Abstract
The monoaminergic neuron, in particular the dopaminergic neuron, is central to mediating the hedonic and addictive properties of drugs of abuse. The effects of amphetamine (AMPH) and cocaine (COC), for example, depend on the ability to increase dopamine in the synapse, by effects on either the plasma membrane transporter DAT or the vesicular transporter for monoamine storage, VMAT2. The potential role of DAT as a target for AMPH and COC has been reviewed extensively. Here, we present VMAT2 as a target that enables the rewarding and addictive actions of these drugs, based on imaging, neurochemical, biochemical, cell biological, genetic, and immunohistochemical evidence. The presence of VMAT2 in noradrenergic, serotoninergic, histaminergic, and potentially trace aminergic neurons invites consideration of a wider role for aminergic neurotransmission in AMPH and COC abuse and addiction.
Keywords: VMAT2, amphetamine, cocaine, addiction, monoamines, vesicular transporter
Introduction
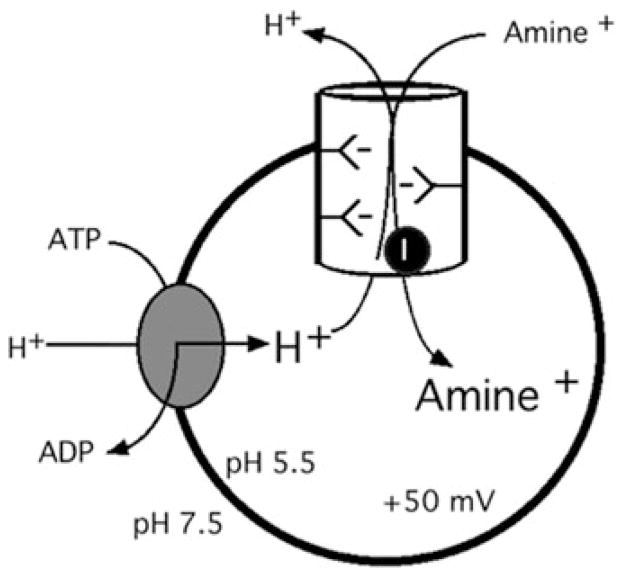
In 1962, Kirshner showed that reserpine and various so-called “indirectly acting sympathomimetic amines” (drugs like amphetamine [AMPH], phenylephrine, and ephedrine with norepinephrine-like pharmacological effects, but without action on adrenergic receptors) could block 14C-epinephrine uptake into a particulate fraction from bovine adrenal medulla (the secretory granule). These experiments established the first molecular link between monoamine uptake into storage vesicles and psychotropic drug action.1 The study of monoamine uptake into storage granules was followed by an extensive characterization of the remarkable amine-accumulating properties of the vesicular monoamine transporter by the laboratories of Scarpa, Johnson, Henry, Schuldiner, and others, showing that the 10,000-fold accumulation was due to an ATP-driven proton gradient, and subsequent proton-monoamine exchange via transport facilitation2–6 (Fig. 1). Subsequently, the laboratories of Eiden and colleagues and Edwards et al. cloned the rodent and human monoamine transporters VMAT1 and VMAT2,7–9 the former found mainly in peripheral neuroendocrine cells and absent from all areas of the central nervous system, and the latter in monoaminergic neurons of the central and peripheral sympathetic nervous systems.10,11 VMAT1 and VMAT2 are members of the solute carrier superfamily and carry the designations Slc18a1 (VMAT1) and Slc18a2 (VMAT2) within that family.4 Fundamental insight into VMAT function can be gained by appreciation of its placement within a superfamily of proteins called TEXANs, for toxin-extruding antiporters.5,12 The vesicular neurotransmitter transporters evolved from bacterial antiporters that exchanged (extruded) cellular toxins in exchange for extracellular protons at the cellular membrane. Their role in vesicular transport represents essentially an “internalization and delayed extrusion” strategy for export of transmitter molecules (by exocytosis) that are in fact toxins (e.g., dopamine) if allowed to accumulate in the neuronal cytoplasm rather than being sequestered in storage vesicles.13–15
Figure 1.
Neurotransmitter accumulation in the synaptic vesicle.
More recently, VMAT2 knockout mice have established the critical role of VMAT2 both in maintaining catecholamine and serotonin levels in CNS, and monoamine availability for exocytotic release from neurons upon depolarization.14,16,17 In the past several years, human variants in VMAT1 have been linked to susceptibility for schizophrenia18 and bipolar depression19 and those in VMAT2 to schizophrenia20 and protection from alcohol neurotoxicity.21 Since available evidence (vide supra) strongly supports the proposition that VMAT2 is the only vesicular monoamine transporter expressed in CNS neurons, this review will focus on it; the general cellular biology and physiology of VMAT1 and VMAT2 together have been reviewed elsewhere.3,22–28
Visualization of VMAT2 binding sites in the brain reveals a target highly susceptible to altered ligand binding (for example, of the [11C]-labeled PET ligand dihydrotetrabenazine, DTBZ) as a function of intracellular amine concentration, and a transporter protein whose expression is far more dynamically regulated by physiological and pharmacological conditions (e.g., stress and drug abuse) than previously appreciated. How have advances in understanding the biochemistry and cell biology of monoamine storage enabled insights into VMAT2 as a target for drugs of abuse? What are the potential roles of VMAT2 in the molecular and cellular mechanisms underlying the rewarding of drug-taking behavior and the maintenance of drug-seeking behavior? Finally, what possibilities might exist for VMAT2 as a target for treatment of addiction? These are the subjects of this review.
Neuroanatomy and cell biology of brain VMAT2
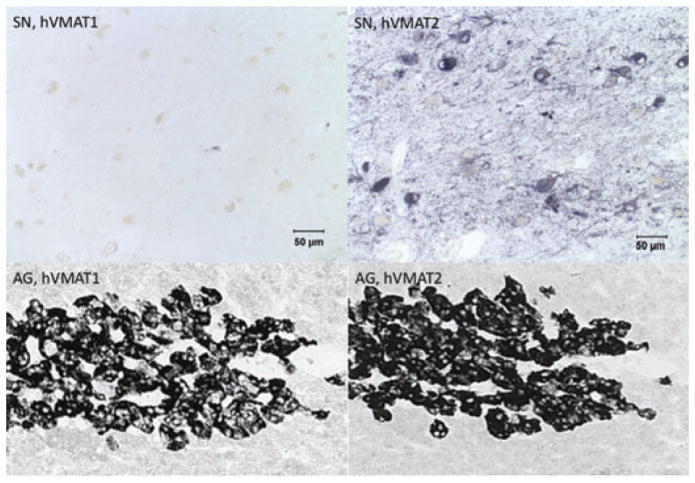
In this section, we review the fundamental neuroscience of the VMATs, with primary emphasis on VMAT2. This is because although VMAT1 and VMAT2 are co-expressed in human adrenal medulla, and thus might both play a role in peripheral adrenaline release by drugs of abuse, the central and peripheral nervous systems of rodents, primates, and humans appear to express only VMAT2. Hansson et al. have reported VMAT1 mRNA expression during development within the sensory nervous system of the rodent.29 However, other studies have failed to substantiate this finding at either the mRNA or protein levels,30,31 and CNS nerve terminals of VMAT2 knockout mice show a complete abolition of reserpine-sensitive monoamine uptake.16 Lohoff et al. have also reported that VMAT1 is present in human brain tissue, and that VMAT1 protein is expressed in neuronal terminals in the hippocampus, striatum, and cortex. Our laboratories and others have been unable to obtain evidence for VMAT1 expression in neurons of adult rodent or primate brain,32 or in the developing rodent (rat) nervous system,30,31 in contrast to the reports cited above. For the purposes of this review, we assume, according to our own published and recently reexamined data (see Fig. 2), that VMAT1 is not expressed at appreciable levels in the brain of adult rodents or humans, the mammalian species in which most data relevant to drug addiction have been obtained. In view of the reported concordance between inheritance of nondominant VMAT1 alleles and prevalence of schizophrenia and affective disorders in certain European populations,18,19,33,34 however, the issue of expression of VMAT1 in human brain should probably be systematically reexamined.
Figure 2.
