanson P, Bronstein MD, Klibanski A, Lamberts S, Casanueva FF, Trainer P, Ghigo E, Ho K, Melmed S, Acromegaly Consensus Group A consensus on criteria for cure of acromegaly. J Clin Endocrinol Metab. 2010;95:3141–3148. doi: 10.1210/jc.2009-2670. [DOI] [PubMed] [Google Scholar]
- 11.Kopchick JJ, Parkinson C, Stevens EC, Trainer PJ. Growth hormone receptor antagonists: discovery, development, and use in patients with acromegaly. Endocr Rev. 2002;23:623–646. doi: 10.1210/er.2001-0022. [DOI] [PubMed] [Google Scholar]
- 12.Funder JW, Carey RM, Fardella C, Gomez-Sanchez CE, Mantero F, Stowasser M, Young WF, Jr, Montori VM, Endocrine Society Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:3266–3281. doi: 10.1210/jc.2008-0104. [DOI] [PubMed] [Google Scholar]
- 13.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ, GRADE Working Group GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kopchick JJ, List EO, Kelder B, Gosney ES, Berryman DE. Evaluation of growth hormone (GH) action in mice: discovery of GH receptor antagonists and clinical indications. Mol Cell Endocrinol. 2014;386:34–45. doi: 10.1016/j.mce.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giustina A, Veldhuis JD. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev. 1998;19:717–797. doi: 10.1210/edrv.19.6.0353. [DOI] [PubMed] [Google Scholar]
- 16.Mazziotti G, Giustina A. Glucocorticoids and the regulation of growth hormone secretion. Nat Rev Endocrinol. 2013;9:265–276. doi: 10.1038/nrendo.2013.5. [DOI] [PubMed] [Google Scholar]
- 17.Kamenický P, Mazziotti G, Lombès M, Giustina A, Chanson P. Growth hormone, insulin-like growth factor-1, and the kidney: pathophysiological and clinical implications. Endocr Rev. 2014;35:234–281. doi: 10.1210/er.2013-1071. [DOI] [PubMed] [Google Scholar]
- 18.Ross RJM, Leung KC, Maamra M, Bennett W, Doyle N, Waters MJ, Ho KKY. Binding and functional studies with the growth hormone receptor antagonist B2036-PEG (Pegvisomant), reveal effects of pegylation and evidence that it binds to a receptor dimer. J Clin Endocrinol Metab. 2001;86:1716–1723. doi: 10.1210/jcem.86.4.7403. [DOI] [PubMed] [Google Scholar]
- 19.de Vos AM, Ultsch M, Kossiakoff AA. Human growth hormone and extracellular domain of its receptor: crystal structure of the complex. Science. 1992;255:306–331. doi: 10.1126/science.1549776. [DOI] [PubMed] [Google Scholar]
- 20.Carter-Su C, Rui L, Herrington J. Role of the tyrosine kinase JAK2 in signal transduction by growth hormone. Pediatr Nephrol. 2000;14:550–557. doi: 10.1007/s004670000366. [DOI] [PubMed] [Google Scholar]
- 21.Muller AF, Kopchick JJ, Flyvbjerg A, Van Der Lely AJ. Growth hormone receptor antagonists. J Clin Endocrinol Metab. 2004;89:1503–1511. doi: 10.1210/jc.2002-022049. [DOI] [PubMed] [Google Scholar]
- 22.Maamra M, Kopchick JJ, Strasburger CJ, Ross RJM. Pegvisomant, a growth hormone-specific antagonist, undergoes cellular internalization. J Clin Endocrinol Metab. 2004;89:4532–4537. doi: 10.1210/jc.2003-031781. [DOI] [PubMed] [Google Scholar]
- 23.Colao A, Arnaldi G, Beck-Peccoz P, Cannavò S, Cozzi R, Degli Uberti E, De Marinis L, De Menis E, Ferone D, Gasco V, Giustina A, Grottoli S, Lombardi G, Maffei P, Martino E, Minuto F, Pivonello R, Ghigo E. Pegvisomant in acromegaly: why, when, how. J Endocrinol Invest. 2007;30:693–699. doi: 10.1007/BF03347452. [DOI] [PubMed] [Google Scholar]
- 24.Hodish I, Barkan A. Long-term effects of Pegvisomant in patients with acromegaly. Nat Clin Pract Endocrinol Metab. 2008;4:324–332. doi: 10.1038/ncpendmet0831. [DOI] [PubMed] [Google Scholar]
- 25.Marazuela M, Paniagua AE, Gahete MD, Lucas T, Alvarez-Escolá C, Manzanares R, Cameselle-Teijeiro J, Luque-Ramirez M, Luque RM, Fernandez-Rodriguez E, Castaño JP, Bernabeu I. Somatotroph tumor progression during Pegvisomant therapy: a clinical and molecular study. J Clin Endocrinol Metab. 2011;96:E251–E259. doi: 10.1210/jc.2010-1742. [DOI] [PubMed] [Google Scholar]
