Abstract
PURPOSE
Cisplatin or cetuximab combined with radiotherapy (RT) each yield superior survival in locally advanced squamous cell head and neck cancer (LA-SCCHN) compared to RT alone. E3303 evaluated the triple combination.
EXPERIMENTAL DESIGN
Patients with stage IV unresectable LA-SCCHN received a loading dose of cetuximab (400mg/m2) followed by 250mg/m2/week and cisplatin 75mg/m2 q 3 weeks x3 cycles concurrent with standard fractionated RT. In the absence of disease progression or unacceptable toxicity, patients continued maintenance cetuximab for 6–12 months. Primary endpoint was 2-year progression-free survival (PFS). Patient tumor and blood correlates, including tumor human papillomavirus (HPV) status, were evaluated for association with survival.
RESULTS
Sixty-nine patients were enrolled; 60 proved eligible and received protocol treatment. Oropharyngeal (OP) primaries constituted the majority (66.7%), stage T4 48.3% and N2–3 91.7%. Median RT dose delivered was 70 Gy, 71.6% received all 3 cycles of cisplatin and 74.6% received maintenance cetuximab. Median PFS was 19.4 months, 2-year PFS 47% (95%CI: 33–61%). 2-year overall survival (OS) was 66% (95%CI: 53–77%); median OS was not reached. Response rate was 66.7%. Most common grade ≥3 toxicities included mucositis (55%), dysphagia (46%) and neutropenia (26%); one attributable grade 5 toxicity occurred. Only tumor HPV status was significantly associated with survival. HPV was evaluable in 29 tumors; 10 (all OP) were HPV+. HPV+ patients had significantly longer OS and PFS (p=0.004 and p=0.036, respectively).
CONCLUSIONS
Concurrent cetuximab, cisplatin and RT were well-tolerated and yielded promising 2-year PFS and OS in LA-SCCHN with improved survival for patients with HPV+ tumors.
Introduction
Conventional chemoradiation (CRT) for locally or regionally advanced squamous cell head and neck cancer (SCCHN) results in 2-year progression-free survival (PFS) rates of only approximately 35% (1–3). The addition of high dose cisplatin (100 mg/m2) every 3 weeks to definitive radiation therapy (RT) improves long-term survival but at the expense of increased toxicity (4–9). Relapse has historically been predominantly locoregional.
Cetuximab, a monoclonal antibody recognizing the epidermal growth factor receptor (EGFR) extracellular domain, has demonstrated synergy with RT and platinum in SCCHN xenograft models (10–21). Cetuximab with RT improved locoregional control and survival compared to RT alone (22, 23). When this study was undertaken, only one reported phase II study incorporated cetuximab into a concomitant boost head and neck radiation regimen with concurrent cisplatin (24). In 21 patients treated for LA-SCCHN, Pfister et al. reported promising results: 3-year PFS of 56%, 3-year locoregional control rate of 71% and 3-year overall survival (OS) of 76%. However, an unexpected rate of unattributable deaths and grade 4 adverse events led to early closure of this study.
In this study we sought to avoid the possibility of greater toxicity and need for RT interruptions by grafting cetuximab onto once daily RT and a lower dose of cisplatin. To test the feasibility of maintenance cetuximab, we continued this agent post CRT for 6 to 12 months. We chose this study design to provide an estimate of treatment activity in this poor prognosis patient group and to mirror the EXTREME trial for recurrent/metastatic SCCHN (25), which employed cetuximab maintenance therapy and was ongoing at the time this study was undertaken. We measured tumor and blood molecular characteristics hypothesized to impact response and tested associations with response to treatment.
Materials and Methods
Patients and Biological Specimens
Eligibility for this phase II Eastern Cooperative Oncology (ECOG) trial E3303 (NCT00096174 ClinicalTrials.gov) stipulated pathologically confirmed stage IV, unresectable locally advanced SCCHN (LA-SCCHN) (excluding nasopharynx, paranasal sinus, parotid gland). Criteria for unresectable disease are provided in Supplemental Table 1. Eligibility also required ECOG performance status (PS) of 0–1 and adequate hematologic, hepatic and renal function. Exclusion criteria included pre-existing cardiac or respiratory conditions precluding treatment; pregnancy or lactation; prior, unrelated malignancy within 3 years; and any prior treatment with RT, chemotherapy, EGFR-targeting agents or chimerized/murine monoclonal antibody. Tissue and blood collection was not mandatory.
Treatment
Initial Administration Schedule
Supplemental Figure 1 illustrates the study schema. The loading dose of cetuximab was 400 mg/m2 intravenously (IV) over 2 hours on day 1. Beginning day 8, cetuximab 250 mg/m2 IV over 1 hour was administered weekly for 8 weeks. Concurrent RT was initiated day 15, simultaneous with cisplatin 75 mg/m2 IV over 60 minutes every 3 weeks [days 15, 36 and 57]. Routine premedication included a 5-HT antagonist and dexamethasone. Cetuximab was administered prior to concurrent chemotherapy and RT. After response evaluation and prior to cetuximab maintenance therapy, patients achieving a complete response (CR) who presented with N2 or N3 disease were considered for elective neck dissection. Allowed dose modifications are described in Supplemental Materials.
Radiation Therapy
The prescribed dose was 70 Gy (1.8–2 Gy daily for 35 fractions over 7 weeks). A sequential cone-down prescription was used defining 3 separate dose planned target volumes (PTV) (PTV50, PTV60, PTV70). Two-dimensional (2D) or three-dimensional (3D) conformal RT planning was permitted.
For 2D techniques +7% and −5% of the prescription dose point variation in the PTV were permitted. For 3D planning ≤20% of the PTV was to receive >110% of the prescribed dose. No more than 1% of any PTV was to receive <93% of the prescribed dose. No more than 1% or 1cc of the tissue outside of the PTVs was to receive >110% of the prescribed dose. Standard immobilization techniques and chemotherapy (CT) treatment planning were mandated. Normal organs at risk for injury within the treated volume were contoured with standard constraints.
Radiation quality assurance was conducted by the Quality Assurance Review Center (QARC). For intensity modulated radiation therapy (IMRT), the institution was required to have completed QARC benchmarks (www.QARC.org). Two separate reviews were conducted for each patient: a rapid review within 3 days of the RT start to provide feedback and facilitate protocol compliance; and a second review at the completion of RT conducted by the radiation oncology co-chair of the study (HQ).
Maintenance Therapy
After completion of concurrent CRT, weekly cetuximab was continued for a minimum of 6 months and permitted for 12 months in patients with no evidence of disease progression (PD) or untoward toxicity. Protocol therapy was halted for PD, withdrawal of consent, unacceptable toxicity or medical co-morbidity prohibiting further treatment.
Dose Modification
Cetuximab was given weekly without interruption. Cisplatin was withheld if absolute neutrophil count was <1500/mm3, platelets <100,000/mm3, or creatinine >1.5 mg/dl. For renal insufficiency, carboplatin at an area under the curve (AUC) of 5 could be substituted for cisplatin on days 36 and 57. In the event of Grade IV mucositis, RT could be interrupted for 3–5 days until resolution to Grade III. Maximum radiation treatment break could not exceed 7 days.