Differential expression of hVMAT1 and hVMAT2 in the CNS and periphery. Staining for VMAT1 and VMAT2 in human substantia nigra (SN) and adrenal gland (AG). Adrenal gland data are taken from Erickson et al.,32 and substantia nigra micrographs were discussed but not shown in that publication. Note the complete absence of specific staining for VMAT1 in human brain tissue, and corresponding expression of both VMAT1 and VMAT2 in human adrenal medulla under conditions of staining and incubation as described in Ref. 32.
Chemical neuroanatomy of VMAT2-expressing central neurons
VMAT2 is expressed in all monoaminergic neurons of the brain, including those expressing dopamine (DA), serotonin (5-HT), norepinephrine (NE), epinephrine (EPI), and histamine (HIS). It is likely that VMAT2 is also the vesicular transporter for neurons synthesizing and storing the so-called trace amines for which cognate receptors have now been identified, including those for tyramine (TYR) and phenylethylamine (PEA).35 Both TYR and PEA have affinity for VMAT2 comparable to that of DA and NE.32 VMAT2 immunohistochemistry allowed the categorization of aminergic neurons in the brain, both during development and in mature mammals, into those that were fully functional for aminergic neurotransmission (e.g., expression of biosynthetic enzymes, VMAT2, and the serotoninergic plasma membrane transporter 5-HTT for 5-HT; of biosynthetic enzymes and co-factors, VMAT2, and the dopamine plasma membrane transporter DAT for DA; biosynthetic enzymes and co-factors, VMAT2, and the norepinephrine plasma membrane transporter NET for NE) and those that might participate in functional neuronal circuitry in other ways. For example, neurons of the primate nucleus tractus solitarius express tyrosine hydroxylase (TH) for DOPA synthesis, but lack dopa decarboxylase (AADC) for DA synthesis and have been termed “DOPAergic” neurons.36 These neurons have no detectable VMAT2 protein, suggesting that neurons unable to produce exocytotically relevant messengers also downregulate or extinguish VMAT2 expression. VMAT2 can also play a significant negative regulatory role in chemical coding of neuro-transmission. For example, sweat gland innervation in the mouse is cholinergic but not functionally monoaminergic due to the selective lack of expression of VMAT2 in these particular postganglionic sympathetic neurons, despite their expression of both TH and dopamine beta-hydroxylase (DBH) (Ref. 37, but see also Ref. 38). In human sweat glands, both cholinergic and noradrenergic functionality is maintained through full expression of the proteins, including VMAT2 and the vesicular acetylcholine transporter VAChT, for both phenotypes.37
The dynamic regulation of VMAT2 expression both in development and upon acute and chronic drug exposure may provide a new dimension in characterizing the cell biological process that occurs during the transition from recreational to compulsive drug use in addiction. Chronic drug use, for example, is already known to produce impairments in monoaminergic circuits in the CNS that include neurotoxicity, and enhanced sensitivity of receptor signaling.39–43 With continued drug use, homeostatic mechanisms can be activated in response to increasingly depleted monoamine stores within specific mesolimbic pathways, an aspect of drug addiction in which VMAT2 is likely to play a central role.
Intriguingly, VMAT2 is expressed transiently in thalamocortical sensory circuits during development, in neurons that otherwise express no biosynthetic enzymes for biogenic amines.31,44 These neurons also express 5-HTT, and have been postulated to scavenge 5-HT from neighboring neurons to create de novo “serotonergic” neurons during CNS development in rodents.44 Whether such neurons exist in human brain, and might be operative in mediating developmental effects of perinatal exposure to drugs of abuse,45 remains an unanswered question.
VMAT2 is the CNS vesicular transporter for not only the biogenic amines DA, NE, EPI, 5-HT, and HIS, but likely also for the trace amines TYR, PEA, and thyronamine (THYR). Tyramine neurons are found in Drosophila where they are essential for sensitization to COC.46 Tyraminergic neurons in mammalian CNS would be identifiable as neurons expressing VMAT2 for storage, and the biosynthetic enzyme aromatic amino acid decarboxylase (AADC).47 Thus neurons identified without TH, but with AADC and VMAT2, are not only potentially “monoenzymatic neurons” (after the nomenclature of Ugrumov48) but also potentially trace amine neurons as well. These would be affected by AMPH and, depending on the presence of postsynaptic trace amine-associated receptors (TAARs)35,49 and trace amine reuptake transporters (i.e., DAT, NET, or 5-HTT), by COC as well.
Substrate specificity of VMAT2
The substrate and inhibitor specificity of VMAT2 for various psychostimulants and/or neurotoxic drugs of abuse was examined by Erickson et al. for human VMAT2 in an heterologous-cell 5-HT uptake assay.32 The affinity for VMAT2 of d-amphetamine, racemic MDMA, fenfluramine, and 1-methyl-4-phenylpyridinium (MPP+) was 2, 7, 5, and 9 uM (compared to 1.4 and 0.9, respectively, for dopamine and 5-HT itself). Thus, these compounds interact with VMAT2 with an apparent affinity similar to that of dopamine, the major neurotransmitter implicated in addiction in the CNS.41 Data for COC interaction with human VMAT2 are not available. However, measurement of VMAT2 interaction with addictive psychotropic agents in comparison with the manifest abilities of such agents to alter VMAT2 expression in vivo after chronic or acute administration could shed light on whether such compounds trigger addiction and/or hedonic reward through direct, or indirect interaction with VMAT2-expressing neuronal circuits in vivo.
The drug specificity of the plasma membrane transporters DAT, NET and 5-HTT have been investigated quite extensively. In contrast, there is no pharmacological specificity expected at the level of vesicular uptake of DA, NE and 5-HT since all are mediated by VMAT2. However, there are distinct differences in DA, NE, and 5-HT metabolism and depletion in DAT as well as VMAT2 knockout mice (see below), suggesting that both chronic and acute effects of agents acting at VMAT2 might well have dramatically different impacts on synaptic transmission in different neurotransmitter systems. There has been little examination of the combinatorial effects of, for example AMPH, COC or MDMA (or even antidepressant NET- and 5-HTT-specific uptake inhibitors) on the DAT/VMAT2, NET/VMAT2, or 5-HTT/VMAT2 neuronal uptake and retention systems upon chronic treatment. However, Gaintedinov and Caron have pointed out that although DA depletion induced by chronic AMPH or COC has “as a rule”50 been interpreted as being due to outright nerve terminal destruction, there is in fact little evidence for this. Vesicular amine depletion of intact nerve terminals, through interactions between drug, DAT and VMAT2, may actually represent a more relevant feature of the process by which individuals become addicted to these compounds, in which case, intervention at the level of vesicular uptake could be envisaged.51,52
Does the substrate specificity of VMAT2, and its role in transmitter uptake of amines besides those mentioned above, play any additional roles in the central neurochemistry of substance abuse and addiction? Duerr et al. have hypothesized that the evolution of a VMAT isoform lacking the ability to store histamine (VMAT1) may have afforded a selective advantage to organisms whose gut enteric nervous system is supplied with serotonin largely from enterochromaffin cells of the gut, which scavenge serotonin from dietary sources and release it presumably to be taken up by “fauxserotonergic” VMAT2-expressing neurons lacking tryptophan hydroxylase for de novo 5-HT synthesis.53 The presence of VMAT2 in mast cells of the central nervous system, in transient transmitter-unidentified somatosensory relay neurons in thalamus, and in histaminergic neurons of the hypothalamus,31 and the pharmacological ability of AMPH and related compounds to release HIS from storage vesicles based on the relative affinity of AMPH and HIS for VMAT2, suggest that HIS-releasing action of addictive amine-releasing drugs should not be overlooked.