- 26.Colao A, Pivonello R, Auriemma RS, De Martino MC, Bidlingmaier M, Briganti F, Tortora F, Burman P, Kourides IA, Strasburger CJ, Lombardi G. Efficacy of 12-month treatment with the GH receptor antagonist Pegvisomant in patients with acromegaly resistant to long-term, high-dose somatostatin analog treatment: effect on IGF-I levels, tumor mass, hypertension and glucose tolerance. Eur J Endocrinol. 2006;154:467–477. doi: 10.1530/eje.1.02112. [DOI] [PubMed] [Google Scholar]
- 27.Neggers SJ, van Aken MO, de Herder WW, Feelders RA, Janssen JA, Badia X, Webb SM, van der Lely AJ. Quality of life in acromegalic patients during long-term somatostatin analog treatment with and without Pegvisomant. J Clin Endocrinol Metab. 2008;93:3853–3859. doi: 10.1210/jc.2008-0669. [DOI] [PubMed] [Google Scholar]
- 28.Herman-Bonert VS, Zib K, Scarlett JA, Melmed S. Growth hormone receptor antagonist therapy in acromegalic patients resistant to somatostatin analogs. J Clin Endocrinol Metab. 2000;85:2958–2961. doi: 10.1210/jcem.85.8.6851. [DOI] [PubMed] [Google Scholar]
- 29.Trainer PJ, Drake WM, Katznelson L, Freda PU, Herman-Bonert V, van der Lely AJ, Dimaraki EV, Stewart PM, Friend KE, Vance ML, Besser GM, Scarlett JA, Thorner MO, Parkinson C, Klibanski A, Powell JS, Barkan AL, Sheppard MC, Malsonado M, Rose DR, Clemmons DR, Johannsson G, Bengtsson BA, Stavrou S, Kleinberg DL, Cook DM, Phillips LS, Bidlingmaier M, Strasburger CJ, Hackett S, Zib K, Bennett WF, Davis RJ. Treatment of acromegaly with the growth hormone-receptor antagonist Pegvisomant. N Engl J Med. 2000;342:1171–1177. doi: 10.1056/NEJM200004203421604. [DOI] [PubMed] [Google Scholar]
- 30.van der Lely AJ, Hutson RK, Trainer PJ, Besser GM, Barkan AL, Katznelson L, Klibanski A, Herman-Bonert V, Melmed S, Vance ML, Freda PU, Stewart PM, Friend KE, Clemmons DR, Johannsson G, Stavrou S, Cook DM, Phillips LS, Strasburger CJ, Hackett S, Zib KA, Davis RJ, Scarlett JA, Thorner MO. Long-term treatment of acromegaly with Pegvisomant, a growth hormone receptor antagonist. Lancet. 2001;358:1754–1759. doi: 10.1016/s0140-6736(01)06844-1. [DOI] [PubMed] [Google Scholar]
- 31.Drake WM, Parkinson C, Akker SA, Monson JP, Besser GM, Trainer PJ. Successful treatment of resistant acromegaly with a growth hormone receptor antagonist. Eur J Endocrinol. 2001;145:451–456. doi: 10.1530/eje.0.1450451. [DOI] [PubMed] [Google Scholar]
- 32.Barkan AL, Burman P, Clemmons DR, Drake WM, Gagel RF, Harris PE, Trainer PJ, van der Lely AJ, Vance ML. Glucose homeostasis and safety in patients with acromegaly converted from long-acting octreotide to Pegvisomant. J Clin Endocrinol Metab. 2005;90:5684–5691. doi: 10.1210/jc.2005-0331. [DOI] [PubMed] [Google Scholar]
- 33.Schreiber I, Buchfelder M, Droste M, Forssmann K, Mann K, Saller B, Strasburger CJ, German Pegvisomant Investigators German Pegvisomant Investigators. Treatment of acromegaly with the GH receptor antagonist Pegvisomant in clinical practice: safety and efficacy evaluation from the German Pegvisomant Observational Study. Eur J Endocrinol. 2007;156:75–82. doi: 10.1530/eje.1.02312. [DOI] [PubMed] [Google Scholar]
- 34.Higham CE, Chung TT, Lawranc J, Drake WM, Trainer PJ. Long-term experience of Pegvisomant therapy as a treatment for acromegaly. Clin Endocrinol. 2009;71:86–91. doi: 10.1111/j.1365-2265.2008.03469.x. [DOI] [PubMed] [Google Scholar]
- 35.Trainer PJ. ACROSTUDY: the first 5 years. Eur J Endocrinol. 2009;161(Suppl 1):S19–S24. doi: 10.1530/EJE-09-0322. [DOI] [PubMed] [Google Scholar]
- 36.Buchfelder M, Schlaffer S, Droste M, Mann K, Saller B, Brübach K, Stalla GK, Strasburger CJ, German Pegvisomant Observational Study The German ACROSTUDY: past and present. Eur J Endocrinol. 2009;161(Suppl 1):S3–S10. doi: 10.1530/EJE-09-0350. [DOI] [PubMed] [Google Scholar]
- 37.Marazuela M, Lucas T, Alvarez-Escolá C, Puig-Domingo M, de la Torre NG, de Miguel-Novoa P, Duran-Hervada A, Manzanares R, Luque-Ramírez M, Halperin I, Casanueva FF, Bernabeu I. Long-term treatment of acromegalic patients resistant to somatostatin analogues with the GH receptor antagonist Pegvisomant: its efficacy in relation to gender and previous radiotherapy. Eur J Endocrinol. 2009;160:535–542. doi: 10.1530/EJE-08-0705. [DOI] [PubMed] [Google Scholar]
