Abstract
Background
There is a growing recognition of the significance of host-pathogen interactions (HPI) in gut biology, leading to a reassessment of the role of bacteria in intestinal anastomotic leak. Understanding the complexities of the early post-surgical gut HPI requires integrating knowledge of both epithelial and bacterial behaviors to generate hypotheses of potential mechanisms of interaction. Agent-based modeling is a computational method well suited to achieving this goal, and we use an agent-based model (ABM) to examine alterations in the HPI affecting re-establishment of the epithelial barrier that may subsequently lead to anastomotic leak.
Methods
Computational agents representing Pseudomonas aeruginosa were added to a previously validated ABM of epithelial restitution. Simulated experiments were performed examining the effect of radiation on bacterial binding to epithelial cells, the plausibility of putative binding targets, and potential mechanisms of epithelial cell killing by virulent bacteria.
Results
Simulation experiments incorporating radiation effects on epithelial monolayers produced binding patterns akin to those seen in-vitro, and suggest that pro-motility integrin-laminin associations represent potential sites for bacterial binding and disruption of restitution. Simulations of potential mechanisms of epithelial cell killing suggested that an injected cytotoxin was the means by which virulent bacteria produced the tissue destruction needed to generate an anastomotic leak, a mechanism subsequently confirmed with genotyping of the virulent P. aeruginosa strain.
Conclusions
This study emphasizes the utility of ABM as an adjunct to traditional research methods, and provides insights into the potentially critical role of HPI in the pathogenesis of anastomotic leak.
Keywords: anastomotic leak, epithelial restitution, agent-based modeling, host-pathogen interaction, Pseudomonas aeruginosa, pre-operative radiation, bacterial virulence
INTRODUCTION
Despite extensive investigation, disruption of gastrointestinal anastomoses leading to leak and sepsis remains as a significant cause of surgical morbidity, with an overall incidence between 2–10%1,2, and greater than 20% in certain high risk situations such as ileorectal anastomosis following total colectomy1. Local factors such as blood supply, presence of infection, immune status of the patient and physical tension at the anastomosis have all been invoked as contributory elements. However, even parsing the pathophysiology of anastomotic leak into defined functional steps is complicated by the degree of complexity of the molecular and cellular interactions present at each level. For example, epithelial restitution (re-apposition of the damaged intestinal epithelium) is known to be a critical early step in restoration of barrier function in the intestinal mucosa3,4. Impairment of restitution of the epithelial barrier may lead to excessive exposure of the underlying tissue layers to detrimental factors in the intestinal lumen (such as bacteria), and can therefore reasonably represent a potential early point of compromise in anastomotic healing. The contribution of this process in the pathogenesis of anastomotic leak is substantiated by the growing recognition that host-pathogen interactions (HPI) significantly influence gut biology. Our laboratory has used in-vitro and in-vivo studies and computational models to demonstrate that environmental host cues can lead to activation of virulent bacteria and resultant tissue damage 5,6. We posit that the HPI at the site of gastrointestinal anastomosis may have significant deleterious effects on the re-establishment of an effective epithelial barrier, and therefore may be a proximal step in how pathogenic organisms contribute to anastomotic leak. We propose to use computational modeling to integrate the extensive knowledge present in literature concerning epithelial and bacterial behaviors to generate new hypotheses with respect to potential mechanisms of interaction7. Used for this purpose, model development using relatively abstracted descriptions of the biological processes can provide a usefully sufficient initial representation of overall system behavior in order to prototype putative hypotheses6–8. Agent-based modeling, an object-oriented, rule-based, spatially-explicit, discrete-event computational modeling technique, is particularly well suited for this role9. ABMs have been previously used to study a wide array of biological processes including sepsis10–12, wound healing 13,14, cancer 15,16, necrotizing enterocolitis17 and inflammatory cell trafficking18. In this study we build on our previously developed in-silico analog of an in-vitro epithelial wound model, the In-Vitro Scratch Agent-based Model (IVSABM)19 by incorporating elements of HPI in order to gain insight into the potential mechanisms of bacterial virulence and interference with epithelial restitution. Our current investigations consist of two steps:
Identifying patterns of HPI and its mechanisms of interaction with a wounded epithelial surface in terms of both positing HPI binding sites as well the how those binding sites lead to inhibition of restitution. The in-vitro experiments that form the reference system for the IVSABM have shown that P. aeruginosa displays different binding patterns when co-incubated with irradiated and non-irradiated cultured intestinal epithelial cells. Because the specific mechanisms underlying these behaviors are unknown, we use the IVSABM to examine the plausibility of a putative hypothesis whereby loss of tight junction integrity, occurring either via scratch or irradiation damage, is required for subsequent binding of bacteria to basolaterally-located epithelial receptors.
Identifying potential mechanisms by which an identified virulent bacterial subtype produces epithelial destruction as involved in the generation of the gross phenotype of anastomotic leak. We have previously shown that exposure of P. aeruginosa to pre-operative radiation and colorectal anastomosis in a rat model of neo-adjuvant radiation and subsequent resection can cause a shift to a more virulent, anastomotic-disrupting phenotype (P2).20 In contrast to the original strain, when the transformed P2 strain is placed back onto scratched monolayers in-vitro there is not only inhibition of healing, but also destruction of the monolayer and epithelial cell death 20 We use the IVSABM to examine the plausibility of several potential mechanisms of bacterially mediated epithelial cell death in order to better understand the contribution of HPI to disruption of epithelial restitution, in the greater context of intestinal anastomotic leak.