VMAT2-regulation of trafficking and transporter activity
A conceptual block to focusing on vesicular transporters as potential regulators of dynamic aspects of synaptic transmission in general has been the understandably ingrained notion that the size of the secretory quantum is fixed and unregulated. This view informs much of the copious research on synaptic plasticity, which has focused, through most of the last 40 years, on mechanisms involving changes in efficiency of presynaptic release, rather than changes in the secretory quantum. Synaptic efficacy is thought to be the cellular substrate for learning and memory including learning associated with addiction.41 However, only recently have activity-dependent changes in synaptic efficacy been sought and found in changes of synaptic scaling involving altered vesicular (quantal) content.54 The view that neurotransmitter quantal content might be a dynamically regulated physiological process was suggested by experiments in which vesicular transporters, such as the vesicular acetylcholine transporter (VAChT) were altered in vivo to affect quantal size.55 It was further demonstrated that the ratio of expression of VAChT to the biosynthetic enzyme for acetylcholine, ChAT, was significantly different in central and peripheral neurons,56 suggesting that biosynthesis and vesicle filling, along with quantal release probability, could regulate the architecture of neurotransmission. In parallel developments in GABAergic and glutamatergic systems, several laboratories found evidence that vesicular filling may be controlled, in addition to the limits set by electrochemical driving forces generated by the vesicular vacuolar H+-generating ATPase (Fig. 1), by accessory proteins that act as quantal size regulators. The Ueda laboratory has identified a fodrin-derived inhibitory protein factor (IPF) that when injected into synaptosomes, decreases quantal size for serotonin, GABA, and glutamate.57,58 No endogenous protein regulator for VMAT has been identified. However, the thyroxine metabolite THYR is a potent inhibitor of monoamine uptake by VMAT2, and has been proposed as an endogenous inhibitor of monoamine uptake and storage in the brain.59
A second potentially important mode of regulation of VMAT2 activity, and thus of vesicular filling and regulation of quantal size, has been identified by Ahnert-Hilger and colleagues in a series of papers that describe linkage between G-protein expression and vesicular filling by VMAT2. VMAT1 or VMAT2 expression in CHO cells allowed uptake of tritiated serotonin. This uptake, modulated by previous store filling with unlabeled biogenic amine, was shown to be dependent on the first intraluminal loop of VMAT.60 Although the relationship between the biogenic amine and VMAT employed (VMAT1 versus VMAT2) is complex, it appears to require G-proteins (Go in platelets and Gi in other cell types) and the calcium-activated protein for secretion (CAPS).61 These intriguing results, collectively, imply an intravesicular monoamine-VMAT interaction, via a GTP-binding protein, that provides a mechanism for modulation of vesicular filling below the set point determined by the vesicular electrochemical gradient. This mechanism may be related to the abolition of cocaine (COC)-induced behavioral sensitization in Go2alpha-knockout mice.62 Completion of crucial details of this mechanism for regulation of VMAT transport activity, including the pathway through which intravesicular amines might affect extravesicular G-protein modulation of VMAT2, should be informative for gaining insight into how COC, METH, AMPH, and perhaps therapeutic agents such as antidepressants, affect neuronal signaling for vesicle filling.
Signaling pathway modulation by addictive drugs may also affect VMAT2 transporter function indirectly, but profoundly, by altering VMAT cellular trafficking. VMAT2 trafficking to SSVs in preference to large dense core vesicles (LDCVs) occurs, at least in PC12 cells, under conditions in which PKA signaling is reduced.63
The relationships between VMAT phosphorylation, G-protein-dependent alterations in vesicle filling, transporter trafficking, and cAMP/PKA signaling are not yet resolved into a coherent picture of signal transduction modulating vesicle filling and quantal size. The effects of addictive drugs on this process, and its role if any in addiction itself, appear to be promising leads in addiction research. These ideas have already led to the proposal that compounds targeting VMAT2, such as lobaline, may be useful in the treatment of methamphetamine addiction by blocking its amine-releasing actions in vivo.64,65 It is especially promising that the effects of addictive drugs on each step of the process of exocytosis, from vesicle filling to neurotransmitter release during synaptic transmission, can be modeled in detail in chromaffin cells and studied in vivo using cyclic voltammetry.66,67 Details of the cellular biology of VMAT2 relevant to both drug action and clinical neuroimaging are shown in Figure 3.
Figure 3.
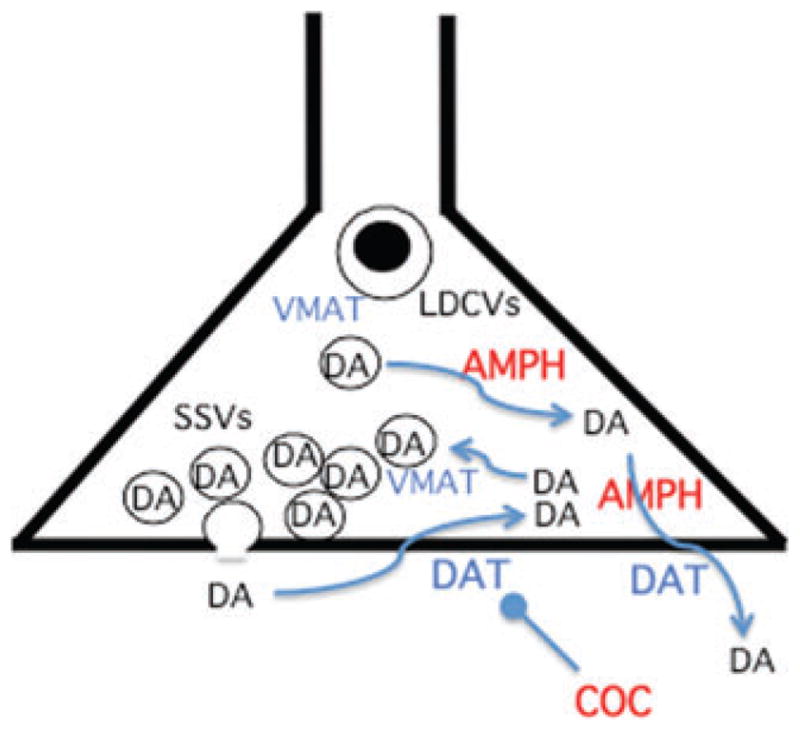
Cellular aspects of VMAT2 trafficking, expression, and targeting by drugs of abuse. Dual site of action of AMPH at the synaptic vesicle and at DAT, and single site of action of COC at DAT, are depicted. VMAT2 trafficking to SSVs and LDCVs is regulated in part by phosphorylation, and VMAT2 transport by G- protein coupling, through signal transduction pathways that are not yet characterized. TBZ binds directly to VMAT2 independently of its cellular location. See text for details.
Genetics of VMAT2
VMAT2 knockouts—clues to VMAT2 brain functions
In 1997, three groups independently reported the generation of knockout mice deficient in VMAT2 expression.14,16,17 Several aspects of VMAT2 function in brain were immediately confirmed. Complete knockout (−/−) of VMAT2 expression is lethal at birth, and accompanied by lack of feeding behavior, and can be partially rescued by administration of amphetamine.16 This suggests that while activity-dependent (vesicular) secretion of a monoamine, centrally or in the periphery, is essential for life, it can be mimicked sufficiently for survival by indirect release via plasma membrane transporter-dependent efflux. Since the restored phenotype appears to be enhanced motor activity allowing feeding, it is most likely dopamine-dependent. Knockout of VMAT2, with preservation of VMAT1, reduced total labeled monoamine accumulation in whole brain vesicular membrane preparations to levels found after reserpine administration.16 This finding provides further functional evidence that, at least in mouse brain, no additional vesicular transporter, such as VMAT1, plays a discernable functional role in vesicular monoamine accumulation. Finally, monoamine (5-HT, DA, and NE) levels are profoundly reduced in VMAT2 (−/−) newborn mice (less than 2% of wild-type) indicating that vesicular storage of monoamines is essential not only for activity-dependent release, but also for biasing the relative rates of synthesis and degradation of monoamines (DA, NE, and 5-HT metabolite levels are as high in knockout as in wild-type brain) in favor of monoamine accumulation, and thus increasing monoamine biosynthetic efficiency.16
Interestingly, VMAT2 heterozygous (+/−) mice also demonstrate about a 50% loss of endogenous DA and 5-HT (though not NE), indicating that gene dosage is critical for VMAT2 function in vivo. These results also offer indirect support for the idea that regulation of VMAT2 activity and/or expression could be a rate-limiting step in monoamine production and release by controlling quantal size in monoaminergic neurons. This notion has been tested directly by Pothos and colleagues by overexpression of VMAT2 in neuroendocrine cell models, in which quantal size is indeed a direct function of vesicular VMAT2 density.68 The VMAT2 heterozygous knockout phenotype has also allowed direct confirmation that vesicular transport, especially in dopaminergic neurons, plays an important role in sequestration of potential neurotoxins like MPP+, as well as the endogenous neurotoxin DA.14,26 These results have direct implications for neurodegeneration and Parkinson-like clinical outcomes associated with environmental exposure to neurotoxins, as well as long-term drug abuse. Finally, altered sensitivity to the locomotor effects of COC, AMPH, and alcohol in VMAT2-deficient mice provide evidence that the effects of drugs of abuse depend directly on ambient amine storage levels, which in turn are set, within fairly tightly constrained boundaries, by expression of VMAT2.14
A point worth considering in somewhat greater detail is the altered sensitivity to drugs of abuse conferred in VMAT2-deficient mice, alluded to in the preceding paragraph. Uhl and colleagues have reported that VMAT2 heterozygous mice displayed both sensitization to AMPH-induced locomotion, and decreased AMPH-induced reward.14,69 The authors concluded that synaptic vesicle function may contribute more to AMPH-induced reward than to locomotion, consistent with an independent report of rescue of the VMAT2 null phenotype from lethality at birth by administration of AMPH, largely restoring locomotor activity required for feeding and survival.16 The implications of this work have been particularly exciting as they not only highlight the possibility that addiction as well as relapse liability may be controllable at the level of the amine storage vesicle,52,64,65 but also provide animal models for testing both potential treatments and hypotheses about processive, long-term neurochemical mechanisms of addiction and addictive behavior.