- 38.García Basavilbaso N, Guitelman M, Nagelberg A, Stalldecker G, Carabelli A, Bruno O, Danilowitz K, Manavela M, Mallea Gil S, Ballarino C, Guelman R, Katz D, Fidalgo S, Leal R, Fideleff H, Servidio M, Bruera D, Librandi F, Chervin A, Vitale M, Basso A. Experience from the Argentine Pegvisomant Observational Study: preliminary data. Front Horm Res. 2010;38:42–49. doi: 10.1159/000318493. [DOI] [PubMed] [Google Scholar]
- 39.van der Lely AJ, Biller BM, Brue T, Buchfelder M, Ghigo E, Gomez R, Hey-Hadavi J, Lundgren F, Rajicic N, Strasburger CJ, Webb SM, Koltowska-Häggström M. Long-term safety of Pegvisomant in patients with acromegaly: comprehensive review of 1288 subjects in ACROSTUDY. J Clin Endocrinol Metab. 2012;97:1589–1597. doi: 10.1210/jc.2011-2508. [DOI] [PubMed] [Google Scholar]
- 40.Gola M, Bonadonna S, Mazziotti G, Amato G, Giustina A. Resistance to somatostatin analogs in acromegaly: an evolving concept? J Endocrinol Invest. 2006;29:86–93. doi: 10.1007/BF03349183. [DOI] [PubMed] [Google Scholar]
- 41.Parkinson C, Burman P, Messig M, Trainer PJ. Gender, body weight, disease activity, and previous radiotherapy influence the response to Pegvisomant. J Clin Endocrinol Metab. 2007;92:190–195. doi: 10.1210/jc.2006-1412. [DOI] [PubMed] [Google Scholar]
- 42.Bianchi A, Giustina A, Cimino V, Pola R, Angelini F, Pontecorvi A, De Marinis L. Influence of growth hormone receptor d3 and full-length isoforms on biochemical treatment outcomes in acromegaly. J Clin Endocrinol Metab. 2009;94:2015–2022. doi: 10.1210/jc.2008-1337. [DOI] [PubMed] [Google Scholar]
- 43.Bianchi A, Mazziotti G, Tilaro L, Cimino V, Veltri F, Gaetani E, Pecorini G, Pontecorvi A, Giustina A, De Marinis L. Growth hormone receptor polymorphism and the effects of Pegvisomant in acromegaly. Pituitary. 2009;12:196–199. doi: 10.1007/s11102-008-0157-8. [DOI] [PubMed] [Google Scholar]
- 44.Bernabeu I, Alvarez-Escolá C, Quinteiro C, Lucas T, Puig-Domingo M, Luque-Ramírez M, de Miguel-Novoa P, Fernandez-Rodriguez E, Halperin I, Loidi L, Casanueva FF, Marazuela M. The exon 3-deleted growth hormone receptor is associated with better response to Pegvisomant therapy in acromegaly. J Clin Endocrinol Metab. 2010;95:222–229. doi: 10.1210/jc.2009-1630. [DOI] [PubMed] [Google Scholar]
- 45.Filopanti M, Olgiati L, Mantovani G, Corbetta S, Arosio M, Gasco V, De Marinis L, Martini C, Bogazzi F, Cannavò S, Colao A, Ferone D, Arnaldi G, Pigliaru F, Peri A, Angeletti G, Jaffrain-Rea ML, Lania AG, Spada A. Growth hormone receptor variants and response to Pegvisomant in monotherapy or in combination with somatostatin analogs in acromegalic patients: a multicenter study. J Clin Endocrinol Metab. 2012;97:E165–E172. doi: 10.1210/jc.2011-1769. [DOI] [PubMed] [Google Scholar]
- 46.Clemmons DR. Consensus statement on the standardization and evaluation of growth hormone and insulin-like growth factor assays. Clin Chem. 2011;57:555–559. doi: 10.1373/clinchem.2010.150631. [DOI] [PubMed] [Google Scholar]
- 47.Milani D, Carmichael JD, Welkowitz J, Ferris S, Reitz RE, Danoff A, Kleinberg DL. Variability and reliability of single serum IGF-I measurements: impact on determining predictability of risk ratios in disease development. J Clin Endocrinol Metab. 2004;89:2271–2274. doi: 10.1210/jc.2003-032150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pokrajac A, Wark G, Ellis AR, Wear J, Wieringa GE, Trainer PJ. Variation in GH and IGF-I assays limits the applicability of international consensus criteria to local practice. Clin Endocrinol. 2007;67:65–70. doi: 10.1111/j.1365-2265.2007.02836.x. [DOI] [PubMed] [Google Scholar]
- 49.Colao A, Ferone D, Marzullo P, Lombardi G. Systemic complications of acromegaly: epidemiology, pathogenesis, and management. Endocr Rev. 2004;25:102–152. doi: 10.1210/er.2002-0022. [DOI] [PubMed] [Google Scholar]
