Abstract
The synthesis of 1,2-diamines has been achieved through a single-step, tandem sequence involving Rh-catalyzed aziridination followed by NaI-promoted rearrangement to an isomeric cyclic sulfamide. Facile ring opening of these products in hot water and pyridine affords differentially protected vicinal diamines. Demonstration of the utility of this method for the syntheses of (±)-enduracididine and (±)-allo-enduracididine is highlighted.
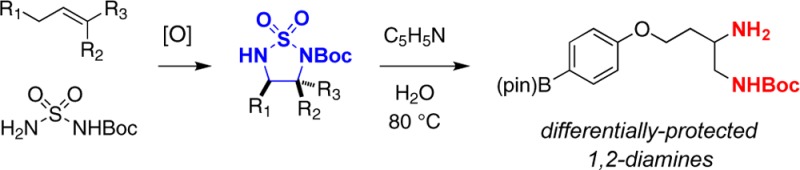
Structures comprising 1,2-diamino functional groups are recurrent in natural products and synthetic materials and find utility across disparate fields of molecular science that include catalysis, supramolecular chemistry, agrochemical and pharmaceutical research.1 The prevalence of 1,2-diamine derivatives in high-value targets has inspired considerable effort to devise expedient methods for crafting such compounds from simple precursors.1f,2 Among the most direct and general approaches for synthesizing 1,2-diamines is the diamination of olefins.3,4 The development of such a process, however, is complicated by several factors, including functional group compatibility with redox reaction conditions and product inhibition of catalyst turnover. In considering basic design criteria, an optimal process for 1,2-diamine assembly would enable differentiation of the two amino groups. Herein, we describe a Rh-catalyzed aziridination/rearrangement method for 1,2-diamine synthesis that offers a solution to these challenges (Figure 1).
Figure 1.
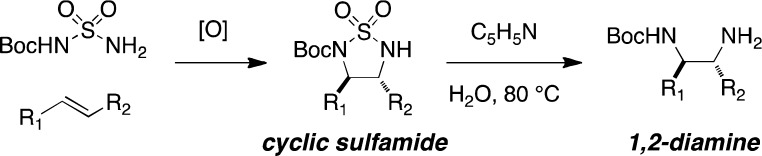
1,2-Diamine synthesis through a two-step sequence.
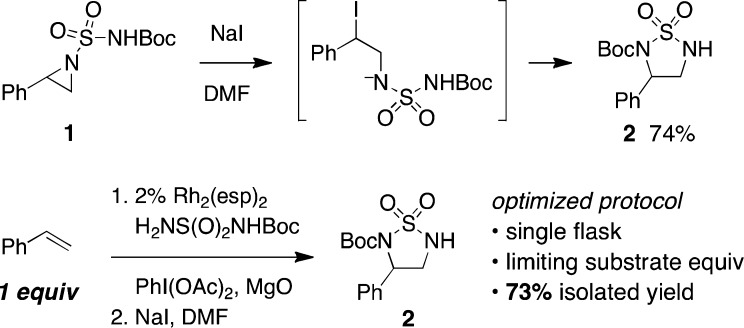
Previous developments in our lab have provided methods for the facile aziridination of alkenes using dirhodium-based catalysts and hypervalent iodine oxidants.5,6 Following this work and related studies on C–H amination with urea, sulfamide, and hydroxylamine-derived sulfamate esters, we recognized the potential of utilizing a bifunctional nitrogen source that could engage in an initial aziridination reaction followed by a simple isomerization to give a diaza-heterocyclic product (Figure 1).7 Ring opening of the heterocycle would then furnish the desired diamine. Reports describing the rearrangement of N-acyl aziridines to the corresponding oxazolines serve as useful precedent for this plan.8
A series of urea, sulfamate, and sulfamide derivatives were tested in initial exploratory reactions with styrene as a model substrate (Table 1).9 Reactions with N-sulfonylated urea and hydroxylamine-derived sulfamate reagents showed little promise and resulted primarily in decomposition of both the nitrogen source and styrene (entries 1–4). By contrast, alkene aziridination occurred smoothly using N-carbamoyl sulfamides (entries 5–7). Experiments with N-benzylsulfamide, however, failed to give any of the desired product (entry 8). In the presence of PhI(OAc)2, MgO, and 2 mol % Rh2(esp)2, reactions with H2NS(O)2NHBoc (entry 6) and the corresponding methylcarbamate derivative (entry 7) performed best and gave the desired aziridine products in yields exceeding 75%.10 Both aziridine derivatives are stable to isolation by silica gel chromatography (the Troc compound, entry 5, is not) and were used in subsequent experiments to optimize the desired rearrangement to the cyclic sulfamide. The Boc-derivative (entry 6) was ultimately selected given the ease of preparation of this reagent and the convenience of the tert-butoxycarbonyl as an amine protecting group. As depicted in Figure 2, the successful rearrangement of the aziridine product furnishes the corresponding cyclic sulfamide in high yield. These heterocycles are broadly useful materials that appear in pharmacologic molecules and serve as precursors to N-Boc-1,2-diamines.11
Table 1. Styrene Aziridination Using Bifunctional Nitrogen Sources.