Study Design
Endpoint Definitions
Response was evaluated using Response Evaluation Criteria in Solid Tumors (RECIST) criteria version 1.1 modified for head and neck cancer (Supplemental Table 2). Specifically, PD was monitored separately for the primary tumor and nodes. PFS, OS and time to locoregional failure were as defined in Supplemental Materials. Toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), Version 3.0.
Endpoints and Sample Size
The primary endpoint was 2-year PFS rate. Secondary endpoints included OS, response rate (RR), and toxicity. Sample size calculation was based on the hypothesis that the addition of cetuximab would increase 2-year PFS rate from 35% to 50%. We posited that if at least 27 of 62 eligible patients were alive and free from progression at two years, the study regimen would warrant further development based on the one-sample 1-sided exact binomial test of 0.35 (null hypothesis) versus 0.50 (target PFS rate). The null hypothesis was based on the observed 2-year disease control rate of 0.35 for the cisplatin plus RT treatment arm of ECOG study E1392 (4, 26). This included type I and II error rates of 0.10 and 0.13, respectively. We projected a 10% ineligibility rate; 68 patients were targeted for accrual.
Tissue Microarray (TMA) Construction
TMAs were constructed from formalin-fixed paraffin-embedded tumor biopsy tissues. 0.6 mm cores were extracted from each tumor block in quadruplicate and arrayed on a recipient paraffin block. Cases received as tissue cores were embedded in a common block.
HPV Status
HPV status was assessed by in situ hybridization (ISH) using an HPV pan-specific DNA probe (Biotinylated Wide Spectrum HPV DNA Probe Cocktail, Dako), recognizing HPV subtypes 6, 11, 16, 18, 31, 33, 35, 45, 51 and 52. Tumors with punctuate nuclear staining were scored HPV positive as previously described (27).
EGFR Gene Amplification
EGFR fluorescence in situ hybridization (FISH) analysis utilized the dual-color EGFR SpectrumOrange/CEP7 SpectrumGreen probe (Vysis). EGFR-FISH positive tumors had >4 gene copies in >40% of cells, >15 gene copies in >10% of cells or a gene:chromosome ratio >2 as previously described (28).
Protein Levels by Immunohistochemical (IHC) Staining
IHC staining was evaluated for cyclin-dependent kinase inhibitor 2A (p16) (G175-405, 1:200, BD PharMingen), EGFR IHC (clone H11 antibody, 1:500, Dako), C-MET (MET) (SC-10,1:150, Santa Cruz Biotechnology), XPF (SPM228, AbCam) or ERCC1 (FL297, Santa Cruz Biotechnology). Cyclin-dependent kinase inhibitor 2A (p16) P16 tumor status was assessed by immunohistochemical (IHC) staining with the monoclonal antibody clone G175-405 (1:200, BD PharMingen, San Diego, CA, USA) and EGFR IHC staining was performed with anti-EGFR antibody clone H11 (1:500, Dako) as previously described (29). Signal amplification was performed using a proprietary micropolymer peroxidase (ImmPRESS™, Vector, Burlingame, CA) conjugated to an anti-mouse antibody. C-MET (MET) tumor levels were assessed by IHC using anti-MET antibody SC-10 (1:150, Santa Cruz Biotechnology, Dallas, TX) followed by incubation with Mach4 Universal HRP-polymer (Biocare Medical, Concord, CA). For p16, EGFR and MET staining immunoreactive cells were visualized with the brown color resulting from incubation with diaminobenzidine (DAB) chromogenic substrate. Sections were counterstained blue with hematoxylin and lithium carbonate to provide morphologic detail.
For XPF and ERCC1 IHC staining, samples were incubated with primary antibody against XPF (SPM228 1:100; AbCam, Cambridge, MA) or ERCC1 (FL297 1:200; Santa Cruz Biotechnology). Primary antibody detection was done using renaissance TSATM (Tyramide Signal Amplification) Biotin System (Perkin Elmer, Bridgeville, PA). Hematoxylin (Vectorlab, Burlingame, CA) was used as nuclear counterstain. Signal intensity found in tumor tissue was scored by a pathologist on an integer scale from 0 (no intensity) to 3+ (strong intensity).
IHC staining intensities were evaluated semi-quantitatively, and an IHC Score was calculated by multiplying the percent tumor stained by the intensity of the staining (integer scale of 0 to +3). IHC scores were averaged for replicate cores to obtain the final IHC score for each tumor. The median IHC Scores for EGFR and MET defined high versus low staining tumors. An IHC score of at least 210 was used to define p16 positive tumors.
Protein Levels by Automated Quantitative Analysis (AQUA)
Tumor protein levels of ERCC1 and XPF were determined using quantitative in situ methods previously described (30). TMA sections were stained by a modified indirect immunofluorescence method. Briefly, sections were deparaffinized in xylene and rehydrated in a graded series of ethanol. The sections were subjected to antigen retrieval by boiling in Tris-EDTA buffer (pH 9.0) for 20 minutes. Endogenous peroxidase activity was blocked by incubating the slides in 3% hydrogen peroxide in methanol for 15 min. After blocking nonspecific reaction with blocking reagent (Background Sniper, Biocare Medical, Cat.# BS966) for 30 min, the sections were incubated overnight with ERCC1 antibody (1:5000, Sigma, Cat.# HPA0297731) or XPF (1:3000, Neomarkers, Cat.# MS-1381-P) and pan-cytokeratin (tumor mask) in antibody diluent (Da Vinci Green, Biocare Medical, PD900) at 4°C overnight. The pan-cytokeratin was probed with an Alexa Fluor 555 labeled secondary antibody (Invitrogen). The primary antibodies were targeted with Envision reagents (DAKO, Carpenteria, CA). Target amplification and visualization was accomplished using a Cy-5-tyramide signal amplification system (TSA, PerkinElmer, Waltham, MA. Cat. AT705A). Prolong Gold mounting medium (P36931; Molecular Probes/Life Technologies, Grand Island, NY) containing 4,6-Diamidino-2-phenylindole (DAPI) was used to stain tissue nuclei.
Automated image capture was performed by PM-2000 (HistoRx, New Haven, CT) using the AQUAsition software. High-resolution monochromatic digital images of the cytokeratin staining visualized with AF555, DAPI and target staining with Cy5 were captured and saved for every tumor histospot on the arrays. Target expression was quantified by calculating Cy5 fluorescent signal intensity. An AQUA score was generated by dividing the sum of target signals within the tumor mask. AQUA scores were normalized to the exposure time and bit depth at which the images were captured, allowing scores collected at different exposure times to be compared directly. Data was analyzed based on the median cut point for nuclear staining of XPF and ERCC1.
Serum Analytes
Custom Searchlight™ (Thermo Scientific) multiplex or singleplex enzyme-linked immunosorbent assays (ELISAs) were used to quantify EGFR, EGF, transforming growth factor-alpha (TGF-α), amphiregulin (AR), epiregulin (EPI), heparin-binding EGF (HB-EGF), hepatocyte growth factor (HGF), IL-6, IL-8 and VEGF serum levels in duplicate. A custom 3-plex assay for EGF, HGF and VEGF, a custom multiplex assay for TGF-α, AR and IL-6 and single-plex assays for EGFR, EPI and HB-EGF were used according to the manufacturer’s instructions. Samples with undetectable analyte were defined as having a level of one-half the limit of assay detection for statistical analyses.
