Abstract
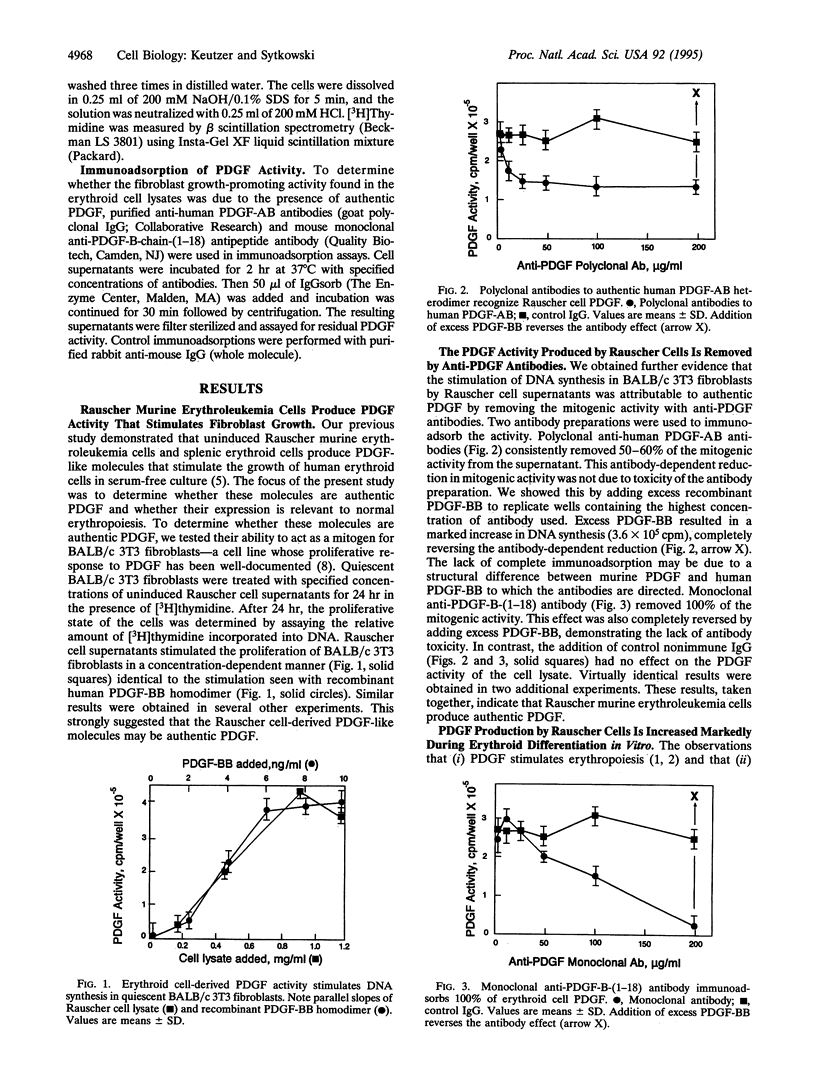
Erythroid progenitor growth in vitro is stimulated by exogenous platelet-derived growth factor (PDGF). We now report that both normal and transformed erythroid progenitor cells produce authentic PDGF in vitro and in vivo. Importantly, this production is highly regulated during erythropoiesis. Addition of soluble lysates from Rauscher murine erythroleukemia cells--an erythropoietin-responsive model progenitor cell line--to quiescent BALB/c 3T3 fibroblasts resulted in a mitogenic response identical to that observed with the addition of authentic recombinant PDGF. Polyclonal and monoclonal anti-PDGF antibodies immunoabsorbed 50-100% of this activity. Induction of Rauscher cell differentiation in vitro with dimethyl sulfoxide or erythropoietin for 48-72 hr markedly upregulated PDGF production by 17- to 18-fold and 14- to 38-fold, respectively. Importantly, stimulation of normal erythropoiesis in vivo in mice treated either with phenylhydrazine or with erythropoietin increased PDGF levels in the spleen by 11- to 48-fold and 20- to 34-fold, respectively. These results strongly suggest a role for erythroid cell-derived PDGF in normal erythropoiesis and provide documentation of the regulated production of a pleiotropic cytokine by erythroid cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auron P. E., Webb A. C., Rosenwasser L. J., Mucci S. F., Rich A., Wolff S. M., Dinarello C. A. Nucleotide sequence of human monocyte interleukin 1 precursor cDNA. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7907–7911. doi: 10.1073/pnas.81.24.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham H., Vannier E., Chi S. Y., Dinarello C. A., Groopman J. E. Cytokine gene expression and synthesis by human megakaryocytic cells. Int J Cell Cloning. 1992 Mar;10(2):70–79. doi: 10.1002/stem.5530100203. [DOI] [PubMed] [Google Scholar]
- Bickel M., Tsuda H., Amstad P., Evequoz V., Mergenhagen S. E., Wahl S. M., Pluznik D. H. Differential regulation of colony-stimulating factors and interleukin 2 production by cyclosporin A. Proc Natl Acad Sci U S A. 1987 May;84(10):3274–3277. doi: 10.1073/pnas.84.10.3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitterman P. B., Wewers M. D., Rennard S. I., Adelberg S., Crystal R. G. Modulation of alveolar macrophage-driven fibroblast proliferation by alternative macrophage mediators. J Clin Invest. 1986 Mar;77(3):700–708. doi: 10.1172/JCI112364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornfeldt K. E., Arnqvist H. J., Norstedt G. Regulation of insulin-like growth factor-I gene expression by growth factors in cultured vascular smooth muscle cells. J Endocrinol. 1990 Jun;125(3):381–386. doi: 10.1677/joe.0.1250381. [DOI] [PubMed] [Google Scholar]
- Canalis E., Pash J., Gabbitas B., Rydziel S., Varghese S. Growth factors regulate the synthesis of insulin-like growth factor-I in bone cell cultures. Endocrinology. 1993 Jul;133(1):33–38. doi: 10.1210/endo.133.1.8319580. [DOI] [PubMed] [Google Scholar]
- Chern Y., Yonekura S., Sytkowski A. J. Potentiation of the erythropoietin response by dimethyl sulfoxide priming of erythroleukemia cells: evidence for interaction of two signaling pathways. Blood. 1990 Dec 1;76(11):2204–2209. [PubMed] [Google Scholar]
- Correa P. N., Eskinazi D., Axelrad A. A. Circulating erythroid progenitors in polycythemia vera are hypersensitive to insulin-like growth factor-1 in vitro: studies in an improved serum-free medium. Blood. 1994 Jan 1;83(1):99–112. [PubMed] [Google Scholar]