We wish to emphasize that these studies are not intended as a comprehensive depiction of anastomotic leak, but that we are evaluating the putative HPI mechanisms on the dynamics of epithelial restitution in order to establish a foundational context upon which subsequent steps, such as collagen deposition and remodeling and angiogenesis, can be placed.
METHODS
Overall Model Architecture
The base ABM used in this study is the In-Vitro Scratch Agent Based Model (IVSABM), an in-silico analog of an in-vitro epithelial cell culture 19. As a brief overview of the IVSABM, computational agents representing individual epithelial cells (IECs) were used to populate a simulated in-vitro cell culture. IECs were created on a simulated background of culture media, containing soluble factors and simulated extracellular matrix (SECM) molecules with which the agents interact. The resultant arrangement represents a single confluent epithelial cell layer, with each IEC agent mapped to a distinct space (patch) on the grid. Intracellular proteins and cell surface receptors were assigned to agents, and diffusible factors were similarly assigned to patches. IECs interact with their environment via surface receptors, and secrete factors that then become patch variables. On ligand-receptor binding, simulated intracellular signaling cascades proceed to produce effector molecules leading to migratory behavior. IECs have intact tight junctions with all of their immediate neighboring cells at the beginning of each simulated experimental run. Tight junctions are broken as IECs move away from neighbors, and are reformed as neighbors come in contact during migration and once apposition is complete. Using these rules, the IVSABM was able to accurately represent epithelial healing dynamics in this context when validated against data from in-vitro experiments19,21. Table 1 lists the general categories of rules, properties and functions of the IECs, for a more comprehensive description of the pathways incorporated into the IECs see Stern, et al19.
Table 1. Rules for Pseudomonas aeruginosa (PA) and epithelial cell agent behavior.
The overall logic of the model is based on the behaviors of each individual agent type. Each agent executes the outlined rules, which manifest as specific behaviors within the model space at both the individual agent and population levels. The epithelial cell agent behaviors are described in considerably more detail in Stern, et al19
| BEHAVIOR | PA AGENT RULE/ACTION |
|---|---|
| Movement | Random |
| Activation | If [ROS] > threshold → activate |
| Binding | If activated and appropriate receptor available, stop moving and attach (Integrin α3β1, LM-332, LM-511) |
| Virulence Expression | If activated, produce virulence factors
|
| BEHAVIOR | EPITHELIALAGENT RULE/ACTION |
|---|---|
| Sensing stimulatory signals (DAMPs, ROS, ATP) | Ligand-receptor binding → signal transduction to effector molecules for migration, spreading |
| Healing | Coordinated cell migration, spreading |
| Tight junction disruption | Wound-, radiation- or toxin-mediated; exposes basolateral receptors |
| Death | When [toxin] > threshold → die (extracellular or intracellular) |
In the current study a second layer of agents representing Pseudomonas aeruginosa bacteria (Pseudomonas Agents = PAs) was added to the IVSABM. PAs are able to move about freely within the simulated culture media, and interact with both IEC agents and soluble factors in the environment. Similar to IECs, each PA maps to a distinct patch space at any given time during simulated experiments. Rules for the behavior of PAs are outlined below, and summarized along with rules for epithelial cell agents in Table 1.
Baseline rules for Pseudomonas Agent (PA) behavior
PAs move about freely within the IVSABM, unless they become bound to an IEC agent or extracellular matrix component based on the hypothetical binding mechanisms placed into the code (described below). PAs contain a bimodal variable to determine whether their virulence is “off” (baseline condition) or “on” (activated); PAs become activated when they are exposed to a threshold value of reactive oxygen species (ROS), a variable in the simulated culture media primarily released by wounded IECs. This phenomenon of ROS-induced bacterial virulence activation is well described in the literature22, and as such was used as a modular variable in the ABM simulating an array of potential virulence-activating factors in the in-vitro condition. PAs can only elaborate virulence factors (see below) or bind to IECs in the activated state.
Simulated radiation effects to examine putative HPI binding sites
These studies were performed to help define potential binding sites between bacteria and epithelial cells. In the IVSABM, simulated radiation induces random epithelial tight junction disruption in a proportion of IECs, a well-described phenomenon in-vitro23,24. These simulations were performed to examine the hypothesis that PAs are able to access “basolaterally” located IEC receptors only when they reach an area where TJs are disrupted, otherwise they remain physically separated from them in the “apical” compartment. Since in-vitro experiments with irradiated monolayer exposed to P. aeruginosa demonstrated more diffuse bacterial adhesion, we hypothesized that these basolateral receptors represent a likely target for P. aeruginosa binding. This hypothetical mechanism was coded into behavioral rules for PA agents in the model, and is outlined in Figure 1. Simulations were performed in the presence and absence of simulated radiation. PAs are able to move about freely unless they encounter an exposed basolateral receptor (Putative Receptor X), at which point they become bound. Binding of PAs to IECs causes cessation of IEC migration, such that binding patterns can be qualitatively assessed. These patterns were compared to in-vitro patterns. A secondary effect of the simulated radiation is to elaborate ROS to a 100-fold higher degree in the system, as this is also known to occur in-vitro25.