Human VMAT genetics and epigenetics and addiction
The primary importance of VMAT2 in monoamine storage and release in the central nervous system provided a strong rationale for examination of single nucleotide polymorphisms (SNPs) in both coding and noncoding regions of VMAT2 that might be linked to psychiatric and neurological diseases in which monoamines are implicated. These include Parkinson’s disease, drug addiction, environmental neurotoxin-mediated neurodegeneration, depression, bipolar disease, and schizophrenia. Heritability is taken as strong evidence for a uni- or oligogenic component of disease, while concordance in identical twins is taken as strong evidence for a genetic component (and may be the only genetic evidence for a multigenic genetic component) for a disease. Finally, association between gene alleles and disease phenotypes or behavioral traits can be identified in case-control studies. The evidence for VMAT2 “defining haplotypes” in various monoamine-associated human diseases, and for VMAT2 haplotypes potentially contributory to addiction or response to addictive drugs in particular, can be considered in this context.
Evidence for VMAT2 as a genetic component in affective disorders is, as for many genetic markers for schizophrenia, mixed. Thus, Uhl and coworkers essentially ruled out VMAT2 allelic variants as genetic contributors to schizophrenia risk,70 while Chu and co-workers using a genomic gene–function approach identify VMAT2 as one of nine genes demonstrating a gene–function correlation in schizophrenia.20 An unexpected correlation has been uncovered between ethanol consumption in male mice and VMAT2 expression, with higher spontaneous ethanol consumption in VMAT2 (+/−) compared to wild-type mice at high ethanol–water ratios,71 while VMAT2-deficient mice show a decreased ethanol preference in a two-bottle preference test.72 This correlation is made more intriguing by the subsequent identification of an hVMAT2 SNP cluster, or defining haplotype, that defines a reduced risk for alcoholism.21 Uncovering the neurochemical phenotype of the haplotype will be an important step forward in understanding what aspect of VMAT2 function confers susceptibility to alcohol addiction. Whether this can be generalized to addiction to substances other than ethanol is of course an even more exciting avenue for continued investigation.
Finally, it should be noted that the work of Barr and colleagues demonstrating modulation of VMAT2 expression during the development of corticosterone-dependent aversion learning in infant rats73 suggests that stable changes in VMAT2 gene expression may be acquired not only genetically, but also epigenetically. However, alterations in VMAT2 expression during the acquisition of addictive behaviors mediated by altered DNA methylation or histone acetylation at this locus have not yet been reported in the literature.
Imaging of VMAT2
Chemical characteristics of VMAT2 ligands
The most-used ligand for VMAT2 binding studies in vivo is [11C]-labeled dihydrotetrabenazine (DTBZ) (Fig. 4). Originally of therapeutic interest due to its antipsychotic properties, tetrabenazine (TBZ) is a benzoquinolizine derivative that is marketed under the trade name Xenazine for treatment of hyperkinetic disorders including chorea associated with Huntington’s disease, and Tourette’s syndrome,74,75 because of its dopamine-depleting properties. DTBZ is its reduced-ketone congener, with decreased chemical reactivity but similar VMAT2 binding characteristics. The affinity of TBZ for VMAT2 is about 0.09 μM, i.e., about 10 times higher than that of DA, and about 10 times less than that for reserpine (RES), as measured under initial-velocity uptake conditions.32 The slow off-rate of RES from VMAT2 makes the latter a quasi-irreversible ligand compared to TBZ. It should be noted that the affinities of TBZ, NE, DA, and 5-HT for rat and human VMAT2a are essentially identical,9 making in vivo imaging studies with DTBZ in rodents pharmacologically quite comparable to results expected in imaging of human subjects.
Figure 4.
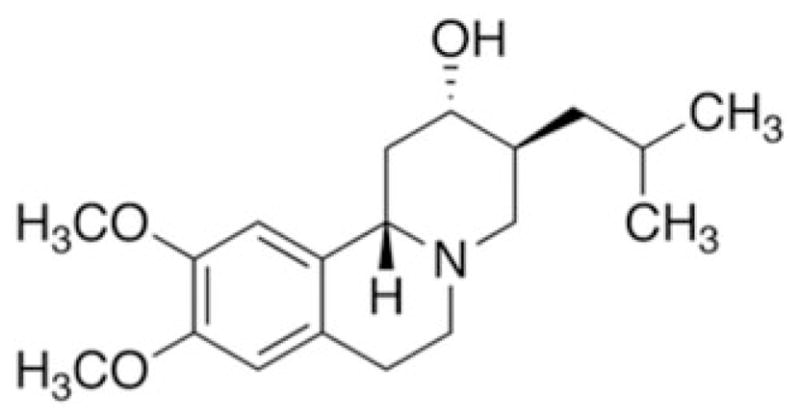
Chemical structure of dihydrotetrabenazine.
Since TBZ does not bind to hVMAT1, while it has a high affinity for hVMAT2,32 analysis of chimeric VMAT1/2 constructs enabled Thiriot and Ruoho to localize TBZ binding to the tenth transmembrane domain of VMAT2, in a region likely to represent part of the vesicle-facing binding site for cat-echolamines.76 This implies that TBZ has a high affinity for VMAT2 relative to DA because the internal site has a lower affinity than the externally facing size for transported catecholamines.5,12 Thus, TBZ as an in vivo ligand could well be sensitive to the relative state of depletion of the dopaminergic vesicle during in vivo conditions (e.g., chronic AMPH usage) in which vesicular DA depletion is likely.
TBZ in brain imaging
[11C]-DTBZ (half-life for positron emission about 11 min) has been employed in a number of pre-clinical (rodent and primate) and clinical positron emission tomography (PET) studies with the goal of imaging monoaminergic nerve terminals in the brain. Initially, the rationale for the use of DTBZ, like that of the vesicular acetylcholine transporter ligand vesamicol, was that VMAT2 was unregulated by drugs, and would therefore function as a marker for synaptic integrity during in vivo imaging of progressive neurodegeneration in neurological diseases such as Parkinson’s disease, Huntington’s chorea, and Alzheimer’s disease.77 However, striatal VMAT2 is decreased not only in advanced Parkinson’s disease,78 but further in patients shortly after administration of levodopa.79 It is also elevated in chronic methamphetamine users shortly after cessation of drug use.80 These findings strongly implied that altered PET signals after DTBZ administration might arise not only as a function of VMAT2 concentration in brain tissue, but also as a function of endogenous DA competition with the tracer ligand during imaging. To test this hypothesis, Boileau et al. used human subjects to measure striatal [11C]-DTBZ binding after an acute oral dose of AMPH. They found a slight decrease in binding, a fact these authors attributed to insufficiency of AMPH dosing to significantly deplete striatal DA in their subjects.81 Kilbourn et al. conducted a more systematic study in rat, in which in vivo PET analysis of [11C]-DTBZ binding was examined as a function of DA depletion with alpha-methyl-para-tyrosine and repletion with L-dopa. These authors concluded that PET-visualized DTBZ binding site density could be altered in vivo as a function of endogenous DA concentrations independently of the actual protein concentration of VMAT2.82 Contribution of mast cell VMAT283,84 to PET signals derived from [11C]-DTBZ binding must also be considered in brain,31 as well as tonsils85 and other tissues. Thus, the picture of precisely what neurochemical information VMAT2 imaging with DTBZ provides is still somewhat unfocused. Altered [11C]-DTBZ may reflect changes in endogenous catecholamine levels with unchanged VMAT2 protein expression, or altered VMAT2 protein expression or trafficking. More systematic preclinical studies will be required to optimally guide the extraction of clinically useful information from [11C]-DTBZ imaging of the human brain.
