Abstract
Cycads are long-lived tropical and subtropical plants that contain azoxyglycosides (e.g., cycasin, macrozamin) and neurotoxic amino acids (notably β-N-methylamino-L-alanine L-BMAA), toxins that have been implicated in the etiology of a disappearing neurodegenerative disease, amyotrophic lateral sclerosis and parkinsonism-dementia complex that has been present in high incidence among three genetically distinct populations in the western Pacific. The neuropathology of amyotrophic lateral sclerosis/parkinsonism-dementia complex includes features suggestive of brain maldevelopment, an experimentally proven property of cycasin attributable to the genotoxic action of its aglycone methylazoxymethanol (MAM). This property of MAM has been exploited by neurobiologists as a tool to study perturbations of brain development. Depending on the neurodevelopmental stage, MAM can induce features in laboratory animals that model certain characteristics of epilepsy, schizophrenia, or ataxia. Studies in DNA repair-deficient mice show that MAM perturbs brain development through a DNA damage-mediated mechanism. The brain DNA lesions produced by systemic MAM appear to modulate the expression of genes that regulate neurodevelopment and contribute to neurodegeneration. Epigenetic changes (histone lysine methylation) have also been detected in the underdeveloped brain after MAM administration. The DNA damage and epigenetic changes produced by MAM and, perhaps by chemically related substances (e.g., nitrosamines, nitrosoureas, hydrazines), might be an important mechanism by which early-life exposure to genotoxicants can induce long-term brain dysfunction.
Keywords: cycasin, methylazoxymethanol, amyotrophic lateral sclerosis–parkinsonism-dementia complex, O6-methylguanine methyltransferase, alkyladenine DNA glycosylase
INTRODUCTION
There is substantial evidence that human exposure to cycad plant toxins is an important etiological trigger of a prototypical neurodegenerative disorder (tauopathy) in the western Pacific with features of amyotrophic lateral sclerosis, atypical parkinsonism, and Alzheimer’s-like dementia (ALS-PD; Spencer et al., 1991; Borenstein et al., 2007; Kisby and Spencer, 2011; Spencer et al., 2012). Among the Chamorro people of Guam, parkinsonism-dementia complex (PDC) and Guam Dementia are strongly associated with exposure to cycad plant toxins during childhood or adolescence, critically important periods of normal development of the human brain. Unknown is whether earlier-life exposure (e.g., in utero, neonatal) to cycad plant genotoxins disrupts the human brain to increase risk for neurodevelopmental disorders (e.g., epilepsy, schizophrenia). Despite a lack of human data, the active metabolite of the principal cycad plant toxin cycasin, methylazoxymethanol (MAM), has been widely used as a tool by neurobiologists to explore the complex mechanisms underlying brain maldevelopment in neurodevelopmental disorders. Depending on the period of brain development, MAM can induce an animal model that exhibits certain features of drug-resistant epilepsy, schizophrenia, or ataxia.
This review describes the opportunities for human contact with MAM, based on the distribution and uses of cycad species, the human disorders linked with cycad exposure, and the variety of neurodevelopmental disorders that can be experimentally induced in laboratory animals exposed systemically to MAM (in the form of MAM acetate) at different periods during prenatal and postnatal development. The mechanisms underlying cycasin-induced central nervous system (CNS) developmental perturbation are linked with MAM-induced DNA lesions that modify cell signaling and change neuronal behavior.
Although this article is focused on cycad toxins, their links with experimental and human brain disorders during development and later life, respectively, the traditional use of cycad seed for medicine or food in the three populations affected by ALS-PDC is disappearing hand in hand with declining disease rates. Although human exposure to cycad toxins has declined and is highly restricted to certain populations, the reader should recall that substances related to MAM are widely distributed worldwide in food, water, and the environment. MAM-like substances include (a) nitrosamines, which are formed in significant concentrations from nitrates and nitrites in preserved foodstuffs, as disinfection by-products in drinking water, in tobacco smoke, in certain industries (rubber), in industrial waste, and (b) hydrazines, which are found in mushrooms, polymer foam, rocket propellant, and antituberculous medicine (isoniazid). The reader will also note that nitrosamines and hydrazines, as well as MAM, are genotoxic substances widely known for their mutagenic and carcinogenic properties. Discussed elsewhere is the relationship at the molecular level between the mutagenic properties of MAM on cycling cells and the toxic properties of MAM on noncycling cells, namely neurons (Spencer et al., 2012). The present review is restricted to the neurotoxic action of cycasin/ MAM on the human and mammalian nervous system.
DISTRIBUTION AND USE OF CYCAD PLANTS
Although these plants are used for ornamental purposes worldwide, living indigenous species of cycads are confined to the tropics and subtropics (Fig. 1). The 11 genera of the single surviving order of the Cycadales include: the Australian genera Macrozamia (east, central, and south-west), Bowenia (northeast Queensland), and Lepidozamia (north-east Queensland and New South Wales); Zamia of the Americas (southern Florida, USA, West Indies, southern Mexico to the Amazon and Peru); Stangeria of southern Africa; and Cycas, which extends from northern Australia, through Oceania, across to the Indian subcontinent, and along the east African coast.
Figure 1.
World-wide distribution of cycads (green). [from Wikipedia, 20 March 2006].
Because cycads have food and medicinal potential, and are resistant to drought, cyclone, and fire, they have been used throughout human history as a subsistence and famine food (Whiting, 1964). Cycad seed and sago (extracted from the overground stem) have been used for food in certain cultures since ancient times. Australian Aboriginal groups have used cycad seed for food for at least 4500 years (Beaton, 1977); in 1996, certain Aboriginal groups in the Northern Territory, Australia, continued their practice of careful seed detoxification for occasional food use. In the Florida Panhandle, Zamia floridana was eaten by Aboriginal people (16th century), subsequently by Seminole Indians, and later by slaves and white settlers; the last cycad-processing plant closed in Miami, Florida, in 1926 (Spencer, 1990). By 1950, nearly six million pounds of sago (prepared from Cycas revoluta) was imported into the United States for use in the preparation of food, syrup, beer, and adhesives, as well as sizing for paper and textiles (Spencer, 1990). Incompletely detoxified cycad flour (Cycas spp.) was an important source of food for the Chamorro people of Guam during and following the Second World War (Whiting, 1963). There is a high correlation between the incidence of ALS (both of males and females) in districts of Guam during the period 1956–1985 and the cycasin content found in cycad flour prepared in those districts in the 1980s (Zhang et al., 1996).
The poisonous properties of cycads were exploited in former times as instruments of chemical warfare (Costa Rica), homicide (Celebes), and state-approved execution (Honduras; Whiting, 1963). In various parts of Oceania (Guam, New Guinea), the highly toxic seed pulp of cycads was used as a poultice to treat superficial and deep wounds (Whiting, 1963; Close and Gelegel, 1975; Spencer et al., 1987). Dried seed of C. revoluta was used to prepare a tonic for young children in Kii peninsula, Honshu Island, Japan (Spencer et al., 1987). Cycad leaves can be found in some apothecaries in China (unpublished data), and the underground caudex of Stangeria eriopus was used by South African ethnic groups as an emetic and ingredient in magical potions (Osborne, 1994).