- Cotton E. W., Means R. T., Jr, Cline S. M., Krantz S. B. Quantitation of insulin-like growth factor-I binding to highly purified human erythroid colony-forming units. Exp Hematol. 1991 May;19(4):278–281. [PubMed] [Google Scholar]
- Dainiak N., Davies G., Kalmanti M., Lawler J., Kulkarni V. Platelet-derived growth factor promotes proliferation of erythropoietic progenitor cells in vitro. J Clin Invest. 1983 May;71(5):1206–1214. doi: 10.1172/JCI110869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dainiak N., Kreczko S. Interactions of insulin, insulinlike growth factor II, and platelet-derived growth factor in erythropoietic culture. J Clin Invest. 1985 Sep;76(3):1237–1242. doi: 10.1172/JCI112079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dainiak N., Najman A., Kreczko S., Baillou C., Mier J., Feldman L., Gorin N. C., Duhamel G. B-lymphocytes as a source of cell surface growth-promoting factors for hematopoietic progenitors. Exp Hematol. 1987 Nov;15(10):1086–1096. [PubMed] [Google Scholar]
- Dainiak N., Sutter D., Kreczko S. L-triiodothyronine augments erythropoietic growth factor release from peripheral blood and bone marrow leukocytes. Blood. 1986 Dec;68(6):1289–1297. [PubMed] [Google Scholar]
- Delwiche F., Raines E., Powell J., Ross R., Adamson J. Platelet-derived growth factor enhances in vitro erythropoiesis via stimulation of mesenchymal cells. J Clin Invest. 1985 Jul;76(1):137–142. doi: 10.1172/JCI111936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid J., Ebert R. F., Gesell M. S., Spivak J. L. Intracellular growth factors in polycythemia vera and other myeloproliferative disorders. Proc Natl Acad Sci U S A. 1987 Jan;84(2):532–536. doi: 10.1073/pnas.84.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleit H. B., Rabinovitch M. Production of interferon by in vitro derived bone marrow macrophages. Cell Immunol. 1981 Jan 15;57(2):495–504. doi: 10.1016/0008-8749(81)90107-6. [DOI] [PubMed] [Google Scholar]
- Gewirtz A. M., Xu W. Y., Mangan K. F. Role of natural killer cells, in comparison with T lymphocytes and monocytes, in the regulation of normal human megakaryocytopoiesis in vitro. J Immunol. 1987 Nov 1;139(9):2915–2924. [PubMed] [Google Scholar]
- Handler J. A., Danilowicz R. M., Eling T. E. Mitogenic signaling by epidermal growth factor (EGF), but not platelet-derived growth factor, requires arachidonic acid metabolism in BALB/c 3T3 cells. Modulation of EGF-dependent c-myc expression by prostaglandins. J Biol Chem. 1990 Mar 5;265(7):3669–3673. [PubMed] [Google Scholar]
- Hara H., Ogawa M. Erthropoietic precursors in mice with phenylhydrazine-induced anemia. Am J Hematol. 1976;1(4):453–458. doi: 10.1002/ajh.2830010410. [DOI] [PubMed] [Google Scholar]
- Hermine O., Beru N., Pech N., Goldwasser E. An autocrine role for erythropoietin in mouse hematopoietic cell differentiation. Blood. 1991 Nov 1;78(9):2253–2260. [PubMed] [Google Scholar]
- June C. H., Ledbetter J. A., Lindsten T., Thompson C. B. Evidence for the involvement of three distinct signals in the induction of IL-2 gene expression in human T lymphocytes. J Immunol. 1989 Jul 1;143(1):153–161. [PubMed] [Google Scholar]
- Kehrl J. H., Wakefield L. M., Roberts A. B., Jakowlew S., Alvarez-Mon M., Derynck R., Sporn M. B., Fauci A. S. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986 May 1;163(5):1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirstein M., Aston C., Hintz R., Vlassara H. Receptor-specific induction of insulin-like growth factor I in human monocytes by advanced glycosylation end product-modified proteins. J Clin Invest. 1992 Aug;90(2):439–446. doi: 10.1172/JCI115879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal G. A simple microassay for erythropoietin based on 3H-thymidine incorporation into spleen cells from phenylhydrazine treated mice. Exp Hematol. 1983 Aug;11(7):649–660. [PubMed] [Google Scholar]