- 50.Clemmons DR. The relative roles of growth hormone and IGF-1 in controlling insulin sensitivity. J Clin Invest. 2004;113:25–27. doi: 10.1172/JCI200420660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mazziotti G, Floriani I, Bonadonna S, Torri V, Chanson P, Giustina A. Effects of somatostatin analogs on glucose homeostasis: a metaanalysis of acromegaly studies. J Clin Endocrinol Metab. 2009;94:1500–1508. doi: 10.1210/jc.2008-2332. [DOI] [PubMed] [Google Scholar]
- 52.Drake WM, Rowles SV, Roberts ME, Fode FK, Besser GM, Monson JP, Trainer PJ. Insulin sensitivity and glucose tolerance improve in patients with acromegaly converted from depot octreotide to Pegvisomant. Eur J Endocrinol. 2003;149:521–527. doi: 10.1530/eje.0.1490521. [DOI] [PubMed] [Google Scholar]
- 53.Urbani C, Sardella C, Calevro A, Rossi G, Scattina I, Lombardi M, Lupi I, Manetti L, Martino E, Bogazzi F (2013) Effects of medical therapies for acromegaly on glucose metabolism. Eur J Endocrinol 169:99–108 [DOI] [PubMed]
- 54.Ghigo E, Biller BM, Colao A, Kourides IA, Rajicic N, Hutson RK, De Marinis L, Klibanski A. Comparison of Pegvisomant and long-acting octreotide in patients with acromegaly naïve to radiation and medical therapy. J Endocrinol Invest. 2009;32:924–933. doi: 10.1007/BF03345774. [DOI] [PubMed] [Google Scholar]
- 55.Lindberg-Larsen R, Møller N, Schmitz O, Nielsen S, Andersen M, Orskov H, Jørgensen JO. The impact of Pegvisomant treatment on substrate metabolism and insulin sensitivity in patients with acromegaly. J Clin Endocrinol Metab. 2007;92:1724–1728. doi: 10.1210/jc.2006-2276. [DOI] [PubMed] [Google Scholar]
- 56.Higham CE, Rowles S, Russell-Jones D, Umpleby AM, Trainer PJ. Pegvisomant improves insulin sensitivity and reduces overnight free fatty acid concentrations in patients with acromegaly. J Clin Endocrinol Metab. 2009;94:2459–2463. doi: 10.1210/jc.2008-2086. [DOI] [PubMed] [Google Scholar]
- 57.Rose DR, Clemmons DR. Growth hormone receptor antagonist improves insulin resistance in acromegaly. Growth Horm IGF Res. 2002;12:418–424. doi: 10.1016/s1096-6374(02)00083-7. [DOI] [PubMed] [Google Scholar]
- 58.Trainer PJ, Ezzat S, D’Souza GA, Layton G, Strasburger CJ. A randomized, controlled, multicentre trial comparing Pegvisomant alone with combination therapy of Pegvisomant and long-acting octreotide in patients with acromegaly. Clin Endocrinol. 2009;71:549–557. doi: 10.1111/j.1365-2265.2009.03620.x. [DOI] [PubMed] [Google Scholar]
- 59.Parkinson C, Drake WM, Wieringa G, Yates AP, Besser GM, Trainer PJ. Serum lipoprotein changes following IGF-I normalization using a growth hormone receptor antagonist in acromegaly. Clin Endocrinol. 2002;56:303–311. doi: 10.1046/j.1365-2265.2002.01460.x. [DOI] [PubMed] [Google Scholar]
- 60.Sesmilo G, Fairfield WP, Katznelson L, Pulaski K, Freda PU, Bonert V, Dimaraki E, Stavrou S, Vance ML, Hayden D, Klibanski A. Cardiovascular risk factors in acromegaly before and after normalization of serum IGF-I levels with the GH antagonist Pegvisomant. J Clin Endocrinol Metab. 2002;87:1692–1699. doi: 10.1210/jcem.87.4.8364. [DOI] [PubMed] [Google Scholar]
- 61.Berg C, Petersenn S, Lahner H, Herrmann BL, Buchfelder M, Droste M, Stalla GK, Strasburger CJ, Roggenbuck U, Lehmann N, Moebus S, Jöckel KH, Möhlenkamp S, Erbel R, Saller B, Mann K, Investigative Group of the Heinz Nixdorf Recall Study and the German Pegvisomant Observational Study Board and Investigators Cardiovascular risk factors in patients with uncontrolled and long-term acromegaly: comparison with matched data from the general population and the effect of disease control. J Clin Endocrinol Metab. 2010;95:3648–3656. doi: 10.1210/jc.2009-2570. [DOI] [PubMed] [Google Scholar]
- 62.Colao A, Pivonello R, Grasso LF, Auriemma RS, Galdiero M, Savastano S, Lombardi G. Determinants of cardiac disease in newly diagnosed patients with acromegaly: results of a 10 year survey study. Eur J Endocrinol. 2011;165:713–721. doi: 10.1530/EJE-11-0408. [DOI] [PubMed] [Google Scholar]
