Abstract
Background: The Control to Range Study was a multinational artificial pancreas study designed to assess the time spent in the hypo- and hyperglycemic ranges in adults and adolescents with type 1 diabetes while under closed-loop control. The controller attempted to keep the glucose ranges between 70 and 180 mg/dL. A set of prespecified metrics was used to measure safety.
Research Design and Methods: We studied 53 individuals for approximately 22 h each during clinical research center admissions. Plasma glucose level was measured every 15–30 min (YSI clinical laboratory analyzer instrument [YSI, Inc., Yellow Springs, OH]). During the admission, subjects received three mixed meals (1 g of carbohydrate/kg of body weight; 100 g maximum) with meal announcement and automated insulin dosing by the controller.
Results: For adults, the mean of subjects' mean glucose levels was 159 mg/dL, and mean percentage of values 71–180 mg/dL was 66% overall (59% daytime and 82% overnight). For adolescents, the mean of subjects' mean glucose levels was 166 mg/dL, and mean percentage of values in range was 62% overall (53% daytime and 82% overnight). Whereas prespecified criteria for safety were satisfied by both groups, they were met at the individual level in adults only for combined daytime/nighttime and for isolated nighttime. Two adults and six adolescents failed to meet the daytime criterion, largely because of postmeal hyperglycemia, and another adolescent failed to meet the nighttime criterion.
Conclusions: The control-to-range system performed as expected: faring better overnight than during the day and performing with variability between patients even after individualization based on patients' prior settings. The system had difficulty preventing postmeal excursions above target range.
Introduction
During the first decade of this century, the combined availability for clinical use of insulin pumps for continuous subcutaneous (SC) insulin infusion (CSII) and systems for online continuous glucose measurement (CGM) from subcutaneously implanted “needle-type” enzymatic glucose sensors has energized the project of closed-loop insulin delivery as a therapy for patients with type 1 diabetes.1 In September 2006, JDRF initiated the Artificial Pancreas Project (APP) and funded a consortium of university centers to carry on closed-loop glucose control research.2 The ultimate goal of the JDRF APP is the development of a commercially available artificial pancreas (AP). The missing link between CSII and CGM is the control algorithm that will drive insulin delivery according to patient needs at all times so that blood glucose can be kept in a near-normal range with minimal patient intervention. Encouraging pilot results have been reported using proportional-integral-derivative control,3–6 model predictive control (MPC),7–9 and fuzzy logic control10 strategies applied to limited numbers of patients. Whereas most studies achieved safe and effective control overnight, the control of glucose in postmeal periods appeared quite challenging. Because of the SC route of insulin infusion, early postmeal spikes can rarely be prevented, and late postmeal lows frequently occur.1 Strategies such as meal announcement,11,12 premeal manual bolus,6 bihormonal use with glucagon13 or amylin,14 and insulin feedback after a priming bolus15 have been used to improve postmeal control with limited success. Using an adaptive controller based on patient body weight and blood glucose inputs every 5 min,16 later extended to use CGM inputs and either a fixed13 or automatically adaptive17 meal priming bolus, the Boston research group has shown that a priming bolus for meal management can result in reduced postmeal hyperglycemia. A similar adaptive identification and control strategy has been recently tested by other authors and demonstrated promising efficacy in preventing hypoglycemia without meal or exercise announcement.18 Nevertheless, most recently reported home or “home-like” studies assessing closed-loop control have been focused on nighttime.19,20
In connection with the control strategy, an important question for AP deployment in home conditions concerns the level of safety of the control system in order to be usable at all times in various populations. Following the development of an MPC algorithm that showed both safety in preventing hypoglycemia and effectiveness in overall glucose control in range and average glucose level in a limited number of adult patients in the clinical research center (CRC),11 we addressed the question of applicability of glucose control in a safe range using this algorithm to larger adult and adolescent populations from various centers in the United States, Europe, and Israel. The main goal of this study was to assess the fulfillment of prespecified safety criteria at both group and individual levels in one full-day inpatient experiment.
Research Design and Methods
This study, conducted at seven clinical centers, was approved by the local institutional review boards/ethics committees. Written informed consent was obtained from adult patients and parents/guardians of minor patients, who provided assent. An independent Data and Safety Monitoring Board provided oversight. The full protocol of this study is available online (www.clinicaltrials.gov/ct2/show/NCT01271023); key aspects are summarized herein.
Major eligibility criteria included 12–65 years of age, type 1 diabetes for at least 1 year, use of an insulin pump for at least 6 months, and hemoglobin A1c level of 5.0–10.5%. Exclusions included current pregnancy, diabetic ketoacidosis in the prior 6 months, severe hypoglycemia with seizure or loss of consciousness in the prior 12 months, and the presence of one of a variety of medical conditions, laboratory abnormalities, or medications that might affect study participation.
Initially, enrollment was limited to participants ≥21 years old; it was then later extended down to 16 years of age and then eventually further down to 12 years of age after initial safety was demonstrated. The first two adults and first two adolescents were regarded as pilot subjects to refine the control algorithm before the study was opened for general enrollment; the data from these pilot subjects were not included in the analyses.
Closed-loop system
The devices used in the closed-loop system were the Dexcom® (San Diego, CA) Seven® Plus CGM device and the OmniPod® insulin pump (Insulet Corp., Bedford, MA). The FreeStyle® Lite® blood glucose meter (Abbott Diabetes Care, Alameda, CA) was used to calibrate the CGM device. The CGM device and pump communicated with a laptop computer that contained the algorithm. The interdevice communication was automated by the Artificial Pancreas System21 developed at the University of California, Santa Barbara and the Sansum Diabetes Research Institute (Santa Barbara, CA). A full closed-loop cycle with a CGM reading, dosing calculation, and insulin dose (if any) nominally occurred every 5 min.
The control algorithm was of control-to-range class,22 and its design was based on modular architecture1,8,11,23 including two interacting modules: the Range Correction Module,24 developed at the University of Pavia, and the Safety Supervision Module,25 developed at the University of Virginia. Added safety was provided by an insulin-on-board constraint developed at the University of California, Santa Barbara and the Sansum Diabetes Research Institute. The algorithm is referred to as “enhanced Control to Range,” and its dosing strategy has been detailed elsewhere.11
CRC protocol
This article reports on the first 22 h, from 9 a.m. to 7 a.m. the following day, of the first admission of the full protocol (see Supplementary Table S1; Supplementary Data are available online at www.liebertonline.com/dia). The admission included three meals with normal announced boluses before each meal and no exercise. Results from the remainder of the admissions, which challenged the system with an exercise session, a meal with a missed bolus, and a meal with an overbolus, will be reported in separate articles elsewhere.