THE CHEMISTRY AND TOXIC PROPERTIES OF CYCAD TOXINS
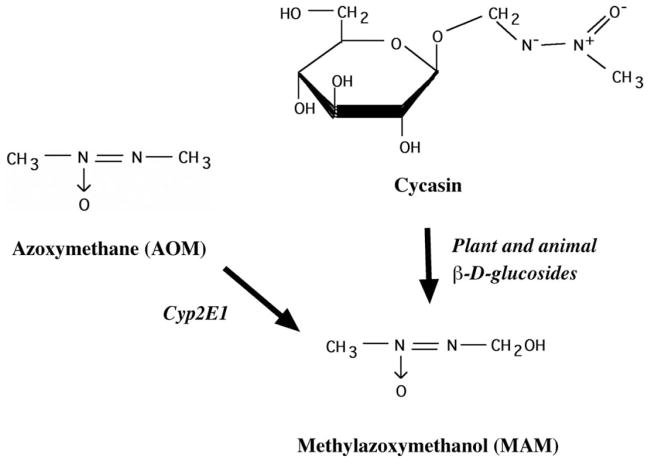
Macrozamia and Cycas contain the primeveroside macrozamin and/or the glucoside cycasin, and macrozamin undergoes conversion to cycasin (Tate, 1995). Cycasin is present in cycad seed in concentrations of 2–4% (w/v; Yagi and Tadera, 1987), and readily undergoes hydrolysis under acidic conditions (e.g., stomach) to yield methanol, formaldehyde, nitrogen, and glucose (Woo, 1988). The β-D-glucopyranosyloxy linkage of cycasin is susceptible to hydrolysis by bacterial, plant, or mammalian β-glucosidases to liberate MAM, a colorless liquid that is unstable in acid or alkali and miscible with water and ethanol (Matsumoto and Strong, 1963; Laqueur and Matsumoto, 1967; Fig. 2). The activity of cytosolic β-glucosidases is reportedly 500-fold higher in the rodent brain than any other organ examined, including the liver (Kisby et al., 1999). Therefore, cycasin that is taken up by the brain would be rapidly converted to MAM. MAM can also be formed from azoxymethane, a synthetic compound used to induce colonic tumors in rodents (Zeller, 1992), an animal model of nonfamilial colorectal cancer (Perse and Cerar, 2011). MAM spontaneously breaks down to release formaldehyde and highly reactive methyladiazonium ions and carbon-centered free radicals (potent alkylating agents) that methylate nucleic acids at the O6-, N7-, and C8 positions of guanine (Matsumoto and Higa, 1966; Shank and Magee, 1967; Nagasawa et al., 1972; Kisby et al., 1995). Although MAM and its glucosides (i.e., azoxyglycosides) are the principal toxic moieties in cycads, these plants also contain a number of other potentially neurotoxic materials, including the nonprotein amino acids, β–N-methylamino-L-alanine (BMAA, Cycas spp.; Chiu et al., 2011) and β-N-oxalylamino-L-alanine (Macrozamia spp.; Beaton, 1977), and the water-insoluble phytosterol glucosides of which β-sitosterol β-D-glucoside (BSSG) is the predominant form (Wilson et al., 2002; Tabata et al., 2008).
Figure 2.
Structure of the cycad genotoxins cycasin (methylazoxymethanol-β-D-glucoside), methylazoxymethanol (MAM), and the related compound azoxymethane (AOM). Cycasin is converted to MAM by plant and animal β-glucosidases, whereas azoxymethane is converted to MAM by mixed function oxidases (i.e., P450, Cyp2E1).
Cycasin and its aglycone MAM have teratogenic, mutagenic, hepatotoxic, and carcinogenic properties in rodents and primates (Kobayashi and Matsumoto, 1965; Sieber et al., 1980). Hepatic and spinal lesions develop in ruminants fed with cycasin (Shimizu et al., 1986). Cycasin is transported by the intestinal Na+/glucose cotransporter, but with a lower affinity than D-glucose (Hirayama et al., 1994). In contrast, cycasin is efficiently (100× lower concentrations) transported by the brain Na+/glucose cotransporter (Matsuoka et al., 1998), suggesting that this CNS transport system is responsible for its entry into the brain of orally fed rats (Dastur and Palekar, 1974; Matsuoka et al., 1998). Cycasin is readily hydrolyzed by gut bacteria or metabolized by the liver to form MAM, which is also able to enter the brain (Kisby et al., 1999).
THE NEUROTOXIC PROPERTIES OF CYCAD PLANT GENOTOXINS
Human Studies
Soon after ingestion of cycad plant products, there is sudden onset of nausea and vomiting, enlargement of the liver, convulsions, loss of consciousness, and death or recovery (Hirono et al., 1970). Chamorro folklore links the practice of handling and consuming cycad materials to a disease known as lytico, many cases of which were diagnosed as ALS (Whiting, 1963, 1964, 1988). Epidemiological studies show that preference for traditional Chamorro food is significantly associated with an increased risk for parkinsonism-dementia (Reed et al., 1987). Other studies show a highly significant positive association between ALS and the cycasin content of flour prepared by Chamorros (Kisby et al., 1992; Zhang et al., 1996). Some Japanese and Guamanian ALS/PDC patients exhibit (in addition to the well-described cerebral neurofilamentous pathology) multinucleated and ectopic neurons in the cerebellum and vestibular nuclei, an observation suggesting that exposure occurred during the later phases of brain development (up to the age of 1 year) to a genotoxic agent (such as MAM) that altered the proliferation and migration of developing neurons (Yase, 1972; Shiraki and Yase, 1975). These data are consistent with more recent studies showing that Guam dementia and PDC are significantly correlated with exposure of Chamorro children or adolescents to cycad plant products (Borenstein et al., 2007). Chamorro migrants from Guam to the United States mainland carry the risk of developing ALS-PDC decades later (but their children born and bred outside of Guam do not), and a few immigrants to Guam who adopted the traditional Chamorro lifestyle developed the disease 10–15 years later. Thus, western Pacific ALS-PDC and dementia are long-latency neurodegenerative disorders that may be acquired years or decades before clinical expression (Spencer et al., 1991; Spencer and Kisby, 1992). The declining patterns of cycad utilization in all western Pacific communities at risk for ALS/PDC suggest a diminishing environmental factor as an etiological link to this disappearing neurodegenerative disorder (Spencer, 1990).
Although there is strong experimental evidence showing that cycasin/MAM are potent developmental neurotoxins in laboratory species (see below), nothing is known about their role in human neurodevelopmental disorders. Since early life exposure to toxic chemicals can precipitate seizures (Jett, 2012), the higher-than-expected rates of epilepsy (excluding febrile convulsions) that were reported on Guam (Lessell et al., 1962; Chen et al., 1968) might be explained by intrauterine exposure to cycad genotoxins (e.g., MAM). The ability of MAM to produce a rodent model of drug-resistant epilepsy following in utero administration is certainly consistent with such a notion (Harrington et al., 2007; Potschka, 2012).