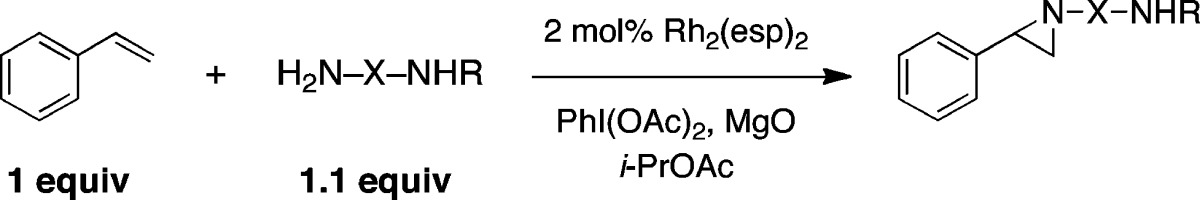
Conversion estimated from integration of the 1H NMR spectrum of the reaction mixture. Parentheses denote isolated yields.
Product could not be isolated due to its instability on SiO2. Tces = 2,2,2-trichloroethoxysulfonyl.
Figure 2.
An optimized, one-flask process for cyclic sulfamide preparation.
Isomerization of aziridine 1 to sulfamide 2 can, in principle, be induced by a Lewis acid catalyst or with an appropriate nucleophilic reagent.8 While the former failed to give any of the desired cyclic sulfamide, treatment of aziridine 1 with NaI in DMF proceeded at room temperature and furnished the desired product in 74% yield as a single isomer.12 The efficiency and selectivity of this process and the preceding aziridination step are sufficiently high that isolation of the intermediate aziridine is superfluous. Accordingly, the optimized protocol for conversion of styrene to 2 is performed in a single flask; once the aziridination reaction is complete, a solution of NaI in DMF is added and the desired isomerization ensues. Under these conditions, the product heterocycle is obtained in 73% yield as a white crystalline solid.
Cyclic sulfamide formation occurs in modest to high yield with a range of mono- and disubstituted alkenes under conditions that employ limiting amounts of substrate (Table 2). Reactions with styrene-type olefins are most efficient and products are furnished as single isomers, consistent with I– opening of the intermediate aziridine at the benzylic carbon center (entries 1–4). Both acyclic and cyclic aliphatic olefins will engage in this diamination reaction (entries 5–12). In general, product yields from oxidation of terminal alkenes are elevated compared to those obtained when cis- or trans-disubstituted olefins are employed as substrates. The rearrangement reaction in these cases proceeds regioselectively to give the sulfamide products as single isomers. For alkenes that engage less effectively in the aziridination reaction, increasing the number of equivalents of the substrate can result in marked improvement in reaction performance.13 Aziridination of both 1,1-disubstituted and trisubstituted olefins is also effective under these reaction conditions. Surprisingly, however, these products have proven recalcitrant to isomerization under a variety of conditions tested.