For 2–3 days prior to CRC admission, two blinded Dexcom Seven Plus sensors were worn. At the time of admission, the investigator chose which CGM device to use as part of the AP system based on accuracy compared with blood glucose from a fingerstick. During the admission, a switch was made to the other CGM device if the primary device failed (no signal in 20 min) or if in the opinion of the study physician the sensor demonstrated persistently poor performance compared with reference blood glucose values. During the inpatient admission, the CGM device was calibrated using fingerstick values 30 min before each meal, at bedtime, and if prompted by the CGM device.
Meal boluses were recommended by the system, based on the estimation of the grams of carbohydrate (CHO) of the meal served by clinical staff, with automated delivery following confirmation. Meal composition consisted of approximately 50% CHO, 20% protein, and 30% fat with 1 g of CHO/kg of body mass for each meal. Meal times were approximately 9 a.m. (breakfast), 1 p.m. (lunch), and 7 p.m. (dinner) with no snacking between meals. Between-meal insulin dosing was automated (without any confirmation).
Plasma glucose was measured using a YSI clinical laboratory analyzer instrument (YSI, Inc., Yellow Springs, OH) every 15 min for 90 min following a meal and every 30 min between meals and overnight. For plasma glucose levels ≤60 mg/dL or if the Safety Monitoring System indicated impending hypoglycemia, treatment was given with approximately 16 g of glucose (juice or glucose tablets) and repeated as necessary, and YSI plasma glucose values were measured every 15 min until the plasma glucose level exceeded 80 mg/dL. For plasma glucose levels ≥300 mg/dL for more than 1 h, a correction bolus of insulin was recommended by protocol.
Adverse events
All serious adverse events were recorded, as were any unexpected medical occurrences related to the study or devices. Hypoglycemic events were to be recorded as adverse events if altered consciousness required assistance of another person to actively administer CHO, glucagon, or other resuscitative actions. Hyperglycemic events were to be recorded as adverse events if the event involved diabetic ketoacidosis.
Statistical methods
Separate analyses were planned for participants ≥18 years old and <18 years old. Sample size was computed to be 25 in each age group such that the margin of error of a one-sided 95% confidence interval (CI) on the percentage of glucose values in the range of 71–180 mg/dL during closed-loop would be 10%, assuming an SD on the mean percentage of glucose values of 30%.
System performance analyses included all available data from participants, whereas analyses of glucose metrics included participants who completed at least 80% of the admission. The main analyses evaluated whether the admission met minimal safety criteria using scheduled YSI glucose measurements. The minimum safety criteria, established a priori from consensus opinion of the participating clinicians, included the following: mean percentage of values 71–180 mg/dL that were greater than 50% with a one-sided 95% CI greater than 40% during the day and overall and that were greater than 60% with a one-sided 95% CI greater than 50% overnight; no admission with less than 30% of values 71–180 mg/dL; no admission with a value >400 mg/dL; and <33% of admissions with a value ≤60 mg/dL.
Secondary outcomes included an assessment of the percentage of admissions with one or more values >300 mg/dL, mean glucose, nadir glucose, peak glucose, and glucose coefficient of variation. Metrics were assessed for daytime (9 a.m.–11 p.m.), overnight (11 p.m.–7 a.m.), and over the entire admission. The bootstrap method was used to compute 95% CIs. Cases where external intervention was necessary to treat hypoglycemia not requested by the controller algorithm were handled by imputing glucose values of 60 mg/dL for 1 h following treatment so as to not artificially inflate the performance metrics (e.g., more values in the target range than would have occurred in the absence of external intervention).
Basic system performance of the closed-loop system and its hardware components was assessed using raw device data and controller log files collected during the study admission. System performance outcomes included reliability of closed-loop glucose control, CGM device and pump operation, and communication channels between component devices.
Results
The study, conducted between July 2011 and July 2012, included 53 individuals with type 1 diabetes who were predominately non-Hispanic whites: 27 were ≥18 years old, and 26 were <18 years old (Table 1). One adolescent (Supplementary Fig. S33) did not complete the study because of recurrent pump failure and was not included in the main analyses. There were no cases of severe hypoglycemia, diabetic ketoacidosis, or adverse events related to use of the system.
Table 1.
Demographic and Clinical Characteristics
| Characteristic | Adults | Adolescents |
|---|---|---|
| Number of participants | 27 | 26 |
| Age (years) | 41±11 | 15±1 |
| Male | 15 (56%) | 13 (50%) |
| Racea | ||
| White non-Hispanic | 17 (85%) | 26 (100%) |
| Hispanic | 2 (10%) | 0 |
| African American | 1 (5%) | 0 |
| BMI (kg/m2) | 24±3 | 23±4 |
| T1D duration (years) | 25±11 | 8±3 |
| HbA1c (%) | 7.7±0.6 | 8.1±0.9 |
| Total daily insulin (U/day) | 43±13 | 56±16 |
Data are number (%) or mean±SD values as indicated.
Ethnicity was not collected for seven adult patients because of French laws.
BMI, body mass index; HbA1c, hemoglobin A1c; T1D, type 1 diabetes.
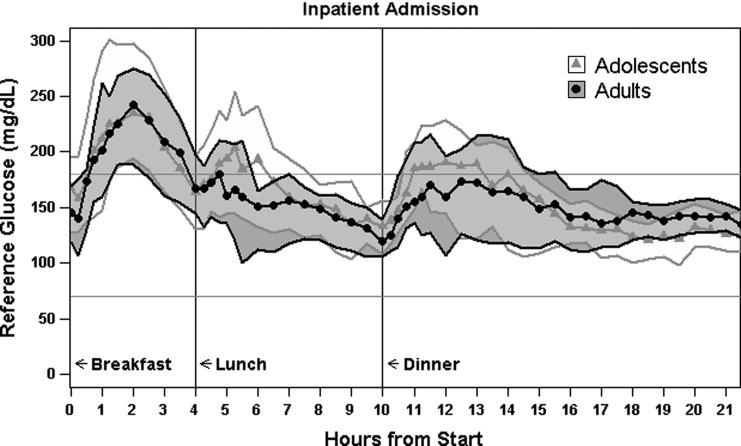
Figure 1 gives the reference glucose levels in both groups during the admission. Table 2 defines the prespecified safety criteria and presents reference glucose outcomes with respect to those criteria at the group and individual levels. Supplementary Tables S2 and S3 provide additional subject-level analysis of glycemic outcomes and associated controller behavior.