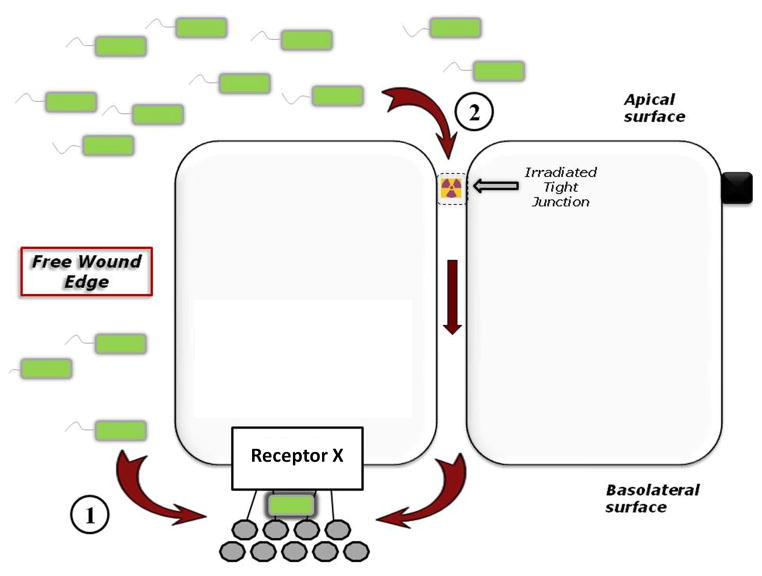
Figure 1. Putative mechanisms of interactions between bacteria and host epithelial cells.
During injury, bacteria are able to access and bind to an as-yet unidentified receptor (Receptor X) on the basolateral surface of epithelial cells via one of two mechanisms: (1) at the free wound edge, or (2) following radiation-induced tight junction disruption. In the absence of injury, this receptor is physically separated from bacteria by intact tight junctions.
Mechanisms of Pseudomonas aeruginosa inhibition of wound healing
We hypothesized several potential points of interaction between PA agents and IEC agents in the IVSABM that could lead to impairment of restitution (Figure 2).
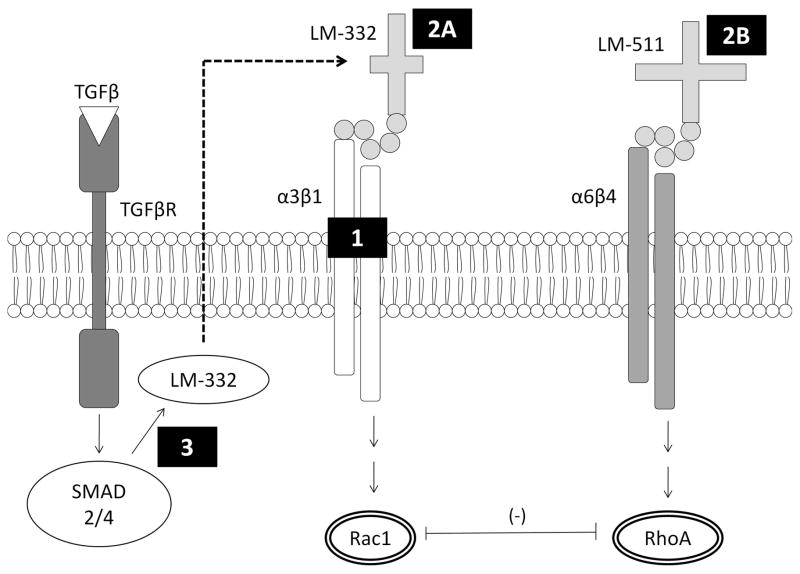
Figure 2. Potential targets for bacterial binding and interference with epithelial restitution.
We hypothesized several points of interaction between P. aeruginosa bacteria and epithelial cells which could lead to inhibition of epithelial restitution. Mechanism 1 involves binding to integrin-α3β1, which is involved in pro-motility signaling through Rac1. Mechanism 2A and 2B involve binding to the extracellular matrix laminins 332 and 511, respectively. Mechanism 3 involves inhibition of LM-332 production by bacteria.
-
Hypothesis 1
PA agents bind to integrin-α3β1 on the basolateral surface of IECs. In the IVSABM, interaction of integrin-α3β1 with laminin-332 (LM-332) in the SECM is critical to IEC motility, as it leads to Rac1 which is known to promote epithelial migration26,27.
-
Hypothesis 2
PA agents bind to either LM-332 or LM-511 in the SECM. Binding of PAs to LM-332 blocks its interaction with integrin-α3β1. Similarly, binding to LM-511 blocks its interaction with integrin-α6β4 in the IVSABM, which is known to lead to pro-stability signals through the GTPase RhoA, which acts an antagonist to Rac128.
-
Hypothesis 3
PAs mediate inhibition of LM-332 production by IECs. Here PAs bind to an as-yet unidentified receptor on the IECs, and specifically inhibit the TGF-β mediated production of LM-332 for deposition in the SECM.
Each of these mechanisms was instantiated separately into the model code, and the results compared to known in-vitro data. Patterns of PA binding to the epithelial layer and resultant effects on epithelial restitution were analyzed, as were quantitative effects on Rac1 as a surrogate for IEC migratory capacity.
