Abstract
The expansion of human settlement into wildland areas, including forests in the eastern United States, has resulted in fragmented forest habitat that has been shown to drive higher entomological risk for Lyme disease. We investigated an alternative pathway between fragmentation and Lyme disease, namely whether increased risk of Lyme disease results in a reduced propensity to settle in high-risk areas at the interface of developed and undeveloped lands. We used longitudinal data analyses at the county level to determine whether Lyme disease incidence (LDI) influences the proportion of the population residing in the wildland–urban interface in 12 high LDI states in the eastern United States. We found robust evidence that a higher LDI reduces the proportion of a county's population residing in the wildland–urban interface in high-LDI states. This study provides some of the first evidence of human behavioral responses to Lyme disease risk via settlement decisions.
Introduction
Globally, emerging infectious disease (EID) events have increased significantly over time and most EIDs over the past 70 years were zoonoses originating in wildlife.1 A primary driver of zoonotic disease emergence, particularly those with wildlife origins, is land use change, which has and continues to drive many zoonotic EIDs from Nipah virus in Malaysia,2,3 to infection with human immunodeficiency virus in sub-Saharan Africa.4 Furthermore, rates of land use change, and associated fragmentation of natural habitats, are steadily increasing across the globe as human population expands.5 These changes in human development and land use patterns have important ecological implications, including for the distribution and abundance of reservoir hosts and vectors of human disease.6 Numerous human diseases are vectored and/or amplified in nonhuman hosts, including leishmaniasis, rabies, and Lyme disease, and many of these nonhuman vectors and hosts are influenced by land use patterns and land use change.7–9 Thus, understanding how current and future changes in land use are expected to impact human disease risk has become an important focus for epidemiologists, disease ecologists, and public health practitioners.5
Of particular interest to this end has been the case of Lyme disease in the United States. Lyme disease, caused by the bacterium Borrelia burgdorferi and vectored by Ixodes scapularis ticks in the east and I. pacificus ticks in the west, was recognized as an important emerging infection in the late 20th century.10 Since its formal recognition more than 30 years ago, Lyme has become the most commonly reported vector-borne disease in North America and Europe, and its prevalence and geographic range continue to increase.11 During this same time, changes in land use (i.e., reforestation and subsequent suburban development), particularly in the northeastern United States, created conditions that promote enzootic B. burgdorferi infection, thus enabling Lyme disease to become zoonotic.12
Since the late 1970s and early 1980s, ecological and epidemiological research has endeavored to explain patterns of Lyme disease emergence and identify human risk factors. It has since been well established that vector tick populations are higher in forested habitats than in adjacent non-forested habitat types, such as grassy lawns or old fields.13–20 Analogous relationships were found between forested habitats more broadly and human cases of Lyme disease. For example, Glass and others21 found that more cases of Lyme disease were associated with residences located in forested areas than in non-forested areas in Baltimore County, Maryland. Similarly, Kitron and Kazmierczak22 found that Wisconsin counties with a higher average normalized difference vegetation index, a remotely sensed vegetation index and surrogate for forest cover, had higher Lyme disease rates than those with a lower average normalized difference vegetation index, suggesting that the presence of forest is an important predictor of human incidence.
Recognition of the importance of forested habitats to the enzootic disease cycle and transmission to humans prompted studies of the effects of fragmentation of forested habitats (often resulting from suburban development in forested landscapes) on entomological risk factors, such as density of nymphs (DON), density of infected nymphs (DIN), or nymphal infection prevalence (NIP), and on patterns of human incidence. Studies exploring the relationship between forest fragmentation and entomologic risk factors have found strong evidence of increased entomologic risk associated with increased forest fragmentation.19,23–26 For example, Allan and others24 found that DON, DIN, and NIP were inversely correlated with forest patch area in Dutchess County, New York. Similarly, Brownstein and others26 found that increasing fragmentation of forest (decreasing patch size and increasing distance between patches) in Connecticut served to increase tick infection prevalence and increase tick density. A higher density of vectors and a higher prevalence of infected vectors suggest that human risk of exposure should be expected to be higher in more fragmented forest habitats than in more contiguous forest habitats.
For zoonotic spillover to occur, susceptible human hosts must be interacting with infected vectors. This interaction should be expected to occur more frequently where suburban neighborhoods intersect with forests that they have fragmented. As a result, the increased entomologic risk factors and increased opportunities for human interaction with infected vectors are expected to result in higher human incidence in more fragmented forest habitat. Surprisingly, empirical evidence for this relationship is much less clear. Studies attempting to link patterns of human incidence directly to forest fragmentation have tended to find the opposite or ambiguous results.26–29 For example, Cromley and others27 found that human incidence is higher in low-density than in medium-density residential developments around Lyme, Connecticut. Brownstein and others26 subsequently expanded this study to the entire state of Connecticut and found that there were fewer cases of Lyme disease in areas where forests were smaller and more isolated (i.e., more fragmented) than in areas with more contiguous forest. Although these two studies found a negative relationship between human incidence and forest fragmentation, Jackson and others28,29 found that incidence rates in Maryland were not influenced by population density or development type, suggesting no effect of fragmentation on Lyme disease incidence (LDI).
This body of research therefore suggests two divergent conclusions: 1) that forest fragmentation is both associated with higher entomological risk, and 2) that forest fragmentation is simultaneously associated with lower human incidence of Lyme disease. Synthesis of these divergent results has been attempted,30 but the discussion is ongoing and unresolved.31,32 The disagreement between these two conclusions and the difficulties associated with reconciling them yields, at best, ambiguous public health recommendations and intervention strategies.
The lack of a clear pathway between entomologic risk and human disease incidence may in part be caused by neglect of the feedback cycle between forest fragmentation and LDI that could be confounding statistical analyses. Suburban development fragments forested habitat, as well as puts persons in closer contact with disease vectors and at higher disease risk. However, the existence of habitat types and particular areas of the landscape associated with higher health risks may themselves modify human development patterns.33,34
The mechanism by which Lyme disease risk could affect human settlement patterns, in this case the population residing in the wildland–urban interface (WUI), is through the effect of risk on housing demand. There is abundant evidence that local demand for housing decreases when residents are exposed to human health risks associated with air and water pollution,35–40 hazardous waste sites,41–43 and nuclear power facilities.44 In turn, lower housing demand results in less land conversion for new housing construction45–47 and reduces migration to the area.48,49 By the same mechanism, counties with higher Lyme disease risk would have lower demand for new housing construction in the WUI and lower growth in the share of the population residing in the WUI.