FIG. 1.
Median (curves) and 25–75% quantiles (shaded areas) of reference glucose levels during admission.
Table 2.
Glycemic Measures
| Reference glucose outcomes | Adults | Adolescents | Prespecified minimal safety criteria | Met criteria |
|---|---|---|---|---|
| Number of admissions | 27 | 25 | ||
| Number measurements per admission [median (IQR)] | 54 (53, 55) | 53 (50, 55) | ||
| % values 71–180 mg/dL [mean (LCL)] | ||||
| Day and night combined | 66% (LCL=62%) | 62% (LCL=55%) | Mean >50% (LCL >40%) | Both groups |
| 0 admissions <30% | 2 admissions <30% | No admissions <30% | Adults only | |
| Day (9:00 a.m.–11:00 p.m.) | 59% (LCL=53%) | 53% (LCL=44%) | Mean >50% (LCL >40%) | Both groups |
| 2 admissions <30% | 6 admissions <30% | No admissions <30% | Neither group | |
| Night (11:00 p.m.–7:00 a.m.) | 82% (LCL=77%) | 82% (LCL=75%) | Mean >60% (LCL >50%) | Both groups |
| 0 admissions <30% | 1 admission <30% | No admissions <30% | Adults only | |
| Mean glucose (mg/dL) [mean±SD] | 159±25 | 166±29 | ||
| Glucose CV [median (IQR)] | 29% (25%, 33%) | 27% (23%, 31%) | ||
| Nadir glucose (mg/dL) [median (IQR)] | 71 (63, 100) | 85 (80, 102) | ||
| % of admissions with glucose ≤60 mg/dL | ||||
| >0 min | 19% | 20% | ≤33% of admissions with a glucose value ≤60 mg/dL | Both groups |
| >60 min | 15% | 0% | ||
| >120 min | 11% | 0% | ||
| Peak glucose (mg/dL) [median (IQR)] | 266 (226, 300) | 256 (237, 310) | ||
| % of admissions with glucose >300 mg/dL | ||||
| >0 min | 22% | 32% | ||
| >60 min | 7% | 20% | ||
| >120 min | 0% | 12% | ||
| % of admissions with glucose >400 mg/dL | 0% | 0% | No admission with a glucose value >400 mg/dL | Both groups |
CV, coefficient of variation (SD/mean) expressed as a percentage; IQR, interquartile range; LCL, one-sided 95% lower confidence limit.
For adults, the mean of subjects' mean glucose levels was 159 mg/dL, and the mean percentage of values 71–180 mg/dL was 66% overall, 59% daytime, and 82% overnight (lower 95% confidence limit [LCL] of 62%, 53%, and 77%, respectively). Although safety criteria of mean time in target range above 60% (LCL>50%) for nighttime and above 50% (LCL>40%) for both daytime and combined day- and nighttime were met in the adult group, two adults (7%) failed individually in keeping glucose levels >30% in target range during daytime (26% and 17% in range). No adult had any glucose values >400 mg/dL, whereas during 22% of admissions at least one YSI value was >300 mg/dL. During 19% of admissions there was at least one glucose value ≤60 mg/dL (i.e., less than the prespecified safety level of 33%). The system requested CHO treatment for anticipated hypoglycemia on 70% of admissions. External intervention not requested by the controller was required to treat hypoglycemia for 15% of admissions.
For adolescents, the mean of subjects' mean glucose levels was 166 mg/dL, and the mean percentage in range was 62% overall, 53% daytime, and 82% overnight (95% LCL, 55%, 44%, and 75%, respectively). Similarly to adults, safety criteria of mean time in target range above 60% (LCL >50%) for nighttime and above 50% (LCL >40%) for both daytime and combined day- and nighttime were met in the adolescent group. Individually, six adolescents (24%) failed to meet the minimal safety criterion of spending at least 30% of time in target during the day (30%, 25%, 22%, 21%, 11%, and 8% in range); two of these adolescents also failed for combined day and night, and one other adolescent (4%) failed this criterion at night only (27% in range). No adolescent had any glucose values >400 mg/dL, whereas 32% of admissions had at least one YSI value >300 mg/dL. During 20% of admissions, there was at least one glucose value ≤60 mg/dL (i.e., less than the predefined safety level of 33%). The controller requested CHO treatment for anticipated hypoglycemia on 60% of admissions. No adolescent required treatment for hypoglycemia not anticipated by the controller.
Assessment of individual glucose profiles from each admission (Supplementary Figs. S1–S53; available online at www.liebertonline.com/dia) showed that most glucose excursions outside the target range during daytime were related to hyperglycemia following meals. This occurred in 40 cases after breakfast, in 22 cases after lunch, and in 29 cases after dinner. In 33 cases, out-of-range excursions only included postmeal hyperglycemia. In 18 cases, hypoglycemia occurred as late postmeal events, following previous hyperglycemia in most cases. An alternative profile, observed in four cases, included several hypoglycemic excursions during day- and/or nighttime without any postmeal hyperglycemia. No relationship between observed glucose profiles and patient characteristics, including hemoglobin A1c level, was evident.
Table 3 gives performance and reliability outcomes of the closed-loop system during the admission. The median intended time the closed-loop control was operational was 99%, with 43% of admissions having at least one unplanned system reset. Reversion to open-loop dosing occurred in 72% of admissions, for a median duration of open-loop dosing of 20 min per 24-h period. A CGM device component was replaced in 28% of admissions, with a mean time between such events of 33 h. CGM device replacement typically involved per-protocol switching to the backup CGM device due to persistent inaccuracy or loss of signal from the primary sensor for at least 20 min, rather than permanent failure of the primary device. A pump component was replaced in 15% of admissions, with a mean time between such events of 142 h. The median time the CGM device recorded a glucose value was 99%, and the median time the controller received a glucose value from at least one of the sensors was 97%. Of note is that no major harmful glucose deviation occurred during device changes or open-loop phases.
Table 3.