- 63.Pivonello R, Galderisi M, Auriemma RS, De Martino MC, Galdiero M, Ciccarelli A, D’Errico A, Kourides I, Burman P, Lombardi G, Colao A. Treatment with growth hormone receptor antagonist in acromegaly: effect on cardiac structure and performance. J Clin Endocrinol Metab. 2007;92:476–482. doi: 10.1210/jc.2006-1587. [DOI] [PubMed] [Google Scholar]
- 64.Auriemma RS, Pivonello R, De Martino MC, Cudemo G, Grasso LF, Galdiero M, Perone Y, Colao A. Treatment with GH receptor antagonist in acromegaly: effect on cardiac arrhythmias. Eur J Endocrinol. 2012;168:15–22. doi: 10.1530/EJE-12-0596. [DOI] [PubMed] [Google Scholar]
- 65.De Martino MC, Auriemma RS, Brevetti G, Vitale G, Schiano V, Galdiero M, Grasso L, Lombardi G, Colao A, Pivonello R. The treatment with growth hormone receptor antagonist in acromegaly: effect on vascular structure and function in patients resistant to somatostatin analogues. J Endocrinol Invest. 2010;33:663–670. doi: 10.1007/BF03346667. [DOI] [PubMed] [Google Scholar]
- 66.Giustina A, Mazziotti G, Canalis E. Growth hormone, insulin-like growth factors, and the skeleton. Endocr Rev. 2008;29:535–559. doi: 10.1210/er.2007-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mazziotti G, Bianchi A, Bonadonna S, Nuzzo M, Cimino V, Fusco A, De Marinis L, Giustina A. Increased prevalence of radiological spinal deformities in adult patients with GH deficiency: influence of GH replacement therapy. J Bone Miner Res. 2006;21:520–528. doi: 10.1359/jbmr.060112. [DOI] [PubMed] [Google Scholar]
- 68.Bonadonna S, Mazziotti G, Nuzzo M, Bianchi A, Fusco A, De Marinis L, Giustina A. Increased prevalence of radiological spinal deformities in active acromegaly: a cross-sectional study in postmenopausal women. J Bone Miner Res. 2005;20:1837–1844. doi: 10.1359/JBMR.050603. [DOI] [PubMed] [Google Scholar]
- 69.Mazziotti G, Gola M, Bianchi A, Porcelli T, Giampietro A, Cimino V, Doga M, Gazzaruso C, De Marinis L, Giustina A. Influence of diabetes mellitus on vertebral fractures in men with acromegaly. Endocrine. 2011;40:102–108. doi: 10.1007/s12020-011-9486-x. [DOI] [PubMed] [Google Scholar]
- 70.Mazziotti G, Bianchi A, Porcelli T, Mormando M, Maffezzoni F, Cristiano A, Giampietro A, De Marinis L, Giustina A. Vertebral fractures in patients with acromegaly: a 3-year prospective study. J Clin Endocrinol Metab. 2013;98:3402–3410. doi: 10.1210/jc.2013-1460. [DOI] [PubMed] [Google Scholar]
- 71.Fairfield WP, Sesmilo G, Katznelson L, Pulaski K, Freda PU, Stavrou S, Kleinberg D, Klibanski A. Effects of a growth hormone receptor antagonist on bone markers in acromegaly. Clin Endocrinol. 2002;57:385–390. doi: 10.1046/j.1365-2265.2002.01624.x. [DOI] [PubMed] [Google Scholar]
- 72.Parkinson C, Kassem M, Heickendorff L, Flyvbjerg A, Trainer PJ. Pegvisomant-induced serum insulin-like growth factor-I normalization in patients with acromegaly returns elevated markers of bone turnover to normal. J Clin Endocrinol Metab. 2003;88:5650–5655. doi: 10.1210/jc.2003-030772. [DOI] [PubMed] [Google Scholar]
- 73.Jimenez C, Ayala-Ramirez M, Liu J, Nunez R, Gagel RF. Inhibition of growth hormone receptor activation by Pegvisomant may increase bone density in acromegaly. Horm Metab Res. 2011;43:55–61. doi: 10.1055/s-0030-1268006. [DOI] [PubMed] [Google Scholar]
- 74.Colao A, Auriemma RS, Lombardi G, Pivonello R. Resistance to somatostatin analogs in acromegaly. Endocr Rev. 2011;32:247–271. doi: 10.1210/er.2010-0002. [DOI] [PubMed] [Google Scholar]
- 75.Murray RD, Melmed S. A critical analysis of clinically available somatostatin analog formulations for therapy of acromegaly. J Clin Endocrinol Metab. 2008;93:2957–2968. doi: 10.1210/jc.2008-0027. [DOI] [PubMed] [Google Scholar]
- 76.Howlett TA, Willis D, Walker G, Wass JAH, Trainer PJ, UK Acromegaly Register Study Group (UKAR-3) Control of growth hormone and IGF1 in patients with acromegaly in the UK: responses to medical treatment with somatostatin analogues and dopamine agonists. Clin Endocrinol. 2013;79:689–699. doi: 10.1111/cen.12207. [DOI] [PubMed] [Google Scholar]