Table 2. Cyclic Sulfamide Formation with Selected Alkenes.
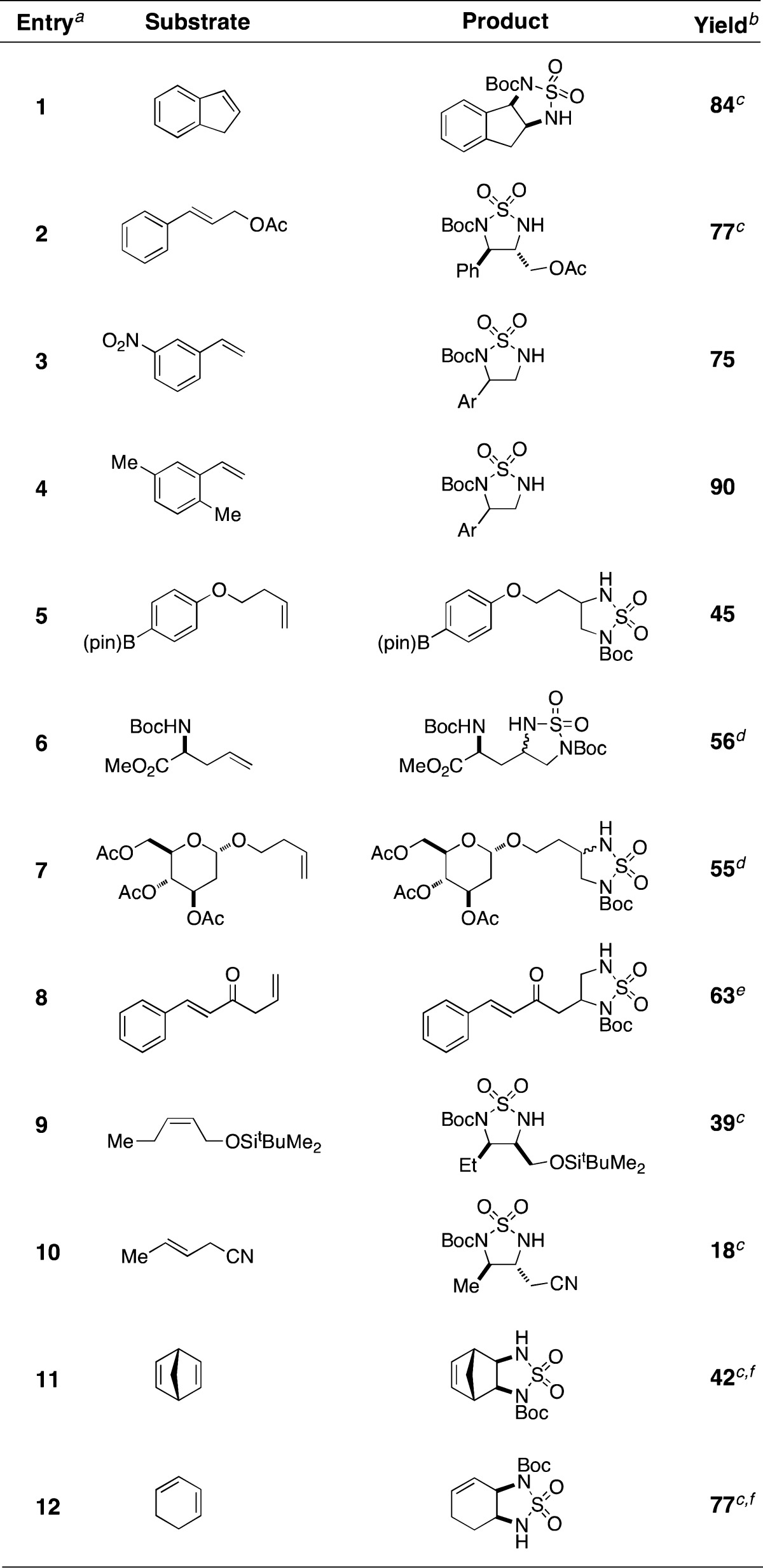
Reactions were performed in i-PrOAc with 1 equiv of substrate, 2 mol % Rh2(esp)2, 1.1 equiv of BocNHSO2NH2, 1.1 equiv of PhI(OAc)2, and 2.3 equiv of MgO. After the completion of the aziridination reaction, 1.1 equiv of NaI and DMF were added to induce rearrangement.