If settlement decisions, and resulting forest fragmentation, are made in response to Lyme disease risk through the above mechanism, and Lyme disease risk is a function of fragmentation, which follows logically from higher entomologic risk in fragmented forests reported in the literature, then statistical analyses attempting to estimate the effect of forest fragmentation on LDI are likely to be biased. This suggestion offers a possible explanation for the ambiguous findings in the literature and the unexpected negative relationships reported.
We explored the existence of a second pathway between LDI and fragmentation. Specifically, we addressed the question of whether LDI is affecting human settlement patterns in the WUI in high LDI states in the northeastern United States. We used multi-year, county-level land use, population, and disease incidence data to provide novel insight into human behavioral modification in response to disease risk and in doing so, elucidated a common statistical problem that may be plaguing broader understanding of what drives human LDI.
Materials and Methods
Data.
Population in the wildland–urban interface.
To understand if Lyme disease risk influences human settlement decisions, we investigated whether LDI changes the share of a county's population residing in the WUI. The WUI is defined as the area where structures and other human development meet or intermingle with undeveloped wildland.50 A census block, the smallest geographic unit used in the population census, is classified as WUI if it contains more than 6.17 housing units/km2 and either 1) more than 50% of the block is covered by wildland vegetation or 2) vegetation covers less than 50% of the block, but it is located within 2.4 km of a block that is heavily vegetated (> 75% cover) and larger than 5 km2.51
Population residing in the WUI in 2000 and 2010 for each county was obtained from the Silvis Laboratory at the University of Wisconsin (http://silvis.forest.wisc.edu/). The Silvis Laboratory website states that the current 2000 and 2010 WUI maps are not comparable. However, the 2000 and 2010 county population totals are comparable (Radeloff V, unpublished data, December 11, 2013). The land covers classified as wildlands include coniferous, deciduous, and mixed forest; shrubland; grasslands/herbaceous; transitional; and woody and emergent herbaceous wetlands. Vegetative cover is measured by using National Land Cover Data for 200152 and 2006.53 For additional methodologic details, see Radeloff and others.50 Total county population was obtained from 2000 and 2010 population censuses. A county's WUI population was obtained by summing the populations of census blocks classified as WUI within a given county. The census block is the smallest geographic unit used in the population census. For the 2010 Census, the average size of census blocks in the contiguous 48 states (excluding water) was approximately 0.70 km2. The proportion of the county residing in the WUI (WUIpop) was then calculated as WUI population divided by total census population.
Lyme disease incidence.
We used county level data on the number of confirmed cases of Lyme disease reported by county of residence in each county for 1992–1996, 1997–2001, 2002–2006, and 2006–2011.54 We define LDI as the number of cases in the county per 100,000 total county population. Total county population is obtained from the most recent census to a given period. Thus, the 1990 population is the base for the 1992–1996 counts, the 2000 population is the base for the 1997–2001 and 2002–2006 counts, and the 2010 population is the base for the 2007–2011 counts.
Additional determinants of WUI population.
The decision to locate in the WUI is likely tied to other attributes beyond disease risk. For instance, higher income households often find it advantageous to locate in exurban areas with larger lot sizes.55 To control for this potential income effect, we obtained the median household income for each county in 1995 and 2005 from the U.S. Bureau of the Census and used the Consumer Price Index to adjust the 1995 values for inflation. Household income data is found at http://www.census.gov/did/www/saipe/data/statecounty/data/1995.html and the Consumer Price Index Inflation Calculator is found at http://data.bls.gov/cgi-bin/cpicalc.pl. We tested models with and without the income covariate (Income).
The decision to locate in the WUI may also be influenced by unobserved characteristics of individual regions. For instance, state-level policies, such as restrictions on land development, may lead to differences among states in the WUI population unrelated to disease risk. Failure to control for these unobserved characteristics would result in biased estimates of the relationship between LDI and WUI population (caused by omitted variable bias). Similarly, temporal effects common to all counties (e.g., home mortgage rates) could influence the decision to locate in the WUI, and as above could result in biased estimates if omitted from the regression. To control for these potential regional and year differences, we tested models with year and state-by-year fixed effects. The year fixed effect (Year_2010) is a dummy variable that takes the value 1 for 2010 and the value 0 for 2000. State-by-year fixed effects are dummy variables that take the value 1 if the observation corresponds to the given state in the year 2010 and the value 0 otherwise.
Our analysis is focused on counties in the northeastern United States with high incidence of Lyme disease. To determine if our results are sensitive to the set of included counties, we conducted robustness tests with two sample regions. Using the predictions of Diuk-Wasser and others56 as a guide, we constructed a small sample of counties, Eastern counties (small sample), that includes all 404 counties in 12 high LDI states (CT, DE, ME, MD, MA, MN, NH, NJ, NY, PA, VT, and WI), and the District of Columbia. A second sample, Eastern counties (large sample), includes an additional 499 counties in five states (IL, IN, MI, OH, and VA). All versions of our model are estimated using the two samples. A third sample, comprised of all counties in the contiguous 48 states except those in the large sample of eastern counties, Non-eastern counties, was used to check whether our main result was caused by spurious correlation.
Methodological challenge.
Because of ethical and logistical constraints, controlled experiments investigating the effect of LDI on human behavior, as with many human health and economics studies, are impossible. Establishing causality in the absence of random assignment into treatment (e.g., into LDI value) often relies on quasi-experimental approaches applied to observational data. These approaches, which include the panel data model we detail below, can identify causal relationships under certain conditions, but are also limited by unobservable bias that precludes a causal interpretation of the regression coefficients. We used a combination of statistical approaches to address two common sources of bias in observational data: omitted variable bias and simultaneity bias. Although there is no way to know if we have eliminated all sources of bias, we showed that our results are robust to changes in sample and model specification.