- 77.Carmichael JD, Bonert VS, Nuno M, Ly D, Melmed S. Acromegaly clinical trial methodology impact on reported biochemical efficacy rates of somatostatin receptor ligand treatments: a meta-analysis. J Clin Endocrinol Metab. 2014;99:1825–1833. doi: 10.1210/jc.2013-3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Amato G, Mazziotti G, Rotondi M, Iorio S, Doga M, Sorvillo F, Manganella G, Di Salle F, Giustina A, Carella C. Long-term effects of lanreotide SR and octreotide LAR on tumour shrinkage and GH hypersecretion in patients with previously untreated acromegaly. Clin Endocrinol. 2002;56:65–71. doi: 10.1046/j.0300-0664.2001.01438.x. [DOI] [PubMed] [Google Scholar]
- 79.Mazziotti G, Giustina A. Effects of lanreotide SR and Autogel on tumor mass in patients with acromegaly: a systematic review. Pituitary. 2010;13:60–67. doi: 10.1007/s11102-009-0169-z. [DOI] [PubMed] [Google Scholar]
- 80.Giustina A, Mazziotti G, Torri V, Spinello M, Floriani I, Melmed S. Meta-analysis on the effects of octreotide on tumor mass in acromegaly. PLoS ONE. 2012;7:e36411. doi: 10.1371/journal.pone.0036411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Salenave S, Boyce AM, Collins MT, Chanson P. Acromegaly and McCune-Albright syndrome. J Clin Endocrinol Metab. 2014;99(6):1955–1969. doi: 10.1210/jc.2013-3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Colao A, Attanasio R, Pivonello R, Cappabianca P, Cavallo LM, Lasio G, Lodrini A, Lombardi G, Cozzi R. Partial surgical removal of growth hormone-secreting pituitary tumors enhances the response to somatostatin analogs in acromegaly. J Clin Endocrinol Metab. 2006;91:85–92. doi: 10.1210/jc.2005-1208. [DOI] [PubMed] [Google Scholar]
- 83.Thorner MO, Strasburger CJ, Wu Z, Straume M, Bidlingmaier M, Pezzoli SS, Zib K, Scarlett JC, Bennett WF. Growth hormone (GH) receptor blockade with a PEG-modified GH (B2036-PEG) lowers serum insulin-like growth factor-I but does not acutely stimulate serum GH. J Clin Endocrinol Metab. 1999;84:2098–2103. doi: 10.1210/jcem.84.6.5732. [DOI] [PubMed] [Google Scholar]
- 84.SOMAVERT EPAR: Available at this URL: http://www.ema.europa.eu/docs/enGB/documentlibrary/EPAR-Summaryforthepublic/human/000409/WC500054622.pdf
- 85.Jen J, LaBadie RR, Liang Y, Crownover PH, Gao X, Hey-Hadavi JH. Pegvisomant bioavability of single30 mg/ml subcutaneous injection compared to two 15 mg/ml subcutaneous injections: a pharmacokinetic, safety and tolerability study. Growth Horm IGF Res. 2013;23:114–119. doi: 10.1016/j.ghir.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 86.Marazuela M, Ramos-Leví A, Sampedro-Núñez M, Bernabeu I. Cabergoline treatment in acromegaly: pros. Endocrine. 2014;46:215–219. doi: 10.1007/s12020-014-0206-1. [DOI] [PubMed] [Google Scholar]
- 87.Kasuki L, Vieira Neto L, Gadelha MR. Cabergoline treatment in acromegaly: cons. Endocrine. 2014;46:220–225. doi: 10.1007/s12020-014-0183-4. [DOI] [PubMed] [Google Scholar]
- 88.Higham CE, Atkinson AB, Aylwin S, Bidlingmaier M, Drake WM, Lewis A, Martin NM, Moyes V, Newell-Price J, Trainer PJ. Effective combination treatment with cabergoline and low-dose Pegvisomant in active acromegaly: a prospective clinical trial. J Clin Endocrinol Metab. 2012;97:1187–1193. doi: 10.1210/jc.2011-2603. [DOI] [PubMed] [Google Scholar]
- 89.Bernabeu I, Alvarez-Escolá C, Paniagua AE, Lucas T, Pavón I, Cabezas-Agrícola JM, Casanueva FF, Marazuela M. Pegvisomant and cabergoline combination therapy in acromegaly. Pituitary. 2013;16:101–108. doi: 10.1007/s11102-012-0382-z. [DOI] [PubMed] [Google Scholar]
- 90.Feenstra J, de Herder WW, ten Have SM, van den Beld AW, Feelders RA, Janssen JA, van der Lely AJ. Combined therapy with somatostatin analogues and weekly Pegvisomant in active acromegaly. Lancet. 2005;365:1644–1646. doi: 10.1016/S0140-6736(05)63011-5. [DOI] [PubMed] [Google Scholar]