Overall System Performance
| System performance metric | Inpatient admission (n=53 admissions) |
|---|---|
| Total duration of CLC | 1,135 h |
| % of study time with successful CLC [median (IQR)]a | 99% (96%, 100%) |
| % of study time without unintended interruption of insulin delivery [median (IQR)] | 99% (97%, 100%) |
| Number of admissions with an unplanned system reset [n (%)] | 23 (43%) |
| Number of unplanned system resets [n (events per 24 h)] | 32 (0.68 events/24 h) |
| Number of operator-initiated shutdowns of CLC | 0 |
| Number of admissions with a reversion to open-loop dosing [n (%)] | 38 (72%) |
| Number of reversions to open-loop dosing [n (events per 24 h)]b | 91 (1.92 events/24 h) |
| Duration of open-loop dosing per 24 h [median (IQR)]c | 20 min (6 min, 33 min) |
| Number of admissions with a replacement of CGM component [n (%)] | 15 (28%) |
| Number of replacements of CGM component [n (events per 24 h)] | 34 (0.72 events/24 h) |
| Mean time between CGM replacementd | 33 h |
| % of study time CGM records a sensor value [median (IQR)]e | 99% (96%, 100%) |
| % of study time controller receives CGM value [median (IQR)] | 97% (94%, 99%) |
| Number of CGM sensor calibrations per 24 h of CLC operation [median (IQR)]e | 4.4 (4.3, 5.1) |
| Number of admissions with a replacement of pump component [n (%)] | 8 (15%) |
| Number of replacements of pump component [n (events per 24 h)] | 8 (0.17 events/24 h) |
| Mean time between pump replacement (h)d | 142 h |
| Number of admissions with pump failing to dose an algorithm recommendation [n (%)]f | 4 (10%) |
| Number of times pump failed to dose algorithm recommendation [n (events per 24 h)] | 5 (0.16 events/24 h) |
Computed as (actual minutes of closed-loop control [CLC])/(intended minutes of CLC).
All reversions to open-loop dosing were unplanned because of system anomalies.
Restricted to admissions with an open-loop dosing.
Computed as (total time of operation)/(number of failures).
Two patients only wore one continuous glucose measurement (CGM) during an admission; all other patients wore two CGM devices for all admissions.
File only available for 40 of 53 admissions. Events per 24 h of pump log file.
IQR, interquartile range.
Discussion
This article reports an assessment of safety and effectiveness of an MPC algorithm designed for closed-loop insulin delivery using an SC route and SC glucose sensing in a large number of adults and adolescents with type 1 diabetes who were investigated in various CRCs over three continents. This variety in recruited patients provides a unique view on AP usage in a controlled environment, which is valuable at a time when trials start moving to “home-like” or home conditions.19,20,26,27 At this step, the concern about safety is a key question.28 Therefore, careful consideration of the challenges for keeping glucose in a safe range is needed to anticipate possible failures related to devices, patients, and algorithms in these outpatient trials.
On the device side, the feasibility of closed-loop insulin delivery using what has become a commonly used AP system configuration is confirmed by the reported study. Closed-loop control was maintained for most of the study admission in all patients but one. However, CGM failures—typically related to persistent inaccuracy or temporary loss of signal—still occurred in 28% of experiments, requiring a switch to the backup sensor. The CGM model used in this trial has been recently assessed versus a new generation of CGM system from the same manufacturer, showing significant improvements in accuracy including the rate of outliers.29 Improved CGM accuracy, combined with robustness improvements in AP research platforms and a shift to 100% wireless signal transmission, is expected to minimize the occurrence of sensor failure in future studies using more current devices. Technical issues related to the infusion system were less frequent, although still noticeable for a short-term trial. Most issues occurred at the infusion site, which was unsurprising given the still-frequent failures of inserted catheters for CSII in clinical practice.30 The lack of experience with the study pump by the patients and most research teams may have increased the occurrence of infusion issues related to misplacements. The algorithm itself drove insulin infusion according to received CGM information for most of the investigation time. Because the system ran on a laptop, it does not allow consideration of issues that may emerge with more mobile platforms as recently reported.27,31 The lessons related to the technical feasibility of closed-loop control that can be drawn from the present experience include the need for simpler, more robust systems and the importance of thorough education of patients in device management before closed-loop experiments so that they will be able to detect and resolve common issues related to CSII and CGM. Recruitment of patients who have no or insufficient knowledge in device management should be discouraged.
Related to the control targets, the prespecified criteria for safety used in this trial were drawn from a consensus of the participating investigators. They considered it would not be wise to proceed to outpatient closed-loop experiments if these minimal criteria could not be reached. Whereas all of these criteria were met in both adults and adolescents as groups of patients, they were not at the individual level, especially in adolescents and mostly in the daytime period. This result points to the variability of closed-loop outcomes between patients using the same algorithm and justifies the performance of trials in larger patient populations than currently included in pilot studies.11 Positive points come from the observation that no patient faced a blood glucose level above 400 mg/dL, ketosis, or severe hypoglycemia, including when CGM device or CSII failures occurred, suggesting that closed-loop control has reached sufficient maturity in development to prevent any major safety issue, at least when running under idealized CRC conditions.
Most failures in reaching prespecified criteria for safety were related to frequent and sometimes prolonged postmeal hyperglycemia. This glucose profile, which occurred at least once in 47 out of 53 subject admissions, led to average blood glucose levels and time spent in target range similar to those reported in studies with no meal announcement.5,13,32 However, average blood glucose levels were higher, and time spent in target range lower, than in some studies that included announced meals, premeal or priming bolus, or adaptive control.6,12,17,18 Of note is that the recently reported CAT trial, which also included a large adult population in various European sites, showed similar results as the present study with two different MPC closed-loop algorithms.33 Several factors could be involved in this failure to control glucose in a safe range after meal consumption. Skills in CHO counting can be influential when meals are announced to the control system. However, this factor did not play a role in the present study because the CHO content of the meals was well defined, and these values were announced to the controller by the investigators. Nevertheless, outcomes on glucose profile related to lipid and protein association with CHO in the ingested meal have been recently underscored as a potential explanation for failures of bolus advisors to suggest the appropriate insulin dose to cover a meal.34 Recent investigations have also demonstrated a clear variability in insulin sensitivity according to the time of meal.35 In our study, breakfast was followed by out-of-range postmeal excursions more frequently than the other meals. This phenomenon could have been exacerbated by the initiation of closed-loop immediately before breakfast (i.e., with no previous “warm-up” phase of the algorithm), which may have contended with impaired insulin sensitivity of most patients at the late night period.35 Because the reference basal insulin infusion rate, upon which the algorithm had to intervene to keep glucose in target range, was computed from basal insulin needs in each patient for the period preceding study enrollment, the conditions were rather challenging for the initiation of the closed-loop before breakfast.