Isolated yields following chromatographic purification.
Product isolated as a single diastereomer.
dr = 1:1.
See ref (14).
Reaction performed with 10 equiv of substrate.
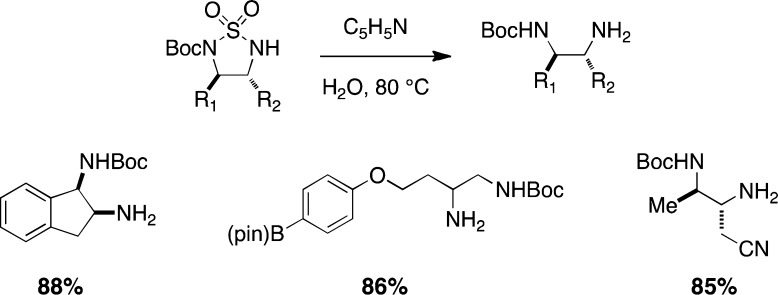
Although cyclic sulfamides generally require forcing conditions of heat and strong acid to induce ring opening, N-Boc-protected derivatives are considerably more labile. When these materials are heated in an aqueous solution of pyridine, the SO2-moiety is excised to give the mono-Boc diamine (Figure 3).9c The product can be isolated following an extractive workup and purified as needed. These conditions have been employed on substrates bearing functional groups that include nitriles, esters, and even pinacolboron. The application of cyclic sulfamides as precursors to 1,2-diamines should enjoy greater use with the availability of this mild method for ring opening.
Figure 3.
Facile hydrolytic ring opening yields differentially protected 1,2-diamines.
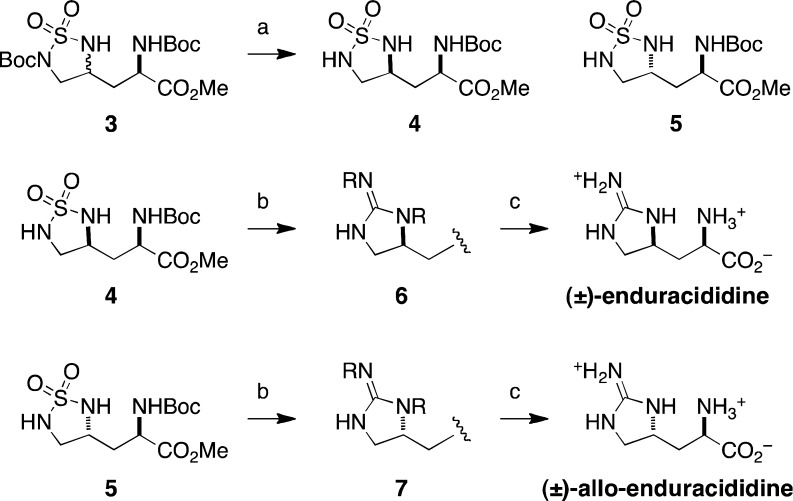
Syntheses of (±)-enduracididine and (±)-allo-enduracididine, amino acid components of the naturally occurring macrocyclic polypeptide antibiotic enduracidin,15,16 are made possible from allylglycine by employing our alkene diamination reaction (Scheme 1). Treatment of 3 with MgBr2·OEt2 promotes selective cleavage of the N-Boc sulfamide.17 Chromatographic separation of the syn and anti diasteromers is followed by a one-step transformation of 4 to Tces-protected guanidine 6. Deprotection of this material is achieved in a single operation that involves ester hydrolysis with LiOH and hydrogenolytic Tces-cleavage to furnish (±)-enduracididine. (±)-Allo-enduracididine can be prepared from sulfamide 5 following an analogous sequence. Overall, the preparation of both natural products requires just four steps from commercially available (±)-allylglycine N-Boc methyl ester, underscoring the effectiveness of the alkene diamination reaction.