Investigation of differential virulent phenotypes of Pseudomonas aeruginosa
Although our laboratory has observed that the P2 phenotype leads to epithelial cell death, the mechanism of killing is unknown. In order to explain this behavior, we assigned two hypothetical P2 mechanisms to PA agents and instantiated them into the IVSABM: a secreted toxin-mediated killer (P2-SEC) where PAs release a soluble patch-variable “toxin” which leads to IEC death when a critical concentration is reached, and a mechanism where PAs can bind and inject a cytotoxic substance, but then release and move on to their next target (P2-INJ). This “molecular syringe” is similar to the known effects of the Type-III secretion system (TTSS)29.
In-vitro bacterial binding assays
Rat intestinal epithelial cells (IEC-18, passages 21–30) were cultured in 75-cm2 plastic culture flasks containing DMEM media (DMEM, high glucose, 4.5g/L containing 5% fetal bovine serum, penicillin streptomycin, and 0.1 U/ml insulin) and kept in a 5% aerated CO2 incubator at 37°C. For experiments involving the assessment of P. aeruginosa adhesion on IECs, antibiotic-free DMEM medium was used. IEC-18 cells were seeded onto collagen coated glass-bottom p35 culture dishes (MatTek, Ashland, MA), grown to confluence, and 5Gy of radiation was delivered at a dose rate of 1.27 Gy/min. Twenty-four hours following radiation, 100 μl of PAO1/EGFP (107 CFU/ml) was added apically onto the cells. Cells were fixed with 4% para-formaldehyde, nuclei were stained with DAPI, and P. aeruginosa adherence to IEC cells was analyzed using fluorescent confocal microscopy. To create wounding, monolayers were scratched with a 10 μl pipette tip, incubated for 1 hour at 37°C, and PAO1/pUCP24- EGFP was introduced as described above.
RESULTS
Effects of simulated radiation on Pseudomonas aeruginosa binding patterns
In the absence of simulated radiation, binding of PA agents to IECs occurs primarily at the wound edge, with some minor binding effects seen within the remainder of the cell layer. However, with radiation there is a dramatic increase in PA binding in general, but this difference is more pronounced in the monolayer away from the wound edge. These data were qualitively similar when compared to our in-vitro results (Figure 3).
Figure 3. Simulated bacterial binding patterns to host epithelial cells are qualitatively similar to in-vitro results.
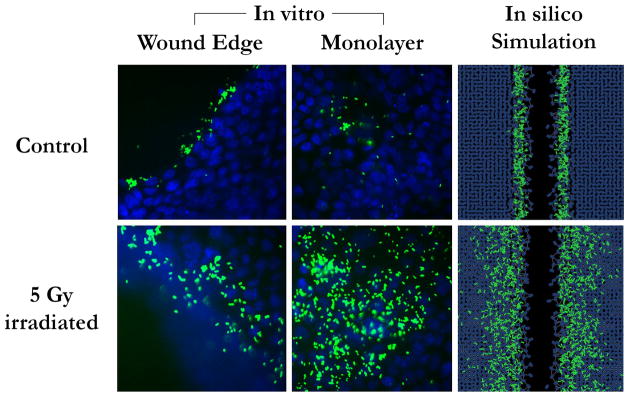
GFP-labeled MPAO-1 strain P. aeruginosa bacteria (green) were incubated with wounded monolayers of IEC-18 intestinal epithelial cells (blue), either previously irradiated or not. Bacteria bind primarily to the wound edge in non-irradiated monolayers, and more diffusely in irradiated conditions. Simulations in the ABM yielded qualitatively similar results using the hypothetical mechanism of host-bacterial interactions outlined in Figure 1.
Effects of Pseudomonas aeruginosa on epithelial restitution
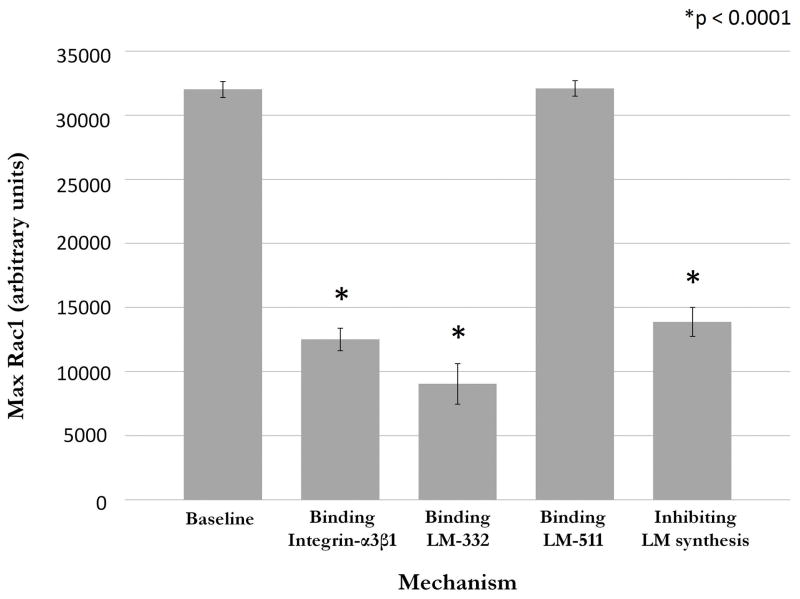
The mean maximum Rac1 level during baseline wound healing conditions (n=100 runs) without bacteria was 32027.16 ± 625.76 units. After addition of P. aeruginosa bacterial agents, maximum Rac1 levels were examined using 100 runs with each of the potential mechanisms of bacterial interference. Binding to integrin-α3β1 (mechanism 1) led to a decrease in max Rac1 to 12513.4 ± 878.17 units. Binding to LM-332 in the SECM (mechanism 2A) reduced max Rac1 to 9039.3 ± 1581.56 units, while binding of P. aeruginosa agents to LM-511 in the SECM (Mechanism 2B) yielded a max Rac1 level of 32101.27 ± 608.48 units, which was not significantly changed from baseline (p = 0.397). Bacterial inhibition of LM-332 production (mechanism 3) reduced levels to 13883.63 ± 1128.58 units. Each of mechanisms 1, 2a and 3 were statistically significant when compared to baseline (unpaired t-test, p < 0.0001). These results are summarized in Figure 4.