- 91.Neggers SJ, van Aken MO, Janssen JA, Feelders RA, de Herder WW, van der Lely AJ. Long-term efficacy and safety of combined treatment of somatostatin analogs and Pegvisomant in acromegaly. J Clin Endocrinol Metab. 2007;92:4598–4601. doi: 10.1210/jc.2007-1234. [DOI] [PubMed] [Google Scholar]
- 92.van der Lely AJ, Bernabeu I, Cap J, Caron P, Colao A, Marek J, Neggers S, Birman P. Coadministration of lanreotide Autogel and Pegvisomant normalizes IGF1 levels and is well tolerated in patients with acromegaly partially controlled by somatostatin analogs alone. Eur J Endocrinol. 2011;164:325–333. doi: 10.1530/EJE-10-0867. [DOI] [PubMed] [Google Scholar]
- 93.Jørgensen JO, Feldt-Rasmussen U, Frystyk J, Chen JW, Kristensen LØ, Hagen C, Ørskov H. Cotreatment of acromegaly with a somatostatin analog and a growth hormone receptor antagonist. J Clin Endocrinol Metab. 2005;90:5627–5631. doi: 10.1210/jc.2005-0531. [DOI] [PubMed] [Google Scholar]
- 94.Leung KC, Doyle N, Ballesteros M, Waters MJ, Ho KK. Insulin regulation of human hepatic growth hormone receptors: divergent effects on biosynthesis and surface translocation. J Clin Endocrinol Metab. 2000;85:4712–4720. doi: 10.1210/jcem.85.12.7017. [DOI] [PubMed] [Google Scholar]
- 95.Bianchi A, Valentini F, Iuorio R, Poggi M, Baldelli R, Passeri M, Giampietro A, Tartaglione L, Chiloiro S, Appetecchia M, Gargiulo P, Fabbri A, Toscano V, Pontecorvi A, De Marinis L. Long-term treatment of somatostatin analog-refractory growth hormone-secreting pituitary tumors with Pegvisomant alone or combined with long-acting somatostatin analogs: a retrospective analysis of clinical practice and outcomes. J Exp Clin Cancer Res. 2013;32:40. doi: 10.1186/1756-9966-32-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.De Marinis L, Bianchi A, Fusco A, Cimino V, Mormando M, Tilaro L, Mazziotti G, Pontecorvi A, Giustina A. Long-term effects of the combination of Pegvisomant with somatostatin analogs (SSA) on glucose homeostasis in non-diabetic patients with active acromegaly partially resistant to SSA. Pituitary. 2007;10:227–232. doi: 10.1007/s11102-007-0037-7. [DOI] [PubMed] [Google Scholar]
- 97.Bonert VS, Kennedy L, Petersenn S, Barkan A, Carmichael J, Melmed S. Lipodystrophy in patients with acromegaly receiving Pegvisomant. J Clin Endocrinol Metab. 2008;93:3515–3518. doi: 10.1210/jc.2008-0833. [DOI] [PubMed] [Google Scholar]
- 98.Maffei P, Martini C, Pagano C, Sicolo N, Corbetti F. Lipohypertrophy in acromegaly induced by the new growth hormone receptor antagonist Pegvisomant. Ann Intern Med. 2006;145:310–312. doi: 10.7326/0003-4819-145-4-200608150-00017. [DOI] [PubMed] [Google Scholar]
- 99.Biering H, Saller B, Bauditz J, Pirlich M, Rudolph B, Johne A, Buchfelder M, Mann K, Droste M, Schreiber I, Lochs H, Strasburger CJ. Elevated transaminases during medical treatment of acromegaly: a review of the German Pegvisomant surveillance experience and a report of a patient with histologically proven chronic mild active hepatitis. Eur J Endocrinol. 2006;154:213–220. doi: 10.1530/eje.1.02079. [DOI] [PubMed] [Google Scholar]
- 100.Neggers SJ, de Herder WW, Janssen JA, Feelders RA, van der Lely AJ. Combined treatment for acromegaly with long-acting somatostatin analogs and Pegvisomant: long-term safety for up to 4.5 years (median 2.2 years) of follow-up in 86 patients. Eur J Endocrinol. 2009;160:529–533. doi: 10.1530/EJE-08-0843. [DOI] [PubMed] [Google Scholar]
- 101.Feenstra J, Van Aken MO, de Herder WW, Feelders RA, Van der Lely AJ. Drug-induced hepatitis in an acromegalic patient during combined treatment with Pegvisomant and octreotide long-acting repeatable attributed to the use of Pegvisomant. Eur J Endocrinol. 2006;154:805–806. doi: 10.1530/eje.1.02160. [DOI] [PubMed] [Google Scholar]
- 102.Trainer PJ, Ezzat S, D’Souza GA, Layton G, Strasburger CJ. A randomized, controlled, multicentre trial comparing Pegvisomant alone with combination therapy of Pegvisomant and long-acting octreotide in patients with acromegaly. Clin Endocrinol. 2009;71:549–557. doi: 10.1111/j.1365-2265.2009.03620.x. [DOI] [PubMed] [Google Scholar]
