Abstract
Introduction
Results from a previous study showed that sexuality was negatively affected in females with untreated obstructive sleep apnea (OSA). Data are sparse on the long-term effects of nocturnal continuous positive airway pressure (CPAP) treatment on sexual difficulties and sexual distress in female patients with OSA.
Aim
The aim of the present study was to investigate the effects after 1 year of CPAP treatment on sexual difficulties, sexual distress, and manifest sexual dysfunction in female patients with OSA. The effect of CPAP on life satisfaction was also investigated.
Methods
Fifty-four therapy-compliant, female patients (age 22–71) received a survey before and after 1 year of nocturnal CPAP treatment. The questions on this survey were drawn from three self-administered questionnaires: two on sexuality and one on life satisfaction. The results were compared with a population sample. The Epworth Sleepiness Scale was used for assessment of daytime sleepiness.
Main Outcome Measures
The Female Sexual Function Index, Female Sexual Distress Scale, Manifest Female Sexual Dysfunction, four questions from Life Satisfaction 11, and the Epworth Sleepiness Scale were all used to measure outcome.
Results
In total, 44 patients responded to the survey (81% response rate). The results were a significant, positive change in manifest female sexual dysfunction, but no significant changes in isolated sexual difficulties or sexual distress. Daytime sleepiness significantly decreased after 1 year. The results from the Life Satisfaction 11 questionnaire remained unchanged after 1 year.
Conclusions
After 1 year of CPAP treatment, female patients with OSA reported reduced manifest sexual dysfunction. However, it cannot be concluded if this result is due to CPAP treatment alone. Furthermore, reduced daytime tiredness was found in the surveyed population. CPAP treatment, per se, does not seem to affect partner relationships. Petersen M, Kristensen E, Berg S, and Midgren B. Long-term effects of continuous positive airway pressure treatment on sexuality in female patients with obstructive sleep apnea. Sex Med 2013;1:62–68.
Keywords: CPAP, OSA, Sexuality, Distress, Female
Introduction
Obstructive sleep apnea (OSA) is characterized by repetitive partial or complete cessation of breathing (hypopnea/apnea) during sleep due to upper airway collapse. OSA may cause sleep fragmentation and daytime tiredness, increases cardiovascular morbidity and mortality, and negatively affects neurocognitive function [1]. Treatment of OSA with continuous positive airway pressure (CPAP) has been shown to reduce these negative effects [2–4]. Furthermore, OSA has been shown to be associated with sexual dysfunction in males, and studies on the effects of CPAP treatment generally suggest a beneficial effect on male sexuality.
Studies focusing on sexual function in female patients with OSA are rare [5–8]. A search of PubMed showed a lack of studies investigating changes in both sexual function and sexual distress in women after long-term CPAP treatment. A previous study found that many aspects of sexuality in women with untreated OSA are negatively affected, as compared with a female normative group, even when confounders, such as age and obesity, were taken into account [5]. The use of psychoactive medication was associated with a higher risk for sexual difficulties, sexual distress, and manifest female sexual dysfunction. These findings are consistent with the hypothesis that female sexual distress is characterized by mental, rather than physical, problems with sexuality, and is not necessarily only linked to sexual dysfunction but also to individual expectations [9,10].
Results from a long-term study of male OSA patients showed a significant improvement in sexual function after 1 year of CPAP treatment [11]. The aim of the present study was to investigate the effects of 1 year of CPAP treatment on sexual difficulties, sexual distress, and manifest sexual dysfunction in female OSA patients. Furthermore, effects on life satisfaction were also investigated. It was hypothesized that female patients with OSA would report improved sexual life and decreased daytime sleepiness after 1 year of CPAP treatment.
Material and Methods
The primary cohort of 92 female patients with OSA was consecutively recruited from October 2005 to January 2008 [5]. Only patients with OSA, who had been recommended for CPAP treatment by an experienced specialist, who were more than 18 years old, and could read and write Danish were included in the study. Exclusion criteria were comorbid sleep disorders and psychiatric conditions. Obesity, depression, diabetes, and/or cardiovascular disorders were not criteria for exclusion.