Controlling for additional determinants of WUI population, such as household income, we can mitigate bias stemming from omitted variables, but estimating the effect of LDI on WUI population faces another critical challenge from simultaneously determined regressors. Although LDI may influence an individual's decision to reside in the WUI, the presence of persons in the WUI may create the conditions for high LDI. In other words, the population living in the WUI and LDI are determined at the same time. To obtain an unbiased coefficient in a least-squares regression framework, the dependent variable cannot be determined simultaneously with any of the regressors because this simultaneity violates the exogeneity assumption (Supplemental Appendix). Simultaneity implies that neither of these two relationships (the effect of LDI on WUI population or the effect of WUI population on LDI) can be identified by separate estimation of either equation.
In some instances, using a time-lagged variable rather than the corresponding contemporaneous measure can address simultaneity problems. In particular, the WUI population in 2000 (2010) cannot affect LDI that had already occurred in 1992–1996 (2002–2006). Furthermore, because there is a delay in the public availability of Lyme disease statistics, settlement decisions cannot respond contemporaneously to disease risk. Although this time delay may eliminate the causal channel from WUI population to LDI, it does not ensure that there is no correlation between the lagged measure of LDI and the error term (i.e., the exogeneity requirement may still fail). For instance, if broad-leaf forests have higher DON and NIP with the Lyme spirochete than conifer forests, and persons have settlement preferences for forest type, then failure to control for forest composition could again result in a biased regression coefficient caused by omitted variable bias. Because forest composition is essentially constant on short time scales, lagged LDI will be correlated with the error terms capturing the effect of forest composition on the current WUI population.
If cross-sectional data is available for multiple periods, then fixed effects can be used to mitigate problems with omitted variables. For our study, this involves estimating a separate intercept term (or dummy variable) for each county. These county-fixed effects control for any time-invariant characteristics of a county (including forest composition) that may otherwise result in correlation between the lagged measure of LDI and the error term. Another way to deal with unobserved differences among counties is the random effects model. However, this model requires the county random effect to be uncorrelated with LDI and all other explanatory variables. The difference between fixed and random effects models is discussed in more detail in the Supplemental Appendix.
Regression models.
To evaluate the potential simultaneity bias, we first regressed the share of the county's population residing in the WUI on the contemporaneous measure of LDI and a constant term. Formally, the cross-sectional models we estimate are specified:
where LDI_00 (LDI_10) is the measure of LDI in 2000 (2010), WUIpop_00 (WUIpop_10) is the share of the population in the WUI in 2000 (2010), β0 is the intercept, β1 is the slope coefficient we are estimating, and ε is the error term.
To address the potential simultaneity between WUIpop and LDI, we pooled the WUIpop variables for 2000 and 2010 (WUIpop_0010) and use the lagged measures of LDI. Formally, the model is specified:
where LDI_lagged includes the pooled LDI measures for 1992–1996 and 2002–2006, and Year_2010 captures any time effects common to all counties. Because (3) specifies a common intercept term, β0, it does not control for the influence of time-invariant county characteristics that may cause lagged LDI to be correlated with the error term.
The last set of models includes county fixed effects to control for any time-invariant factors that affect the WUI population. The lagged LDI model with county and year fixed effects is formally specified as
where the intercept, βi, now takes a different value for each county i. The final model is the same as (4) except that it also includes the income variable (Income) and state-by-year fixed effects.
Results
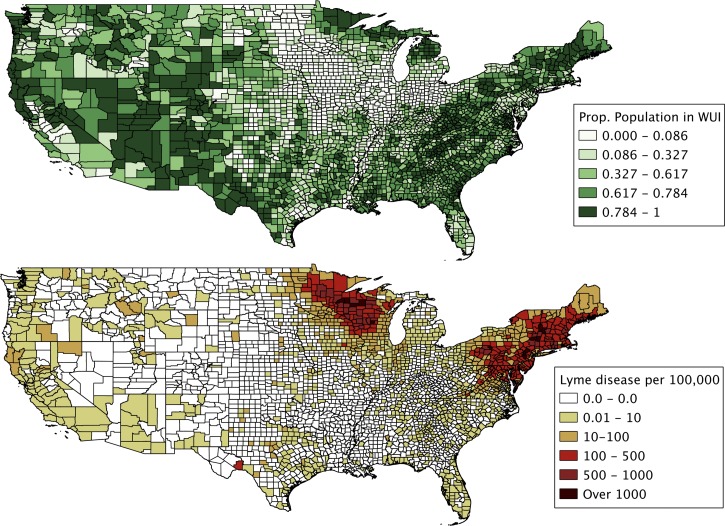
The variables used in the analysis are summarized in Table 1 and shown in Figure 1. The mean county share of the population living in the WUI increased during 2000–2010 in all three samples, although the gain was smallest in the small sample of eastern counties. Mean LDI was highest for the small sample of eastern counties, although it increased steadily from the early 1990s to the late 2000s in the small and large samples of eastern counties. Finally, average household income in inflation-adjusted dollars increased slightly during 1995–2005 in all samples. We mapped the 2010 measure of WUI population and the 2007–2011 measure of LDI (Figure 1). It is evident that counties with high LDI tend to have a large share of its population residing in the WUI. However, many counties with large WUI populations, such as those in the Rocky Mountain region, have relatively low LDI, suggesting that factors other than LDI are important for explaining variation in WUI population shares.
Table 1.
Summary statistics for variables used in the analysis*
| Variable | Period | Eastern counties (large sample) | Eastern counties (small sample) | Non-eastern counties | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| WUlpop | 2000 | 0.377 | 0.324 | 0.429 | 0.321 | 0.478 | 0.303 |
| WUlpop | 2010 | 0.384 | 0.329 | 0.432 | 0.324 | 0.489 | 0.306 |
| LDI | 1992–1996 | 39.9 | 202.8 | 83.7 | 297.3 | 3.9 | 13.4 |
| LDI | 1997–2001 | 54.3 | 224.4 | 117.0 | 324.8 | 2.3 | 6.2 |
| LDI | 2002–2006 | 84.4 | 259.7 | 180.6 | 365.4 | 2.2 | 7.1 |
| LDI | 2007–2011 | 114.9 | 232.2 | 238.3 | 300.9 | 2.3 | 13.7 |
| Income | 1995 | 44,255 | 10,213 | 45,400 | 10,591 | 36,500 | 8,351 |
| Income | 2005 | 44,885 | 11,146 | 46,841 | 11,522 | 36,662 | 8,455 |
| No. observations | 903 | 404 | 2,204 | ||||
WUIpop is the share of the county population living in the wildland-urban interface; LDI is the number of confirmed cases of Lyme disease in a county per 100,000 population; Income is mean household income in 2005 U.S. dollars. Eastern counties (large sample), Eastern counties (small sample), and Non-eastern counties represent the three different samples evaluated.