- 103.Madsen M, Poulsen PL, Ørskov H, Møller N, Jørgensen JOL. Cotreatment with Pegvisomant and a Somatostatin Analog (SA) in SA-Responsive Acromegalic Patients. J Clin Endocrinol Metab. 2011;96:2405–2413. doi: 10.1210/jc.2011-0654. [DOI] [PubMed] [Google Scholar]
- 104.Bernabeu I, Marazuela M, Lucas T, Loidi L, Alvarez-Escola´ C, Luque-Ramírez M, Fernandez-Rodriguez E, Paniagua AE, Quinteiro C, Casanueva FF. Pegvisomant-induced liver injury is related to the UGT1A1*28 polymorphism of Gilbert’s syndrome. J Clin Endocrinol Metab. 2010;95:2147–2154. doi: 10.1210/jc.2009-2547. [DOI] [PubMed] [Google Scholar]
- 105.Jimenez C, Burman P, Abs R, Clemmons DR, Drake WM, Hutson KR, Messig M, Thorner MO, Trainer PJ, Gagel RF. Follow-up of pituitary tumor volume in patients with acromegaly treated with Pegvisomant in clinical trials. Eur J Endocrinol. 2008;159:517–523. doi: 10.1530/EJE-08-0205. [DOI] [PubMed] [Google Scholar]
- 106.Buchfelder M, Weigel D, Droste M, Mann K, Saller B, Brubach K, Stalla GK, Bidlingmaier M, Strasburger CJ. Pituitary tumor size in acromegaly during Pegvisomant treatment: experience from MR re-evaluations of the German Pegvisomant Observational Study. Eur J Endocrinol. 2009;161:27–35. doi: 10.1530/EJE-08-0910. [DOI] [PubMed] [Google Scholar]
- 107.Buhk JH, Jung S, Psychogios MN, Goricke S, Hartz S, Schulz-Heise S, Klingebiel R, Forsting M, Bruckmann H, Dorfler A, Jordan M, Buchfelder M, Knauth M. Tumor volume of growth hormone secreting pituitary adenomas during treatment with Pegvisomant: a prospective multicenter study. J Clin Endocrinol Metab. 2010;95:552–558. doi: 10.1210/jc.2009-1239. [DOI] [PubMed] [Google Scholar]
- 108.Jehle S, Reyes CM, Sundeen RE, Freda PU. Alternate-day administration of Pegvisomant maintains normal serum insulin-like growth factor-i levels in patients with acromegaly. J Clin Endocrinol Metab. 2005;90:1588–1593. doi: 10.1210/jc.2004-1967. [DOI] [PubMed] [Google Scholar]
- 109.Brian SR, Bidlingmaier M, Wajnrajch MP, Weinzimer SA, Inzucchi SE. Treatment of acromegaly with Pegvisomant during pregnancy: maternal and fetal effects. J Clin Endocrinol Metab. 2007;92:3374–3377. doi: 10.1210/jc.2007-0997. [DOI] [PubMed] [Google Scholar]
- 110.Didoni G, Grottoli S, Gasco V, Battistini M, Ferone D, Giusti M, Ragazzoni F, Ruffo P, Ghigo E, Minuto F. Cost-of-illness study in acromegalic patients in Italy. J Endocrinol Invest. 2004;27:1034–1039. doi: 10.1007/BF03345306. [DOI] [PubMed] [Google Scholar]
- 111.Ben-Shlomo A, Sheppard MC, Stephens JM, Pulgar S, Melmed S. Clinical, quality of life, and economic value of acromegaly disease control. Pituitary. 2011;14:284–294. doi: 10.1007/s11102-011-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Moore DJ, Adi Y, Connock MJ, Bayliss S. Clinical effectiveness and cost-effectiveness of Pegvisomant for the treatment of acromegaly: a systematic review and economic evaluation. BMC Endocrinol Disorder. 2009;9:20. doi: 10.1186/1472-6823-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.De Marinis L, Bonadonna S, Bianchi A, Maira G, Giustina A. Primary empty sella. J Clin Endocrinol Metab. 2005;90:5471–5477. doi: 10.1210/jc.2005-0288. [DOI] [PubMed] [Google Scholar]
- 114.Sandret L, Maison P, Chanson P. Place of cabergoline in acromegaly: a meta-analysis. J Clin Endocrinol Metab. 2011;96:1325–1335. doi: 10.1210/jc.2010-2443. [DOI] [PubMed] [Google Scholar]
- 115.Giustina A, Karamouzis I, Patelli I, Mazziotti G. Octreotide for acromegaly treatment: a reappraisal. Expert Opin Pharmacother. 2013;14:2433–2447. doi: 10.1517/14656566.2013.847090. [DOI] [PubMed] [Google Scholar]
- 116.Maison P, Tropeano AI, Macquin-Mavier I, Giustina A, Chanson P. Impact of somatostatin analogs on the heart in acromegaly: a metaanalysis. J Clin Endocrinol Metab. 2007;92:1743–1747. doi: 10.1210/jc.2006-2547. [DOI] [PubMed] [Google Scholar]