Informed consent was obtained, and the study was reviewed and approved by the local ethical committee. Female participants from a cross-sectional, national survey of Danish women's sexual life were used as controls. A total of 1,996 Danish women were identified from the central health registry. Selection criteria were age 20–65 years and zip codes, which ensured that the group reflected the population regarding the part of the country in which they lived. The women were mailed the questionnaires. Two hundred forty age-matched women were selected as the control group to the OSA group—three from the control group for each OSA patient. Data on age, body mass index (BMI), permanent sexual relationship, and education were collected for both patients and normal subjects.
The two questionnaires on sexuality in women were the following: (i) The Female Sexual Function Index (FSFI) (Appendix S1), which is only for women with partners [12], scores ranging 2–36, where a total score cutoff of ≤26.55 indicates sexual difficulties [13]; (ii) the Female Sexual Distress Scale (FSDS) (Appendix S2) [14], scores ranging 0–72, where a total score cutoff of ≥15 indicates sexual distress. Furthermore, The Manifest Female Sexual Dysfunction (MFSD) [15,16] was used, which is a combined index from the cutoff scores in FSFI (≤26.55) and FSDS (≥15), indicating that both sexual difficulties and sexual distress are present. MFSD has not been validated but is described and tested by Oberg et al. [15].
Four questions (life as a whole, family life, relationship to partner, and sexual life) were selected from the questionnaire, Life Satisfaction 11 (LiSat-11) (Appendix S3) [17], after consultation with the author (Dr. A. Fugl-Meyer, pers. comm.), each score ranging 1–6. The Epworth Sleepiness Scale (ESS) (Appendix S4) assesses the likelihood of the patient to doze off or fall asleep in eight daily situations, using a score between 0 and 3, score ranging 0–24, and has a cutoff of ≥10, which indicates daytime sleepiness [18,19]. After 1 year of CPAP treatment, questionnaires were given to those OSA patients who were still using CPAP. To ensure anonymity, the questionnaires were coded in order to retrieve baseline data for each patient, but the coding did not reveal the identity of the patient. Thus, reminders could not be sent.
Menopause was considered a relevant variable, but since the state of menopause was not known for all subjects, the data were subdivided according to age, with a cutoff value of 45 years as a proxy for menopause.
All patients were investigated for OSA using identical, portable devices (EMBLETTA, Embla, Broomfield, CO, USA). The recording montage included nasal airflow and snoring, using a nasal pressure catheter, respiratory movement with thoracic and abdominal bands (XactTrace, Embla, Broomfield, CO, USA), pulse oximetry, and body position. Apneas were diagnosed as the cessation of breathing of >10 seconds. Hypopneas were diagnosed as the reduction in airflow >30%, associated with a desaturation of ≥4%. The apnea and hypopnea index (AHI) was calculated as the total number of apneas and hypopneas, divided by the estimated sleep time, in hours. AHI 5–15 is considered mild sleep apnea, AHI 15–30 is moderate sleep apnea, and AHI >30 is severe sleep apnea. Patients, who, after 1 year, had used their CPAP for less than an average of 4 hours/night were considered noncompliant to CPAP treatment, and excluded.
Statistical Analysis
Descriptive statistics (mean and standard deviation [SD]) were used to summarize the clinical and sociodemographic data. Paired t-tests were used when comparing numerical study data before and during CPAP treatment. The McNemar's test (for changes in proportion of categorical data) was used when investigating the changes in FSFI (cutoff ≤26.55), FSDS (cutoff ≥15), and MFSD. Pearson's correlation analysis was used to relate age (≥45 years), BMI (≥30), ESS, FSFI, FSDS, and LiSat-11. All tests were performed on the whole group, as well as on each subgroup, divided into two: <45 years old and ≥45 years old. A significance level of P < 0.05 was used for all statistical analyses.
Results
In total, 92 female patients, aged 22–71 years, were included in the baseline study [5]. Of these, 54 (59%) continued to use CPAP after 1 year. These patients were given the follow-up questionnaires. Forty-four (81% response rate) patients returned the forms. All of the respondents used their CPAP for >4 hours/night, on average. AHI was not collected after 1 year. Sociodemographic and medical data in the respondent group and population sample are shown in Table 1. Significant correlations were calculated between BMI (≥30) and age (≥45 years), family life and BMI (≥30), life as a whole and age (≥45 years), and life as a whole and ESS. The 10 women who did not return the 1-year evaluation questionnaires did not show any statistically significant differences in sociodemographics or most of their medical data, in comparison with the responding 44 CPAP users. The only differences that they showed were lower BMI (28.4 ± 5.8 vs. 34.7 ± 7.9; P = 0.02) and lower ESS (4.0 ± 1.0 vs. 11.0 ± 3.7; P = 0.003).