Figure 1.
Proportion of the United States population residing in the wildland–urban interface (WUI) in 2010 and Lyme disease incidence per 100,000 county residents for 2007–2011.
Estimation results are shown in Table 2 for the cross-sectional and pooled models (equations 1–3) and the two eastern samples. To avoid small coefficient estimates and standard errors, we multipled the WUIpop variable by 1,000 before estimation. In each of the six models, we estimate a positive coefficient on the contemporaneous measure of LDI. All estimates are significantly different from zero and have a high degree of confidence. These results are counterintuitive because one would expect high LDI to deter persons from locating in the WUI. Use of the time-lagged incidence measure (equation 3) does not change the qualitative nature of the results. The coefficients on the LDI variable are still positive and significantly different from zero. The adjusted R2 measures for these regressions are small (2.2–10.7%), suggesting that additional explanatory variables are needed.
Table 2.
Cross-sectional models (equations 1 and 2) and pooled models (equation 3) of the county population share living in the wildland-urban interface*
| Variable | Coefficient | SE | P | Variable | Coefficient | SE | P |
|---|---|---|---|---|---|---|---|
| Eastern counties (large sample) | Eastern counties (small sample) | ||||||
| 2000 | 2000 | ||||||
| LDI_00 | 0.232 | 0.047 | < 0.001 | LDI_00 | 0.188 | 0.048 | < 0.001 |
| Constant | 364.2 | 10.951 | < 0.001 | Constant | 407.1 | 16.693 | < 0.001 |
| Adjusted R2 = 0.025 | Adjusted R2 = 0.034 | ||||||
| 2010 | 2010 | ||||||
| LDI_10 | 0.372 | 0.046 | < 0.001 | LDI_10 | 0.356 | 0.051 | < 0.001 |
| Constant | 341.7 | 11.790 | < 0.001 | Constant | 347.6 | 19.417 | < 0.001 |
| Adjusted R2 = 0.068 | Adjusted R2 = 0.107 | ||||||
| 2000 and 2010 pooled | 2000 and 2010 pooled | ||||||
| LDI_lagged | 0.211 | 0.033 | < 0.001 | LDI_lagged | 0.170 | 0.034 | < 0.001 |
| Year_2010 | −1.718 | 15.258 | 0.910 | Year_2010 | −13.131 | 22.581 | 0.561 |
| Constant | 368.4 | 10.819 | < 0.001 | Constant | 414.9 | 16.049 | < 0.001 |
| Adjusted R2 = 0.022 | Adjusted R2 = 0.028 | ||||||
| Dependent variable = WUIpop × 1,000 | |||||||
Cross-sectional and pooled models with contemporaneous (LDI_00 or LDI_10) and lagged (LDI_lagged) measure of Lyme disease incidence in the small sample and large sample of Eastern counties. LDI is the number of confirmed cases of Lyme disease in a county per 100,000 population and WUIpop is the share of the county population living in the wildland–urban interface. In both samples and both years, we found a counterintuitive positive relationship that was significant (P < 0.05) when contemporaneous LDI was used. Using lagged LDI did not change the sign or statistical significance of the result.
Results for the fixed effects models are shown in Table 3. The first four columns in Table 3 show the estimates for equation (4) (the county fixed effects are not reported). We include a constant term in the model, which requires that the fixed effect for one county be omitted. For the large and small samples of eastern counties, the estimated coefficients on the LDI measure are negative, significantly different from zero, and show a high degree of confidence. The coefficient estimate changes only slightly when the more restrictive sample is used. The negative coefficients on LDI_lagged conform with expectations, indicating that as LDI in a county increases, the WUI population decreases, all else equal. The fixed effects models explain a large share of the variation (approximately 99%) in the WUI population among counties and over time. The high adjusted R2 statistics are due primarily to the county fixed effects, not to the LDI variable, as suggested by a comparison with the pooled model results in Table 2.
Table 3.
Fixed effects models (equation 4) of the county population share living in the wildland–urban interface*
| Variable | Coefficient | SE | P | Variable | Coefficient | SE | P |
|---|---|---|---|---|---|---|---|
| Eastern counties (large sample) | |||||||
| LDI_lagged | −0.019 | 0.007 | 0.006 | LDI_lagged | −0.016 | 0.007 | 0.018 |
| Year_2010 | 8.525 | 1.508 | < 0.001 | Year_2010 | 0.554 | 43.271 | 0.990 |
| Constant | 377.6 | 1.079 | < 0.001 | Income | 0.001 | 0.000 | 0.175 |
| Constant | 347.7 | 21.959 | < 0.001 | ||||
| State-by-year effects: No | State-by-year effects: Yes | ||||||
| Adjusted R2 = 0.991 | Adjusted R2 = 0.991 | ||||||
| Eastern counties (small sample) | |||||||
| LDI_lagged | −0.015 | 0.006 | 0.012 | LDI_lagged | −0.016 | 0.006 | 0.008 |
| Year_2010 | 4.817 | 2.026 | 0.018 | Year_2010 | −0.646 | 21.734 | 0.976 |
| Constant | 430.4 | 1.463 | < 0.001 | Income | 0.001 | 0.001 | 0.091 |
| Constant | 375.8 | 32.243 | < 0.001 | ||||
| State-by-year effects: No | State-by-year effects: Yes | ||||||
| Adjusted R2 = 0.993 | Adjusted R2 = 0.993 | ||||||
| Non-eastern counties | |||||||
| LDI_lagged | −0.098 | 0.092 | 0.287 | LDI_lagged | −0.116 | 0.095 | 0.219 |
| Year_2010 | 11.947 | 1.305 | < 0.001 | Year_2010 | 23.945 | 15.619 | 0.125 |
| Constant | 477.9 | 0.984 | < 0.001 | Income | 0.0005 | 0.0005 | 0.295 |
| Constant | 460.2 | 17.005 | < 0.001 | ||||
| State-by-year effects: No | State-by-year effects: Yes | ||||||
| Adjusted R2 = 0.980 | Adjusted R2 = 0.980 | ||||||
| Dependent variable = WUIpop × 1,000 | |||||||
Year and county fixed effects models with lagged Lyme disease incidence for the small sample and large sample of eastern counties, and a model robustness check with non-eastern counties. Including county and year fixed effects we find a negative and significant relationship between LDI and WUI population in both eastern samples. LDI is the number of confirmed cases of Lyme disease in a county per 100,000 population and WUIpop is the share of the county population living in the wildland–urban interface. This relationship holds when state-by-year fixed effects and income are included, and, as expected, is not observed in non-eastern counties where LDI is much lower. The high R2 is caused by the inclusion of fixed effects, as can be determined by comparing the fixed effects model to the cross-sectional models in Table 2.