Beyond this specific breakfast challenge, the method for individualization of algorithms based on usual basal insulin needs raises questions when one considers the various closed-loop glucose profiles. Besides the 33 cases in which glucose was typically in the upper part of the target range or above this range, four patients were conversely often in the lower part of the target range or below it. Moreover, overcompensation for hyperglycemia could lead to hypoglycemia, as shown in 18 cases in our study that experienced at least one late postmeal hypoglycemia, sometimes nocturnal, following a previous excursion above target range. After a few days, it is likely the algorithm would better accommodate the individual insulin needs, thanks to a stabilized fasting prebreakfast glucose level.
Recent reports have illustrated the efficacy of adaptive identification and control strategy and priming-bolus based on insulin needs for previous meals in minimizing out-of-range glucose excursions with no meal or exercise announcements.17,18 This control mode is based on both data collected in open-loop configuration before closing the loop and online adjustments according to CGM data and computed “insulin on board.”18 From our study results, we think better individualization of the control algorithm using previously recorded individual “open-loop” data would have been valuable to obtain higher time of glucose in the target range and lower average glucose levels, as well as improved management of postmeal hyperglycemia. The adaptive control strategy at mealtimes would likely benefit adolescents most because of the higher variability of insulin sensitivity in this population.17
Related to the occurrence of hypoglycemia, especially at late postmeal periods, the challenge is to combine sufficient “aggressiveness” to prevent early postmeal peaks with prevention of secondary hypoglycemia. Adaptive control including insulin-on-board constraints can provide advantages for this challenge with no need for meal announcement.18 The dual-hormone concept allowing glucagon delivery appears to be a valuable and effective option when hypoglycemia is predicted by CGM data and insulin-on-board,17 although concerns remain regarding dual-chambered pump availability and the limited stability of glucagon solutions.
In conclusion, our study points to several challenges that need to be overcome in order to keep the glucose level in a safe range with a closed-loop system, even if major risks of harmful glucose deviations can already be efficiently prevented. All improvements in accuracy and reliability of glucose sensing and insulin infusion will be valuable to reach better closed-loop performance. Simpler, more robust devices and effective patient training in the management of these devices are critical preliminary conditions when considering outpatient experiments. Training should include meal content estimation for experiments with announced meals. A more precise identification of basal insulin needs of investigated patients before switching to closed-loop is expected to help algorithm performance, at least during the initial period of time. A preliminary step of 24-h open-loop recording of insulin doses and glucose levels would likely be a valuable option in order to capture individual characteristics of insulin action. Bolus advice according to meal announcement needs tight connection with premeal insulin delivery according to the algorithm, and the algorithm itself needs more precise consideration of the amount of insulin delivered as meal bolus to reach a better postmeal glucose control. This importance of the coordination between bolus and basal insulin delivery has already been underscored for optimal use of insulin pumps in the open-loop mode.36 Recent advances in this area have been tested in pilot closed-loop experiments demonstrating significant improvements in postmeal glucose control.37 The demonstrated variability of glucose control outcomes between patients while using the same closed-loop algorithm strongly argues for paying more attention to the individualization of closed-loop parameters in the perspective of AP development as a therapy for type 1 diabetes. Remote monitoring of glucose control through real-time data transmission to cloud-based systems could help researchers adjust algorithm parameters for improved performance during prolonged use of closed-loop systems in home conditions. Such communication flows from and to the control system have been described as a “new diabetes ecosystem” that may apply to long-term use of the AP.38
Appendix
Clinical Centers
Listings are by clinical center name, city, and state. Personnel are listed as Principal Investigator (PI), co-Investigator (I), Coordinator (C), Engineer (E), Biostatistician (B), and other personnel positions (O).
Sansum Diabetes Research Institute, Santa Barbara, CA
Howard Zisser, MD (PI), Lois Jovanovic, MD (I), Alison Wollitzer, PhD (I), Wendy Bevier, PhD (I), Eyal Dassau, PhD (I, E), Kristin Castorino, DO (I), Kateryna Markova, MD (C), Jacqueline Wiley, MA (O), Erin Beveridge, BS (O), Nicolas Santibanez, RN (O), Alexandra Sales, BS (O), Maia Bradley, BS (O), and Adam Castorino, AS (O).
University of Virginia, Charlottesville, VA
Boris Kovatchev, PhD (PI), Stacey M. Anderson, MD (I), Susan Demartini, MD (I), Sue Brown, MD (I), William Clarke, MD (I), Marc Breton, PhD (I, E), Stephen Patek, PhD (E), Patrick Keith-Hynes, PhD (E), Colleen Hughes-Karvetski, PhD (O), Molly McElwee, RN, CDE (C), Mary Oliveri, CCRP (C), and Christian Wakeman, BS (O).
Department of Endocrinology, Diabetes, Nutrition and INSERM 1001 Clinical Investigation Center, Montpellier University Hospital, Institute of Functional Genomics, UMR CNRS 5203/INSERM U661, University of Montpellier, Montpellier, France
Eric Renard, MD, PhD (PI), Anne Farret, MD, PhD (I), Marie-Josee Pelletier, MD (I), Hugues Chevassus, PharmD (O), and Jerome Place, MSc (E, C).
Jesse Z and Sara Lea Shafer Institute for Endocrinology and Diabetes, National Center for Childhood Diabetes, Schneider Children's Medical Center of Israel, Sackler Faculty of Medicine, Tel-Aviv University, Israel
Moshe Phillip, MD (PI), Eran Atlas, MSc (I, E), Revital Nimri, MD (I), Tal Oron, MD (I), Alon Farfel, MD (I), Sharon Demol, MD (I), Eran Mel, MD (I), Tal Ben-Ari, MD (I), Michael Gilon, PhD (O), Ayele Parnes (C), Ido Muller, BSc (E), Shahar Miller, BSc (I, E), Alona Hamou, MSc (C), Orna Hermon, BSc (C), Galit Shiovitch-Mantzuri, RN (O), and Galia Fayman, BSc (O).