Endpoint Definitions
PFS was defined as time from registration to first documentation of PD or to death without PD. If date of death was >3 months after date of last disease assessment, the patient was censored at the time of last disease assessment. Patients without documented progression were censored at the time they were last known to be free of progression. OS was defined as time from registration to death from any cause. Patients who were alive at the time of analysis were censored at the date last known alive. Time to locoregional failure was defined as the time from study registration to loco (L), regional (R), or loco-regional (L/R) disease progression, censored at the date of last disease assessment for those who did not have L, R, or L/R disease progression.
Analysis Method
Exact binomial 90% confidence intervals (CI) were computed for the objective RR (complete response (CR) plus partial response rate). PFS and OS time-to-event distributions were estimated by the Kaplan-Meier Method and compared using log rank tests. For continuous variables, two classification groups were defined by the median biomarker level except for p16, which used 210 as the cutoff point. Univariate and multivariable Cox proportional hazards regression models evaluated marker effect on time-to-event distributions. Multivariable models were adjusted for age, sex, race, ECOG PS, weight loss within 6 months prior to enrollment, tumor site and smoking history. Associations between markers were evaluated using Fisher’s exact tests. Eligible patients who started protocol treatment and provided written consent to laboratory studies were included in the marker analysis. No multiple comparisons adjustment was made due to the exploratory nature of the marker analysis. P-values were two-sided and considered statistically significant if <0.05.
Results
Patient and Disease Characteristics
Sixty-nine patients were accrued between December 2004 and July 2006. Among those who started treatment, 6 were deemed ineligible. Three patients who never started protocol treatment were also ineligible. All outcome analyses were based on 60 eligible and treated patients, with the exception of toxicity analysis, which included all 66 treated patients.
As shown in Table 1, eligible and treated patients were mostly male with a smoking history. Oropharynx was the predominant primary site, and most patients had N2 or N3 disease. All patients but one had tumors with squamous cell carcinoma histology; one laryngeal tumor had non-small cell carcinoma histology, which in the absence of a pulmonary primary met inclusion criteria.
Table 1.
Clinical Characteristics (N=60)
| Age (years) | |
| Median (Range) | 54.8 (42.0–78.5) |
| Sex, N (%) | |
| Male | 51 (85%) |
| Female | 9 (15%) |
| Ethnicity, N (%) | |
| White | 47 (78%) |
| Black | 12 (20%) |
| Asian | 1 (2%) |
| Performance Status, N (%) | |
| 0 | 26 (43%) |
| 1 | 34 (57%) |
| Weight Loss (prior 6 months), N (%) | |
| < 5% | 35 (59%) |
| 5–10% | 11 (18%) |
| 10–20% | 8 (13%) |
| ≥ 20% | 6 (10%) |
| Smoking Status, N (%) | |
| Never | 15 (25%) |
| Former | 30 (50%) |
| Current | 15 (25%) |
| Histology, N (%) | |
| Non-small cell carcinoma | 1 (2%) |
| Squamous cell | 59 (98%) |
| Tumor Site, N (%) | |
| Oral Cavity | 3 (5%) |
| Oropharynx | 40 (67%) |
| Hypopharynx | 6 (10%) |
| Larynx | 9 (15%) |
| Other | 2 (3%) |
| T Stage, N (%) | |
| T1 | 2 (3%) |
| T2 | 16 (27%) |
| T3 | 13 (22%) |
| T4 | 29 (48%) |
| N Stage, N (%) | |
| N0 | 2 (3%) |
| N1 | 3 (5%) |
| N2 | 46 (77%) |
| N3 | 9 (15%) |
| Disease Stage, N (%) | |
| IV A (AJCC 5th edition) | 6 (10%) |
| IV A (AJCC 6th edition) | 42 (70%) |
| IV B (AJCC 6th edition) | 12 (20%) |
| Prior Surgery, N (%) | |
| No | 42 (70%) |
| Yes | 18 (30%) |
Treatment Administration
The median administered RT dose was 70 Gy; five patients (8%) received <50 Gy. Two patients received total doses of 50 and 52 Gy, respectively; 53 patients (88%) received 66–74 Gy. 56 patients (93%) received cisplatin during concurrent treatment. Carboplatin was substituted for cisplatin on days 36 and/or 57 because of toxicity in 3 patients (5%). One patient was removed from protocol prior to the first dose of cisplatin because of PD during the cetuximab run-in. Among all 60 eligible and treated patients, the majority received all 3 scheduled doses of platinum (Table 2). Fifty-four patients (90%) were able to complete the first 9 weeks of cetuximab per protocol. Of 59 eligible and treated patients with maintenance cetuximab data, 44 (75%) received maintenance therapy. Median maintenance duration was 5.5 months. 23 (39%) received at least 6 months of maintenance; 8 (14%) received a full year of maintenance therapy.
Table 2.
Treatments and Outcomes (N=60)
| Treatment Completed, N (%) | |
| Yes | 7 (12%) |
| No | 53 (88%) |
| Reason Therapy Discontinued | |
| Disease progression | 9 (15%) |
| Toxicity | 15 (25%) |
| Death on study | 6 (10%) |
| Patient withdrawal/refusal | 15 (25%) |
| Other | 8 (13%) |
| Cycles Cisplatin Received, N (%)a | |
| 0 | 1 (1%) |
| 1 | 3 (5%) |
| 2 | 10 (17%) |
| 3 | 46 (77%) |
| Maintenance Cetuximab Received, N (%)b | |
| Yes | 44 (75%) |
| No | 15 (25%) |
| Progression-Free Survival | |
| median months (95% CI) | 19.4 (14.4-∞) |
| 1-year (95% CI) | 73% (59–83%) |
| 2-year (95% CI) | 47% (33–61%) |
| 5-year (95% CI) | 41% (27–55%) |
| Response, N(%) | |
| Complete Response | 23 (39%) |
| Partial Response | 17 (28%) |
| Stable Disease | 11 (18%) |
| Progressive Disease | 3 (5%) |
| Unevaluable | 6 (10%) |
| Sites of Disease Progression, N (%) | |
| No Disease Progression | 29 (48%) |
| Local | 1 (2%) |
| Regional | 4 (7%) |
| Distant | 14 (23%) |
| Local-Regional | 2 (3%) |
| Local/Regional/Local-Regional & Distant | 4 (7%) |
| Unevaluable | 6 (10%) |
| Overall Survival | |
| Median months (95% CI) | _ c |
| 1-year (95% CI) | 78% (65–87%) |
| 2-year (95% CI) | 66% (53–77%) |
| 5-year (95% CI) | 54% (41–66%) |
Carboplatin could be substituted in lieu of cisplatin.
No maintenance information available for 1 patient.
Median OS not reached.
Toxicities
Table 2 summarizes the disposition of all 60 eligible and treated patients. Nine (15%) stopped treatment because of PD with a median time to progression of 6.9 months. Thirty patients (50%) stopped therapy for either toxicity (25%) or withdrawal of consent (25%); eight patients stopped during cetuximab maintenance therapy, most commonly because of cutaneous toxicity.