For the final specification, we augment equation (4) by including a measure of household income and state-by-year fixed effects (columns 5–8 of Table 3). The coefficient on Income is positive for all three samples, but not significantly different from zero at standard confidence levels. Including Income and state-by-year fixed effects (not reported) has only a small effect on the estimated LDI_lagged coefficient. Importantly, the coefficient estimate remains negative and significantly different from zero for the two eastern samples.
As a final robustness check, we estimate the two fixed effects models using the sample of non-eastern counties (bottom panel of Table 3). If the effects we find in northeastern counties truly measure a human response to Lyme disease risk, those effects should disappear in areas where Lyme disease risk is low. As shown in Table 1, mean LDI is close to zero in the non-eastern sample of counties. When we estimated the fixed effects models with this sample, the coefficients on LDI_lagged became much larger in absolute value and the standard errors increase by a factor of 13. The estimates are no longer significantly different from zero.
To understand the magnitude of the effects, consider that the average population for counties in the Eastern counties (small sample) was 183,132 persons in 2010, of which 43.2% (79,113 person) lived in the WUI on average. Over the period 2007–2011, there was an average of 238 Lyme disease cases per 100,000 population. Our results indicate that, for the average county, a 10% increase in Lyme disease cases per 100,000 population resulted in 70 fewer persons locating in the WUI (70 = 0.10 × 238 × 0.000016 × 183132). Thus, a 10% increase in LDI translates into approximately a 0.1% decrease in the WUI population.
Discussion
Approximately 60% of human pathogens are zoonotic,57,58 and many of these are closely intertwined with land use and land use change.5 Land use patterns and land use change can drive environmental modifications that promote vector or reservoir host populations (e.g., increased standing water for irrigation, reduced predation on reservoir hosts). These same patterns and changes can also influence human encounter rates with vectors and reservoir hosts of disease as human settlements encroach upon, and intermingle with, natural habitat. There is strong evidence that habitat modification, in the form of forest fragmentation, is a driver of entomologic risk for Lyme disease,19,23–26 and it follows logically that increased entomologic risk, coupled with increased human population in high-risk areas, should increase LDI. However, empirical studies testing this theory have found surprisingly divergent results, often reporting the opposite of the expected relationship.26–29
One possible cause of the divergent results reported in the literature is a simultaneous relationship between LDI and human settlement in high-risk areas, which often results in fragmentation of natural habitat. We provide evidence of an alternative pathway from LDI to forest fragmentation, namely that LDI has a negative and statistically significant effect on the population residing in the WUI. Rather than using forest fragmentation metrics as in earlier studies, we instead examine the share of a county's population residing in the WUI to provide a more precise measure of human exposure to entomologic risk because forest fragmentation can occur without human settlement.
We isolated a significant negative effect of LDI on human population residing in the WUI only after we included controls for the feedback from WUI population to LDI. When this feedback is ignored, LDI is found to have a counterintuitive, positive effect on population residing in the WUI. This feedback is the root cause of simultaneity bias, which, as the name suggests, is caused by the dependent and one or more right-hand side variables being determined simultaneously. The counterintuitive result in our case is consistent with there being a simultaneous relationship between WUI population and LDI. Beyond biasing the estimated effect of LDI on the WUI population, this relationship would also bias the estimated effect of WUI population (or forest fragmentation, as in previous studies) on LDI.
Several previous studies have estimated the effect of forest fragmentation on LDI and reported counterintuitive findings. For instance, Brownstein and others26 used fragmentation metrics, mean patch size and mean patch isolation, calculated for towns in Connecticut to predict human incidence rates of Lyme disease by using Poisson regression models. The authors found a significant positive relationship between mean patch size and LDI and a significant negative relationship between mean patch isolation and LDI, suggesting that human cases are more common in areas with larger, less isolated forest patches.26 This result is in contrast with field-collected data on tick density and infection prevalence from the same study.26
Similarly, Jackson and others28 used calculated land-cover metrics (e.g., landscape area, percentage of landscape in forest, length of edge habitat around forest patches) to predict reported LDI in a 12-county area of Maryland by using regression analysis. The authors found ambiguous relationships, ranging from weakly positive to weakly negative, between number of forest patches < 2 hectares (a measure of fragmentation) and LDI depending on which other variables were included in the model. As a result, this fragmentation metric was dropped from the final model, suggesting that fragmentation had no explanatory power in the analysis. Furthermore, neither development type nor population density were significant predictors of LDI in the study area.29
As in these studies, we also obtained unexpected results when the feedback from forest fragmentation to LDI was not accounted for. Intuitively, high LDI would not be expected to increase the human population residing in the WUI where entomologic risk is high. To control for the feedback from WUI population to LDI, we used a time-lagged measure of LDI and included county fixed effects in the model. Although lagging LDI eliminates the simultaneity with WUI population, it does not ensure that the exogeneity condition (i.e., no correlation between lagged LDI and the disturbance terms) will be satisfied. If there are determinants of WUI population that change little over time, and they are not measured by independent variables, their influence on WUI population will be captured in the model's disturbance terms. If these time-invariant factors are also correlated with LDI, then there will be correlation between the disturbance terms and lagged LDI, which is a potential source of omitted-variable bias.