Animal Studies
Cycasin and MAM reproducibly induce pronounced changes in rodent brain that vary with the neurodevelopmental stage (Ferguson, 1996; Ferguson et al., 1996; Cattabeni and Di Luca, 1997; Colacitti et al., 1999; Fig. 3). Between days 13 and 15 of gestation, fetal exposure to MAM via treatment of dams induces microencephaly of the cerebral cortex (Balduini et al., 1986; Cattabeni and Di Luca, 1997) and displaced hippocampal neurons (i.e., heterotopias), leading to an increased susceptibility to seizures (Calcagnotto and Baraban, 2005; Harrington et al., 2007). The disruption of DNA by MAM during fetal brain development is reportedly responsible for the abnormal patterns of cell formation and migration that culminate in heterotopic neuronal clusters (Schwartzkroin and Wenzel, 2012). The increased sensitivity of these heterotopic clusters to seizure generation has been ascribed to alterations in the expression of glutamate receptors/transporters (Harrington et al., 2007), the dampening of inhibitory neurotransmission (i.e., GABA) via increased extracellular GABA (Karlsson et al., 2011), and/or a direct antiangiogenic effect in heterotopic areas of the adult brain that are caused by persistent cerebrovascular dysfunction (Bassanini et al., 2007). Thus, MAM induces both direct and indirect persistent effects on early brain development to produce an animal model that is prone to seizures. Since drug-resistant epilepsy frequently occurs in pediatric patients with neuronal migration disorders, the MAM-E13–15 animal model might be particularly useful for elucidating the underlying mechanisms of drug resistance and developing novel therapeutic agents (Potschka, 2012).
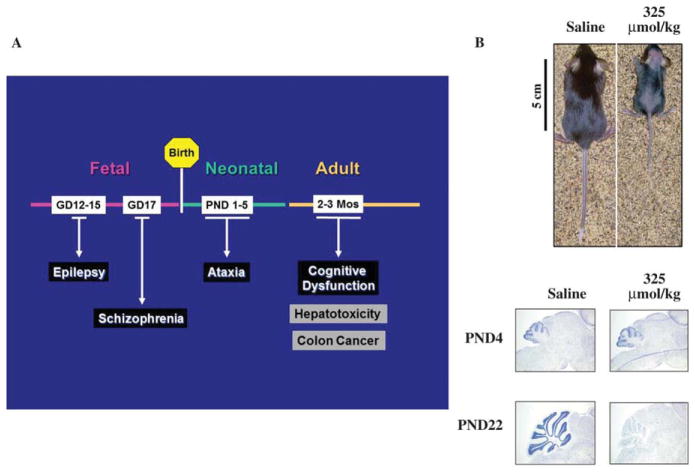
Figure 3.
Effect of MAM on rodent brain development. (A) Intrauterine exposure to a single injection of MAM produces an animal model with neuroanatomical and neurobehavioral features of drug-resistant epilepsy or schizophrenia. After birth, neonatal or adult rodents that have been treated with single or multiple injections of MAM develop motor dysfunction (i.e., ataxia) or cognitive dysfunction, respectively. Adult rodents that have been treated with azoxymethane (metabolic precursor of MAM) daily for several weeks also develop hepatotoxicity or colon cancer. (B) Growth of neonatal (PND22) mice after treatment on postnatal day 3 with a single injection of saline or MAM (325 μmol/kg; top). Note the pronounced effect of the cycad genotoxin on development. Light micrographs of representative areas from cresyl violet stained parasaggittal sections (10 μm) of the cerebellum from postnatal day 4 (PND4) and 22 (PND22) C57BL/6 mice pups similarly treated at PND3 with either saline or MAM (bottom). Note that the staining in the granule cell layer is significantly reduced (cerebellar atrophy) only in PND22 mice. [modified from (Kisby et al., 2009)]
Schizophrenia is generally considered to be a neurodevelopmental disorder because several neuroanatomical and cytoarchitectural abnormalities (e.g., misplaced/clustered neurons, fewer dendritic spines/ arborizations, reductions in interneurons and oligodendrocytes) have been identified in the brains of patients with the disease (Fatemi and Folsom, 2009; Insel, 2010). The detection of these neurodevelopmental changes prompted the development of animal models that focus on disrupted neurogenesis to elucidate the underlying neurobiological processes of schizophrenia. The MAM rat model is widely thought by neurobiologists to be a valid neurodevelopmental disruption animal model of schizophrenia (Jongen-Relo et al., 2004; Lodge and Grace, 2009; Powell, 2010; Sanderson et al., 2012; Lodge, 2013). When MAM is administered to rat dams on gestational day 17 (E17), the cycad genotoxin induces neuroanatomical, pharmacological, and neurobehavioral changes in the adolescent offspring that are consistent with those observed in schizophrenia (Chin et al., 2011; Flagstad et al., 2004; Moore et al., 2006; Lodge and Grace, 2009; Lodge, 2013). In the MAM-E17 animal model, the plant genotoxin specifically targets the developing prefrontal cortex, entorhinal cortex, and hippocampus, brain regions that also show prominent deficits in schizophrenia patients. MAM reduces cerebral cortical volume, causing an increase in neuronal density without neuronal loss, and induces heterotopias and reduces spine density in hippocampal pyramidal neurons, subtle neuroanatomical changes also observed in the schizophrenia brain (Chin et al., 2011; Flagstad et al., 2004; Moore et al., 2006; Lodge and Grace, 2009). Adult MAM-E17 rats also exhibit impaired social interaction and increased locomotor deficits in response to dopamine or psychostimulants (Le Pen et al., 2006; Moore et al., 2006). As in schizophrenia, these neurobehavioral changes emerge during adolescence in the MAM-E17 rat model.
How the cycad plant toxin MAM induces subtle neurodevelopmental changes in E17 rats is unknown. MAM might disrupt the expression of genes that regulate neurodevelopment either through DNA damage (Kisby et al., 2009; Kisby et al., 2011) and/or epigenetic mechanisms (Kisby and Spencer, 2011; Mackowiak et al., 2013). In support of a DNA damage-mediated mechanism, adult rats given O6-benzylguanine (a DNA-repair inhibitor) before MAM treatment at E17 showed more pronounced hippocampal dysgenesis than E17 rats treated only with MAM (Fig. 4; H. Moore, unpublished data). Moreover, the hippocampus of adult MAM-E17 rats showed changes in the expression of genes involved in DNA repair and neuronal and glial development (Merker et al., 2009). An examination of the medial prefrontal cortex of adult MAM-E17 rats also revealed a pronounced effect on the methylation of histone proteins (i.e., H3K4, and H3K9). H3K9me2 levels were decreased before puberty (PND 15 and PND 45) and H3K4me3 levels were significantly decreased when rats reached adulthood (PND 60 and PND 70). Therefore, MAM administration at E17 reduced H3K9me2 and H3K4me3 levels (markers of histone H3 methylation) in the prefrontal cortex before and following puberty, respectively (Mackowiak et al., 2013). These histone H3 methylation changes were also associated with altered levels of enzymes that methylate (ASH2L) and demethylate (LSD1, JARID1c) histone proteins. Since histone lysine methylation and altered levels of methyltransferases and demethylases are also a molecular signature of the schizophrenia brain (Akbarian, 2009), the MAM-E17 animal model might provide important clues about the contribution of environmental and epigenetic factors in the molecular pathogenesis of schizophrenia.