Scheme 1. Synthesis of Enduracididine and Allo-enduracididine.
Conditions: (a) MgBr2·OEt2, CH3CN, 60 °C, 61% (separable 1:1 mixture of diastereomers); (b) TcesN=C(SMe)Cl, iPr2NEt, DMAP, CH3CN, −25 °C; then Cl3CCH2OH, 50 °C, 42%; (c) LiOH (aq), THF/H2O; then CF3CO2H, cat. Pd/C, H2, 44%. R = Tces.
An efficient and selective process has been delineated for the synthesis of differentially protected 1,2-diamine derivatives through a two-step sequence involving alkene oxidation/isomerization and sulfamide ring opening. This chemistry is easily performed and applicable for use with a range of structurally disparate mono- and disubstituted alkenes. The effectiveness of such technologies for the construction of C–N bonds is displayed in a concise preparation of the guanidinium natural products, (±)-enduracididine and (±)-allo-enduracididine.
Acknowledgments
We thank Arun Thottumkara for helpful discussions. D.E.O. gratefully acknowledges Eli Lilly for a graduate fellowship. This work was supported in part by the National Institute of Health (R01NS045684) and the National Science Foundation under the CCI Center for Selective C–H Functionalization (CHE-1205646).
Supporting Information Available
Experimental details and characterization data are available in the supporting online material. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Present Address
† Dr. David E. Olson, Stanley Center for Psychiatric Research, Broad Institute of MIT and Harvard, Cambridge, Massachusetts 02142.
Author Present Address
‡ Mr. D. Allen Roberts, Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington 98121.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- For general reviews on 1,2-diamines, see:; a Bennani Y. L.; Hanessian S. Chem. Rev. 1997, 97, 3161. [DOI] [PubMed] [Google Scholar]; b Lucet D.; Le Gall T.; Mioskowski C. Angew. Chem., Int. Ed. 1998, 37, 2580. [DOI] [PubMed] [Google Scholar]; c Viso A.; de la Pradilla R. F.; García A.; Flores A. Chem. Rev. 2005, 105, 3167. [DOI] [PubMed] [Google Scholar]; d Bogatcheva E.; Hanrahan C.; Nikonenko B.; Samala R.; Chen P.; Gearhart J.; Barbosa F.; Einck L.; Nacy C. A.; Protopova M. J. Med. Chem. 2006, 49, 3045. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Kizirian J.-C. Chem. Rev. 2008, 108, 140. [DOI] [PubMed] [Google Scholar]; f Kotti S. R. S. S.; Timmons C.; Li G. Chem. Biol. Drug Des. 2006, 67, 101. [DOI] [PubMed] [Google Scholar]; g Grygorenko O. O.; Radchenko D. S.; Volochnyuk D. M.; Tomalchev A. A.; Komarov I. V. Chem. Rev. 2011, 111, 5506. [DOI] [PubMed] [Google Scholar]
- For selected reports of diamine synthesis, see:; a Okada A.; Shibuguchi T.; Ohshima T.; Masu H.; Yamaguchi K.; Shibasaki M. Angew. Chem., Int. Ed. 2005, 44, 4564. [DOI] [PubMed] [Google Scholar]; b Savoia D. Top. Organomet. Chem. 2005, 15, 1. [Google Scholar]; c Uraguchi D.; Koshimoto K.; Ooi T. J. Am. Chem. Soc. 2008, 130, 10878. [DOI] [PubMed] [Google Scholar]; d Handa S.; Gnanadesikan V.; Matsunaga S.; Shibasaki M. J. Am. Chem. Soc. 2010, 132, 4925. [DOI] [PubMed] [Google Scholar]; e Davis T. A.; Johnston J. N. Chem. Sci. 2011, 2, 1076. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Olson D. E.; Roberts D. A.; Du Bois J. Org. Lett. 2012, 14, 6174. [DOI] [PubMed] [Google Scholar]; g Kano T.; Sakamoto R.; Akakura M.; Maruoka K. J. Am. Chem. Soc. 2012, 134, 7516. [DOI] [PubMed] [Google Scholar]; h Liew S. K.; He Z.; St. Denis J. D.; Yudin A. K. J. Org. Chem. 2013, 78, 11637. [DOI] [PubMed] [Google Scholar]; Also, see:; i Noble A.; Anderson J. C. Chem. Rev. 2013, 113, 2887. [DOI] [PubMed] [Google Scholar]
- For recent reviews on alkene diamination, see:; a de Figueiredo R. M. Angew. Chem., Int. Ed. 2009, 48, 1190. [DOI] [PubMed] [Google Scholar]; b Cardona F.; Goti A. Nat. Chem. 2009, 1, 269. [DOI] [PubMed] [Google Scholar]; c Ramirez T. A.; Zhao B.; Shi Y. Chem. Soc. Rev. 2012, 41, 931. [DOI] [PubMed] [Google Scholar]; d Muñiz K.; Martínez C. J. Org. Chem. 2013, 78, 2168. [DOI] [PubMed] [Google Scholar]
- For recent examples of alkene diamination, see:; a Bar G. L. J.; Lloyd-Jones G. C.; Booker-Milburn K. I. J. Am. Chem. Soc. 2005, 127, 7308. [DOI] [PubMed] [Google Scholar]; b Zhao B.; Peng X.; Zhu Y.; Ramirez T. A.; Cornwall R. G.; Shi Y. J. Am. Chem. Soc. 2011, 133, 20890. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Röben C.; Souto J. A.; González Y.; Lishchynskyi A.; Muñiz K. Angew. Chem., Int. Ed. 2011, 50, 9478. [DOI] [PubMed] [Google Scholar]; d Kim H. J.; Cho S. H.; Chang S. Org. Lett. 2012, 14, 1424. [DOI] [PubMed] [Google Scholar]; e Ingalls E. L.; Sibbald P. A.; Kaminsky W.; Michael F. E. J. Am. Chem. Soc. 2013, 135, 8854. [DOI] [PubMed] [Google Scholar]; f Cornwall R. G.; Zhao B.; Shi Y. Org. Lett. 2013, 15, 796. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Turnpenny B. W.; Chemler S. R. Chem. Sci. 2014, 5, 1786. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Zhang B.; Studer A. Org. Lett. 2014, 16, 1790. [DOI] [PubMed] [Google Scholar]
- For representative examples, see:; a Guthikonda K.; Du Bois J. J. Am. Chem. Soc. 2002, 124, 13672. [DOI] [PubMed] [Google Scholar]; b Guthikonda K.; Wehn P. M.; Caliando B. J.; Du Bois J. Tetrahedron 2006, 62, 11331. [Google Scholar]; c Olson D. E.; Maruniak A.; Malhotra S.; Trost B. M.; Du Bois J. Org. Lett. 2011, 13, 3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For recent reviews on alkene aziridination, see:; a Aggarwal V. K.; Badine D. M.; Moorthie V. A. In Aziridines and Epoxides in Organic Synthesis; Yudin A. K., Ed.; Wiley-VCH: Weinheim, Germany, 2006; pp 1–35. [Google Scholar]; b Pellissier H. Tetrahedron 2010, 66, 1509. [Google Scholar]; c Karila D.; Dodd R. H. Curr. Org. Chem. 2011, 15, 1507. [Google Scholar]; d Jiang H.; Zhang X. P. In Comprehensive Chirality; Carreira E. M., Yamamoto H., Eds.; Elsevier: Amsterdam, 2012; Vol. 5, pp 168–182. [Google Scholar]
- For alternative preparations of cyclic sulfamides, see:; a Zabawa T. P.; Chemler S. R. Org. Lett. 2007, 9, 2035. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Zhao B.; Yuan W.; Du H.; Shi Y. Org. Lett. 2007, 9, 4943. [DOI] [PubMed] [Google Scholar]; c McDonald R. I.; Stahl S. S. Angew. Chem., Int. Ed. 2010, 49, 5529. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Chávez P.; Kirsch J.; Hövelmann C. H.; Streuff J.; Martínez-Belmonte M.; Escudero-Adán E. C.; Martin E.; Muñiz K. Chem. Sci. 2012, 3, 2375. [Google Scholar]