Grade 2–4 treatment-attributable toxicities for 66 treated patients are summarized in Table 3. A single grade 5 event was attributed to neutropenic fever and pulmonary infection. A second patient experienced sudden death in the absence of obvious toxicity; this was not attributed to treatment. Four additional treatment-unrelated deaths were attributed to the following causes: systemic deterioration with multi-organ failure, death cause not specified, cardiac ischemia, and disease progression. Seventeen patients (26%) sustained grade 4 worst toxicity, most commonly neutropenia. Forty-six patients (70%) had at least one grade 3 non-hematologic toxicity; the most common grade 3 toxicities included mucositis, dysphagia, and anorexia.
Table 3.
Relevant Toxicities Observed Among Enrolled Subjects
| Toxicitya N (%) | Grade | ||
|---|---|---|---|
| 2 | 3 | 4 | |
| Anemia | 21 (32%) | 3 (5%) | 1 (1.5%) |
| Neutropenia | 16 (24%) | 11 (17%) | 6 (9%) |
| Thrombocytopenia | 1 (1.5%) | - | - |
| Hypoalbuminemia | 18 (27%) | 3 (5%) | - |
| Hypomagnesemia | 10 (15%) | 4 (6%) | - |
| Renal (creatinine) | - | 1 (1.5%) | - |
| Hypersensitivity Reaction | 1 (1.5%) | - | - |
| Tinnitus | 5 (7.5%) | - | - |
| Fatigue | 29 (44%) | 13 (20%) | 2 (3%) |
| Xeroderma | 14 (21%) | 1 (1.5%) | - |
| Acneiform rash | 27 (41%) | 17 (26%) | - |
| Dehydration | 9 (14%) | 13 (20%) | - |
| Anorexia | 15 (23%) | 22 (33%) | 1 (1.5%) |
| Dysphagia | 16 (24%) | 29 (44%) | 1 (1.5%) |
| Xerostomia | 14 (21%) | 11 (17%) | - |
| Mucositis | 19 (29%) | 34 (52%) | 2 (3%) |
| Nausea + Vomiting | 20 (30%) | 14 (21%) | - |
| Neurosensory | 2 (3%) | - | - |
One grade 5 treatment-related event occurred as a result of infection.
Clinical Outcomes
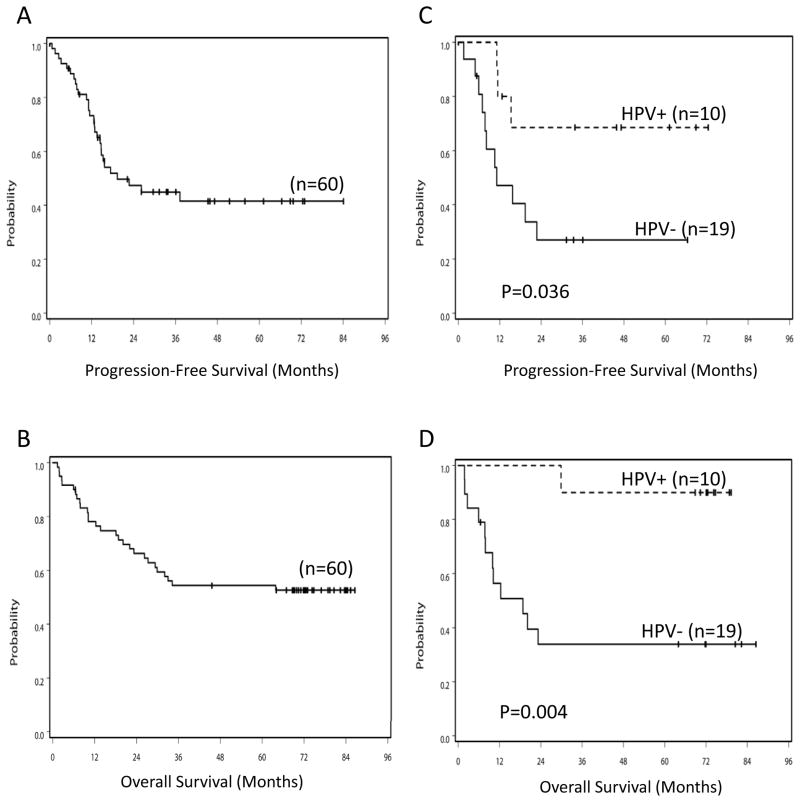
Among 60 eligible and treated patients, 54 were evaluable. Of these, 67% (95% CI 55–77%) experienced an objective response (Table 2). Three patients (5%) experienced PD as the best overall response. Six patients (10%) were unevaluable: 4 did not have a follow-up measurement and 2 were evaluated using an alternative method. Sixteen patients underwent neck dissection: 15 had N2 and 1 had N3 stage disease at diagnosis. Pathologic nodal involvement was detected in 5 of 15 patients who presented initially with N2 disease; the patient with N3 disease at presentation had a negative neck dissection. Figure 1A displays the Kaplan-Meier estimate of PFS. Median PFS for all 60 analyzable patients was 19.4 months (95%CI 14.4-∞).
Figure 1.
Progression-free survival (PFS) and overall survival (OS) Kaplan Meier plots for all eligible and treated subjects (A and B, respectively) and by tumor HPV status (C and D, respectively). Log rank p values comparing survival for patients with HPV+ versus HPV− tumors are provided.
We posited that if at least 27 of 62 eligible patients were alive and free from progression at two years, the study regimen would warrant further development. Among 60 eligible and treated patients, 39 patients were alive at 2 years after registration. For those with follow-up evaluations at 2 years, 28 patients were progression-free (20 with documented clinical evidence and 8 with undocumented clinical evidence). Thus, the total number of patients alive and progression free at 2 years met the minimum criterion of 27. The two-year PFS rate was 47% (95%CI 33–61%). PFS rate details are provided in Table 2. This study was designed using a 1-sided type I error rate of 10% for 2-year PFS rate, this error rate corresponded to an 80% CI of 2-year PFS rate of 38–56% with a two-sided test. Thus, the null hypothesis of 35% 2-year PFS was rejected. Although the study was not powered to detect such differences, neither sex nor race had any effect on PFS. Of 60 analyzable patients, 39 (65%) were still alive ≥2 years after registration.
Twenty-five patients (42%) experienced PD. In 14 (23%) distant relapse was the first event, while 4 others (7%) experienced distant progression synchronously with local or local-regional progression. Table 2 lists the pattern and frequency of progression sites for all evaluable patients. We performed a post hoc analysis to evaluate locoregional failure (Supplemental Figure 2A). Two-year locoregional control rate was 72% (95%CI 54–83%). The predominant site of progression was lung; in nine this was the only site. Four patients have reported a second primary cancer (1 prostate, 3 non-melanomatous skin cancer).
Of the 60 eligible and treated patients, 31 (52%) were alive with a median follow-up time of 72.9 months (range 45.5–86.5 months) (Figure 1B and Table 2). Median OS has not yet been observed. Men had better OS than women (median OS not observed vs. 10.0 months [95%CI 1.9-∞], p=0.027). Whites had superior OS compared to African-Americans (median OS not observed vs. 15.1 months [95%CI 2.6-∞], p=0.029). We did not collect data regarding whether patients subsequently underwent salvage surgery.