University of Padova, Padova, Italy
Claudio Cobelli, PhD (PI), Chiara Dalla Man, PhD (I), Angelo Avogaro, MD, PhD (I), Daniela Bruttomesso, MD, PhD (I), Alberto Maran, MD (C), Michele Schiavon (O), Simone Del Favero, PhD (E, C), Roberto Vistenin, MSc (O), Rachele Scotton, MD (O), and Alessio Filippi, MD (O).
University of Pavia, Pavia, Italy
Lalo Magni, PhD (PI), Chiara Toffanin, PhD (I, E), Giuseppe De Nicolao, PhD (E), Simone Mancini, BSc (O), and Davide Martino Raimondo, PhD (E).
Division of Pediatric Endocrinology and Diabetes, Stanford University, Stanford, CA
Bruce A. Buckingham, MD (PI), Darrell M. Wilson, MD (I), Kari Benassi, NP, CDE (C), Paula Clinton, RD, CDE (C), Breanne P. Harris, BS (C), Satya Shanmugham, BS (O), and Kimberly Caswell, NP, CDE (O).
Barbara Davis Center for Childhood Diabetes, University of Colorado, Denver, CO
H. Peter Chase, MD (PI), David M. Maahs, MD, PhD (I), Robert Slover, MD (I), Sally Sullivan, BSN, CDE (C), Laurel Messer, RN, CDE (C), Victoria Gage, BSN, CDE (C), Jaime Realsen, BS (C), Emily Westfall, BA, BS (O), and Hannah Goettle, BS (O).
Department of Chemical Engineering, University of California, Santa Barbara, Santa Barbara, CA
Francis J. Doyle III, PhD (PI).
Coordinating Center. Jaeb Center for Health Research, Tampa, FL
Roy W. Beck, MD, PhD (PI), John Lum, MS (I), Craig Kollman, PhD (B), Peter Calhoun, MA (B), Judy Sibayan, MPH (C), Nelly M. Njeru, (C), and Werner Sauer (E).
Data and Safety Monitoring Board
John C. Pickup, BM, DPhil (chair), Irl Hirsch, MD, and Howard Wolpert, MD.
Supplementary Material
Acknowledgments
We would like to recognize the efforts of the participants and their families and thank them. The project described was supported by grant 22-2011-643 from the JDRF. Continuous glucose monitors and sensors were purchased at a bulk discount price from Insulet Corp. (Bedford, MA) and Dexcom (San Diego, CA). Home glucose meters and test strips were provided to the study by Abbott Diabetes Care, Inc. The companies had no involvement in the design, conduct, or analysis of the trial or the manuscript preparation.
Author Disclosure Statement
The study was designed and conducted by the investigators. The Writing Group collectively wrote the manuscript and vouch for the data. The investigators had complete autonomy to analyze and report the trial results. There were no agreements concerning confidentiality of the data among the JDRF, the authors, or their institutions. The Jaeb Center for Health Research had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
H.Z. reports grants, personal fees, and nonfinancial support from Insulet, grants and nonfinancial support from LifeScan, and grants from Dexcom during the conduct of the study, as well as grants, personal fees, and nonfinancial support from Animas, grants and personal fees from Roche, personal fees from Cellnovo, personal fees from MannKind, grants and nonfinancial support from Abbott, grants and nonfinancial support from Eli Lilly, grants and nonfinancial support from GluMetrics, grants and nonfinancial support from Medtronic, grants and nonfinancial support from Novo Nordisk, and grants and nonfinancial support from Sanofi outside the submitted work. E.R. reports personal fees from A. Menarini Diagnostics, personal fees and nonfinancial support from Abbott Diabetes Care, personal fees from Cellnovo, personal fees and nonfinancial support from Dexcom, personal fees from Eli Lilly, personal fees from Animas, personal fees from Medtronic, personal fees from NovoNordisk, personal fees and nonfinancial support from Roche Diagnostics, personal fees from Sanofi-Aventis, nonfinancial support from Insulet, and personal fees from LifeScan outside the submitted work. B.K. reports grants from JDRF, nonfinancial support from Insulet Corp., and nonfinancial support from Dexcom during the conduct of the study, as well as nonfinancial support from Abbott, nonfinancial support from LifeScan, nonfinancial support from Tandem, nonfinancial support and personal fees from Sanofi-Aventis, and personal fees from Animas outside the submitted work. In addition, B.K. has a U.S. patent (application number 2012-0059353-A1) pending. C.C. reports receiving grants and material support from Dexcom, Insulet, and Roche Diagnostics. A.A. reports honoraria for advisory work and lectures from Astra Zeneca, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly & Co., Merck Sharpe & Dohme, Novartis Pharmaceutical Co., Novo Nordisk, Sanofi, Recordati, and Servier and reports research support from Bristol Myers Squibb, Merck Sharpe & Dohme, Novartis Pharmaceutical Co., Novo Nordisk, and Boehringer. L.M. reports grants from the Italian MIUR during the conduct of the study. B.A.B. reports grants from JDRF during the conduct of the study, as well as grants, personal fees, and nonfinancial support from Medtronic Diabetes, personal fees and nonfinancial support from Dexcom, personal fees from BD, personal fees from Sanofi-Aventis, personal fees from Roche, and personal fees from Glysense outside the submitted work. B.A.B. also has a U.S. patent issued (number 60/234,632: Method and apparatus for real-time control of glucose). In addition, B.A.B and H.P.C have a U.S. patent pending (number 61/197230: Predictive low glucose suspend). H.P.C. reports grants from Dexcom outside the submitted work. F.J.D. III reports receiving nonfinancial support from Animas, nonfinancial support from Dexcom, and nonfinancial support from Insulet during the course of the study. C.K. reports grants from JDRF during the conduct of the study, as well as personal fees from Diabetes Technology Management outside the submitted work. E.D. reports receiving nonfinancial support from Dexcom and nonfinancial support from Insulet during the course of the study, as well as nonfinancial support and personal fees from Animas outside the submitted work. M.B. has received research support from Insulet, Animas, Sanofi, Abbott, Dexcom, Tandem, Roche, BD, and LifeScan and honoraria from Roche GmBH and Abbott, has performed consulting for Senseonics and The Epsilon Group, and is an equity holder in TypeZero Technologies and Inspark. In addition, M.B. reports authoring several U.S. patents (numbers 8,562,587 and 8,585,593) related to the submitted work. S.A. reports grants from JDRF during the conduct of the study, as well as grants from Animas and Medtronic Diabetes, and other support from Senseonics outside the submitted work. M.P. reports personal fees and nonfinancial support from Sanofi, personal fees from Bristol Myers-Squibb, personal fees from AstraZeneca, personal fees, nonfinancial support, and other support from Medtronic, personal fees and nonfinancial support from Eli Lilly, personal fees and other support from CGM3 Ltd., personal fees from D-Medical, personal fees and nonfinancial support from Andromeda, nonfinancial support from Novo Nordisk, personal fees and nonfinancial support from Roche, personal fees from Johnson & Johnson-Animas, and personal fees from Dexcom outside the submitted work. R.W.B reports grants from JDRF, during the course of the study. R.N., J.L., P.C., A.F., J.P., C.D.M., S.D.F., D.B., A.F., R.S., E.A., I.M., S.M., C.T., D.M.R., and G.D.N. declare no competing financial interests exist.
