Abstract
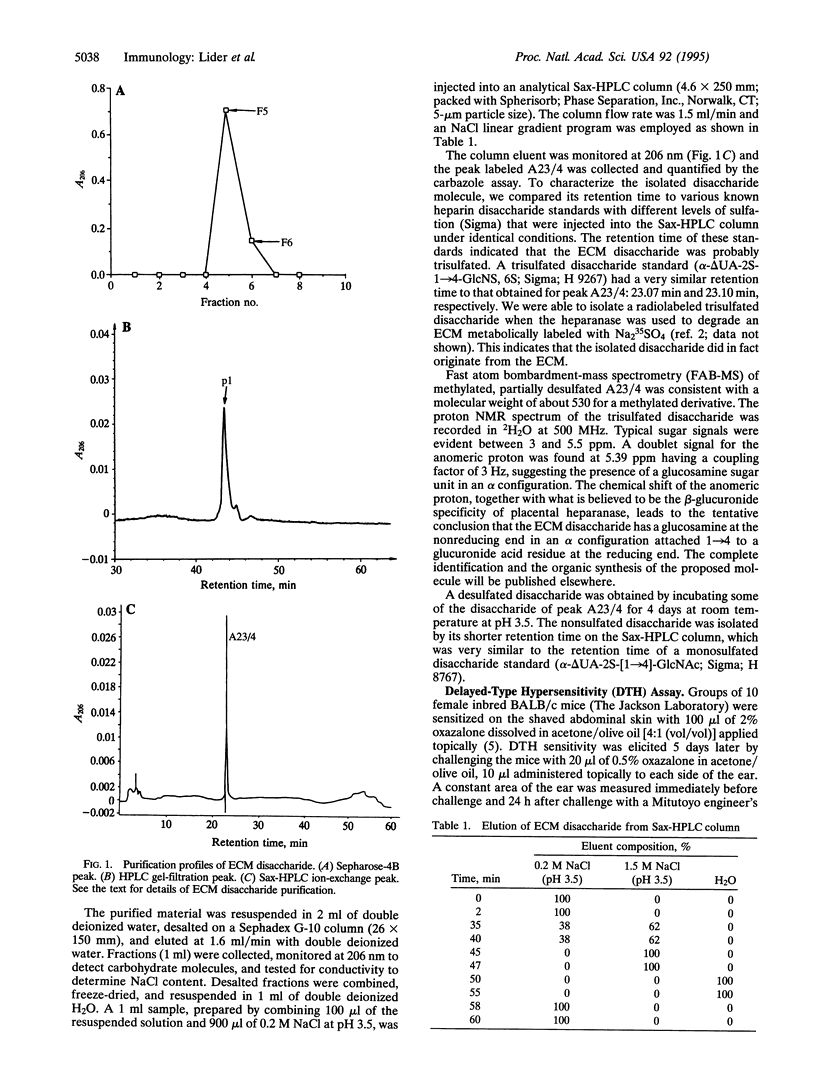
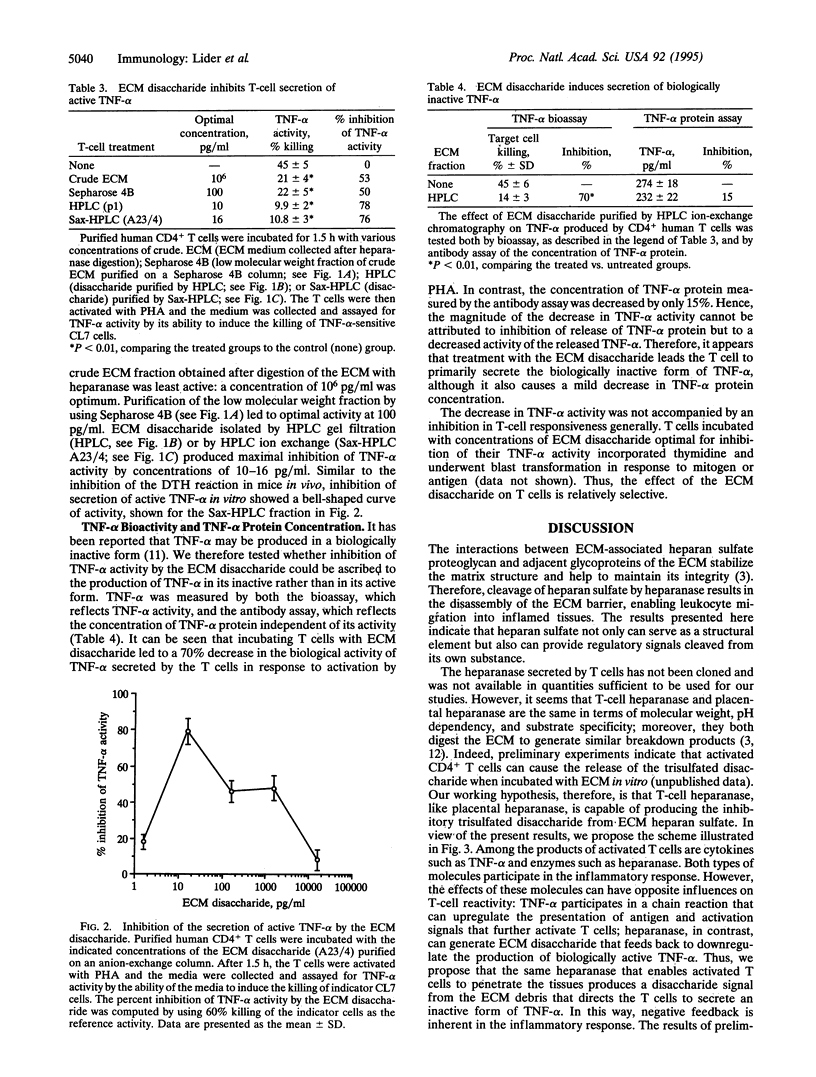
The activation of T cells by antigens or mitogens leads to the secretion of cytokines and enzymes that shape the inflammatory response. Among these molecular mediators of inflammation is a heparanase enzyme that degrades the heparan sulfate scaffold of the extracellular matrix (ECM). Activated T cells use heparanase to penetrate the ECM and gain access to the tissues. We now report that among the breakdown products of the ECM generated by heparanase is a trisulfated disaccharide that can inhibit delayed-type hypersensitivity (DTH) in mice. This inhibition of T-cell mediated inflammation in vivo was associated with an inhibitory effect of the disaccharide on the production of biologically active tumor necrosis factor alpha (TNF-alpha) by activated T cells in vitro; the trisulfated disaccharide did not affect T-cell viability or responsiveness generally. Both the in vivo and in vitro effects of the disaccharide manifested a bell-shaped dose-response curve. The inhibitory effects of the trisulfated disaccharide were lost if the sulfate groups were removed. Thus, the disaccharide, which may be a natural product of inflammation, can regulate the functional nature of the response by the T cell to activation. Such a feedback control mechanism could enable the T cell to assess the extent of tissue degradation and adjust its behavior accordingly.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alon R., Cahalon L., Hershkoviz R., Elbaz D., Reizis B., Wallach D., Akiyama S. K., Yamada K. M., Lider O. TNF-alpha binds to the N-terminal domain of fibronectin and augments the beta 1-integrin-mediated adhesion of CD4+ T lymphocytes to the glycoprotein. J Immunol. 1994 Feb 1;152(3):1304–1313. [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bar-Ner M., Eldor A., Wasserman L., Matzner Y., Cohen I. R., Fuks Z., Vlodavsky I. Inhibition of heparanase-mediated degradation of extracellular matrix heparan sulfate by non-anticoagulant heparin species. Blood. 1987 Aug;70(2):551–557. [PubMed] [Google Scholar]
- Cohen I. R. Language, meaning and the immune system. Isr J Med Sci. 1995 Jan;31(1):36–37. [PubMed] [Google Scholar]
- Cohen I. R. The cognitive paradigm and the immunological homunculus. Immunol Today. 1992 Dec;13(12):490–494. doi: 10.1016/0167-5699(92)90024-2. [DOI] [PubMed] [Google Scholar]
- Cseh K., Beutler B. Alternative cleavage of the cachectin/tumor necrosis factor propeptide results in a larger, inactive form of secreted protein. J Biol Chem. 1989 Sep 25;264(27):16256–16260. [PubMed] [Google Scholar]