Molecular Correlate Analyses
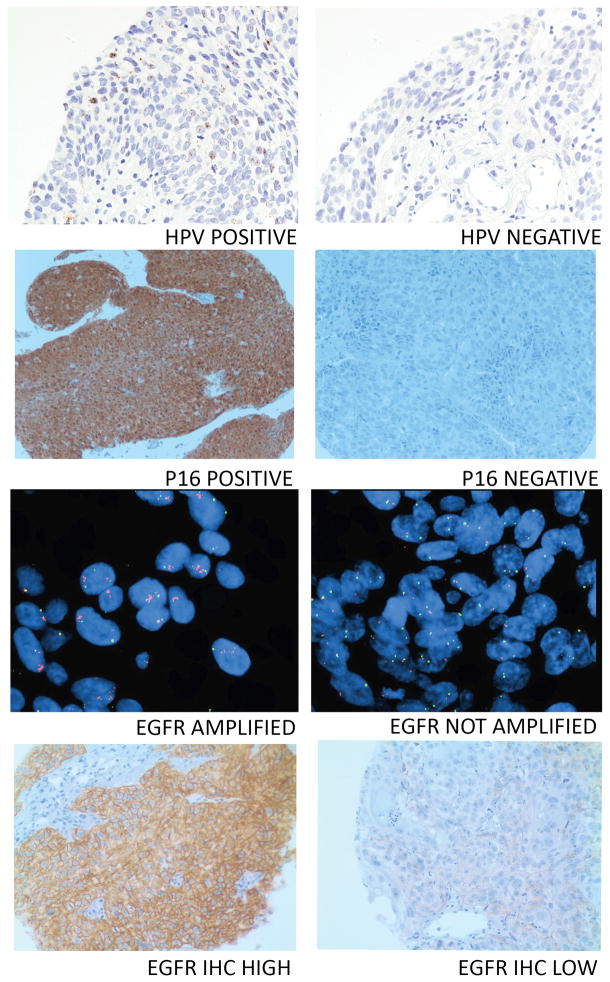
We selected molecular correlates for analysis based on clinical and/or preclinical data supporting their role as either prognostic or predictive indicators (31–36). Baseline tissue and blood specimens of adequate quantity and quality for analysis were collected for 32 and 27 eligible patients, respectively. HPV and p16 status were determined for 29 and 30 tumors, respectively. Of the 29 tumors assessed for HPV status, 17 were OP; all 10 HPV+ tumors arose in the OP in males. In univariate analysis, HPV+ status was associated with significantly longer PFS (Table 4 and Figure 1C) and significantly longer OS (Table 4 and Figure 1D). Time to locoregional failure did not differ significantly for HPV+ and HPV− disease (p=0.30) (Supplemental Figure 2B). Representative tumor sections positive or negative for HPV ISH are provided in Figure 2. Tumor p16 status was not significantly associated with PFS or OS although HPV and P16 status were 79% concordant and significantly associated (tau b=0.53, p=0.005). Representative tumor sections positive or negative for p16 are provided in Figure 2. EGFR gene amplification status and EGFR protein levels were evaluated in 26 and 31 tumors, respectively (Figure 2). Lower tumor EGFR protein levels were significantly associated with improved PFS but not OS (Table 4). Neither tumor HPV status nor tumor EGFR level remained predictive of PFS in multivariable models. HPV status remained a significant predictor of OS in the multivariable model (Table 4).
Table 4.
Hazard Ratio (low vs. high or positive vs. negative) in Univariate and Multivariable Models
| Model | Predictor(s)d | N | Progression-Free Survival | Overall Survival | ||||
|---|---|---|---|---|---|---|---|---|
| HRa | (95% CI) | P | HRa | (95% CI) | P | |||
| Univariate | ||||||||
| Tumor HPV by ISH | 29 | 0.28 | (0.08–1.00) | 0.05 | 0.09 | (0.01–0.71) | 0.02 | |
| Tumor P16 by IHC | 30 | 1.71 | (0.55–5.3) | 0.36 | 2.2 | (0.61–7.93) | 0.23 | |
| Tumor EGFR by IHC | 31 | 0.27 | (0.09–0.86) | 0.03 | 0.72 | (0.26–2.03) | 0.53 | |
| Tumor EGFR copy number by ISH | 26 | 1.31 | (0.45–3.82) | 0.62 | 1.45 | (0.47–4.50) | 0.52 | |
| Tumor Met by IHC | 30 | 0.64 | (0.24–1.73) | 0.38 | 0.71 | (0.25–2.06) | 0.53 | |
| Tumor ERCC1 by AQUA | 32 | 0.55 | (0.17–1.80) | 0.32 | 0.87 | (0.30–2.79) | 0.87 | |
| Tumor ERCC1 by IHC | 27 | 0.61 | (0.22–1.69) | 0.34 | 0.61 | (0.22–1.73) | 0.36 | |
| Tumor XPF by AQUA | 31 | 0.69 | (0.26–1.84) | 0.45 | 0.41 | (0.14–1.21) | 0.11 | |
| Tumor XPF by IHC | 25 | 0.52 | (0.16–1.64) | 0.26 | 0.99 | (0.32–3.08) | 0.99 | |
| Plasma EGFR | 27 | 0.35 | (0.09–1.33) | 0.12 | 0.79 | (0.22–2.79) | 0.71 | |
| Plasma Amphiregulin | 27 | 0.46 | (0.14–1.50) | 0.19 | 0.45 | (0.13–1.59) | 0.22 | |
| Plasma TGF-alpha | 27 | 0.62 | (0.19–2.04) | 0.43 | 0.57 | (0.16–2.01) | 0.38 | |
| Plasma EGF | 27 | 0.35 | (0.09–1.31) | 0.12 | 0.66 | (0.19–2.34) | 0.52 | |
| Plasma Epiregulinb | 27 | 1.89 | (0.24–14.78) | 0.54 | - | - | - | |
| Plasma HGF | 27 | 0.81 | (0.25–2.68) | 0.73 | 0.33 | (0.09–1.29) | 0.11 | |
| Plasma IL-8 | 27 | 0.99 | (0.30–3.27) | 0.99 | 1.13 | (0.33–3.92) | 0.84 | |
| Plasma IL-6 | 27 | 0.67 | (0.20–2.19) | 0.50 | 0.34 | (0.09–1.32) | 0.12 | |
| Plasma VEGF | 27 | 1.02 | (0.31–3.34) | 0.98 | 0.99 | (0.29–3.41) | 0.98 | |
| Multivariablec | ||||||||
| Tumor HPV Status | 29 | 0.31 | (0.06–1.43) | 0.11 | 0.06 | (0.01–0.80) | 0.03 | |
Low-expression level vs. high-expression level or positive status vs. negative status, depending on the marker.
No statistic was reported for OS because no death event was observed in the high expression group.
Multivariable model adjusted for age, sex, race, performance status, weight loss prior 6 months (<5% vs. ≥5%), tumor primary site (oropharynx vs. non-oropharynx), smoking history (ever vs. never).