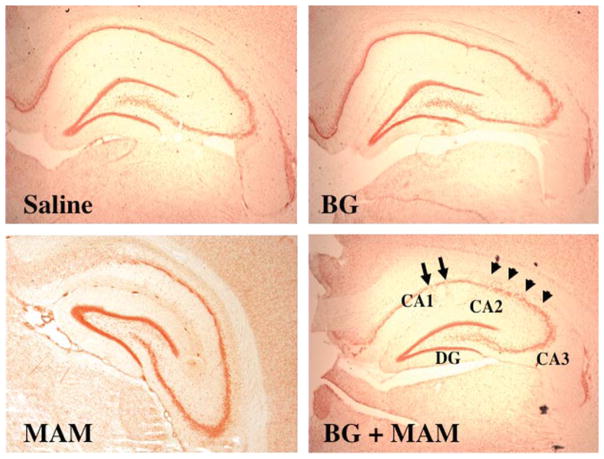
Figure 4.
The neurogenesis of pyramidal neurons is more severely disrupted in the adult rat hippocampus after treatment of E17 rats with MAM plus a DNA-repair inhibitor, than with MAM alone. E17 rat dams were pretreated with the MGMT inhibitor O6-benzylguanine (BG; 50 mg/kg, i.p.) and, 12 h later, given a single injection of MAM (22 mg/kg, i.p.). The hippocampus of the offspring was examined at PND 60 for morphological changes. Note the more pronounced effect of MAM on the migration of CA1 and CA2 pyramidal neurons (arrows) in E17 rats pretreated with BG. (H. Moore, unpublished data)
Postnatal exposure (days 1–5) to MAM leads to microencephaly (e.g., reduced folia and fissures) of the cerebellum that is characterized by specific targeting of glutamatergic and GABAergic precursor cells (especially granule cells) resulting in misalignment of Purkinje cells, and ectopic and multinucleated granule cells (Sullivan-Jones et al., 1994; Ferguson, 1996). However, repetitive administration of lower concentrations of MAM (1–5 mg/kg vs. 20–25 mg/ kg) to adolescent mice induced neurodevelopmental changes, consisting of reductions in GABAergic hippocampal interneurons and sensorimotor gating deficits (Guo et al., 2013). An enriched environment produces long-lasting effects on rodents by epigenetic mechanisms (Arai and Feig, 2011). The ability of environmental enrichment to reverse the sensorimotor deficits and improve learning in young adult rodents after injection of neonatal mice (Guo et al., 2013) or fetal rats (Wallace et al., 2003; Ueda et al., 2010) with MAM suggests that this plant genotoxin induces neurobehavioral changes in part by an epigenetic mechanism (e.g., histone methylation).
MAM has been administered to young adult rodents to determine the role of neurogenesis in learning, memory, and ischemia. Neurogenesis was reduced by 80% in the dentate gyrus of adult rats after daily administration of MAM (7 mg/kg, s.c.) for 14 days (Shors et al., 2001). A substantial reduction of newly generated dentate gyrus neurons in the adult rat impaired some but not all types of hippocampus-dependent memories. When young adult mice (6–8 weeks old) were injected subcutaneously for 2 weeks with “several doses” of MAM (3 mg/ kg), the plant genotoxin severely reduced hippocampal neurogenesis and associated formation of (contextual fear) memory (Ko et al., 2009).
MAM has also been used to clarify the importance of neurogenesis in ischemia (Maysami et al., 2008). Ischemia-induced neurogenesis was severely impaired in adult mice (7–9 week old) that had been pretreated daily with MAM for 1 week before occlusion of the middle cerebral artery. The size of the infarct was significantly larger in adult mice that had been pre-treated with MAM prior to occluding the middle cerebral artery.
NEURODEVELOPMENTAL MECHANISM OF CYCAD PLANT GENOTOXINS
Our studies and those of other investigators show that MAM, the active metabolite of the plant toxin cycasin, has a pronounced influence on both brain (Cattabeni and Di Luca, 1997; Germano and Sperber, 1998; Kisby et al., 2005) and neuronal development (Hoffman et al., 1996; Kisby et al., 2006). Yet, the mechanism by which MAM alters brain development is not well-understood. It has long been thought that MAM alters brain development by indirectly killing dividing neurons or supporting cells (e.g., radial glia) by a DNA damage-mediated mechanism (Nagata and Matsumoto, 1969; Matsumoto et al., 1972). This hypothesis was tested by comparing the effect of MAM on developing neurons from transgenic mice that are proficient or deficient in DNA repair (Kisby et al., 2009). The development of “knock-out” and transgenic mice for DNA repair (Friedberg and Meira, 2004) provides a novel approach for assessing the influence of different types of MAM-induced DNA lesions (i.e., N7-methylguanine, O6-methylguanine) on brain development. Alkyladenine DNA glycosylase (Aag) repairs N7-mG DNA lesions, whereas O6-methylguanine methyltransferase (Mgmt) repairs O6-mG DNA lesions (Bugni et al., 2009; Lee et al., 2009). We compared the sensitivity of neurons from the cerebellum of neonatal mice that are deficient in base-excision repair (BER; i.e., alkyladenine alkyltransferase, Aag−/−) and O6-methylguanine methyltransferase (Mgmt−/−), or that overexpress MGMT (MgmtTg+) to MAM-induced N7-mG or O6-mG DNA lesions. The in vitro studies showed that immature cerebellar granule cell neurons from Mgmt KO mice were more vulnerable to MAM than neurons from either wild type or Aag KO mice. Granule cell development and motor function were also shown to be more severely disturbed by MAM in Mgmt KO mice, whereas both of these neurotoxic features were preserved in comparably treated MgmtTg+ mice (Fig. 5). The O6-mG DNA lesions formed by MAM in the neonatal brain (Kisby et al., 2009) appear to play a major role in altering the intrinsic processes that regulate neuronal development (mitosis and migration). The correlation of specific brain DNA lesions (e.g., O6-mG) with the differential expression of genes in several cell-signaling pathways (i.e., MAPK, Akt) in MAM-treated young adult mice is further evidence that MAM targets cell-signaling pathways by a DNA damage-mediated mechanism (Kisby et al., 2011). These findings indicate that MAM predominantly perturbs molecular pathways that regulate neuronal maturation and migration, changes that are consistent with the neurodevelopmental changes observed in Mgmt KO mice (Kisby et al., 2009) and in some individuals with western Pacific ALS-PDC (Yase, 1972; Shiraki and Yase, 1975). More specifically, MAM appears to perturb the expression of genes that regulate the development of neuronal processes (i.e., axons, dendrites), with the result that the pathfinding function and motility of neuronal growth cones are impaired (Hoffman et al., 1996; Hatten, 1999). The preferential targeting of developing neurons by MAM is also consistent with the ability of this plant genotoxin to disrupt unique molecular networks during brain development, either at the fetal stage (Merker et al., 2009) or postnatally (Kisby et al., 2005). These studies with transgenic mice also show that DNA repair plays a key role in protecting the genome of neurons from genotoxicant insult during critical periods of brain development. However, since the repair of O6-mG DNA lesions is particularly low (comparable to Mgmt KO mice) during the fetal, neonatal, and adolescent periods of human brain development (Silber et al., 1996; Bobola et al., 2007), such factors are pertinent to understanding how early-life exposure to environmental genotoxicants might perturb neurodevelopment to induce long-term brain injury.