Physiological and pathophysiological plasticity of VMAT2 expression
As mentioned above, using TBZ as a marker for occupancy of brain VMAT2 binding sites is confounded by two often-opposing effects of chronic drug use. Changes in the ambient levels of endogenous transmitters or drugs at time of imaging cause changes in competitive inhibition of TBZ binding during the imaging event. Changes in the actual number of VMAT2 molecules expressed in brain tissues also alter TBZ binding. Can these two variously additive and opposing effects be distinguished to allow mechanistic insight into drug addiction from descriptive studies of DTBZ binding in vivo? Knowledge of the relative affinities of endogenous transmitters, drugs of abuse, and imaging ligands in vitro, while invaluable, are insufficient for this purpose, since the concentrations of competing ligands in vivo are rarely known, and the amount of VMAT2 protein per unit brain tissue cannot be assumed to be constant across individuals in different experimental groups. Rigorous preclinical studies are still too few to resolve this issue (vide supra), and provide a solid basis for interpretation of the existing clinical studies already reported. Nevertheless, some principles are emerging from this work that allow the tentative conclusion that VMAT2 protein expression is dynamically regulated as a function of drug administration.
Drug administration modulates VMAT2 activity
It was initially thought that DAT was highly regulated by neuronal trafficking and signaling pathways, which are sensitive to perturbation by drugs such as COC and AMPH, as well as some antide-pressants86 and, conversely, VMAT2 expression and transport activity were relatively unregulated and unperturbed by drug treatment. For example, profound depletion of brain monoamines with reserpine depresses TBZ binding because reserpine binds irreversibly to the same site that TBZ occupies reversibly, albeit with high affinity.87 However, this treatment has no effect on striatal VMAT2 protein levels, as measured by quantitative immunohistochemistry with VMAT2 antibodies whose binding is unaffected by reserpine attachment to the transporter.87 With respect to transport parameters associated with VMAT2 function, Wilson and Kish found no alteration in TBZ binding site number (VMAT2 Bmax) in striatal slices from rodents chronically treated with COC.88 These investigators and their colleagues extended these findings in rodent to postmortem analysis of human striatal tissue, obtained at autopsy from chronic COC or methamphetamine (METH) users.89,90 In both cases, while striatal DA was depleted, VMAT2 levels were affected in chronic COC (17–22% decrease) but not in chronic METH users. It is noteworthy that these investigators used TBZ binding rather than quantitative Western blotting as an index of VMAT2 levels.
VMAT2 is clearly required for the rewarding pharmacological effects of both AMPH and COC—and perhaps also for their abuse liability. AMPH release of DA from synapses requires both an action at VMAT2 to release DA to the cytoplasm and a concerted release of DA from the cytoplasm via “reverse transport” through DAT.40 COC action likewise requires exocytotic release of DA from vesicles filled through the action of VMAT2 before it can fulfill its pharmacological action of inhibiting DA reuptake through DAT.50 A consensus developed in the imaging literature that neither VMAT2 protein nor VMAT2 transporter function was dynamically regulated by acute or chronic treatment with drugs of abuse, making DTBZ imaging an attractive candidate for assessing monoaminergic neurodegeneration in disease and after chronic drug use.82 However, this consensus discouraged the view that VMAT2 could be a therapeutic target in disease. Recently, however, the notion that VMAT2 could be regulated dynamically by drugs of abuse (and therefore might represent a potential site of pharmacological intervention) was proposed by Fleck-enstein et al., based on their observations that COC administration in rodents rapidly and reversibly increased VMAT2 Vmax and Bmax in striatal purified vesicle preparations, while AMPH administration has the opposite effect (decreased VMAT2 Vmax and Bmax). Schwartz et al. have also reported that short term COC treatment (5 days), but not AMPH treatment, elevates VMAT2 density as measured in membrane particulate fractions of rat striatal homogenates.91 Since AMPH increases cytoplasmic DA (through intracellular vesicular release) while COC decreases cytoplasmic DA (through inhibition of reuptake of extra-cellular DA), these results imply that VMAT2 function may be directly regulated by cytoplasmic levels of neurotransmitter. The discrepancy between the results of Wilson and Kish obtained via quantitative autoradiography of 3H-TBZ binding in striatal slices, in which VMAT2 density did not change,88 and results obtained by direct measurement of TBZ binding in striatal vesicular preparations, where it did, may reflect regulation through redistribution of VMAT2 from small synaptic vesicles to large vesicles, other organelles, or to nonvesicular locations.40
Altered VMAT2 expression in stress
Sabban and co-workers have recently reported that VMAT2 is up-regulated in chromaffin cells in vivo following immobilization stress (Sabban et al., in press, 2010). Barr and co-workers have observed that developmentally dependent aversion learning requires amygdalar dopaminergic up-regulation that includes increases in both DAT and VMAT2 expression along with another marker for dopaminergic function, aldehyde dehydrogenase 1a1.73
In addition to altered VMAT2 protein expression secondary to altered gene transcription, VMAT2 activity in neurons may be regulated by changes in trafficking to multiple vesicle types,92 as postulated by Fleckenstein et al. for measured changes in VMAT2 Bmax and Vmax specific to the small synaptic vesicle compartment after AMPH treatment.40 G-protein-specific regulation of vesicle filling may also involve molecular changes in VMAT2, including phosphorylation, with dramatic effects on sorting between small and large vesicle compartments of the cell.63 These changes may occur on the order of hours, days and weeks. They are therefore especially worthy of further investigation in light of the potential for understanding short- and long-term regulation of quantal size, and location of neurotransmitter release, that might underlie the development of addictive behavior, its maintenance, and its waning and recrudescence in withdrawal and relapse behaviors. The finding of altered VMAT2 expression in aversive learning and stress, both likely components of drug-seeking behavior, withdrawal, and relapse, may provide a critical link in monoaminergic mediation of rewarding and addictive properties of AMPH, COC, and other drugs of abuse.
Perspectives in drug abuse and addiction
Despite what is known about VMAT2 basic biology in brain, and the ability to image it, knowledge about its role in drug addiction and neonatal susceptibility to maternal drug use, potential translational prospects for therapeutics of drug addiction remain rather primitive. Is it possible to blunt reward pathway vulnerability with anti-VMAT2 drugs without causing depression? Is it possible to offer neonatal protection against addictive liability with similar drugs? Can we learn more about the mechanisms of drug addiction and drug-related hedonic behavior with VMAT2 pharmacological experiments and TBZ imaging? What have we really learned so far about monoamine synaptic dynamics by imaging either DAT or VMAT2, given that altered ligand binding for these proteins can involve both changes in protein levels, and altered competition for the imaging ligand by both endogenous amines and administered drugs?
Perhaps the major development of the last decade concerning vesicular neurotransmitter storage is the level of control and regulation at the level of the transporters themselves. Not only VMATs, but GABA, glutamate and acetylcholine vesicular transporters seem to be actors in the dynamic control of vesicular transmitter content as a function of neuronal activity and intracellular signaling by calcium, kinases, and phosphatases. Clearly, VMAT2 is an important effector for DA release by AMPH, COC, and other drugs of abuse. Whether its dynamic regulation, especially in the dopaminergic system, might thus be an important contributor to drug addiction is an intriguing question that is only now being definitively explored. Of particular interest is the linkage between prolonged stress and VMAT2 expression in adrenal. Whether this extends from rodent to primate species, and from peripheral to central neurons is a key question, since altered expression of VMAT2 could play a role in enhanced susceptibility to drug-seeking, maintaining, or relapse behavior as a function of stress.