- Kurtz A., Härtl W., Jelkmann W., Zapf J., Bauer C. Activity in fetal bovine serum that stimulates erythroid colony formation in fetal mouse livers is insulinlike growth factor I. J Clin Invest. 1985 Oct;76(4):1643–1648. doi: 10.1172/JCI112149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary J. F., Farley B. A., Giuliano R., Kosciolek B. A., La Bella S., Rowley P. T. Induction of megakaryocytic characteristics in human leukemic cell line K562: polyploidy, inducers, and secretion of mitogenic activity. J Biol Regul Homeost Agents. 1987 Apr-Jun;1(2):73–80. [PubMed] [Google Scholar]
- Levitt L. J., Nagler A., Lee F., Abrams J., Shatsky M., Thompson D. Production of granulocyte/macrophage-colony-stimulating factor by human natural killer cells. Modulation by the p75 subunit of the interleukin 2 receptor and by the CD2 receptor. J Clin Invest. 1991 Jul;88(1):67–75. doi: 10.1172/JCI115306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. B., Prickett K. S., Larsen A., Grabstein K., Weaver M., Wilson C. B. Restricted production of interleukin 4 by activated human T cells. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9743–9747. doi: 10.1073/pnas.85.24.9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinkiewicz J., Chain B. Antigen-specific inhibition of IL-2 and IL-3 production in contact sensitivity to TNP. Immunology. 1989 Oct;68(2):185–189. [PMC free article] [PubMed] [Google Scholar]
- Merchav S., Graif Z., Skottner A. In-vitro response of erythroid progenitors from children with thalassaemia major to human growth hormone and insulin-like growth factor I. Clin Endocrinol (Oxf) 1993 Aug;39(2):207–211. doi: 10.1111/j.1365-2265.1993.tb01775.x. [DOI] [PubMed] [Google Scholar]
- Miura M., Li S. W., Dumenil G., Baserga R. Platelet-derived growth factor-induced expression of messenger RNA for the proliferating cell nuclear antigen requires a functional receptor for the insulin-like growth factor I. Cancer Res. 1994 May 1;54(9):2472–2477. [PubMed] [Google Scholar]
- Muta K., Krantz S. B. Apoptosis of human erythroid colony-forming cells is decreased by stem cell factor and insulin-like growth factor I as well as erythropoietin. J Cell Physiol. 1993 Aug;156(2):264–271. doi: 10.1002/jcp.1041560207. [DOI] [PubMed] [Google Scholar]
- Papayannopoulou T., Raines E., Collins S., Nakamoto B., Tweeddale M., Ross R. Constitutive and inducible secretion of platelet-derived growth factor analogs by human leukemic cell lines coexpressing erythroid and megakaryocytic markers. J Clin Invest. 1987 Mar;79(3):859–866. doi: 10.1172/JCI112895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel H. R., Choi H. S., Sytkowski A. J. Activation of two discrete signaling pathways by erythropoietin. J Biol Chem. 1992 Oct 25;267(30):21300–21302. [PubMed] [Google Scholar]
- Piacibello W., Lu L., Wachter M., Rubin B., Broxmeyer H. E. Release of granulocyte-macrophage colony stimulating factors from major histocompatibility complex class II antigen-positive monocytes is enhanced by human gamma interferon. Blood. 1985 Dec;66(6):1343–1351. [PubMed] [Google Scholar]
- Pistoia V., Zupo S., Corcione A., Roncella S., Matera L., Ghio R., Ferrarini M. Production of colony-stimulating activity by human natural killer cells: analysis of the conditions that influence the release and detection of colony-stimulating activity. Blood. 1989 Jul;74(1):156–164. [PubMed] [Google Scholar]