As discussed above, county forest composition is one such time-invariant factor that could induce correlation between lagged LDI and errors in WUI population. Road densities are a second potential source of omitted-variable bias. Higher road densities increase human access to the WUI and, therefore, are likely to be associated with higher WUI populations. Roads can also be the cause of forest fragmentation and may affect how easily host species move between forest fragments (i.e., the permeability of non-forest habitats), which could in turn affect important entomologic risk factors such as DON or NIP within forest fragments.28,29 Because county road densities change little within a 10-year time period, LDI measures for 1992–1996 (2002–2006) are likely to be correlated with road densities in 2000 (2010). Thus, if the WUI population model does not include road density as an independent variable (see equation 3), there can be correlation between the model's disturbance terms and the lagged LDI measure.
The solution to this omitted variable bias problem is to control for the time-invariant determinants of WUI population. In this case, their effects are no longer captured by the model's disturbance terms, thereby eliminating the induced correlation with the lagged LDI measure. Because it is difficult to identify, much less measure precisely, all of the potential time-invariant determinants of WUI population, we include county fixed effects in the model. A county fixed effect is a catch-all variable that measures the combined influence of all time-invariant factors on a county's WUI population. When we include county fixed effects in the model (equation 4), the lagged LDI measure has the expected negative effect on WUI population. This finding is robust to two alternative samples of eastern counties and, as expected, the coefficient on lagged LDI becomes statistically insignificant when we use a sample of counties with low LDI.
In addition to identifying a possible explanation of the divergent results presented in the literature on forest fragmentation as a driver of entomologic risk but not LDI, our results imply that humans respond to risk of acquiring Lyme disease through settlement choice. Humans respond to risk through behavioral changes aimed at risk reduction. This response is particularly true of risk of acquiring infectious diseases, behavioral responses to which range from washing hands to avoid rhinovirus infection, to using bed nets and insecticides to reduce malaria transmission. Larger scale measures such as draining wetlands59 or altering animal husbandry practices60 have also been undertaken in efforts to reduce human disease risk. One would expect humans to also respond to Lyme disease risk through behavioral changes. There is abundant evidence that demand for housing decreases in areas where human health risks are greater. Given the strong association between forested, especially fragmented forest, habitats and increased entomologic risk, reduced propensity to reside in the WUI is one way for humans to avoid this increased risk.
Societies and persons have correctly associated particular habitats with increased disease risk and have been avoiding these habitats since before the modern germ theory of disease became widely accepted. The name malaria came from a pre-germ theory association between the symptoms of Plasmodium infection and the bad air emanating from swamps and marshes where the malaria vector breeds. As the miasma theory of disease, which produced these early associations, gave way to the germ theory and modern science, avoidance of swamps and associated bad air gave way to the use of bed nets, insecticides, and destruction of mosquito breeding habitat. Having eradicated malaria in addition to many other infectious diseases, from smallpox, to polio, to hookworm, throughout the developed world, the possibility that infectious diseases could be altering behavior or changing settlement patterns at such a scale in the developed world is easily overlooked. However, in this study we found that humans make settlement decisions, altering larger scale patterns of development, in response to Lyme disease risk.
This alternative pathway between LDI and forest fragmentation has not yet been investigated,61 and although the results obtained here are novel, they may not be limited to Lyme disease. Many other zoonotic diseases are influenced by land use and land use change from Nipah virus and leishmaniasis to malaria and infection with human immunodeficiency virus.1–9,62 With the increase in zoonotic disease emergence and spread globally, it is necessary to understand what land use configurations inhibit or exacerbate disease incidence. Further, accounting for human behavioral responses to risk will be critical to identifying relationships between land use and human disease into the future.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Cheryl Briggs for providing insightful comments on an earlier version of this manuscript and Dr. Volker Radeloff for providing county data.
Disclaimer: All authors contributed equally to this study. Order of authorship is alphabetical.
Footnotes
Financial support: Ashley E. Larsen was supported by the University of California, Santa Barbara Graduate Division. Andrew J. MacDonald was supported by a Department of Ecology, Evolution, and Marine Biology block grant.
Authors' addresses: Ashley E. Larsen and Andrew J. MacDonald, Department of Ecology, Evolution, and Marine Biology, University of California, Santa Barbara, CA, E-mails: alarsen@lifesci.ucsb.edu and andrew.macdonald@lifesci.ucsb.edu. Andrew J. Plantinga, Bren School of Environmental Science and Management, University of California, Santa Barbara, CA, E-mail: plantinga@bren.ucsb.edu.
References
- 1.Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chua KB, Goh KJ, Wong KT, Kamarulzaman A, Tan PSK, Ksiazek TG, Zaki SR, Paul G, Lam SK, Tan CT. Fatal encephalitis due to Nipah virus among pig-farmers in Malaysia. Lancet. 1999;354:1257–1259. doi: 10.1016/S0140-6736(99)04299-3. [DOI] [PubMed] [Google Scholar]