- For selected examples of the rearrangement of N-acyl aziridines to oxazolines, see:; a Cardillo G.; Tolomelli A. Tetrahedron 1999, 55, 15151. [Google Scholar]; b Ferraris D.; Drury W. J. III; Cox C.; Lectka T. J. Org. Chem. 1998, 63, 4568. [DOI] [PubMed] [Google Scholar]; c Martin A.; Casto K.; Morris W.; Morgan J. B. Org. Lett. 2011, 13, 5444. [DOI] [PubMed] [Google Scholar]; d Okazaki H.; Hanaya K.; Shoji M.; Hada N.; Sugai T. Tetrahedron 2013, 69, 7931. [Google Scholar]
- a Kim M.; Mulcahy J. V.; Espino C. G.; Du Bois J. Org. Lett. 2006, 8, 1073. [DOI] [PubMed] [Google Scholar]; b Olson D. E.; Du Bois J. J. Am. Chem. Soc. 2008, 130, 11248. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Kurokawa T.; Kim M.; Du Bois J. Angew. Chem., Int. Ed. 2009, 48, 2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rh2(esp)2 = Rh2(α,α,α′,α′-1,3-benzenediproprionate)2 and is commercially available from Aldrich.
- For representative examples, see:; a Winum J.-Y.; Scozzafava A.; Montero J.-L.; Supuran C. T. Expert Opin. Ther. Patents 2006, 16, 27. [DOI] [PubMed] [Google Scholar]; b Benltifa M.; García Moreno M. I.; Mellet C. O.; García Fernández J. M.; Wadouachi A. Bioorg. Med. Chem. Lett. 2008, 18, 2805. [DOI] [PubMed] [Google Scholar]; c Dou D.; Mandadapu S. R.; Alliston K. R.; Kim Y.; Chang K.-O.; Groutas W. C. Eur. J. Med. Chem. 2012, 47, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Brodney M. A.; Barreiro G.; Ogilvie K.; Hajos-Korcsok E.; Murray J.; Vajdos F.; Ambroise C.; Christofferson C.; Fisher K.; Lanyon L.; Liu J.; Nolan C. E.; Withka J. W.; Borzilleri K. A.; Efremov I.; Oborski C. E.; Varghese A.; O’Neill B. T. J. Med. Chem. 2012, 55, 9224. [DOI] [PubMed] [Google Scholar]
- Efforts to isomerize aziridine 1 by heating or by treatment with Lewis acids such as BF3·(OEt)2 or Sc(OTf)3 resulted in decomposition of starting material.
- Reactions performed with 1 equiv of norbornadiene (entry 11) or 1 equiv of cyclohexadiene (entry 12) afford the corresponding cyclic sulfamide products in 11% and 31% yields, respectively. In general, reactions that underperform do so in the aziridination step; recovered starting alkene accounts for the mass balance.
- The mechanism for isomerization in this example likely involves an E1cb elimination reaction followed by 1,4 addition. This same process can be effected in good yield by iPr2NEt in place of NaI.
- For the original isolation of enduracididine and allo-enduracididine, see:Horii S.; Kameda Y. J. Antibiot. 1968, 11, 665. [PubMed] [Google Scholar]
- For previous synthetic studies on enduracididine, see:; a Tsuji S.; Kusumoto S.; Shiba T. Chem. Lett. 1975, 1281. [Google Scholar]; b Sanière L.; Leman L.; Bourguignon J.; Dauban P.; Dodd R. H. Tetrahedron 2004, 60, 5889. [Google Scholar]
- Stafford J. A.; Brackeen M. F.; Karanewsky D. S.; Valvano N. L. Tetrahedron Lett. 1993, 34, 7873. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.