- Pledger W. J., Stiles C. D., Antoniades H. N., Scher C. D. Induction of DNA synthesis in BALB/c 3T3 cells by serum components: reevaluation of the commitment process. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4481–4485. doi: 10.1073/pnas.74.10.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders M., Sorba S., Dainiak N. Insulin-like growth factors stimulate erythropoiesis in serum-substituted umbilical cord blood cultures. Exp Hematol. 1993 Jan;21(1):25–30. [PubMed] [Google Scholar]
- Sawada K., Krantz S. B., Dessypris E. N., Koury S. T., Sawyer S. T. Human colony-forming units-erythroid do not require accessory cells, but do require direct interaction with insulin-like growth factor I and/or insulin for erythroid development. J Clin Invest. 1989 May;83(5):1701–1709. doi: 10.1172/JCI114070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokado K., Raines E. W., Madtes D. K., Barrett T. B., Benditt E. P., Ross R. A significant part of macrophage-derived growth factor consists of at least two forms of PDGF. Cell. 1985 Nov;43(1):277–286. doi: 10.1016/0092-8674(85)90033-9. [DOI] [PubMed] [Google Scholar]
- Spangler R., Bailey S. C., Sytkowski A. J. Erythropoietin increases c-myc mRNA by a protein kinase C-dependent pathway. J Biol Chem. 1991 Jan 15;266(2):681–684. [PubMed] [Google Scholar]
- Spivak J. L., Toretti D., Dickerman H. W. Effect of phenylhydrazine-induced hemolytic anemia on nuclear RNA polymerase activity of the mouse spleen. Blood. 1973 Aug;42(2):257–266. [PubMed] [Google Scholar]
- Sytkowski A. J., O'Hara C., Vanasse G., Armstrong M. J., Kreczko S., Dainiak N. Characterization of biologically active, platelet-derived growth factor-like molecules produced by murine erythroid cells in vitro and in vivo. J Clin Invest. 1990 Jan;85(1):40–46. doi: 10.1172/JCI114431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sytkowski A. J., Salvado A. J., Smith G. M., McIntyre C. J., deBoth N. J. Erythroid differentiation of clonal Rauscher erythroleukemia cells in response to erythropoietin or dimethyl sulfoxide. Science. 1980 Oct 3;210(4465):74–76. doi: 10.1126/science.6932101. [DOI] [PubMed] [Google Scholar]
- Uckun F. M., Vallera D. A., Wee S. L. B lymphocyte regulation of human hematopoiesis. J Immunol. 1985 Dec;135(6):3817–3822. [PubMed] [Google Scholar]
- Ververis J. J., Ku L., Delafontaine P. Regulation of insulin-like growth factor I receptors on vascular smooth muscle cells by growth factors and phorbol esters. Circ Res. 1993 Jun;72(6):1285–1292. doi: 10.1161/01.res.72.6.1285. [DOI] [PubMed] [Google Scholar]
- Yan X. Q., Brady G., Iscove N. N. Platelet-derived growth factor (PDGF) activates primitive hematopoietic precursors (pre-CFCmulti) by up-regulating IL-1 in PDGF receptor-expressing macrophages. J Immunol. 1993 Mar 15;150(6):2440–2448. [PubMed] [Google Scholar]
- de Both N. J., Vermey M., van't Hull E., Klootwijk-van-Dijke E., van Griensven L. J., Mol J. N., Stoof T. J. A new erythroid cell line induced by Rauscher murine leukaemia virus. Nature. 1978 Apr 13;272(5654):626–628. doi: 10.1038/272626a0. [DOI] [PubMed] [Google Scholar]
- van Lier R. A., Brouwer M., Rebel V. I., van Noesel C. J., Aarden L. A. Immobilized anti-CD3 monoclonal antibodies induce accessory cell-independent lymphokine production, proliferation and helper activity in human T lymphocytes. Immunology. 1989 Sep;68(1):45–50. [PMC free article] [PubMed] [Google Scholar]