Table 1.
Anthropometric, social, and medical data in the population sample and in female patients with obstructive sleep apnea at baseline, and after 1 year of CPAP treatment
| Before CPAP | After 1 year | P values | ||||
|---|---|---|---|---|---|---|
| Population sample | Nonrespondents to the survey after 1 year | Respondents to the survey after 1 year | In CPAP treatment | Before CPAP nonrespondents vs. respondents | Before vs. after 1 year in CPAP treatment | |
| n | 240 | 10 | 44 | 44 | 54 | 44 |
| Age, mean (SD) | 51.3 (8.8) | 57.0 (7.5) | 52.0 (10.9) | NA | 0.179* | NA |
| Age ≥45, n (%) | 182 (76) | 9 (90) | 31 (70) | NA | 0.263† | NA |
| Having a sexual partner (%) | 204 (85) | 7 (70) | 34 (77) | 34 (77) | 0.627† | 1.000† |
| Education ≥10 years, n (%) | 219 (91) | 7 (70) | 32 (82) | NA | 0.767† | NA |
| Medication | ||||||
| Cardiovascular medication‡, n (%) | 56 (23) | 6 (60) | 21 (48) | NA | 0.363† | NA |
| Psychopharmaca§, n (%) | 26 (11) | 2 (20) | 12 (27) | NA | 0.699† | NA |
| Antidiabetics¶, n (%) | 9 (4) | 0 | 4 (9) | NA | 0.335† | NA |
| BMI, mean (SD) | 25.0 (4.3) | 28.4 (5.8) | 34.7 (7.9) | 33.6 (6.3) | 0.022* | 0.307* |
| BMI range | 18–46 | 23–39 | 23–64 | 23–47 | NA | NA |
| BMI ≥30, n (%) | 29 (12) | 3 (30) | 31 (70) | 26 (59) | 0.019† | 0.186† |
| AHI, mean (SD) | NA | 37.5 (21.6) | 35.7 (25.3) | NA | 0.854* | NA |
| ESS, mean (SD) | NA | 4.0 (1.0) | 11.0 (3.7) | 7.0 (4.3) | 0.003* | 0.000* |
Student's t-test.
χ2-test.
Digoxin, antihypertensive, diuretics, beta blockers, calcium antagonists, ACE inhibitors.
Antipsychotics, anxiolytics, hypnotics, antidepressants.
Given orally or as injections.
NA = not analyzed; BMI = body mass index; AHI = apnea hypopnea index; ESS = Epworth Sleepiness Scale
FSFI
Female Sexual Function Index only included women with a regular partner (n = 32). Paired t-tests showed no significant improvements for any of the components of FSFI: desire (P = 0.69), arousal (P = 0.97), lubrication (P = 0.85), orgasm (P = 0.90), satisfaction (P = 0.96), pain (P = 0.94) or total score (P = 0.89) (Table 2). The proportion of patients without sexual difficulties (FSFI ≤ 26.55) did not improve significantly (Figure 1). Splitting the group into age < or ≥45 years showed no subgroup effects.
Table 2.
Scores of Female Sexual Function Index (FSFI) and Female Sexual Distress Scale (FSDS) for women with obstructive sleep apnea (OSA) at baseline, and after 1 year of CPAP treatment, with the population sample scores for comparison
| OSA baseline—Population sample | OSA baseline—1 year | OSA 1 year—Population sample | ||||
|---|---|---|---|---|---|---|
| OSA, baseline | OSA, 1 year | Population sample | P value | P value | P value | |
| FSDS, total score mean (SD) | 16.59 (12.23) | 13.77 (11.37) | 10.15 (10.07) | <0.001 | 0.06 | <0.05 |
| FSFI, total score mean (SD) | 20.34 (9.60) | 20.94 (9.22) | 24.36 (9.78) | <0.05 | 0.89 | <0.01 |
P values were calculated using the Student's t-test.
Figure 1.