Immunohistochemical staining (IHC), in situ hybridization (ISH).
Figure 2.
Representative tumor cores from tumors with HPV+ or HPV− by ISH, P16 positive or negative by IHC, EGFR gene amplification positive or negative and EGFR high or low by IHC.
Tumor levels of ERCC1 and XPF by AQUA™ (Supplemental Figure 3) and IHC (Supplemental Figure 4), and MET by IHC (Supplemental Figure 4), were not independently associated with PFS or OS in this small sample (Table 4). Different antibodies were used for the ERCC1 analysis by AQUA and IHC with previously described different specificities (37, 38). The agreement between AQUA and IHC staining results for ERCC1 were modest and not significantly correlated (Spearman’s rho=0.37, p=0.07); XPF analyses results by AQUA and IHC were significantly but modestly correlated (Spearman’s rho=0.44, p=0.03).
We successfully measured blood analytes in baseline blood samples from 27 eligible and treated patients except for HB-EGF, which was not detected in any sample tested (Supplemental Table 3). None of the measured blood analytes were significantly associated with PFS or OS (Table 4).
We tested tumor and blood markers for differences by tumor HPV status. HPV+ tumors were more frequently p16 high (p=0.01) while high tumor MET levels by IHC and high tumor XPF levels by AQUA were both independently associated with HPV− tumor status (p=0.04 and p=0.01, respectively). No other tumor marker and no analyzed blood marker differed by tumor HPV status (all p>0.5). We estimated that we had at least 80% power with one-sided alpha of 0.05 to detect a HR (favorable versus unfavorable biomarker status) of 0.19 or lower with 30 samples available for molecular correlate analyses.
Discussion
This study (E3303) demonstrated cetuximab, RT and reduced dose cisplatin treatment was feasible with acceptable toxicity in fit patients with LA-SCCHN. The study met its primary endpoint, rejecting the null hypothesis with 80% confidence: 2-year PFS was 47%. In addition, our results demonstrated that 6 months of cetuximab maintenance therapy was feasible in 50% of patients.
Though caution is warranted when comparing results across trials, these results compare favorably to the cetuximab plus RT arm of the Phase III randomized trial by Bonner et al., which reported a 5-year OS rate of 45.6% for those 211 patients with stage III/IV LA-SCCHN (23). The ECOG 1392 and Southwest Oncology Group (SWOG) 9059 Intergroup phase III study INT 0126 arm with planned concurrent cisplatin at 100 mg/m2 and 70 Gy RT yielded a complete response (CR) rate of 40.2% and reported 3-year projected OS rate of 37% in 87 analyzable patients with LA-SCCHN (4); all but 2 patients had stage IV disease and 60% had OP primaries, similar to the current trial (67%). Because HPV+ SCCHN is generally associated with improved prognosis compared to HPV− SCCHN and OP cancers are enriched for HPV+ cancers, the paucity of HPV tumor status data further limits direct comparisons. Nonetheless, the INT 0126 trial results provide some context for this trial. Though the CR rate in the INT-0126 study arm was superior to the CR rate observed in E3303, the OS rate of E3303 compares favorably with the Intergroup study. Disease eligibility in E3303 was restricted to stage IV disease but otherwise identical to INT 0126. Unique toxicities in E3303 included acneiform rash (expected with cetuximab) and a possible increase in mucositis; neither proved dose-limiting. The incidence of nephrotoxicity, anemia, and neutropenia was lower in E3303 compared to the Intergroup study, likely due to modification of the cisplatin dose. Eligibility criteria were nearly identical and the proportion of stage IV disease and OP cancer were similar in the two studies. To date, cisplatin at a dose of 75 mg/m2 has not been formally compared to 100 mg/m2 in the context of CRT. Stage migration and other factors, such as the increasing incidence of HPV expression in OP cancer, likely compromise historic comparisons (39–42).
The RTOG recently completed a 940 patient, prospective, randomized, phase III trial [0522] comparing CRT with concomitant boost RT to identical CRT plus cetuximab with two doses of cisplatin at 100 mg/m2 every three weeks during RT (43). Maintenance cetuximab was not included. With a median follow-up of 2.4 years for surviving patients, the 2-year OS rate for the RTOG 0522 arm combining cetuximab with CRT was reported to be 83% and did not differ significantly from the comparator arm (44). P16 status was determined for 51% of 628 RTOG 0522 OP tumors; with limited reported follow up thus far, cetuximab was determined to provide no benefit for patients with either p16 positive or negative OP SCCHN (45).
In our trial, the incidence of distant relapse as first site of progression eclipsed local-regional recurrence. This finding may reflect modern imaging techniques for patient follow-up, be related to improved local control when cetuximab is added to CRT or reflect the high percentage of patients with N3 disease. The frequency of distant recurrences highlights the need for more effective systemic strategies..
HPV DNA was detected in 59% of the OP tumors in the current study; tumors tested from other sites were HPV−. Though our findings were limited by the small number of specimens, consistent with prior studies evaluating different treatments and SCCHN patient populations, individuals with HPV+ tumors had improved survival compared to patients with HPV− tumors (46). HPV status out-performed p16 tumor status as a prognostic marker in this study. This is likely in part a result of p16 being an imperfect surrogate for HPV status (47). In addition, we employed our previously reported definition for p16 positivity (27), which sets a comparatively rigorous threshold for p16 positivity (48). No other molecular correlate analyzed was significantly associated with either PFS or OS in multivariable models. Tumor MET and XPF levels were significantly higher in HPV− tumors compared to HPV+ tumors, suggesting MET and/or XPF may contribute to HPV− SCCHN.
In conclusion, though we cannot ascribe apparent improved survival to the addition of cetuximab therapy, the addition of cisplatin and cetuximab to once daily RT appears to be well tolerated and therapeutically promising. Further studies will be necessary to identify biomarkers of response in addition to tumor HPV status.
Supplementary Material
Statement of Translational Relevance.
The survival for patients with unresectable stage IV head and neck squamous cell carcinoma is poor. This reports the results of the first phase II trial combining the epidermal growth factor receptor (EGFR)-targeted therapy, cetuximab, with platinum and radiation therapy followed by maintenance cetuximab therapy for patients with unresectable stage IV locally advanced head and neck squamous cell carcinoma (LA-SCCHN). Sixty eligible and treated patients with LA-SCCHN were evaluated in this cooperative group study; the treatment was generally well tolerated and associated with encouraging progression-free and overall survival rates. Tumor molecular characteristics and blood analyte levels were evaluated for association with survival using Cox proportional hazards models. Of the assessed molecular markers, only tumor human papillomavirus (HPV) positivity was associated with significantly improved survival in multivariable models. Elevated tumor levels of c-MET and XPF were observed among HPV− tumors compared to HPV+ tumors, suggesting possible contributors to HPV− disease.
Acknowledgments
Funding Acknowledgment: This study was conducted by the Eastern Cooperative Oncology Group (Robert L. Comis, M.D., Chair) and supported in part by Public Health Service Grants CA23318, CA66636, CA21115, CA27525, CA39229, CA15488, CA16116 and CA137140 and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services.