VMAT2, despite its occurrence in all monoaminergic neurons, may be quite specifically regulated by physiological stressors and drugs in each of them. In the brain, this appears to be especially the case in the dopaminergic neuronal population. The functional relationship between the rewarding effects of drugs of abuse and neurotransmitter availability in nerve terminal vesicles and cytoplasm is a crucial one. Changes in nerve terminal monoamine stores driven by chronic use of drugs like METH and COC are likely to be responsible for tolerance and frank failure-to-reward. Less directly, such mechanisms may also impact the development of tolerance and addiction to opiates.43 Understanding the dynamics of VMAT2 adaptation as VMAT2- and DAT-directed drugs deplete brain monoamines in addiction may provide a unique entrée to the neurochemistry of drug seeking, addiction, and postwithdrawal relapse.
Footnotes
VMAT2 was originally termed ‘VMAT1’ or ‘CNS VMAT’ (see [9]). VMAT2 is now the accepted name for the VMAT expressed predominantly in neurons (Slc18A2), and VMAT1 is the accepted name for the VMAT expressed predominantly in neuroendocrine cells (Slc18A1; [4,10,11, 32]).
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Kirshner N. Uptake of catecholamines by a particulate fraction of the adrenal medulla. J Biol Chem. 1962;237:2311–2317. [PubMed] [Google Scholar]
- 2.Johnson RG., Jr Accumulation of biological amines into chromaffin granules: a model for hormone and neuro-transmitter transport. Physiol Revs. 1988;68:232–307. doi: 10.1152/physrev.1988.68.1.232. [DOI] [PubMed] [Google Scholar]
- 3.Eiden LE. The vesicular neurotransmitter transporters: current perspectives and future prospects. FASEB J. 2000;14:2396–2400. doi: 10.1096/fj.00-0817rev. [DOI] [PubMed] [Google Scholar]
- 4.Eiden LE, et al. The vesicular amine transporter family (SLC18): amine/proton antiporters required for vesicular accumulation and regulated exocytotic secretion of monoamines and acetylcholine. Pflugers Arch. 2004;447:636–640. doi: 10.1007/s00424-003-1100-5. [DOI] [PubMed] [Google Scholar]
- 5.Schuldiner S, Shirvan A, Linial M. Vesicular neuro-transmitter transporters: from bacteria to humans. Physiol Rev. 1995;75:369–392. doi: 10.1152/physrev.1995.75.2.369. [DOI] [PubMed] [Google Scholar]
- 6.Henry JP, et al. The vesicular monoamine transporter: from chromaffin granule to brain. Neurochem Int. 1998;32:227–246. doi: 10.1016/s0197-0186(97)00092-2. [DOI] [PubMed] [Google Scholar]
- 7.Erickson JD, Eiden LE, Hoffman B. Expression cloning of a reserpine-sensitive vesicular monoamine transporter. Proc Natl Acad Sci USA. 1992;89:10993–10997. doi: 10.1073/pnas.89.22.10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, et al. A cDNA that suppresses MPP+ toxicity encodes a vesicular amine transporter. Cell. 1992;70:539–551. doi: 10.1016/0092-8674(92)90425-c. [DOI] [PubMed] [Google Scholar]
- 9.Erickson JD, Eiden LE. Functional identification and molecular cloning of a human brain vesicle monoamine transporter. J Neurochem. 1993;61:2314–2317. doi: 10.1111/j.1471-4159.1993.tb07476.x. [DOI] [PubMed] [Google Scholar]
- 10.Peter D, et al. Differential expression of two vesicular monoamine transporters. J Neurosci. 1995;15:6179–6188. doi: 10.1523/JNEUROSCI.15-09-06179.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weihe E, et al. Localization of vesicular monoamine transporter isoforms (VMAT1 and VMAT2) to endocrine cells and neurons in rat. J Mol Neurosci. 1994;5:149–164. doi: 10.1007/BF02736730. [DOI] [PubMed] [Google Scholar]
- 12.Vardy E, et al. Structural conservation in the major facilitator superfamily as revealed by comparative modeling. Protein Sci. 2004;13:1832–1840. doi: 10.1110/ps.04657704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kariya S, et al. Increased vulnerability to L-DOPA toxicity in dopaminergic neurons from VMAT2 heterozygote knockout mice. J Mol Neurosci. 2005;27:277–279. doi: 10.1385/JMN:27:3:277. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi N, et al. VMAT2 knockout mice: heterozygotes display reduced amphetamine-conditioned reward, enhanced amphetamine locomotion, and enhanced MPTP toxicity. Proc Natl Acad Sci USA. 1997;94:9938–9943. doi: 10.1073/pnas.94.18.9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaudhry FA, Edwards RH, Fonnum F. Vesicular neurotransmitter transporters as targets for endogenous and exogenous toxic substances. Annu Rev Pharmacol Toxicol. 2008;48:277–301. doi: 10.1146/annurev.pharmtox.46.120604.141146. [DOI] [PubMed] [Google Scholar]
- 16.Fon EA, et al. Vesicular transport regulates monoamine storage and release but is not essential for amphetamine action. Neuron. 1997;19:1271–1283. doi: 10.1016/s0896-6273(00)80418-3. [DOI] [PubMed] [Google Scholar]
- 17.Wang YM, et al. Knockout of the vesicular monoamine transporter 2 gene results in neonatal death and supersensitivity to cocaine and amphetamine. Neuron. 1997;19:1285–1296. doi: 10.1016/s0896-6273(00)80419-5. [DOI] [PubMed] [Google Scholar]
- 18.Lohoff FW, et al. Association between polymorphisms in the vesicular monoamine transporter 1 gene (VMAT1/SLC18A1) on chromosome 8p and schizophrenia. Neuropsychobiology. 2008;57:55–60. doi: 10.1159/000129668. [DOI] [PubMed] [Google Scholar]
- 19.Lohoff FW, et al. Variations in the vesicular monoamine transporter 1 gene (VMAT1/SLC18A1) are associated with bipolar i disorder. Neuropsychopharmacology. 2006;31:2739–2747. doi: 10.1038/sj.npp.1301196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chu TT, Liu Y. An integrated genomic analysis of gene-function correlation on schizophrenia susceptibility genes. J Hum Genet. 2010;55:285–292. doi: 10.1038/jhg.2010.24. [DOI] [PubMed] [Google Scholar]
- 21.Lin Z, et al. SLC18A2 promoter haplotypes and identification of a novel protective factor against alcoholism. Hum Mol Genet. 2005;14:1393–1404. doi: 10.1093/hmg/ddi148. [DOI] [PubMed] [Google Scholar]
- 22.Efange SMN. In vivo imaging of the vesicular acetylcholine transporter and the vesicular monoamine transporter. FASEB J. 2000;14:2401–2413. doi: 10.1096/fj.00-0204rev. [DOI] [PubMed] [Google Scholar]
- 23.Erickson JD, Varoqui H. Molecular analysis of vesicular amine transporter function and targeting to secretory organelles. FASEB J. 2000;14:2450–2458. doi: 10.1096/fj.00-0206rev. [DOI] [PubMed] [Google Scholar]
- 24.Parsons SM. Transport mechanisms in acetylcholine and monoamine storage. FASEB J. 2000;14:2423–2434. doi: 10.1096/fj.00-0203rev. [DOI] [PubMed] [Google Scholar]
- 25.Rand JB, Duerr JS, Frisby DL. Neurogenetics of vesicular transporters in C. elegans. FASEB J. 2000;14:2414–2422. doi: 10.1096/fj.00-0313rev. [DOI] [PubMed] [Google Scholar]
- 26.Uhl GR, et al. The VMAT2 gene in mice and humans: amphetamine responses, locomotion, cardiac arrhythmias, aging, and vulnerability to dopaminergic toxins. FASEB J. 2000;14:2459–2476. doi: 10.1096/fj.00-0205rev. [DOI] [PubMed] [Google Scholar]
- 27.Weihe E, Eiden LE. Vesicular amine transporter expression in amine-handling cells of the nervous, endocrine and inflammatory systems. FASEB J. 2000;14:2435–2449. doi: 10.1096/fj.00-0202rev. [DOI] [PubMed] [Google Scholar]