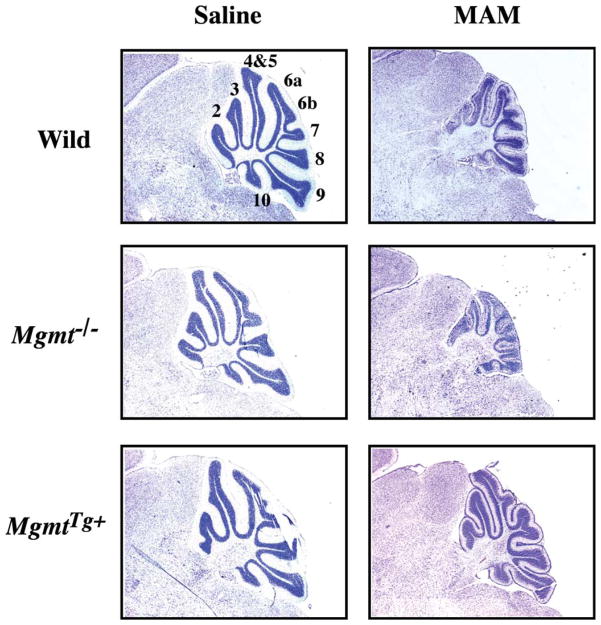
Figure 5.
Cytoarchitecture of the cerebellum of Mgmt KO and Mgmt overexpressing mice treated with MAM. Light micrographs of representative areas from cresyl violet stained parasagittal sections (10 μm) of the PND22 cerebellum from C57BL/6J (Wild), Mgmt KO (Mgmt−/−), or Mgmt-overexpressing (MgmtTg+) mice treated on PND3 with a single subcutaneous injection of saline (left panels) or MAM (325 μmol, right panels). Mag × 3.85. [modified from (Kisby et al., 2009)]
SUMMARY
There is substantial evidence to support the hypothesis that human exposure to cycad plant toxins is an important etiological trigger of a prototypical neurodegenerative disorder in the western Pacific with features of ALS, atypical parkinsonism, and Alzheimer’s-like dementia (ALS-PD; Spencer et al., 1991; Borenstein et al., 2007; Kisby and Spencer, 2011; Spencer et al., 2012). Among the Chamorro people of Guam, PDC and Guam Dementia are strongly associated with exposure to cycad plant toxins during childhood or adolescence, critically important periods of normal development of the human brain. Unknown is whether earlier-life exposure (e.g., in utero, neonatal) to cycad plant genotoxins disrupts human brain development to increase risk for neurodevelopmental disorders (e.g., epilepsy, schizophrenia). Despite a lack of human data, the genotoxic metabolite (MAM) of the principal cycad plant toxin cycasin has been widely used (as MAM acetate) by neurobiologists as a tool to explore the complex mechanisms underlying brain maldevelopment in neurodevelopmental disorders. Depending on the developmental stage at which the brain is exposed to MAM, this substance can induce animal models with certain features of drug-resistant epilepsy, schizophrenia, or ataxia. If MAM is given to adult animals, its effect is primarily restricted to brain regions (e.g., hippocampus) where neurons are still actively proliferating (i.e., neurogenesis). There is evidence from these studies that mature animals treated with MAM also develop cognitive deficits or are especially prone to ischemia.
The use of transgenic mice with different DNA-repair deficits has provided insight into the molecular mechanism by which MAM perturbs brain development. These studies demonstrate that MAM-induced DNA damage (i.e., O6-mG, N7-mG lesions) plays an important role in disrupting neurodevelopment, and multiple DNA-repair pathways are required to protect the developing brain from insult by environmental genotoxic agents (e.g., MAM). The pronounced influence of MAM on genes that regulate neuronal development in Mgmt KO mice (Kisby et al., 2011) and in mature neurons from the cerebellum of wild-type mice (Kisby et al., 2006), also support this notion. Since epigenetic changes (i.e., histone lysine methylation) were recently observed in the adolescent rodent brain after in utero MAM administration, this cycad toxin might disrupt neurodevelopment by inflicting both DNA damage and epigenetic changes. Thus, MAM is an important tool to investigate mechanisms underlying neurodevelopmental disorders, as well as late-life neurodegenerative diseases.
Acknowledgments
Supported by a grant from the U.S. Army Medical Research Materiel Command under Contract/Grant/Intergovernmental Project Order DAMD 17-98-1-8625 (GEK) and, in part, by NIH grants 5P42-ES10338-02 (National Institute of Environmental Health Sciences’ Toxicogenomics Consortium) (GEK, PSS).
Footnotes
The authors declare that they have no conflict of interest.
Contributor Information
Glen E. Kisby, Department of Basic Medical Sciences, Western University of Health Sciences, College of Osteopathic Medicine of the Pacific Northwest, Lebanon, Oregon, 97355.
Holly Moore, Department of Psychiatry, Columbia University and Department of Integrative Neuroscience, New York State Psychiatric Institute, New York, NY.