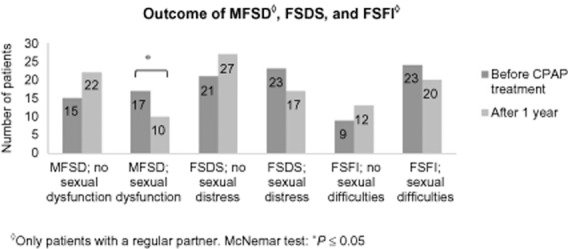
Manifest Female Sexual Dysfunction (MFSD), Female Sexual Distress Scale (FSDS), and Female Sexual Function Index (FSFI) scores in female patients with obstructive sleep apnea (OSA) before and after 1 year of CPAP treatment. MFSD scores indicate that both sexual difficulties and sexual distress are present (n = 32). Score ≥15 in FSDS indicates sexual distress (n = 44). Score ≤26.55 in FSFI indicates sexual difficulties (n = 32).
FSDS
Female Sexual Distress Scale included 44 women. Paired t-tests showed no significant improvement for FSDS (P = 0.06). Total scores are shown in Table 2. The number of patients without sexual distress (FSDS ≥ 15) did not improve significantly (Figure 1). Splitting the group into age <45 or ≥45 years showed no subgroup effects.
MFSD
Manifest Female Sexual Dysfunction included only women with regular partner (n = 32). The proportion of patients with MFSD (the secondary index derived from FSFI and FSDS) was significantly lower after 1 year on CPAP treatment (Figure 1). There was a tendency to a stronger effect in the older (≥45 years) age group (P = 0.06) as compared with the younger (<45 years) (P = 0.63).
LiSat-11
Paired t-tests showed no significant improvements for any of the tested parameters of the LiSat-11: life as a whole (P = 0.59), family life (P = 0.73), partner relationship (P = 1.00), and sexual life (P = 0.92). Splitting the group into age < or ≥45 years showed no subgroup effects.
ESS
Paired t-tests showed that ESS improved significantly after 1 year of CPAP treatment (P < 0.001) (Table 1). The effect was present in both subgroups (<45 years P = 0.014 and ≥45 years; P = 0.004).
Discussion
The main result from this study is that the previous observation of a beneficial effect of CPAP on sexuality in males with OSA can also be extended to females, although the effect seems to be less pronounced [11].
The strength of this study is that it was sufficiently long to allow for adaptation to CPAP treatment, which often takes at least a couple of months. Many patients may be reluctant to use a treatment involving the use of a mask, a tube, and machine every night for many years to come. However, one of the main findings is that CPAP treatment, per se, does not negatively affect family life or partner relationships. Sharing this information with patients may be important when initiating CPAP treatment.
FSFI and FSDS did not change significant after 1 year in CPAP treatment. MFSD demonstrated a significant improvement. However, it cannot be concluded if this result is due to CPAP treatment. Whereas men improved their sexuality in several specific aspects [11], the effect in women could only be demonstrated as an improvement of MFSD, which is a composite measure indicating women being sexual dysfunctional (measured by FSFI) and having sexual distress (measured by FSDS). Whether this result represents a true gender difference or if it is merely due to underpowering of our study is a topic for further research.
ESS was used as a measure of sleepiness/daytime tiredness. ESS improved significantly, indicating a positive effect on daytime tiredness. The magnitude of this effect was similar for men and women. AHI was not collected after 1 year, but a decrease in ESS could be indicative of a reduction in AHI. It is important to underline that all patients in the study of male sexuality [11], as well as in this study, underwent the same diagnostic procedures, performed, and received the same instructions, follow-up, and treatment contacts. There were no significant changes in the three items investigating closeness in the LiSat-11 (family life, partner relationship, and sexual life). However, in a previous study [5], results at baseline showed no significant differences in the untreated OSA group compared with the normative group, providing no indication that closeness is impaired by OSA or necessarily impaired by its treatment by CPAP. Thus, our results suggest that CPAP does not cause changes in closeness.
The lack of validity of the MFSD is a limitation of the study. The decision to use MFSD was taken because it has been used meaningfully in other studies and the definition that both sexual difficulties and sexual distress should be present before talking about sexual dysfunction makes sense. Conditions in one's life can result in decreased sexual function without necessarily meaning sexual distress. Conversely, you can experience sexual distress without necessarily meaning decreased sexual function. The significant improvement in MFSD, but not in FSFI and FSDS (though there was a trend for FSDS), indicates that an Error 2 may explain these differences. However, using the baseline number of 80 participants for the analysis, the result showed a significant improvement in both FSFI and FSDS. One might speculate that a better-powered study would yield different findings. Gender differences may also explain the results. A number of studies have shown that gender has a significant influence on sexuality, and that there are marked differences in sexuality between genders. Laumann et al. [20] found that sexual problems were associated with physical health among men more often than women.