- 3.Lam SK, Chua KB. Nipah virus encephalitis outbreak in Malaysia. Clin Infect Dis. 2002;34:S48–S51. doi: 10.1086/338818. [DOI] [PubMed] [Google Scholar]
- 4.Wolfe ND, Eitel MN, Gockowski J, Muchaal PK, Nolte C, Tassy Prosser A, Ndongo Torimiro J, Weise SF, Burke DS. Deforestation, hunting and the ecology of microbial emergence. Glob Change Hum Health. 2000;1:10–25. [Google Scholar]
- 5.Patz JA, Daszak P, Tabor GM, Aguirre AA, Pearl M, Epstein J, Wolfe ND, Kilpatrick AM, Foufopoulos J, Molyneux D, Bradley DJ. Members of the Working Group on Land Us Change and Disease Emergence Unhealthy landscapes: policy recommendations on land use change and infectious disease emergence. Environ Health Perspect. 2004;112:1092–1098. doi: 10.1289/ehp.6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones BA, Grace D, Kock R, Alonso S, Rushton J, Said MY, McKeever D, Mutua F, Young J, McDermott J, Pfeiffer DU. Zoonosis emergence linked to agricultural intensification and environmental change. Proc Natl Acad Sci USA. 2013;110:8399–8404. doi: 10.1073/pnas.1208059110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desjeux P. The increase in risk factors for leishmaniasis worldwide. Trans R Soc Trop Med Hyg. 2001;95:239–243. doi: 10.1016/s0035-9203(01)90223-8. [DOI] [PubMed] [Google Scholar]
- 8.Singh J, Jain DC, Bhatia R, Ichhpujani RL, Harit AK, Panda RC, Tewari KN, Sokhey J. Epidemiological characteristics of rabies in Delhi and surrounding areas, 1998. Indian Pediatr. 2001;38:1354–1360. [PubMed] [Google Scholar]
- 9.Ostfeld RS, Keesing F. Biodiversity and disease risk: the case of Lyme disease. Conserv Biol. 2000;14:722–728. [Google Scholar]
- 10.Steere AC. Medical progress: Lyme disease. N Engl J Med. 2001;345:115–125. doi: 10.1056/NEJM200107123450207. [DOI] [PubMed] [Google Scholar]
- 11.Bacon RM, Kugeler KJ, Mead PS. Surveillance for Lyme disease–United States, 1992–2006. MMWR Morb Mortal Wkly Rep. 2008;57:1–9. [PubMed] [Google Scholar]
- 12.Spielman A. The emergence of Lyme disease and human babesiosis in a changing environment. Ann N Y Acad Sci. 1994;740:146–156. doi: 10.1111/j.1749-6632.1994.tb19865.x. [DOI] [PubMed] [Google Scholar]
- 13.Kitron U, Bouseman JK, Jones CJ. Use of the ARC/INFO GIS to study the distribution of Lyme disease ticks in an Illinois county. Prev Vet Med. 1991;11:243–248. [Google Scholar]
- 14.Maupin GO, Fish D, Zultowsky J, Campos EG, Piesman J. Landscape ecology of Lyme disease in a residential area of Westchester County, New York. Am J Epidemiol. 1991;133:1105–1113. doi: 10.1093/oxfordjournals.aje.a115823. [DOI] [PubMed] [Google Scholar]
- 15.Stafford KC, Magnarelli LA. Spatial and temporal patterns of Ixodes scapularis (Acari: Ixodidae) in southeastern Connecticut. J Med Entomol. 1993;30:762–771. doi: 10.1093/jmedent/30.4.762. [DOI] [PubMed] [Google Scholar]
- 16.Duffy DC, Clark DD, Campbell SR, Gurney S, Perello R, Simon N. Landscape patterns of abundance of Ixodes scapularis (Acari: Ixodidae) on Shelter Island, New York. J Med Entomol. 1994;31:875–879. doi: 10.1093/jmedent/31.6.875. [DOI] [PubMed] [Google Scholar]
- 17.Ostfeld RS, Cepeda OM, Hazler KR, Miller MC. Ecology of Lyme disease: habitat associations of ticks (Ixodes scapularis) in a rural landscape. Ecol Appl. 1995;5:353–361. [Google Scholar]
- 18.Frank DH, Fish D, Moy FH. Landscape features associated with Lyme disease risk in a suburban residential environment. Landscape Ecol. 1998;13:27–36. [Google Scholar]
- 19.Guerra M, Walker E, Jones C, Paskewitz S, Cortinas MR, Stancil A, Beck L, Bobo M, Kitron U. Predicting the risk of Lyme disease: habitat suitability for Ixodes scapularis in the north central United States. Emerg Infect Dis. 2002;8:289–297. doi: 10.3201/eid0803.010166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisen RJ, Eisen L, Lane RS. Predicting density of Ixodes pacificus nymphs in dense woodlands in Mendocino County, California, based on geographic information systems and remote sensing versus field-derived data. Am J Trop Med Hyg. 2006;74:632–640. [PubMed] [Google Scholar]
- 21.Glass GE, Schwartz BS, Morgan JM, III, Johnson DT, Noy PM, Israel E. Environmental risk factors for Lyme disease identified with geographic information systems. Am J Public Health. 1995;85:944–948. doi: 10.2105/ajph.85.7.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitron U, Kazmierczak JJ. Spatial analysis of the distribution of Lyme disease in Wisconsin. Am J Epidemiol. 1997;145:558–566. doi: 10.1093/oxfordjournals.aje.a009145. [DOI] [PubMed] [Google Scholar]
- 23.Glass GE, Amerasinghe FP, Morgan JM, Scott TW. Predicting Ixodes scapularis abundance on white-tailed deer using geographic information systems. Am J Trop Med Hyg. 1994;51:538–544. doi: 10.4269/ajtmh.1994.51.538. [DOI] [PubMed] [Google Scholar]
- 24.Allan BF, Keesing F, Ostfeld RS. Effect of forest fragmentation on Lyme disease risk. Conserv Biol. 2003;17:267–272. [Google Scholar]
- 25.Lubelczyk CB, Elias SP, Rand PW, Holman MS, Lacombe EH, Smith RP. Habitat associations of Ixodes scapularis (Acari: Ixodidae) in Maine. Environ Entomol. 2004;33:900–906. [Google Scholar]
- 26.Brownstein JS, Skelly DK, Holford TR, Fish D. Forest fragmentation predicts local scale heterogeneity of Lyme disease risk. Oecologia. 2005;146:469–475. doi: 10.1007/s00442-005-0251-9. [DOI] [PubMed] [Google Scholar]
- 27.Cromley EK, Cartter ML, Mrozinski RD, Ertel S-H. Residential setting as a risk factor for Lyme disease in a hyperendemic region. Am J Epidemiol. 1998;147:472–477. doi: 10.1093/oxfordjournals.aje.a009473. [DOI] [PubMed] [Google Scholar]
- 28.Jackson LE, Hilborn ED, Thomas JC. Towards landscape design guidelines for reducing Lyme disease risk. Int J Epidemiol. 2006;35:315–322. doi: 10.1093/ije/dyi284. [DOI] [PubMed] [Google Scholar]
- 29.Jackson LE, Levine JF, Hilborn ED. A comparison of analysis units for associating Lyme disease with forest-edge habitat. Community Ecol. 2006;7:189–197. [Google Scholar]