References
- 1.Cobelli C, Renard E, Kovatchev B: Artificial pancreas: past, present, future. Diabetes 2011;60:2672–2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Juvenile Diabetes Research Foundation: Emerging technologies in diabetes research. The JDRF e-Newsletter 2006; (Sept):10 [Google Scholar]
- 3.Abbes IB, Richard PY, Lefebvre MA, Guilhem I, Poirier JY: A closed-loop artificial pancreas using a proportional integral derivative with double phase lead controller based on a new nonlinear model of glucose metabolism. J Diabetes Sci Technol 2013;7:699–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Grady MJ, Retterath AJ, Keenan DB, Kurtz N, Cantwell M, Spital G, Kremliovsky MN, Roy A, Davis EA, Jones TW, Ly TT: The use of an automated, portable glucose control system for overnight glucose control in adolescents and young adults with type 1 diabetes. Diabetes Care 2012;35:2182–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steil GM, Rebrin K, Darwin C, Hariri F, Saad MF: Feasibility of automating insulin delivery for the treatment of type 1 diabetes. Diabetes 2006;55:3344–3350 [DOI] [PubMed] [Google Scholar]
- 6.Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV: Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care 2008;31:934–939 [DOI] [PubMed] [Google Scholar]
- 7.Hovorka R, Kumareswaran K, Harris J, Allen JM, Elleri D, Xing D, Kollman C, Nodale M, Murphy HR, Dunger DB, Amiel SA, Heller SR, Wilinska ME, Evans ML: Overnight closed loop insulin delivery (artificial pancreas) in adults with type 1 diabetes: crossover randomised controlled studies. BMJ 2011;342:d1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kovatchev B, Cobelli C, Renard E, Anderson S, Breton M, Patek S, Clarke W, Bruttomesso D, Maran A, Costa S, Avogaro A, Dalla Man C, Facchinetti A, Magni L, De Nicolao G, Place J, Farret A: Multinational study of subcutaneous model-predictive closed-loop control in type 1 diabetes mellitus: summary of the results. J Diabetes Sci Technol 2010;4:1374–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parker R, Doyle F, III, Peppas N: A model-based algorithm for blood glucose control in type 1 diabetic patients. IEEE Trans Biomed Eng 1999;46:148–157 [DOI] [PubMed] [Google Scholar]
- 10.Atlas E, Nimri R, Miller S, Grunberg EA, Phillip M: MD-Logic artificial pancreas system: a pilot study in adults with type 1 diabetes. Diabetes Care 2010;33:1072–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breton M, Farret A, Bruttomesso D, Anderson S, Magni L, Patek S, Dalla Man C, Place J, Demartini S, Del Favero S, Toffanin C, Hughes-Karvetski C, Dassau E, Zisser H, Doyle FJ, 3rd, De Nicolao G, Avogaro A, Cobelli C, Renard E, Kovatchev B: Fully integrated artificial pancreas in type 1 diabetes: modular closed-loop glucose control maintains near normoglycemia. Diabetes 2012;61:2230–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elleri D, Allen JM, Kumareswaran K, Leelarathna L, Nodale M, Caldwell K, Cheng P, Kollman C, Haidar A, Murphy HR, Wilinska ME, Acerini CL, Dunger DB, Hovorka R: Closed-loop basal insulin delivery over 36 hours in adolescents with type 1 diabetes: randomized clinical trial. Diabetes Care 2013;36:838–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russell SJ, El-Khatib FH, Nathan DM, Magyar KL, Jiang J, Damiano ER: Blood glucose control in type 1 diabetes with a bihormonal bionic endocrine pancreas. Diabetes Care 2012;35:2148–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinzimer SA, Sherr JL, Cengiz E, Kim G, Ruiz JL, Carria L, Voskanyan G, Roy A, Tamborlane WV: Effect of pramlintide on prandial glycemic excursions during closed-loop control in adolescents and young adults with type 1 diabetes. Diabetes Care 2012;35:1994–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steil GM, Palerm CC, Kurtz N, Voskanyan G, Roy A, Paz S, Kandeel FR: The effect of insulin feedback on closed loop glucose control. J Clin Endocrinol Metab 2011;96:1402–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Khatib FH, Russell SJ, Nathan DM, Sutherlin RG, Damiano ER: A bihormonal closed-loop artificial pancreas for type 1 diabetes. Sci Transl Med 2010;2:27ra27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Khatib FH, Russell SJ, Magyar KL, Sinha M, McKeon K, Nathan DM, Damiano ER: Autonomous and continuous adaptation of a bihormonal bionic pancreas in adults and adolescents with type 1 diabetes. J Clin Endocrinol Metab 2014;99:1701–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turksoy K, Bayrak ES, Quinn L, Littlejohn E, Cinar A: Multivariable adaptive closed-loop control of an artificial pancreas without meal and activity announcement. Diabetes Technol Ther 2013;15:386–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nimri R, Danne T, Kordonouri O, Atlas E, Bratina N, Biester T, Avbelj M, Miller S, Muller I, Phillip M, Battelino T: The “Glucositter” overnight automated closed loop system for type 1 diabetes: a randomized crossover trial. Pediatr Diabetes 2013;14:159–167 [DOI] [PubMed] [Google Scholar]
- 20.Phillip M, Battelino T, Atlas E, Kordonouri O, Bratina N, Miller S, Biester T, Stefanija MA, Muller I, Nimri R, Danne T: Nocturnal glucose control with an artificial pancreas at a diabetes camp. N Engl J Med 2013;368:824–833 [DOI] [PubMed] [Google Scholar]