- 28.Weihe E, Eiden LE. Chemical neuroanatomy of the vesicular amine transporters. FASEB J. 2000;14:2435–2449. doi: 10.1096/fj.00-0202rev. [DOI] [PubMed] [Google Scholar]
- 29.Hansson SR, Hoffman BJ, Mezey E. Ontogeny of vesicular monoamine transporter mRNAs VMAT1 and VMAT2. I. The developing rat central nervous system. Dev Brain Res. 1998;110:135–158. doi: 10.1016/s0165-3806(98)00104-7. [DOI] [PubMed] [Google Scholar]
- 30.Schütz B, et al. Ontogeny of vesicular amine transporter expression in the rat: new perspectives on aminergic neuronal and neuroendocrine differentiation. Adv Pharmacol. 1998;42:903–908. doi: 10.1016/s1054-3589(08)60893-5. [DOI] [PubMed] [Google Scholar]
- 31.Schütz B, et al. Vesicular amine transporter expression and isoform selection in developing brain, peripheral nervous system and gut. Dev Brain Res. 1998;106:181–204. doi: 10.1016/s0165-3806(97)00196-x. [DOI] [PubMed] [Google Scholar]
- 32.Erickson JD, et al. Distinct pharmacological properties and distribution in neurons and endocrine cells of two isoforms of the human vesicular monoamine transporter. Proc Natl Acad Sci USA. 1996;93:5166–5171. doi: 10.1073/pnas.93.10.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lohoff FW. Genetic variants in the vesicular monoamine transporter 1 (VMAT1/SLC18A1) and neuropsychiatric disorders. Methods Mol Biol. 2010;637:165–180. doi: 10.1007/978-1-60761-700-6_9. [DOI] [PubMed] [Google Scholar]
- 34.Lohoff FW, et al. Association between variation in the vesicular monoamine transporter 1 gene on chromosome 8p and anxiety-related personality traits. Neurosci Lett. 2008;434:41–45. doi: 10.1016/j.neulet.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 35.Borowsky B, et al. Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci USA. 2001;98:8966–8971. doi: 10.1073/pnas.151105198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weihe E, et al. Three types of tyrosine hydroxylase-positive CNS neurons distinguished by dopa decarboxylase and VMAT2 co-expression. Cell Mol Neurobiol. 2006;26:659–678. doi: 10.1007/s10571-006-9053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weihe E, et al. Co-expression of cholinergic and noradrenergic phenotypes in human and non-human autonomic nervous system. J Comp Neurol. 2005;492:370–379. doi: 10.1002/cne.20745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Habecker BA, et al. Norepinephrine transporter expression in cholinergic sympathetic neurons: differential regulation of membrane and vesicular transporters. Dev Biol. 2000;220:85–96. doi: 10.1006/dbio.2000.9631. [DOI] [PubMed] [Google Scholar]
- 39.Chang L, et al. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007;102(Suppl 1):16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- 40.Fleckenstein AE, et al. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- 41.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 42.Laakso A, et al. Experimental genetic approaches to addiction. Neuron. 2002;36:213–228. doi: 10.1016/s0896-6273(02)00972-8. [DOI] [PubMed] [Google Scholar]
- 43.Nestler EJ, Aghajanian GK. Molecular and cellular basis of addiction. Science. 1997;278:58–63. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- 44.Lebrand C, et al. Transient developmental expression of monoamine transporters in the rodent forebrain. J Comp Neurol. 1998;401:506–524. [PubMed] [Google Scholar]
- 45.Rokyta R, et al. Prenatal and perinatal factors influencing nociception, addiction and behavior during ontogenetic development. Physiol Res. 2008;57(Suppl 3):S79–S88. doi: 10.33549/physiolres.931602. [DOI] [PubMed] [Google Scholar]
- 46.McClung C, Hirsh J. The trace amine tyramine is essential for sensitization to cocaine in Drosophila. Curr Biol. 1999;9:853–860. doi: 10.1016/s0960-9822(99)80389-3. [DOI] [PubMed] [Google Scholar]
- 47.Boulton AA. The tyramines: functionally significant biogenic amines or metabolic accidents? Life Sci. 1978;23:659–671. doi: 10.1016/0024-3205(78)90064-4. [DOI] [PubMed] [Google Scholar]
- 48.Ugrumov M, et al. Tyrosine hydroxylase- and/or aromatic L-amino acid decarboxylase-expressing neurons in the rat arcuate nucleus: ontogenesis and functional significance. Psychoneuroendocrinology. 2002;27:533–548. doi: 10.1016/s0306-4530(01)00091-9. [DOI] [PubMed] [Google Scholar]
- 49.Branicky R, Schafer WR. Tyramine: a new receptor and a new role at the synapse. Neuron. 2009;62:458–460. doi: 10.1016/j.neuron.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 50.Gainetdinov RR, Caron MG. Monoamine transporters: from genes to behavior. Annu Rev Pharmacol Toxicol. 2003;43:261–284. doi: 10.1146/annurev.pharmtox.43.050802.112309. [DOI] [PubMed] [Google Scholar]
- 51.Truong JG, et al. Pramipexole increases vesicular dopamine uptake: implications for treatment of Parkinson’s neurodegeneration. Eur J Pharmacol. 2003;474:223–226. doi: 10.1016/s0014-2999(03)02080-6. [DOI] [PubMed] [Google Scholar]
- 52.Dwoskin LP, Crooks PA. A novel mechanism of action and potential use for lobeline as a treatment for psychostimulant abuse. Biochem Pharmacol. 2002;63:89–98. doi: 10.1016/s0006-2952(01)00899-1. [DOI] [PubMed] [Google Scholar]
- 53.Duerr JS, et al. The cat-1 gene of Caenorhabditis elegans encodes a vesicular monoamine transporter required for specific monoamine-dependent behaviors. J Neurosci. 1999;19:72–84. doi: 10.1523/JNEUROSCI.19-01-00072.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Gois S, et al. Homeostatic scaling of vesicular glutamate and GABA transporter expression in rat neocortical circuits. J Neurosci. 2005;25:7121–7133. doi: 10.1523/JNEUROSCI.5221-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Song H, et al. Expression of a putative vesicular acetylcholine transporter facilitates quantal transmitter packaging. Neuron. 1997;18:815–826. doi: 10.1016/s0896-6273(00)80320-7. [DOI] [PubMed] [Google Scholar]
- 56.Schutz B, Weihe E, Eiden LE. Independent patterns of transcription for the products of the rat cholinergic gene locus. Neuroscience. 2001;104:633–642. doi: 10.1016/s0306-4522(01)00100-2. [DOI] [PubMed] [Google Scholar]
- 57.Ozkan ED, Lee FS, Ueda T. A protein factor that inhibits ATP-dependent glutamate and gamma-aminobutyric acid accumulation into synaptic vesicles: purification and initial characterization. Proc Natl Acad Sci USA. 1997;94:4137–4142. doi: 10.1073/pnas.94.8.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tamura Y, et al. IPF, a vesicular uptake inhibitory protein factor, can reduce the Ca(2+)-dependent, evoked release of glutamate, GABA and serotonin. J Neurochem. 2001;76:1153–1164. doi: 10.1046/j.1471-4159.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- 59.Snead AN, et al. Thyronamines inhibit plasma membrane and vesicular monoamine transport. ACS Chem Biol. 2007;2:390–398. doi: 10.1021/cb700057b. [DOI] [PubMed] [Google Scholar]
- 60.Brunk I, et al. The first luminal domain of vesicular monoamine transporters mediates G-protein-dependent regulation of transmitter uptake. J Biol Chem. 2006;281:33373–33385. doi: 10.1074/jbc.M603204200. [DOI] [PubMed] [Google Scholar]
- 61.Brunk I, et al. Ca2+-dependent activator proteins of secretion promote vesicular monoamine uptake. J Biol Chem. 2009;284:1050–1056. doi: 10.1074/jbc.M805328200. [DOI] [PubMed] [Google Scholar]