Research funding was received by A. Argiris (Bristol-Myers Squibb (BMS)) and J.R. Grandis (BMS, Novartis and OSI Pharmaceuticals). Compensated consultant or advisory roles were filled by C. Langer (BMS and Lilly/ImClone), R. Mehra (BMS and Novartis) and B. Burtness (BMS, Lilly/ImClone and Genmab). Honoraria were received by R. Mehra (Pfizer), and R. Mehra has an immediate family member employed by Glaxo Smith Kline.
Footnotes
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
This study has been presented in part at the following meeting: American Society of Clinical Oncology 2010
The authors have the following potential conflicts of interest to declare:
References
- 1.Vokes E, Weichselbaum RR, Lippman SM, Hong WK. Head and neck cancer. NEJM. 1993;328:184–94. doi: 10.1056/NEJM199301213280306. [DOI] [PubMed] [Google Scholar]
- 2.Adelstein DJ, Tan EH, Lavertu P. Treatment of head and neck cancer: the role of chemotherapy. Crit Rev Oncol Hematol. 1996;24:97–116. doi: 10.1016/1040-8428(96)00215-6. [DOI] [PubMed] [Google Scholar]
- 3.Haddad RI, Shin DM. Recent advances in head and neck cancer. N Engl J Med. 2008;359:1143–54. doi: 10.1056/NEJMra0707975. [DOI] [PubMed] [Google Scholar]
- 4.Adelstein DJ, Li Y, Adams GL, Wagner H, Jr, Kish JA, Ensley JF, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003;21:92–8. doi: 10.1200/JCO.2003.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Adelstein DJ, Saxton JP, Lavertu P, Tuason L, Wood BG, Wanamaker JR, et al. A phase III randomized trial comparing concurrent chemotherapy and radiotherapy with radiotherapy alone in resectable stage III and IV squamous cell head and neck cancer: preliminary results. Head Neck. 1997;19:567–75. doi: 10.1002/(sici)1097-0347(199710)19:7<567::aid-hed2>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 6.Wendt TG, Grabenbauer GG, Rodel CM, Thiel HJ, Aydin H, Rohloff R, et al. Simultaneous radiochemotherapy versus radiotherapy alone in advanced head and neck cancer: a randomized multicenter study. J Clin Oncol. 1998;16:1318–24. doi: 10.1200/JCO.1998.16.4.1318. [DOI] [PubMed] [Google Scholar]
- 7.Brizel DM, Albers ME, Fisher SR, Scher RL, Richtsmeier WJ, Hars V, et al. Hyperfractionated irradiation with or without concurrent chemotherapy for locally advanced head and neck cancer. N Engl J Med. 1998;338:1798–804. doi: 10.1056/NEJM199806183382503. [DOI] [PubMed] [Google Scholar]
- 8.Calais G, Alfonsi M, Bardet E, Sire C, Germain T, Bergerot P, et al. Randomized trial of radiation therapy versus concomitant chemotherapy and radiation therapy for advanced-stage oropharynx carcinoma. J Natl Cancer Inst. 1999;91:2081–6. doi: 10.1093/jnci/91.24.2081. [DOI] [PubMed] [Google Scholar]
- 9.Pignon JP, Bourhis J, Domenge C, Designe L. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet. 2000;355:949–55. [PubMed] [Google Scholar]
- 10.Mendelsohn J, Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol. 2003;21:2787–99. doi: 10.1200/JCO.2003.01.504. [DOI] [PubMed] [Google Scholar]
- 11.Rubin Grandis J, Melhem MF, Gooding WE, Day R, Holst VA, Wagener MM, et al. Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst. 1998;90:824–32. doi: 10.1093/jnci/90.11.824. [DOI] [PubMed] [Google Scholar]
- 12.Ang KK, Andratschke NH, Milas L. Epidermal growth factor receptor and response of head-and-neck carcinoma to therapy. Int J Radiat Oncol Biol Phys. 2004;58:959–65. doi: 10.1016/j.ijrobp.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Eriksen JG, Steiniche T, Askaa J, Alsner J, Overgaard J. The prognostic value of epidermal growth factor receptor is related to tumor differentiation and the overall treatment time of radiotherapy in squamous cell carcinomas of the head and neck. Int J Radiat Oncol Biol Phys. 2004;58:561–6. doi: 10.1016/j.ijrobp.2003.09.043. [DOI] [PubMed] [Google Scholar]
- 14.Ang KK, Berkey BA, Tu X, Zhang HZ, Katz R, Hammond EH, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Research. 2002;62:7350–6. [PubMed] [Google Scholar]
- 15.Liang K, Ang KK, Milas L, Hunter N, Fan Z. The epidermal growth factor receptor mediates radioresistance. Int J Radiat Oncol Biol Phys. 2003;57:246–54. doi: 10.1016/s0360-3016(03)00511-x. [DOI] [PubMed] [Google Scholar]
- 16.Fan Z, Lu Y, Wu X, Mendelsohn J. Antibody-induced epidermal growth factor receptor dimerization mediates inhibition of autocrine proliferation of A431 squamous carcinoma cells. J Biol Chem. 1994;269:27595–602. [PubMed] [Google Scholar]
- 17.Bonner JA, Maihle NJ, Folven BR, Christianson TJ, Spain K. The interaction of epidermal growth factor and radiation in human head and neck squamous cell carcinoma cell lines with vastly different radiosensitivities. Int J Radiat Oncol Biol Phys. 1994;29:243–7. doi: 10.1016/0360-3016(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 18.Saleh MN, Raisch KP, Stackhouse MA, Grizzle WE, Bonner JA, Mayo MS, et al. Combined modality therapy of A431 human epidermoid cancer using anti-EGFr antibody C225 and radiation. Cancer Biother Radiopharm. 1999;14:451–63. doi: 10.1089/cbr.1999.14.451. [DOI] [PubMed] [Google Scholar]
- 19.Huang SM, Bock JM, Harari PM. Epidermal growth factor receptor blockade with C225 modulates proliferation, apoptosis, and radiosensitivity in squamous cell carcinomas of the head and neck. Cancer Res. 1999;59:1935–40. [PubMed] [Google Scholar]
- 20.Huang SM, Harari PM. Modulation of radiation response after epidermal growth factor receptor blockade in squamous cell carcinomas: inhibition of damage repair, cell cycle kinetics, and tumor angiogenesis. Clin Cancer Res. 2000;6:2166–74. [PubMed] [Google Scholar]
- 21.Milas L, Mason K, Hunter N, Petersen S, Yamakawa M, Ang K, et al. In vivo enhancement of tumor radioresponse by C225 antiepidermal growth factor receptor antibody. Clin Cancer Res. 2000;6:701–8. [PubMed] [Google Scholar]
- 22.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–78. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 23.Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11:21–8. doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 24.Pfister DG, Su YB, Kraus DH, Wolden SL, Lis E, Aliff TB, et al. Concurrent cetuximab, cisplatin, and concomitant boost radiotherapy for locoregionally advanced, squamous cell head and neck cancer: a pilot phase II study of a new combined-modality paradigm. J Clin Oncol. 2006;24:1072–8. doi: 10.1200/JCO.2004.00.1792. [DOI] [PubMed] [Google Scholar]