- 21.Dassau E, Zisser H, Palerm CC, Buckingham BA, Jovanovic L, Doyle F: Modular artificial B-cell system: a prototype for clinical research. J Diabetes Sci Technol 2008;2:863–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kovatchev B, Patek S, Dassau E, Doyle FJ, III, Magni L, De Nicolao G, Cobelli C; the Juvenile Diabetes Research Foundation Artificial Pancreas Consortium: Control to range for diabetes: functionality and modular architecture. J Diabetes Sci Technol 2009;3:1058–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patek SD, Magni L, Dassau E, Karvetski C, Toffanin C, De Nicolao G, Del Favero S, Breton M, Man CD, Renard E, Zisser H, Doyle FJ, 3rd, Cobelli C, Kovatchev BP: Modular closed-loop control of diabetes. IEEE Trans Biomed Eng 2012;59:2986–2999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magni L, Raimondo DM, Bossi L, Dalla Man C, De Nicolao G, Kovatchev B, Cobelli C: Model predictive control of type 1 diabetes: an in silico trial. J Diabetes Sci Technol 2007;1:804–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hughes CS, Patek SD, Breton MD, Kovatchev BP: Hypoglycemia prevention via pump attenuation and red-yellow-green “traffic” lights using continuous glucose monitoring and insulin pump data. J Diabetes Sci Technol 2010;4:1146–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cobelli C, Renard E, Kovatchev BP, Keith-Hynes P, Ben Brahim N, Place J, Del Favero S, Breton M, Farret A, Bruttomesso D, Dassau E, Zisser H, Doyle FJ, 3rd, Patek SD, Avogaro A: Pilot studies of wearable outpatient artificial pancreas in type 1 diabetes. Diabetes Care 2012;35:e65–e67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kovatchev BP, Renard E, Cobelli C, Zisser HC, Keith-Hynes P, Anderson SM, Brown SA, Chernavvsky DR, Breton MD, Farret A, Pelletier MJ, Place J, Bruttomesso D, Del Favero S, Visentin R, Filippi A, Scotton R, Avogaro A, Doyle FJ: Feasibility of outpatient fully integrated closed-loop control: first studies of wearable artificial pancreas. Diabetes Care 2013;36:1851–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Renard E, Cobelli C, Kovatchev BP. Closed loop developments to improve glucose control at home. Diabetes Res Clin Pract 2013;102:79–85 [DOI] [PubMed] [Google Scholar]
- 29.Christiansen M, Bailey T, Watkins E, Liljenquist D, Price D, Nakamura K, Boock R, Peyser T: A new-generation continuous glucose monitoring system: improved accuracy and reliability compared with a previous-generation system. Diabetes Technol Ther 2013;15:881–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Renard E, Guerci B, Leguerrier AM, Boizel R: Lower rate of initial failures and reduced occurrence of adverse events with a new catheter model for continuous subcutaneous insulin infusion: prospective, two-period, observational, multicenter study. Diabetes Technol Ther 2010;12:769–773 [DOI] [PubMed] [Google Scholar]
- 31.Elleri D, Allen JM, Biagioni M, Kumareswaran K, Leelarathna L, Caldwell K, Nodale M, Wilinska ME, Acerini CL, Dunger DB, Hovorka R: Evaluation of a portable ambulatory prototype for automated overnight closed-loop insulin delivery in young people with type 1 diabetes. Pediatr Diabetes 2012;13:449–453 [DOI] [PubMed] [Google Scholar]
- 32.Mauseth R, Hirsch IB, Bollyky J, Kircher R, Matheson D, Sanda S, Greenbaum C: Use of a “fuzzy logic” controller in a closed-loop artificial pancreas. Diabetes Technol Ther 2013;15:628–633 [DOI] [PubMed] [Google Scholar]
- 33.Luijf YM, DeVries JH, Zwinderman K, Leelarathna L, Nodale M, Caldwell K, Kumareswaran K, Elleri D, Allen JM, Wilinska ME, Evans ML, Hovorka R, Doll W, Ellmerer M, Mader JK, Renard E, Place J, Farret A, Cobelli C, Del Favero S, Dalla Man C, Avogaro A, Bruttomesso D, Filippi A, Scotton R, Magni L, Lanzola G, Di Palma F, Soru P, Toffanin C, De Nicolao G, Arnolds S, Benesch C, Heinemann L: Day and night closed-loop control in adults with type 1 diabetes: a comparison of two closed-loop algorithms driving continuous subcutaneous insulin infusion versus patient self-management. Diabetes Care 2013;36:3882–3887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smart CEM, Evans M, O'Connell SM, McElduff P, Lopez PE, Jones TWJ, Davis EA, King BR: Both dietary protein and fat increase postprandial glucose excursions in children with type 1 diabetes, and the effect is additive. Diabetes Care 2013;36:3897–3902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hinshaw L, Dalla Man C, Nandy DK, Saad A, Bharucha AE, Levine JA, Rizza RA, Basu R, Carter RE, Cobelli C, Kudva YC, Basu A: Diurnal pattern of insulin action in type 1 diabetes: implications for a closed-loop system. Diabetes 2013;62:2223–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bondia J, Dassau E, Zisser H, Calm R, Vehí J, Jovanovič L, Doyle FJ, 3rd: Coordinated basal-bolus infusion for tighter postprandial glucose control in insulin pump therapy. J Diabetes Sci Technol 2009;3:89–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Del Favero S, Bruttomesso D, Di Palma F, Lanzola G, Visentin R, Filippi A, Scotton R, Toffanin C, Messori M, Scarpellini S, Keith-Hynes P, Kovatchev BP, Devries JH, Renard E, Magni L, Avogaro A, Cobelli C; AP@home Consortium: First use of model predictive control in outpatient wearable artificial pancreas. Diabetes Care 2014;37:1212–1215 [DOI] [PubMed] [Google Scholar]
- 38.Renard E, Cobelli C, Zisser HC, Kovatchev BP: Artificial pancreas goes outpatient: a new diabetes ecosystem. J Diabetes Sci Technol 2013;7:1411–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.