Figure 4. Effect of bacterial binding to putative epithelial targets on expression of Rac1 in the ABM.
Rac1 is a surrogate for epithelial capacity for restitution in the ABM. When bacterial agents were coded to bind to integrin-α3β1 or LM-332, or to inhibit synthesis of LM-332, there is a significant decrease in Rac1 levels when compared to baseline conditions (t-test, p < 0.001). Binding to LM-511 did not significantly affect Rac1 levels. These correspond to the mechanisms seen in Figure 2.
Epithelial restitution was also examined with each of the putative mechanisms. Binding to integrin-α3β1 and LM-332 each demonstrated a decrease in IEC ability to migrate into the simulated wound space, as was the case for inhibition of LM-332 production. However, bacterial adherence to LM-511 did not appear to inhibit IEC migration and restitution. Bacterial agent binding to LM-511 (mechanism 2B) or integrin-α3β1 (mechanism 1) showed strong binding at the wound edge, with some additional minor attachment scattered throughout the bulk of the uninjured monolayer. Binding was completely limited to the wound edge in the case of LM-332 binding (mechanism 2A) (Figure 5). Mechanism 3 is not shown, as the effect is not based primarily on binding to IECs.
Figure 5. Bacterial binding patterns on interaction with putative epithelial cell targets in the ABM.
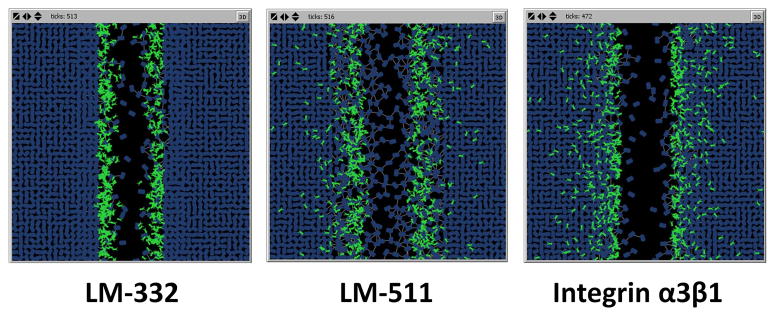
When PA agents are coded to interact with LM-332, binding occurs only to the exposed matrix adjacent to the wound edge. Binding to LM-511 leads to a more diffuse binding pattern, primarily in the matrix at the wound edge as well as through a small number of disrupted tight junctions. Binding to integrin-α3β1 leads to binding to epithelial cell agents both at the wound edge and, to a smaller extent, through the remaining monolayer.
Killing of IECs by P2 phenotypes
When both the P2-SEC and the P2-INJ phenotypes were simulated in the IVSABM, there was qualitatively similar epithelial cell killing observed (Figure 6), and in both conditions there was complete destruction of the epithelial monolayer. However, the time course and killing dynamics were different between the two phenotypes. The P2-SEC led to complete epithelial death in approximately 450 ticks (arbitrary time units). There is an early loss of tight junctions beginning at 100 ticks, with a stable number of epithelial cells. At 400 ticks there is a dramatic killing effect as a critical level of toxin is reached in the system (Figure 7). In the P2-INJ phenotype, there was also an early loss of tight junctions at around 100–200 ticks. Epithelial cell death occurs logarithmically over a much longer time course, with complete destruction at approximately 2000 ticks (Figure 7). This is in contrast to the P2-SEC simulations, where the majority of the monolayer (> 80%) is destroyed at approximately 450 ticks.
Figure 6. Epithelial cell destruction with addition of P2 bacteria in the ABM.
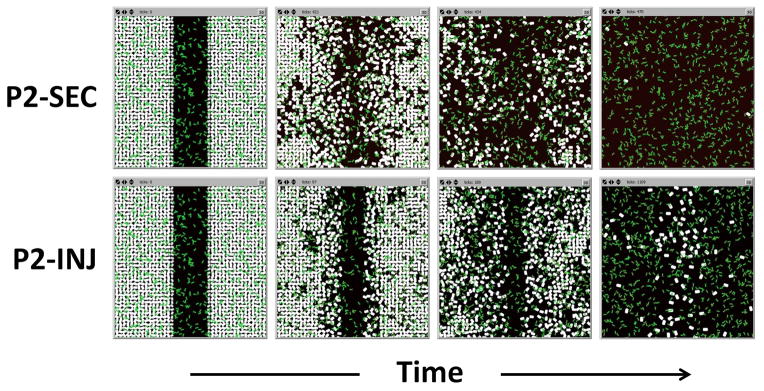
Putative mechanisms of P2 cytotoxicity were coded into the ABM. Both the secreted toxin (P2-SEC) and the injected cytotoxic (P2-INJ) mechanisms of P2 bacteria (green) lead to widespread epithelial cell death (white) and complete destruction of the simulated monolayer.