Other studies have found that mood and mental well-being impact female sexuality. In one study, Hartmann et al. [21] found that life stressors, contextual factors, past sexuality, and mental health problems are important predictors of low sexual desire. In a large study, Colson et al. [22] found that 63% of the women reported decreased sexual desire during periods of work-related stress. The main causes for the absence of sexual activity were tiredness (43%) and no desire (21%). A national survey in America found that the best predictors of sexual distress were markers of low general emotional well-being and emotional relationship with partner during sexual activity [23]. A review by McCabe et al. [24] showed that neither treatment with psychotherapy alone nor medical intervention alone is sufficient to help women with low sexual desire, and suggests that therapy should be supplemented with a biopsychosocial approach.
It is well known that depression influences female sexuality [25–28], and this may contribute to the findings in this study, in which 27% of participants were using psychotropic medications at baseline. Similar results have been found for other chronic illnesses. In a study of diabetes and sexuality in women, Nowosielski et al. [29] showed that “the role of partner-related factors and the presence of depressive symptoms in sexual functioning of women seem to be greater than biological.” Giraldi et al. [30] found that sexual dysfunction in women with diabetes is less linked to organic factors and more to psychological factors. Duncan et al. [31] investigated quality of sexual function in women with hypertension; they found that there were no statistically significant differences in reported sexual functioning between the medicated and unmedicated group. The same results were found in a study of quality of life in hypertensive patients, where no significant difference was found between treated and untreated women with hypertension [32]. Furthermore, Cavalcante et al. [32] did not find any relation to the number and type of cardiovascular medication with the quality of sex life. These results are consistent with the findings in this study. Despite the fact that 48% of participants used cardiovascular medication at baseline in this study, there was no correlation to sexual dysfunction.
Conclusion
After 1 year of CPAP treatment, female patients with OSA reported reduced manifest sexual dysfunction. However, it cannot be concluded if this result is due to CPAP treatment alone. Furthermore, reduced daytime tiredness was found in the surveyed population. CPAP treatment, per se, does not seem to affect partner relationships. Sharing these findings with patients may be important for future CPAP users.
Conflict of Interest
Marian Petersen has no conflict of interest. Ellids Kristensen has received honoraria from Pfizer, Bristol-Myers Squibb, Eli Lilly, and Servier for lectures, and from Eli Lilly for consultant commitments. Søren Berg is one of the owners and managers of ScanSleep, and has received honoraria from ResMed Denmark and Sweden for lectures and consultant commitments. Bengt Midgren has received honoraria from Philips Respironics Sweden, ResMed Sweden, Denmark, and Norway, and from BREAS Medical Sweden for lectures and consultant commitments.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Appendix S1 The Female Sexual Function Index.
Appendix S2 The Female Sexual Distress Scale—Revised.
Appendix S3 The LiSat-11 checklist.
Appendix S4 Epworth Sleepiness Scale (ESS).