- Fridman R., Lider O., Naparstek Y., Fuks Z., Vlodavsky I., Cohen I. R. Soluble antigen induces T lymphocytes to secrete an endoglycosidase that degrades the heparan sulfate moiety of subendothelial extracellular matrix. J Cell Physiol. 1987 Jan;130(1):85–92. doi: 10.1002/jcp.1041300113. [DOI] [PubMed] [Google Scholar]
- Hershkoviz R., Gilat D., Miron S., Mekori Y. A., Aderka D., Wallach D., Vlodavsky I., Cohen I. R., Lider O. Extracellular matrix induces tumour necrosis factor-alpha secretion by an interaction between resting rat CD4+ T cells and macrophages. Immunology. 1993 Jan;78(1):50–57. [PMC free article] [PubMed] [Google Scholar]
- Ishai-Michaeli R., Eldor A., Vlodavsky I. Heparanase activity expressed by platelets, neutrophils, and lymphoma cells releases active fibroblast growth factor from extracellular matrix. Cell Regul. 1990 Oct;1(11):833–842. doi: 10.1091/mbc.1.11.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lider O., Baharav E., Mekori Y. A., Miller T., Naparstek Y., Vlodavsky I., Cohen I. R. Suppression of experimental autoimmune diseases and prolongation of allograft survival by treatment of animals with low doses of heparins. J Clin Invest. 1989 Mar;83(3):752–756. doi: 10.1172/JCI113953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lider O., Mekori Y. A., Miller T., Bar-Tana R., Vlodavsky I., Baharav E., Cohen I. R., Naparstek Y. Inhibition of T lymphocyte heparanase by heparin prevents T cell migration and T cell-mediated immunity. Eur J Immunol. 1990 Mar;20(3):493–499. doi: 10.1002/eji.1830200306. [DOI] [PubMed] [Google Scholar]
- Lortat-Jacob H., Kleinman H. K., Grimaud J. A. High-affinity binding of interferon-gamma to a basement membrane complex (matrigel). J Clin Invest. 1991 Mar;87(3):878–883. doi: 10.1172/JCI115093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naparstek Y., Cohen I. R., Fuks Z., Vlodavsky I. Activated T lymphocytes produce a matrix-degrading heparan sulphate endoglycosidase. Nature. 1984 Jul 19;310(5974):241–244. doi: 10.1038/310241a0. [DOI] [PubMed] [Google Scholar]
- Oosta G. M., Favreau L. V., Beeler D. L., Rosenberg R. D. Purification and properties of human platelet heparitinase. J Biol Chem. 1982 Oct 10;257(19):11249–11255. [PubMed] [Google Scholar]
- Vlodavsky I., Eldor A., Haimovitz-Friedman A., Matzner Y., Ishai-Michaeli R., Lider O., Naparstek Y., Cohen I. R., Fuks Z. Expression of heparanase by platelets and circulating cells of the immune system: possible involvement in diapedesis and extravasation. Invasion Metastasis. 1992;12(2):112–127. [PubMed] [Google Scholar]