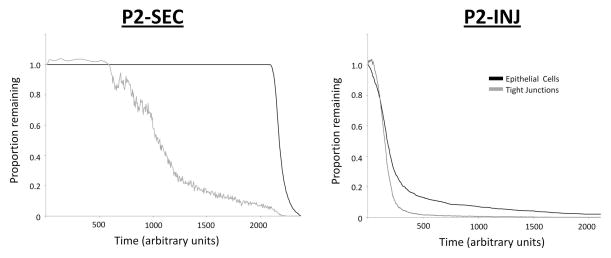
Figure 7. Loss of tight junctions and epithelial cell death with addition of virulent P2 bacterial phenotypes in the ABM.
With the toxin-producing P2 phenotype (P2-SEC, left) there is loss of tight junctions (gray) at approximately 200 ticks (arbitrary time), with a stable population of epithelial cells (black) until approximately 400 ticks when the population is quickly eliminated. With the injected cytotoxic phenotype (P2-INJ, right), there is loss of tight junctions at a similar time point (approximately 200 ticks), with a more logarithmic decrease in epithelial cell population.
DISCUSSION
The restoration of the epithelial barrier (including epithelial restitution) represents one of the most proximal events in healing, and while impairment of restitution in and of itself may not be sufficient to generate a leak, we posit that a delay in re-establishment of the epithelial barrier increases the failure rate at subsequent steps in healing such as cellular migration, deposition of extracellular matrix and angiogenesis. The goal of this study is to attempt a characterization of factors present at and associated with potential impairment of the initial step of restitution. Given the near ubiquitous presence of bacteria adjacent to the anastomosis, we focus on examining differences between the functional capabilities of those bacteria that might be present.
The first of these functional capabilities presumes that bacterial binding to the epithelial cells impairs restitution, and therefore our initial investigations involved trying to identify these interaction points in a manner consistent with existing knowledge and data. Simulated radiation effects implemented within the IVSABM provided some important information with regard to potential P. aeruginosa binding sites and their subsequent effect on epithelial migration. The bacterial binding pattern seen using in-vitro scratch assays suggested that access to the basolateral membrane of epithelial cells may be essential for bacterial binding. Binding was primarily seen only where bacteria can access this compartment: at the wound edge in the absence of radiation (as intact tight junctions would otherwise prevent bacteria from accessing the compartment), and more diffusely in the presence of radiation (due to radiation-induced tight junction disruption). When simulations were run using a generic basolateral receptor (Putative Receptor X), the resultant binding patterns were comparable to those seen in the original in-vitro experiments. This validates the plausibility of the hypothesis, and prompted further investigation into potential molecular binding targets. Our previous work on simulating epithelial restitution has demonstrated the importance of epithelial integrin receptors α3β1 and α6β4 and their interaction with laminins 332 and 511 in the extracellular matrix, respectively19. These interactions occur on the basolateral surface of epithelial cells in order to effect migration, and are therefore represent good candidates for bacterial binding targets which lead to disruption of restitution. Binding to integrin-α3β1 or its ligand LM-332 led to a significant decrease in Rac1 levels (indicative of migratory capacity) and qualitatively decreased restitution. Furthermore, inhibition of LM-332 production by bacteria led to the same. In contrast, bacterial binding to LM-511 did not affect Rac1 levels or restitution. These data suggest that the pro-motility interaction between these two molecules is indeed a reasonable target for bacterial binding, as simulation of this hypothesis yielded a result that is consistent with phenomena observed in-vitro with bacterial inhibition of restitution.
However, given that most anastomoses heal successfully even in the face of bacterial interactions with the epithelium, we need to invoke an additional insult in order to plausibly connect the pathophysiological dots en route to an anastomotic leak. This leads to our second bacterial functional capability of interest: the cytotoxic mechanisms resulting from virulent bacterial transformation. Our laboratory has previously shown that host factors induced by neo-adjuvant radiation and subsequently present at anastomotic sites can transform P. aeruginosa to an anastomotic-disrupting phenotype (P2) in a rat model 20. Our simulations suggest that the cytotoxic effects of the P2 phenotype - associated with anastomotic leak in our rat model - may represent the additional tissue damage component required to produce clinical anastomotic failure. Simulations of both direct cytotoxic (P2-INJ) and secreted toxin-mediated (P2-SEC) mechanisms of epithelial cell killing yielded tight junction disruption, followed by epithelial cell death to complete monolayer destruction. However, the rapidity of the tissue destruction seen in the secreted toxin P2-SEC phenotype would seem to argue against this mechanism; it is too destructive, as least given the time course normally associated with the development of a leak. Rather, the injected toxin P2-INJ phenotype provides the type of progressive tissue destruction that might be associated with the sequential systems failure likely associated with the development of a leak. This is consistent with our subsequent finding that P2 has a mutation in the MexT gene20, which normally represses production of Type-III secretion system (TTSS) products30. TTSS-mediated epithelial cell killing occurs via an injected toxin through a syringe-like protrusion29, which would be more analogous to the direct cytotoxic mechanism (P2-INJ) in simulations.