Peter S. Spencer, Department of Neurology, School of Medicine, Center for Research on Occupational and Environmental Toxciology; and Global Health Center, Oregon Health and Science University, Portland, Oregon, 97201
References
- Akbarian S. The molecular pathology of schizophrenia—focus on histone and DNA modifications. Brain Res Bull. 2009;83:103–107. doi: 10.1016/j.brainresbull.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai JA, Feig LA. Long-lasting and transgenerational effects of an environmental enrichment on memory formation. Brain Res Bull. 2011;85:30–35. doi: 10.1016/j.brainresbull.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balduini W, Cimino M, Lombardelli G, et al. Microencephalic rats as a model for cognitive disorders. Clin Neuropharmacol. 1986;9:S8–S18. [PubMed] [Google Scholar]
- Bassanini S, Hallene K, Battaglia G, et al. Early cerebrovascular and parenchymal events following prenatal exposure to the putative neurotoxin methylazoxymethanol. Neurobiol Dis. 2007;26:481–495. doi: 10.1016/j.nbd.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaton JM. PhD thesis. Australian National University; Canberra: 1977. Dangerous Harvest: investigations in the late prehistoric occupation of southeast central Queeensland. [Google Scholar]
- Bobola MS, Blank A, Berger MS, Silber JR. O6-methylguanine-DNA methyltransferase deficiency in developing brain: implications for brain tumorigenesis. DNA Repair (Amst) 2007;6:1127–1133. doi: 10.1016/j.dnarep.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein AR, Mortimer JA, Schofield E, et al. Cycad exposure and risk of dementia, MCI, and PDC in the Chamorro population of Guam. Neurology. 2007;68:1764–1771. doi: 10.1212/01.wnl.0000262027.31623.b2. [DOI] [PubMed] [Google Scholar]
- Bugni JM, Meira LB, Samson LD. Alkylation-induced colon tumorigenesis in mice deficient in the Mgmt and Msh6 proteins. Oncogene. 2009;28:734–741. doi: 10.1038/onc.2008.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcagnotto ME, Baraban SC. Prolonged NMDA-mediated responses, altered ifenprodil sensitivity, and epileptiform-like events in the malformed hippocampus of methylazoxymethanol exposed rats. J Neurophysiol. 2005;94:153–162. doi: 10.1152/jn.01155.2004. [DOI] [PubMed] [Google Scholar]
- Cattabeni F, Di Luca M. Developmental models of brain dysfunctions induced by targeted cellular ablations with methylazoxymethanol. Physiol Rev. 1997;77:199–215. doi: 10.1152/physrev.1997.77.1.199. [DOI] [PubMed] [Google Scholar]
- Chen KM, Brody JA, Kurland LT. Patterns of neurologic diseases on Guam. Arch Neurol. 1968;19:573–578. doi: 10.1001/archneur.1968.00480060043005. [DOI] [PubMed] [Google Scholar]
- Chin CL, Curzon P, Schwartz AJ, et al. Structural abnormalities revealed by magnetic resonance imaging in rats prenatally exposed to methylazoxymethanol acetate parallel cerebral pathology in schizophrenia. Synapse. 2011;65:393–403. doi: 10.1002/syn.20857. [DOI] [PubMed] [Google Scholar]
- Chiu AS, Gehringer MM, Welch JH, Neilan BA. Does α-amino-β-methylaminopropionic acid (BMAA) play a role in neurodegeneration? Int J Environ Res Public Health. 2011;8:3728–3746. doi: 10.3390/ijerph8093728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close KCA, Gelegel N. Medicinal plants of the Maprik Area. Papua New Guinea Med J. 1975;18:153. [PubMed] [Google Scholar]
- Colacitti C, Sancini G, DeBiasi S, et al. Prenatal methylazoxymethanol treatment in rats produces brain abnormalities with morphological similarities to human developmental brain dysgeneses. J Neuropathol Exp Neurol. 1999;58:92–106. doi: 10.1097/00005072-199901000-00010. [DOI] [PubMed] [Google Scholar]
- Dastur DK, Palekar RS. The experimental pathology of cycad toxicity with special reference to oncogenic effects. Indian J Cancer. 1974;2:33–49. [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD. The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophr Bull. 2009;35:528–548. doi: 10.1093/schbul/sbn187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SA. Neuroanatomical and functional alterations resulting from early postanatal cerebellar insults in rodents. Pharmacol Biochem Behav. 1996;55:663–671. doi: 10.1016/s0091-3057(96)00253-5. [DOI] [PubMed] [Google Scholar]
- Ferguson SA, Paule MG, Holson RR. Functional effects of methylzoxymethanol-induced cerebellar hypoplasia in rats. Neurotoxicol Teratol. 1996;18:529–537. doi: 10.1016/0892-0362(96)00083-9. [DOI] [PubMed] [Google Scholar]
- Flagstad P, Mork A, Glenthoj BY, et al. Disruption of neurogenesis on gestational day 17 in the rat causes behavioral changes relevant to positive and negative schizophrenia symptoms and alters amphetamine-induced dopamine release in nucleus accumbens. Neuropsychopharmacology. 2004;29:2052–2064. doi: 10.1038/sj.npp.1300516. [DOI] [PubMed] [Google Scholar]
- Friedberg EC, Meira LB. Database of mouse strains carrying targeted mutations in genes affecting biological responses to DNA damage (Version 6) DNA Repair (Amst) 2004;3:1617–1638. doi: 10.1016/j.dnarep.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Germano IM, Sperber EF. Transplacentally induced neuronal migration disorders: an animal model for the study of the epilepsies. J Neurosci Res. 1998;51:473–488. doi: 10.1002/(SICI)1097-4547(19980215)51:4<473::AID-JNR7>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Guo N, Yoshizaki K, Kimura R, et al. A sensitive period for GABAergic interneurons in the dentate gyrus in modulating sensorimotor gating. J Neurosci. 2013;33:6691–6704. doi: 10.1523/JNEUROSCI.0032-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington EP, Moddel G, Najm IM, Baraban SC. Altered glutamate receptor: transporter expression and spontaneous seizures in rats exposed to methylazoxymethanol in utero. Epilepsia. 2007;48:158–168. doi: 10.1111/j.1528-1167.2006.00838.x. [DOI] [PubMed] [Google Scholar]
- Hatten ME. Central nervous system neuronal migration. Annu Rev Neurosci. 1999;22:511–539. doi: 10.1146/annurev.neuro.22.1.511. [DOI] [PubMed] [Google Scholar]
- Hirayama B, Hazama A, Loo DF, et al. Transport of cycasin by the intestinal Na+ /glucose cotransporter. Biochim Biophys Acta. 1994;1193:151–154. doi: 10.1016/0005-2736(94)90344-1. [DOI] [PubMed] [Google Scholar]
- Hirono I, Kachi H, Kato T. A survey of acute toxicity of cycads and mortality rate from cancer in the Miyako Islands, Okinawa. Acta Pathol Jpn. 1970;20:327–337. doi: 10.1111/j.1440-1827.1970.tb03074.x. [DOI] [PubMed] [Google Scholar]