References
- 1.Al Lawati NM, Patel SR, Ayas NT. Epidemiology, risk factors, and consequences of obstructive sleep apnea and short sleep duration. Prog Cardiovasc Dis. 2009;51:285–293. doi: 10.1016/j.pcad.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 2.El-Sherbini AM, Bediwy AS, El-Mitwalli A. Association between obstructive sleep apnea (OSA) and depression and the effect of continuous positive airway pressure (CPAP) treatment. Neuropsychiatr Dis Treat. 2011;7:715–721. doi: 10.2147/NDT.S26341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antic NA, Catcheside P, Buchan C, Hensley M, Naughton MT, Rowland S, Williamson B, Windler S, McEvoy RD. The effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA. Sleep. 2011;34:111–119. doi: 10.1093/sleep/34.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doherty LS, Kiely JL, Swan V, McNicholas WT. Long-term effects of nasal continuous positive airway pressure therapy on cardiovascular outcomes in sleep apnea syndrome. Chest. 2005;127:2076–2084. doi: 10.1378/chest.127.6.2076. [DOI] [PubMed] [Google Scholar]
- 5.Petersen M, Kristensen E, Berg S, Giraldi A, Midgren B. Sexual function in female patients with obstructive sleep apnea. J Sex Med. 2011;8:2560–2568. doi: 10.1111/j.1743-6109.2011.02358.x. [DOI] [PubMed] [Google Scholar]
- 6.Onem K, Erol B, Sanli O, Kadioglu P, Yalin AS, Canik U, Cuhadaroglu C, Kadioglu A. Is sexual dysfunction in women with obstructive sleep apnea-hypopnea syndrome associated with the severity of the disease? A pilot study. J Sex Med. 2008;5:2600–2609. doi: 10.1111/j.1743-6109.2008.00934.x. [DOI] [PubMed] [Google Scholar]
- 7.Subramanian S, Bopparaju S, Desai A, Wiggins T, Rambaud C, Surani S. Sexual dysfunction in women with obstructive sleep apnea. Sleep Breath. 2010;14:59–62. doi: 10.1007/s11325-009-0280-4. [DOI] [PubMed] [Google Scholar]
- 8.Koseoglu N, Koseoglu H, Itil O, Oztura I, Baklan B, Ikiz AO, Esen AA. Sexual function status in women with obstructive sleep apnea syndrome. J Sex Med. 2007;4:1352–1357. doi: 10.1111/j.1743-6109.2006.00302.x. [DOI] [PubMed] [Google Scholar]
- 9.McCall K, Meston C. Differences between pre- and postmenopausal women in cues for sexual desire. J Sex Med. 2007;4:364–371. doi: 10.1111/j.1743-6109.2006.00421.x. [DOI] [PubMed] [Google Scholar]
- 10.Basson R, Rees P, Wang R, Montejo AL, Incrocci L. Sexual function in chronic illness. J Sex Med. 2010;7(1 Pt 2):374–388. doi: 10.1111/j.1743-6109.2009.01621.x. [DOI] [PubMed] [Google Scholar]
- 11.Petersen M, Kristensen E, Berg S, Midgren B. Sexual function in male patients with obstructive sleep apnoea after 1 year of CPAP treatment. Clin Respir J. 2012 doi: 10.1111/j.1752-699X.2012.00307.x. 2012 July 3. doi: 10.1111/j.1752-699X.2012.00307.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Rosen R, Brown C, Heiman J, Leiblum S, Meston C, Shabsigh R, Ferguson D, D'Agostino R., Jr The Female Sexual Function Index (FSFI): A multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 13.Wiegel M, Meston C, Rosen R. The Female Sexual Function Index (FSFI): Cross-validation and development of clinical cutoff scores. J Sex Marital Ther. 2005;31:1–20. doi: 10.1080/00926230590475206. [DOI] [PubMed] [Google Scholar]
- 14.Derogatis LR, Rosen R, Leiblum S, Burnett A, Heiman J. The Female Sexual Distress Scale (FSDS): Initial validation of a standardized scale for assessment of sexually related personal distress in women. J Sex Marital Ther. 2002;28:317–330. doi: 10.1080/00926230290001448. [DOI] [PubMed] [Google Scholar]
- 15.Oberg K, Fugl-Meyer AR, Fugl-Meyer KS. On categorization and quantification of women's sexual dysfunctions: An epidemiological approach. Int J Impot Res. 2004;16:261–269. doi: 10.1038/sj.ijir.3901151. [DOI] [PubMed] [Google Scholar]
- 16.Petersen CD, Giraldi A, Lundvall L, Kristensen E. Botulinum toxin type A-a novel treatment for provoked vestibulodynia? Results from a randomized, placebo controlled, double blinded study. J Sex Med. 2009;6:2523–2537. doi: 10.1111/j.1743-6109.2009.01378.x. [DOI] [PubMed] [Google Scholar]