- 25.Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. The New England journal of medicine. 2008;359:1116–27. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Gates MG. Phase III comparison of standard radiotherapy, versus radiotherapy plus simultaneous cisplatin, versus split-course radiotherapy plus simultaneous cisplatin and 5-fluorouracil, in patients with unresectable squamous cell carcinoma of the head and neck 2001. Report No.: 1041E [Google Scholar]
- 27.Argiris A, Heron DE, Smith RP, Kim S, Gibson MK, Lai SY, et al. Induction docetaxel, cisplatin, and cetuximab followed by concurrent radiotherapy, cisplatin, and cetuximab and maintenance cetuximab in patients with locally advanced head and neck cancer. J Clin Oncol. 2010;28:5294–300. doi: 10.1200/JCO.2010.30.6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung CH, Ely K, McGavran L, Varella-Garcia M, Parker J, Parker N, et al. Increased epidermal growth factor receptor gene copy number is associated with poor prognosis in head and neck squamous cell carcinomas. J Clin Oncol. 2006;24:4170–6. doi: 10.1200/JCO.2006.07.2587. [DOI] [PubMed] [Google Scholar]
- 29.Wheeler S, Siwak DR, Chai R, LaValle C, Seethala RR, Wang L, et al. Tumor epidermal growth factor receptor and EGFR PY1068 are independent prognostic indicators for head and neck squamous cell carcinoma. Clin Cancer Res. 2012;18:2278–89. doi: 10.1158/1078-0432.CCR-11-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Psyrri A, Yu Z, Weinberger PM, Sasaki C, Haffty B, Camp R, et al. Quantitative determination of nuclear and cytoplasmic epidermal growth factor receptor expression in oropharyngeal squamous cell cancer by using automated quantitative analysis. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11:5856–62. doi: 10.1158/1078-0432.CCR-05-0420. [DOI] [PubMed] [Google Scholar]
- 31.Jun HJ, Acquaviva J, Chi D, Lessard J, Zhu H, Woolfenden S, et al. Acquired MET expression confers resistance to EGFR inhibition in a mouse model of glioblastoma multiforme. Oncogene. 2012;31:3039–50. doi: 10.1038/onc.2011.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olaussen KA, Dunant A, Fouret P, Brambilla E, Andre F, Haddad V, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355:983–91. doi: 10.1056/NEJMoa060570. [DOI] [PubMed] [Google Scholar]
- 33.Jacobs B, De Roock W, Piessevaux H, Van Oirbeek R, Biesmans B, De Schutter J, et al. Amphiregulin and epiregulin mRNA expression in primary tumors predicts outcome in metastatic colorectal cancer treated with cetuximab. J Clin Oncol. 2009;27:5068–74. doi: 10.1200/JCO.2008.21.3744. [DOI] [PubMed] [Google Scholar]
- 34.Khambata-Ford S, Garrett CR, Meropol NJ, Basik M, Harbison CT, Wu S, et al. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol. 2007;25:3230–7. doi: 10.1200/JCO.2006.10.5437. [DOI] [PubMed] [Google Scholar]
- 35.Xie Q, Bradley R, Kang L, Koeman J, Ascierto ML, Worschech A, et al. Hepatocyte growth factor (HGF) autocrine activation predicts sensitivity to MET inhibition in glioblastoma. Proc Natl Acad Sci U S A. 2012;109:570–5. doi: 10.1073/pnas.1119059109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Argiris A, Lee SC, Feinstein T, Thomas S, Branstetter BFt, Seethala R, et al. Serum biomarkers as potential predictors of antitumor activity of cetuximab-containing therapy for locally advanced head and neck cancer. Oral Oncol. 2011;47:961–6. doi: 10.1016/j.oraloncology.2011.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhagwat NR, Roginskaya VY, Acquafondata MB, Dhir R, Wood RD, Niedernhofer LJ. Immunodetection of DNA repair endonuclease ERCC1-XPF in human tissue. Cancer Res. 2009;69:6831–8. doi: 10.1158/0008-5472.CAN-09-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehra R, Zhu F, Yang DH, Cai KQ, Weaver J, Singh MK, et al. Quantification of excision repair cross-complementing group 1 and survival in p16-negative squamous cell head and neck cancers. Clin Cancer Res. 2013;19:6633–43. doi: 10.1158/1078-0432.CCR-13-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 40.Milas L, Fang FM, Mason KA, Valdecanas D, Hunter N, Koto M, et al. Importance of maintenance therapy in C225-induced enhancement of tumor control by fractionated radiation. Int J Radiat Oncol Biol Phys. 2007;67:568–72. doi: 10.1016/j.ijrobp.2006.09.044. [DOI] [PubMed] [Google Scholar]
- 41.Vermorken JB, Trigo J, Hitt R, Koralewski P, Diaz-Rubio E, Rolland F, et al. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy.[see comment] Journal of Clinical Oncology. 2007;25:2171–7. doi: 10.1200/JCO.2006.06.7447. [DOI] [PubMed] [Google Scholar]
- 42.Trigo J, Hitt R, Koralewski P, Diaz-Rubio E, Rolland F, Knecht R, et al. Cetuximab monotherapy is active in patients (pts) with platinum-refractory recurrent/metastatic squamous cell carcinoma of the head and neck (SCCHN): Results of a phase II study. Proc Am Soc Clin Oncol. 2004;22:A5502. [Google Scholar]
- 43.RTOG 0522: a randomized phase III trial of concurrent accelerated radiation and cisplatin versus concurrent accelerated radiation, cisplatin, and cetuximab [followed by surgery for selected patients] for Stage III and IV head and neck carcinomas. Clin Adv Hematol Oncol. 2007;5:79–81. [PubMed] [Google Scholar]
- 44.Ang KK, Zhang QE, Rosenthal DI, Nguyen-Tan P, Sherman EJ, Weber RS, et al. A randomized phase III trial (RTOG 0522) of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III-IV head and neck squamous cell carcinomas (HNC) J Clin Oncol. 2011;29 [Google Scholar]
- 45.Oncology ASoC. A randomized phase III trial (RTOG 0522) of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III-IV head and neck squamous cell carcinomas (HNC) ASCO 2011 Annual Meeting Library. 2011 [cited 2014 April 2]; Available from: http://meetinglibrary.asco.org/content/63118?media=vm.
- 46.Langer CJ. Exploring biomarkers in head and neck cancer. Cancer. 2012;118:3882–92. doi: 10.1002/cncr.26718. [DOI] [PubMed] [Google Scholar]
- 47.Stephen JK, Divine G, Chen KM, Chitale D, Havard S, Worsham MJ. Significance of p16 in Site-specific HPV Positive and HPV Negative Head and Neck Squamous Cell Carcinoma. Cancer Clin Oncol. 2013;2:51–61. doi: 10.5539/cco.v2n1p51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jordan RC, Lingen MW, Perez-Ordonez B, He X, Pickard R, Koluder M, et al. Validation of methods for oropharyngeal cancer HPV status determination in US cooperative group trials. Am J Surg Pathol. 2012;36:945–54. doi: 10.1097/PAS.0b013e318253a2d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.