- 62.Brunk I, et al. Deletion of Go2alpha abolishes cocaine-induced behavioral sensitization by disturbing the striatal dopamine system. FASEB J. 2008;22:3736–3746. doi: 10.1096/fj.08-111245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yao J, Erickson JD, Hersh LB. Protein kinase A affects trafficking of the vesicular monoamine transporters in PC12 cells. Traffic. 2004;5:1006–1016. doi: 10.1111/j.1600-0854.2004.00240.x. [DOI] [PubMed] [Google Scholar]
- 64.Beckmann JS, et al. The Novel Pyrrolidine nor-Lobelane Analog UKCP-110 (cis-2,5-di-(2-Phenethyl)-pyrrolidine Hydrochloride) Inhibits VMAT2 Function, Methamphetamine-evoked Dopamine Release,and Methamphetamine Self-administration in Rats. J Pharmacol Exp Ther. 2010 doi: 10.1124/jpet.110.172742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nickell JR, et al. Lobelane inhibits methamphetamine-evoked dopamine release via inhibition of the vesicular monoamine transporter-2. J Pharmacol Exp Ther. 2010;332:612–621. doi: 10.1124/jpet.109.160275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Montesinos MS, et al. The crucial role of chromogranins in storage and exocytosis revealed using chromaffin cells from chromogranin A null mouse. J Neurosci. 2008;28:3350–3358. doi: 10.1523/JNEUROSCI.5292-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Venton BJ, et al. Cocaine increases dopamine release by mobilization of a synapsin-dependent reserve pool. J Neurosci. 2006;26:3206–3209. doi: 10.1523/JNEUROSCI.4901-04.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pothos EN, et al. Synaptic vesicle transporter expression regulates vesicle phenotype and quantal size. J Neurosci. 2000;20:7297–7306. doi: 10.1523/JNEUROSCI.20-19-07297.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fukushima S, et al. Methamphetamine-induced locomotor activity and sensitization in dopamine transporter and vesicular monoamine transporter 2 double mutant mice. Psychopharmacology (Berl) 2007;193:55–62. doi: 10.1007/s00213-007-0749-4. [DOI] [PubMed] [Google Scholar]
- 70.Persico AM, et al. Exclusion of close linkage between the synaptic vesicular monoamine transporter locus and schizophrenia spectrum disorders. Am J Med Genet. 1995;60:563–565. doi: 10.1002/ajmg.1320600616. [DOI] [PubMed] [Google Scholar]
- 71.Hall FS, Sora I, Uhl GR. Sex-dependent modulation of ethanol consumption in vesicular monoamine transporter 2 (VMAT2) and dopamine transporter (DAT) knockout mice. Neuropsychopharmacology. 2003;28:620–628. doi: 10.1038/sj.npp.1300070. [DOI] [PubMed] [Google Scholar]
- 72.Savelieva KV, Caudle WM, Miller GW. Altered ethanol-associated behaviors in vesicular monoamine transporter heterozygote knockout mice. Alcohol. 2006;40:87–94. doi: 10.1016/j.alcohol.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 73.Barr GA, et al. Transitions in infant learning are modulated by dopamine in the amygdala. Nat Neurosci. 2009;12:1367–1369. doi: 10.1038/nn.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fasano A, Bentivoglio AR. Tetrabenazine Expert Opin Pharmacother. 2009;10:2883–2896. doi: 10.1517/14656560903386292. [DOI] [PubMed] [Google Scholar]
- 75.Jankovic J. Treatment of hyperkinetic movement disorders. Lancet Neurol. 2009;8:844–856. doi: 10.1016/S1474-4422(09)70183-8. [DOI] [PubMed] [Google Scholar]
- 76.Thiriot DS, Sievert MK, Ruoho AE. Identification of human vesicle monoamine transporter (VMAT2) lumenal cysteines that form an intramolecular disulfide bond. Biochemistry. 2002;41:6346–6353. doi: 10.1021/bi015779j. [DOI] [PubMed] [Google Scholar]
- 77.Suzuki M, et al. Vesicular neurotransmitter transporters in Huntington’s disease: initial observations and comparison with traditional synaptic markers. Synapse. 2001;41:329–336. doi: 10.1002/syn.1089. [DOI] [PubMed] [Google Scholar]
- 78.Bohnen NI, et al. Positron emission tomography of monoaminergic vesicular binding in aging and Parkinson disease. J/Cereb Blood Flow Metab. 2006;26:1198–1212. doi: 10.1038/sj.jcbfm.9600276. [DOI] [PubMed] [Google Scholar]
- 79.de la Fuente-Fernandez R, et al. Visualizing vesicular dopamine dynamics in Parkinson’s disease. Synapse. 2009;63:713–716. doi: 10.1002/syn.20653. [DOI] [PubMed] [Google Scholar]
- 80.Boileau I, et al. Increased vesicular monoamine transporter binding during early abstinence in human methamphetamine users: is VMAT2 a stable dopamine neuron biomarker? J Neurosci. 2008;28:9850–9856. doi: 10.1523/JNEUROSCI.3008-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boileau I, et al. Influence of a low dose of amphetamine on vesicular monoamine transporter binding: a PET (+)[11C]DTBZ study in humans. Synapse. 2010;64:417–420. doi: 10.1002/syn.20743. [DOI] [PubMed] [Google Scholar]
- 82.Kilbourn MR, et al. In vivo [11C]dihydrotetrabenazine binding in rat striatum: sensitivity to dopamine concentrations. Nucl Med Biol. 2010;37:3–8. doi: 10.1016/j.nucmedbio.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Anlauf M, et al. The vesicular monoamine transporter 2 (VMAT2) is expressed by normal and tumor cutaneous mast cells and Langerhans cells of the skin but is absent from Langerhans cell histiocytosis. J Histochem Cytochem. 2004;52:779–788. doi: 10.1369/jhc.4A6264.2004. [DOI] [PubMed] [Google Scholar]
- 84.Weihe E, et al. VMAT2 is the transporter mediating sequestration of monoamines in rat and human platelets, mast cells, and cutaneous dendritic cells. Soc Neurosci Abstr. 1998 Nov 7–12;:301. [Google Scholar]
- 85.Anlauf M, et al. Vesicular Monoamine Transporter 2 (VMAT2) Expression in Hematopoietic Cells and in Patients with Systemic Mastocytosis. J Histochem Cytochem. 2006;54:201–213. doi: 10.1369/jhc.5A6739.2005. [DOI] [PubMed] [Google Scholar]
- 86.Schmitt KC, Reith ME. Regulation of the dopamine transporter: aspects relevant to psychostimulant drugs of abuse. Ann N Y Acad Sci. 2010;1187:316–340. doi: 10.1111/j.1749-6632.2009.05148.x. [DOI] [PubMed] [Google Scholar]
- 87.Naudon L, et al. Reserpine affects differentially the density of the vesicular monoamine transporter and di-hydrotetrabenazine binding sites. Eur J Neurosci. 1996;8:842–846. doi: 10.1111/j.1460-9568.1996.tb01271.x. [DOI] [PubMed] [Google Scholar]
- 88.Wilson JM, Kish SJ. The vesicular monoamine transporter, in contrast to the dopamine transporter, is not altered by chronic cocaine self-administration in the rat. J Neurosci. 1996;16:3507–3510. doi: 10.1523/JNEUROSCI.16-10-03507.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wilson JM, et al. Striatal dopamine, dopamine transporter, and vesicular monoamine transporter in chronic cocaine users. Ann Neurol. 1996;40:428–439. doi: 10.1002/ana.410400312. [DOI] [PubMed] [Google Scholar]
- 90.Wilson JM, et al. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- 91.Schwartz K, et al. Cocaine, but not amphetamine, short term treatment elevates the density of rat brain vesicular monoamine transporter 2. J Neural Transm. 2007;114:427–430. doi: 10.1007/s00702-006-0549-8. [DOI] [PubMed] [Google Scholar]
- 92.Li H, et al. Sorting of vesicular monoamine transporter 2 to the regulated secretory pathway confers the somatodendritic exocytosis of monoamines. Neuron. 2005;48:619–633. doi: 10.1016/j.neuron.2005.09.033. [DOI] [PubMed] [Google Scholar]