- 30.Wood CL, Lafferty KD. Biodiversity and disease: a synthesis of ecological perspectives on Lyme disease transmission. Trends Ecol Evol. 2013;28:239–247. doi: 10.1016/j.tree.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 31.Ostfeld RS, Keesing F. Straw men don't get Lyme disease: response to Wood and Lafferty. Trends Ecol Evol. 2013;28:502–503. doi: 10.1016/j.tree.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Lafferty KD, Wood CL. It's a myth that protection against disease is a strong and general service of biodiversity conservation: response to Ostfeld and Keesing. Trends Ecol Evol. 2013;28:503–504. doi: 10.1016/j.tree.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 33.Sawyer D. Economic and social consequences of malaria in new colonization projects in Brazil. Soc Sci Med. 1993;37:1131–1136. doi: 10.1016/0277-9536(93)90252-y. [DOI] [PubMed] [Google Scholar]
- 34.Sachs J, Malaney P. The economic and social burden of malaria. Nature. 2002;415:680–685. doi: 10.1038/415680a. [DOI] [PubMed] [Google Scholar]
- 35.Smith VK, Deyak TA. Measuring the impact of air pollution on property values. J Reg Sci. 1975;15:277–288. [Google Scholar]
- 36.Kiel KA, McClain KT. House prices during siting decision stages: the case of an incinerator from rumor through operation. J Environ Econ Manage. 1995;28:241–255. [Google Scholar]
- 37.Chattopadhyay S. Estimating the demand for air quality: new evidence based on the Chicago housing market. Land Econ. 1999;75:22–38. [Google Scholar]
- 38.Beron K, Murdoch J, Thayer M. The benefits of visibility improvement: new evidence from the Los Angeles metropolitan area. J Real Estate Financ. 2001;22:319–337. [Google Scholar]
- 39.Won Kim C, Phipps TT, Anselin L. Measuring the benefits of air quality improvement: a spatial hedonic approach. J Environ Econ Manage. 2003;45:24–39. [Google Scholar]
- 40.Hoehn JP, Berger MC, Blomquist GC. A hedonic model of interregional wages, rents, and amenity values. J Reg Sci. 1987;27:605–620. [Google Scholar]
- 41.Kohlhase JE. The impact of toxic waste sites on housing values. J Urban Econ. 1991;30:1–26. [Google Scholar]
- 42.Nelson AC, Genereux J, Genereux MM. Price effects of landfills on different house value strata. J Urban Plan D-ASCE. 1997;123:59–67. [Google Scholar]
- 43.Hite D, Chern W, Hitzhusen F, Randall A. Property value impacts of an environmental disamenity: the case of landfills. J Real Estate Financ. 2001;22:185–202. [Google Scholar]
- 44.Folland S, Hough R. Externalities of nuclear power plants: further evidence. J Reg Sci. 2000;40:735–753. [Google Scholar]
- 45.Bockstael NE. Modeling economics and ecology: the importance of a spatial perspective. Am J Agric Econ. 1996;78:1168–1180. [Google Scholar]
- 46.Carrión-Flores C, Irwin EG. Determinants of residential land-use conversion and sprawl at the rural-urban fringe. Am J Agric Econ. 2004;86:889–904. [Google Scholar]
- 47.Lubowski RN, Plantinga AJ, Stavins RN. What drives land-use change in the United States? A national analysis of landowner decisions. Land Econ. 2008;84:529–550. [Google Scholar]
- 48.Greenwood MJ, Hunt GL, Rickman DS, Treyz GI. Migration, regional equilibrium, and the estimation of compensating differentials. Am Econ Rev. 1991;81:1382–1390. [Google Scholar]
- 49.Plantinga AJ, Detang-Dessendre C, Hunt GL, Piguet V. Housing prices and inter-urban migration. Reg Sci Urban Econ. 2013;43:296–306. [Google Scholar]
- 50.Radeloff VC, Hammer RB, Stewart SI, Fried JS, Holcomb SS, McKeefry JF. The wildland-urban interface in the United States. Ecol Appl. 2005;15:799–805. [Google Scholar]
- 51.Agriculture USDO, Interior USDOT Urban wildland interface communities within the vicinity of federal lands that are at high risk of wildfire. Fed Regist. 2001;66:751–777. [Google Scholar]
- 52.Homer C, Dewitz J, Fry J, Coan M, Hossain N, Larson C, Herold N, McKerrow A, VanDriel JN, Wickham J. Completion of the 2001 National Land Cover Database for the conterminous United States. Photogramm Eng Remote Sensing. 2007;73:337–341. [Google Scholar]
- 53.Fry JA, Xian G, Jin S, Dewitz JA, Homer CG, Yang L, Barnes CA, Herold ND, Wickham JD. Completion of the 2006 National Land Cover Database for the conterminous United States. Photogramm Eng Remote Sensing. 2011;77:858–864. [Google Scholar]
- 54.Centers for Disease Control and Prevention County-Level Reported Cases of Lyme Disease, 1992–2011. 2014. http://www.cdc.gov/lyme/stats/index.html Available at. Accessed February 4, 2014.
- 55.Wu JJ, Plantinga AJ. The influence of public open space on urban spatial structure. J Environ Econ Manage. 2003;46:288–309. [Google Scholar]
- 56.Diuk-Wasser MA, Hoen AG, Cislo P, Brinkerhoff R, Hamer SA, Rowland M, Cortinas R, Vourc'h G, Melton F, Hickling GJ, Tsao JI, Bunikis J, Barbour AG, Kitron U, Piesman J, Fish D. Human risk of infection with Borrelia burgdorferi, the Lyme disease agent, in eastern United States. Am J Trop Med Hyg. 2012;86:320–327. doi: 10.4269/ajtmh.2012.11-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taylor LH, Latham SM, Woolhouse M. Risk factors for human disease emergence. Philos Trans R Soc Lond B Biol Sci. 2001;356:983–989. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Woolhouse M, Gowtage-Sequeria S. Host range and emerging and reemerging pathogens. Emerg Infect Dis. 2005;11:1842–1847. doi: 10.3201/eid1112.050997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keiser J, Singer BH, Utzinger J. Reducing the burden of malaria in different eco-epidemiological settings with environmental management: a systematic review. Lancet Infect Dis. 2005;5:695–708. doi: 10.1016/S1473-3099(05)70268-1. [DOI] [PubMed] [Google Scholar]
- 60.Sims LD, Peiris M. One health: the Hong Kong experience with avian influenza. Curr Top Microbiol Immunol. 2012;365:281–298. doi: 10.1007/82_2012_254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ostfeld R. Lyme Disease: The Ecology of a Complex System. New York: Oxford University Press; 2011. [Google Scholar]
- 62.Coluzzi M. Malaria and the Afrotropical ecosystems: impact of man-made environmental changes. Parassitologia. 1994;36:223–227. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.