- Hoffman JR, Boyne LJ, Levitt P, Fischer I. Short exposure of methylazoxymethanol causes a long-term inhibition of axonal outgrowth from cultured embryonic rat hippocampal neurons. J Neurosci Res. 1996;46:349–359. doi: 10.1002/(SICI)1097-4547(19961101)46:3<349::AID-JNR8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–193. doi: 10.1038/nature09552. [DOI] [PubMed] [Google Scholar]
- Jett DA. Chemical toxins that cause seizures. Neurotoxicology. 2012;33:1473–1475. doi: 10.1016/j.neuro.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Jongen-Relo AL, Leng A, Luber M, et al. The prenatal methylazoxymethanol acetate treatment: a neurodevelopmental animal model for schizophrenia? Behav Brain Res. 2004;149:159–181. doi: 10.1016/s0166-4328(03)00228-6. [DOI] [PubMed] [Google Scholar]
- Karlsson A, Lindquist C, Malmgren K, Asztely F. Altered spontaneous synaptic inhibition in an animal model of cerebral heterotopias. Brain Res. 2011;1383:54–61. doi: 10.1016/j.brainres.2011.01.080. [DOI] [PubMed] [Google Scholar]
- Kisby GE, Eizirik D, Sweatt C, Spencer PS. Reactive oxygen species produced by the cycad toxin methylazoxymethanol, a candidate etiological factor for western Pacific ALS/P-D. J Cell Biochem. 1995;21B:99. [Google Scholar]
- Kisby GE, Ellison M, Spencer PS. Content of the neurotoxins cycasin (methylazoxymethanol β-D-glucoside) and BMAA (β-N-methylamino-L-alanine) in cycad flour prepared by Guam Chamorros. Neurology. 1992;42:1336–1340. doi: 10.1212/wnl.42.7.1336. [DOI] [PubMed] [Google Scholar]
- Kisby GE, Fry RC, Lasarev MR, et al. The cycad genotoxin MAM modulates brain cellular pathways involved in neurodegenerative disease and cancer in a DNA damage-linked manner. PLoS One. 2011;6:e20911. doi: 10.1371/journal.pone.0020911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisby GE, Kabel H, Hugon J, Spencer P. Damage and repair of nerve cell DNA in toxic stress. Drug Metab Rev. 1999;31:589–618. doi: 10.1081/dmr-100101937. [DOI] [PubMed] [Google Scholar]
- Kisby GE, Olivas A, Park T, et al. DNA repair modulates the vulnerability of the developing brain to alkylating agents. DNA Repair (Amst) 2009;8:400–412. doi: 10.1016/j.dnarep.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisby GE, Olivas A, Standley M, et al. Genotoxicants target distinct molecular networks in neonatal neurons. Environ Health Perspect. 2006;114:1703–1712. doi: 10.1289/ehp.9073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisby GE, Spencer PS. Is neurodegenerative disease a long-latency response to early-life genotoxin exposure? Int J Environ Res Public Health. 2011;8:3889–3921. doi: 10.3390/ijerph8103889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisby GE, Standley M, Lu X, et al. Molecular networks perturbed in a developmental animal model of brain injury. Neurobiol Dis. 2005;19:108–118. doi: 10.1016/j.nbd.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Ko HG, Jang DJ, Son J, et al. Effect of ablated hippocampal neurogenesis on the formation and extinction of contextual fear memory. Mol Brain. 2009;2:1. doi: 10.1186/1756-6606-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A, Matsumoto H. Studies on methylazoxymethanol, the aglycone of cycasin. Isolation, biological, and chemical properties. Arch Biochem Biophys. 1965;110:373–380. doi: 10.1016/0003-9861(65)90137-2. [DOI] [PubMed] [Google Scholar]
- Laqueur GL, Matsumoto H. Neoplasms in female Fischer rats following intraperitoneal injection of methylazoxymethanol. J Natl Cancer Inst. 1967;124:691–697. [PubMed] [Google Scholar]
- Lee CY, Delaney JC, Kartalou M, et al. Recognition and processing of a new repertoire of DNA substrates by human 3-methyladenine DNA glycosylase (AAG) Biochemistry. 2009;48:1850–1861. doi: 10.1021/bi8018898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Pen G, Gourevitch R, Hazane F, et al. Peri-pubertal maturation after developmental disturbance: a model for psychosis onset in the rat. Neuroscience. 2006;143:395–405. doi: 10.1016/j.neuroscience.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Lessell S, Torres JM, Kurland LT. Seizure disorders in a Guamanian village. Arch Neurol. 1962;7:37–44. doi: 10.1001/archneur.1962.04210010043004. [DOI] [PubMed] [Google Scholar]
- Lodge DJ. The MAM rodent model of schizophrenia. Curr Protoc Neurosci. 2013;Chapter 9(Unit 9.43) doi: 10.1002/0471142301.ns0943s63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Gestational methylazoxymethanol acetate administration: a developmental disruption model of schizophrenia. Behav Brain Res. 2009;204:306–312. doi: 10.1016/j.bbr.2009.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackowiak M, Bator E, Latusz J, et al. Prenatal MAM administration affects histone H3 methylation in postnatal life in the rat medial prefrontal cortex. Eur Neuropsychopharmacol. doi: 10.1016/j.euroneuro.2013.05.013. in press. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Higa HH. Studies on methylazoxymethanol, the aglycone of cycasin: methylation of nucleic acids in vitro. Biochem J. 1966;98:20C–22C. doi: 10.1042/bj0980020c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H, Spatz M, Laqueur GL. Quantitative changes with age in the DNA content of methylazoxymethanol-induced microencephalic rat brain. J Neurochem. 1972;19:297–306. doi: 10.1111/j.1471-4159.1972.tb01339.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Strong FM. The occurrence of methylazoxymethanol in cycas circinalis L. Arch Biochem Biophys. 1963;101:299–310. [Google Scholar]
- Matsuoka T, Nishizaki T, Kisby GE. Na+-dependent and phlorizin-inhibitable transport of glucose and cycasin in brain endothelial cells. J Neurochem. 1998;70:772–777. doi: 10.1046/j.1471-4159.1998.70020772.x. [DOI] [PubMed] [Google Scholar]
- Maysami S, Lan JQ, Minami M, Simon RP. Proliferating progenitor cells: a required cellular element for induction of ischemic tolerance in the brain. J Cereb Blood Flow Metab. 2008;28:1104–1113. doi: 10.1038/jcbfm.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merker RJ, Graham P, Cressman V, et al. Abnormal neonatal and adult gene expression patterns in the hippocampus and prefrontal cortex of offspring of rat dams exposed to MAM on embryonic day 17. Soc Neurosci Abstr. 2009;341:5. [Google Scholar]
- Moore H, Jentsch JD, Ghajarnia M, et al. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biol Psychiatry. 2006;60:253–264. doi: 10.1016/j.biopsych.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa HT, Shirota FN, Matsumoto H. Decomposition of methylazoxymethanol, the algycone of cycasin, in D2O. Nature. 1972;236:234–235. doi: 10.1038/236234a0. [DOI] [PubMed] [Google Scholar]
- Nagata Y, Matsumoto H. Studies on methylazoxymethanol: methylation of nucleic acids in the fetal rat brain. Proc Soc Exp Biol Med. 1969;132:383–385. doi: 10.3181/00379727-132-34220. [DOI] [PubMed] [Google Scholar]