This study utilizes agent-based modeling to explain phenomena seen during traditional experimental techniques in the examination of HPIs as associated with one proximal step in anastomotic healing. We recognize that development of a clinically significant anastomotic leak is certainly multifactorial, and as such our findings do not represent an all-encompassing explanation for this phenomenon; we are clearly focusing on one single component of many involved in the pathogenesis of anastomotic leak, the HPI. Mechanical contributions to leak such as tension on the anastomosis or technical errors are not represented here, nor are host factors and co-morbidities that may alter tissue healing capacity; these factors may likely operate independently from the HPI. There may also be molecular details of critical importance that have not been included in the IVSABM. Furthermore, we have not yet incorporated existing measures, such as peri-operative antibiotics or bowel preps (either antimicrobial or mechanical), used to forestall leaks. However, effective use of the IVSABM, and all computational models in general, requires the recognition that all such models are by necessity incomplete, only reflecting the limited knowledge used in their creation. Therefore, the goal of such models is not the attainment of ontological truth, but rather of pragmatic utility 31. This study emphasizes the utility of ABM as a complement to traditional in-vitro and in-vivo studies by providing dynamic representation of known data and new hypotheses, being able to invalidate candidate hypotheses, and guiding further research endeavors in the laboratory. Since alterations in the gut microenvironment can lead to factors which induce virulence expression in pathogenic organisms leading to tissue damage5, we propose that modulation of the effect of these alterations on the HPI could represent a novel pathway to prevent leaks and/or reduce the associated morbidity and mortality. Using computational models that extend the IVSABM can provide a “virtual sandbox” in which researchers can propose, implement and evaluate potential therapies, which, by altering local conditions at the anastomotic site or in the pre-operatively irradiated bowel or shifting, could prevent bacterial transformation to virulent phenotypes and help reduce anastomotic leak.
References
- 1.Hyman N, Manchester TL, Osler T, Burns B, Cataldo PA. Anastomotic leaks after intestinal anastomosis: it’s later than you think. Ann Surg. 2007;245(2):254–258. doi: 10.1097/01.sla.0000225083.27182.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Telem D, Chin EH, Nguyen SQ, Divino CM. Risk factors for anastomotic leak following colorectal surgery: a case-control study. Arch Surg. 2010;145(4):371–376. doi: 10.1001/archsurg.2010.40. [DOI] [PubMed] [Google Scholar]
- 3.Mammen J, Matthews JB. Mucosal repair in the gastrointestinal tract. Crit Care Med. 2003;31(8 Suppl):S532–537. doi: 10.1097/01.CCM.0000081429.89277.AF. [DOI] [PubMed] [Google Scholar]
- 4.Blikslager A, Moeser AJ, Gookin JL, Jones SL, Odle J. Restoration of barrier function in injured intestinal mucosa. Physiol Rev. 2007;87(2):545–564. doi: 10.1152/physrev.00012.2006. [DOI] [PubMed] [Google Scholar]
- 5.Wu L, Holbrook C, Zaborina O, Ploplys E, Rocha F, Pelham D, Chang E, Musch M, Alverdy J. Pseudomonas aeruginosa expresses a lethal virulence determinant, the PA-I lectin/adhesin, in the intestinal tract of a stressed host: the role of epithelia cell contact and molecules of the Quorum Sensing Signaling System. Ann Surg. 2003;238(5):754–764. doi: 10.1097/01.sla.0000094551.88143.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seal J, Alverdy JC, Zaborina O, An G. Agent-based dynamic knowledge representation of Pseudomonas aeruginosa virulence activation in the stressed gut: Towards characterizing host-pathogen interactions in gut-derived sepsis. Theor Biol Med Model. 2011;8:33. doi: 10.1186/1742-4682-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.An G. Dynamic knowledge representation using agent-based modeling: ontology instantiation and verification of conceptual models. Methods Mol Biol. 2009;500:445–468. doi: 10.1007/978-1-59745-525-1_15. [DOI] [PubMed] [Google Scholar]
- 8.An G. A model of TLR4 signaling and tolerance using a qualitative, particle-event-based method: introduction of spatially configured stochastic reaction chambers (SCSRC) Math Biosci. 2008;217(1):43–52. doi: 10.1016/j.mbs.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 9.An G, Mi Q, Dutta-Moscato J, Vodovotz Y. Agent-based models in translational systems biology. [Accessed June 25, 2009];Wiley Interdisciplinary Reviews: Systems Biology and Medicine. 2009 doi: 10.1002/wsbm.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.An G. Agent-based computer simulation and sirs: building a bridge between basic science and clinical trials. Shock. 2001;16(4):266–273. doi: 10.1097/00024382-200116040-00006. [DOI] [PubMed] [Google Scholar]
- 11.An G. In silico experiments of existing and hypothetical cytokine-directed clinical trials using agent-based modeling. Critical care medicine. 2004 Oct;32(10):2050–2060. doi: 10.1097/01.ccm.0000139707.13729.7d. [DOI] [PubMed] [Google Scholar]