- 17.Fugl-Meyer AR, Melin R, Fugl-Meyer KS. Life satisfaction in 18- to 64-year-old Swedes: In relation to gender, age, partner and immigrant status. J Rehabil Med. 2002;34:239–246. doi: 10.1080/165019702760279242. [DOI] [PubMed] [Google Scholar]
- 18.Johns MW. A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 19.Weaver TE, Laizner AM, Evans LK, Maislin G, Chugh DK, Lyon K, Smith PL, Schwartz AR, Redline S, Pack AI, Dinges DF. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20:835–843. [PubMed] [Google Scholar]
- 20.Laumann EO, Nicolosi A, Glasser DB, Paik A, Gingell C, Moreira E, Wang T. Sexual problems among women and men aged 40–80 y: Prevalence and correlates identified in the Global Study of Sexual Attitudes and Behaviors. Int J Impot Res. 2005;17:39–57. doi: 10.1038/sj.ijir.3901250. [DOI] [PubMed] [Google Scholar]
- 21.Hartmann U, Philippsohn S, Heiser K, Ruffer-Hesse C. Low sexual desire in midlife and older women: Personality factors, psychosocial development, present sexuality. Menopause. 2004;11(6 Pt 2):726–740. doi: 10.1097/01.gme.0000143705.42486.33. [DOI] [PubMed] [Google Scholar]
- 22.Colson MH, Lemaire A, Pinton P, Hamidi K, Klein P. Sexual behaviors and mental perception, satisfaction and expectations of sex life in men and women in France. J Sex Med. 2006;3:121–131. doi: 10.1111/j.1743-6109.2005.00166.x. [DOI] [PubMed] [Google Scholar]
- 23.Bancroft J, Loftus J, Long JS. Distress about sex: A national survey of women in heterosexual relationships. Arch Sex Behav. 2003;32:193–208. doi: 10.1023/a:1023420431760. [DOI] [PubMed] [Google Scholar]
- 24.McCabe M, Althof SE, Assalian P, Chevret-Measson M, Leiblum SR, Simonelli C, Wylie K. Psychological and interpersonal dimensions of sexual function and dysfunction. J Sex Med. 2010;7(1 Pt 2):327–336. doi: 10.1111/j.1743-6109.2009.01618.x. [DOI] [PubMed] [Google Scholar]
- 25.Fabre LF, Smith LC. The effect of major depression on sexual function in women. J Sex Med. 2012;9:231–239. doi: 10.1111/j.1743-6109.2011.02445.x. [DOI] [PubMed] [Google Scholar]
- 26.Tagliabue M, Gottero C, Zuffranieri M, Negro M, Carletto S, Picci RL, Tomelini M, Bertaina S, Pucci E, Trento M, Ostacoli L. Sexual function in women with type 1 diabetes matched with a control group: Depressive and psychosocial aspects. J Sex Med. 2011;8:1694–1700. doi: 10.1111/j.1743-6109.2011.02262.x. [DOI] [PubMed] [Google Scholar]
- 27.Lai CH. Major depressive disorder: Gender differences in symptoms, life quality, and sexual function. J Clin Psychopharmacol. 2011;31:39–44. doi: 10.1097/JCP.0b013e318205a670. [DOI] [PubMed] [Google Scholar]
- 28.Schnatz PF, Whitehurst SK, O'Sullivan DM. Sexual dysfunction, depression, and anxiety among patients of an inner-city menopause clinic. J Womens Health (Larchmt) 2010;19:1843–1849. doi: 10.1089/jwh.2009.1800. [DOI] [PubMed] [Google Scholar]
- 29.Nowosielski K, Drosdzol A, Sipinski A, Kowalczyk R, Skrzypulec V. Diabetes mellitus and sexuality—Does it really matter? J Sex Med. 2010;7(2 Pt 1):723–735. doi: 10.1111/j.1743-6109.2009.01561.x. [DOI] [PubMed] [Google Scholar]
- 30.Giraldi A, Kristensen E. Sexual dysfunction in women with diabetes mellitus. J Sex Res. 2010;47:199–211. doi: 10.1080/00224491003632834. [DOI] [PubMed] [Google Scholar]
- 31.Duncan LE, Lewis C, Jenkins P, Pearson TA. Does hypertension and its pharmacotherapy affect the quality of sexual function in women? Am J Hypertens. 2000;13(6 Pt 1):640–647. doi: 10.1016/s0895-7061(99)00288-5. [DOI] [PubMed] [Google Scholar]
- 32.Cavalcante MA, Bombig MT, Luna FB, Carvalho AC, Paola AA, Povoa R. Quality of life of hypertensive patients treated at an outpatient clinic. Arq Bras Cardiol. 2007;89:245–250. doi: 10.1590/s0066-782x2007001600006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 The Female Sexual Function Index.
Appendix S2 The Female Sexual Distress Scale—Revised.
Appendix S3 The LiSat-11 checklist.
Appendix S4 Epworth Sleepiness Scale (ESS).