- Osborne R, Grove A, Oh P, et al. The magical and medicinal usage of Stangeria eriopus in South Africa. J Ethnopharmacol. 1994;43:67–72. doi: 10.1016/0378-8741(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Perse M, Cerar A. Morphological and molecular alterations in 1,2 dimethylhydrazine and azoxymethane induced colon carcinogenesis in rats. J Biomed Biotechnol. 2011;2011:473964. doi: 10.1155/2011/473964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potschka H. Animal models of drug-resistant epilepsy. Epileptic Disord. 2012;14:226–234. doi: 10.1684/epd.2012.0532. [DOI] [PubMed] [Google Scholar]
- Powell SB. Models of neurodevelopmental abnormalities in schizophrenia. Curr Top Behav Neurosci. 2010;4:435–481. doi: 10.1007/7854_2010_57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed D, Labarthe D, Chen KM, Stallones R. A cohort study of amyotrophic lateral sclerosis and Parkinsonism-dementia on Guam and Rota. Am J Epidemiol. 1987;125:92–100. doi: 10.1093/oxfordjournals.aje.a114515. [DOI] [PubMed] [Google Scholar]
- Sanderson TM, Cotel MC, O’Neill MJ, et al. Alterations in hippocampal excitability, synaptic transmission and synaptic plasticity in a neurodevelopmental model of schizophrenia. Neuropharmacology. 2012;62:1349–1358. doi: 10.1016/j.neuropharm.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin PA, Wenzel HJ. Are developmental dysplastic lesions epileptogenic? Epilepsia. 2012;53:35–44. doi: 10.1111/j.1528-1167.2012.03473.x. [DOI] [PubMed] [Google Scholar]
- Shank RC, Magee PN. Similarities between the biochemical actions of cycasin and dimethylnitrosamine. Biochem J. 1967;105:521–527. doi: 10.1042/bj1050521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Yasuda N, Kono I, et al. Hepatic and spinal lesions in goats chronically intoxicated with cycasin. Jpn J Vet Sci. 1986;48:1291–1295. doi: 10.1292/jvms1939.48.1291. [DOI] [PubMed] [Google Scholar]
- Shiraki H, Yase Y. ALS in Japan. In: Vinken PJ, Bruyn GW, editors. Handbook of clinical neurology. New York: Elsevier; 1975. pp. 353–419. [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, et al. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Sieber SM, Correa P, Dalgard DW, et al. Carcinogenicity and hepatotoxicity of cycasin and its aglycone methylazoxymethanol acetate in nonhuman primates. J Natl Cancer Inst. 1980;65:177–189. [PubMed] [Google Scholar]
- Silber JR, Blank A, Bobola MS, et al. Lack of the DNA repair protein O6-methylguanine-DNA methyltransferase in histologically normal brain adjacent to primary human brain tumors. Proc Natl Acad Sci USA. 1996;93:6941–6946. doi: 10.1073/pnas.93.14.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer PS. Are neurotoxins driving us crazy? Planetary observations of neurodegenerative diseases of old age. In: Russell RW, Flattau PE, Pope AM, editors. Behavioral measures in neurotoxicity. Washington, DC: National Academy Press; 1990. p. 11. [Google Scholar]
- Spencer PS, Fry RC, Palmer VS, Kisby GE. Western Pacific ALS-PDC: a prototypical neurodegenerative disorder linked to DNA damage and aberrant proteogenesis? Front Neurol. 2012;3:180. doi: 10.3389/fneur.2012.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer PS, Kisby GE. Slow toxins and western Pacific amyotrophic lateral sclerosis. In: Smith RA, editor. Handbook of amyotrophic lateral sclerosis. New York: Marcel Dekker; 1992. pp. 575–585. [Google Scholar]
- Spencer PS, Kisby GE, Ludolph AC. Slow toxins, biologic markers, and long-latency neurodegenerative disease in the western Pacific region. Neurology. 1991;41:62–66. doi: 10.1212/wnl.41.5_suppl_2.62. [DOI] [PubMed] [Google Scholar]
- Spencer PS, Ohta M, Palmer V. Cycad use and motor neuron disease in Kii peninsula of Japan. Lancet. 1987;2:1462–1463. doi: 10.1016/s0140-6736(87)91159-7. [DOI] [PubMed] [Google Scholar]
- Sullivan-Jones P, Gouch AB, Holson RR. Postnatal methylazoxymethanol: sensitive periods and regional selectivity of effects. Neurotoxicol Teratol. 1994;16:631–637. doi: 10.1016/0892-0362(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Tabata RC, Wilson JM, Ly P, et al. Chronic exposure to dietary sterol glucosides is neurotoxic to motor neurons and induces an ALS-PDC phenotype. Neuromolecular Med. 2008;10:24–39. doi: 10.1007/s12017-007-8020-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate ME, Delaere IM, Jones GP, Tierkink ERT. Crystal and molecular structure of (Z)-β-O-D-glucopyranosyloxy-NNO-azoxymethane. Aust J Chem. 1995;48:1059. [Google Scholar]
- Ueda S, Yoshimoto K, Kadowaki T, et al. Improved learning in microencephalic rats. Congenit Anom (Kyoto) 2010;50:58–63. doi: 10.1111/j.1741-4520.2009.00265.x. [DOI] [PubMed] [Google Scholar]
- Wallace CS, Reitzenstein J, Withers GS. Diminished experience-dependent neuroanatomical plasticity: evidence for an improved biomarker of subtle neurotoxic damage to the developing rat brain. Environ Health Perspect. 2003;111:1294–1298. doi: 10.1289/ehp.6088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting M. Food practices in ALS foci in Japan, the Marianas, and New Guinea. Fed Proc. 1964;23:1343–1345. [PubMed] [Google Scholar]
- Whiting M. Toxicity of cycads: implications for neurodegenerative diseases and cancer. Transcripts of Four Cycad Conferences; Third World Medical Research Foundation; NY. 1988. [Google Scholar]
- Whiting MG. Toxicity of cycads. Econ Bot. 1963;17:271–302. [Google Scholar]
- Wilson JM, Khabazian I, Wong MC, et al. Behavioral and neurological correlates of ALS-parkinsonism dementia complex in adult mice fed washed cycad flour. Neuromol Med. 2002;1:207–221. doi: 10.1385/NMM:1:3:207. [DOI] [PubMed] [Google Scholar]
- Woo YT, Lai DY, Arcos JC, Argus MF. Structural basis and biological mechanisms. San Diego: Academic Press; 1988. Natural, metal, fiber and macromolecular carcinogens (Section 5.3.2.2. Cycasin and related compounds). Chemical induction of cancer; p. 178. [Google Scholar]
- Yagi F, Tadera K. Azoxyglycoside contents in seeds of several cycad species and various parts of Japanese cycad. Agric Biol Chem. 1987;51:1719–1721. [Google Scholar]
- Yase Y. The pathogenesis of amyotrophic lateral sclerosis. Lancet. 1972;2:292–296. doi: 10.1016/s0140-6736(72)92903-0. [DOI] [PubMed] [Google Scholar]
- Zeller WJ. Neurotrophic carcinogenesis. In: Herken H, Hucho F, editors. Selective neurotoxicity. Vol. 102. Berlin: Springer-Verlag; 1992. pp. 193–224. [Google Scholar]
- Zhang ZX, Anderson DW, Mantel N, Roman GC. Motor neuron disease on Guam: geographic and familial occurrence, 1956–85. Acta Neurol Scand. 1996;94:51–59. doi: 10.1111/j.1600-0404.1996.tb00039.x. [DOI] [PubMed] [Google Scholar]