- 12.An G. Introduction of an agent-based multi-scale modular architecture for dynamic knowledge representation of acute inflammation. Theoretical biology & medical modelling. 2008 May 27;5(1):11. doi: 10.1186/1742-4682-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker DC, Hill G, Wood SM, Smallwood RH, Southgate J. Agent-based computational modeling of wounded epithelial cell monolayers. IEEE transactions on nanobioscience. 2004 Sep;3(3):153–163. doi: 10.1109/tnb.2004.833680. [DOI] [PubMed] [Google Scholar]
- 14.Mi Q, Riviere B, Clermont G, Steed DL, Vodovotz Y. Agent-based model of inflammation and wound healing: insights into diabetic foot ulcer pathology and the role of transforming growth factor-beta1. Wound Repair Regen. 2007 Sep-Oct;15(5):671–682. doi: 10.1111/j.1524-475X.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- 15.Deisboeck TS, Berens ME, Kansal AR, Torquato S, Stemmer-Rachamimov AO, Chiocca EA. Pattern of self-organization in tumour systems: complex growth dynamics in a novel brain tumour spheroid model. Cell proliferation. 2001 Apr;34(2):115–134. doi: 10.1046/j.1365-2184.2001.00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mansury Y, Diggory M, Deisboeck TS. Evolutionary game theory in an agent-based brain tumor model: exploring the ‘Genotype-Phenotype’ link. Journal of theoretical biology. 2006 Jan 7;238(1):146–156. doi: 10.1016/j.jtbi.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 17.Kim M, Christley S, Alverdy JC, Liu D, An G. Immature oxidative stress management as a unifying principle in the pathogenesis of necrotizing enterocolitis: insights from an agent-based model. Surgical infections. 2012 Feb;13(1):18–32. doi: 10.1089/sur.2011.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bailey A, Thorne BC, Peirce SM. Multi-cell agent-based simulation of the microvasculature to study the dynamics of circulating inflammatory cell trafficking. Ann Biomed Eng. 2007;35(6):916–936. doi: 10.1007/s10439-007-9266-1. [DOI] [PubMed] [Google Scholar]
- 19.Stern JR, Christley S, Zaborina O, Alverdy JC, An G. Integration of TGF-β and EGFR based signaling pathways using an agent-based model of epithelial restitution. Wound Repair and Regeneration. 2012;20(6):862–863. doi: 10.1111/j.1524-475X.2012.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olivas A, Shogan B, Valuckaite V, et al. Intestinal tissues induce an SNP mutation in Pseudomonas aeruginosa that enhances its virulence: possible role in anastomotic leak. PLoS ONE. 2012;7(8):e44326. doi: 10.1371/journal.pone.0044326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kheradmand F, Folkesson HG, Shum L, Derynk R, Pytela R, Matthay MA. Transforming growth factor-α enhances alveolar epithelial cell repair in a new in vitro model. Am J Physiol. 1994;267:L728–L738. doi: 10.1152/ajplung.1994.267.6.L728. Lung Cell Mol Physiol 11. [DOI] [PubMed] [Google Scholar]
- 22.Lan L, Murray TS, Kazmierczak BI, He C. Pseudomonas aeruginosa OspR is an oxidative stress sensing regulator that affects pigment production, antibiotic resistance and dissemination during infection. Mol Microbiol. 2010;75(1):76–91. doi: 10.1111/j.1365-2958.2009.06955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Somosy Z, Bognár G, Thuróczy G, Köteles GJ. Biological responses of tight junction to ionizing radiation and electromagnetic field expostion. Cell Mol Biol (Noisy-le-grand) 2002;48(5):571–575. [PubMed] [Google Scholar]
- 24.Moyes S, Killick EM, Morris JF, Kadhim MA, Hill MA, Carr KE. Changes produced by external radiation in parameters influencing intestinal permeability and microparticle uptake in vitro. Int J Radiat Biol. 2008;84(6):467–486. doi: 10.1080/09553000802078388. [DOI] [PubMed] [Google Scholar]
- 25.Riley P. Free radicals in biology: oxidative stress and the effects of ionizing radiation. Int J Radiat Biol. 1994;65(1):27–33. doi: 10.1080/09553009414550041. [DOI] [PubMed] [Google Scholar]
- 26.Dise R, Frey MR, Whitehead RH, Polk DB. Epidermal growth factor stimulates Rac activation through Src and phosphatidylinositol 3-kinase to promote colonic epithelial cell migration. Am J Physiol Gastrointest Liver Physiol. 2008;294:G276–G285. doi: 10.1152/ajpgi.00340.2007. [DOI] [PubMed] [Google Scholar]
- 27.Choma D, Pumiglia K, DiPersio CM. Integrin alpha3beta1 directs the stabilization of a polarized lamellipodium in epithelial cells through activation of Rac1. J Cell Sci. 2004;117(Pt 17):3947–3959. doi: 10.1242/jcs.01251. [DOI] [PubMed] [Google Scholar]
- 28.Russell A, Fincher EF, Millman L, Smith R, Vela V, Waterman EA, Dey CN, Guide S, Weaver VM, Marinkovich MP. Alpha 6 beta 4 integrin regulates keratinocyte chemotaxis through differential GTPase activation and antagonism of alpha 3 beta 1 integrin. J Cell Sci. 2003;116(Pt 17):3543–3556. doi: 10.1242/jcs.00663. [DOI] [PubMed] [Google Scholar]
- 29.Hauser A. The Type III Secretion System of Pseudomonas aeruginosa: Infection by Injection. Nat Rev Microbiol. 2009;7(9):654–665. doi: 10.1038/nrmicro2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin Y, Yang H, Qiao M, Jin S. MexT regulates the type III secretion system through MexS and PtrC in Pseudomonas aeruginosa. J Bacteriol. 2011;193(2):399–410. doi: 10.1128/JB.01079-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.An G. Closing the scientific loop: bridging correlation and causality in the petaflop age. Sci Transl Med. 2010 Jul 21;2(41):41ps34. doi: 10.1126/scitranslmed.3000390. [DOI] [PubMed] [Google Scholar]